Sustainable Adsorption of Antibiotics in Water: The Role of Biochar from Leather Tannery Waste and Sargassum Algae in Removing Ciprofloxacin and Sulfamethoxazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorbent Preparation
2.3. Characterization Techniques
2.4. Adsorption Experiments
2.4.1. Batch Adsorption
2.4.2. Adsorption Isotherm and Kinetic Study
2.4.3. Regeneration Test
3. Results and Discussion
3.1. Adsorbents Characterization
3.2. Adsorption Test
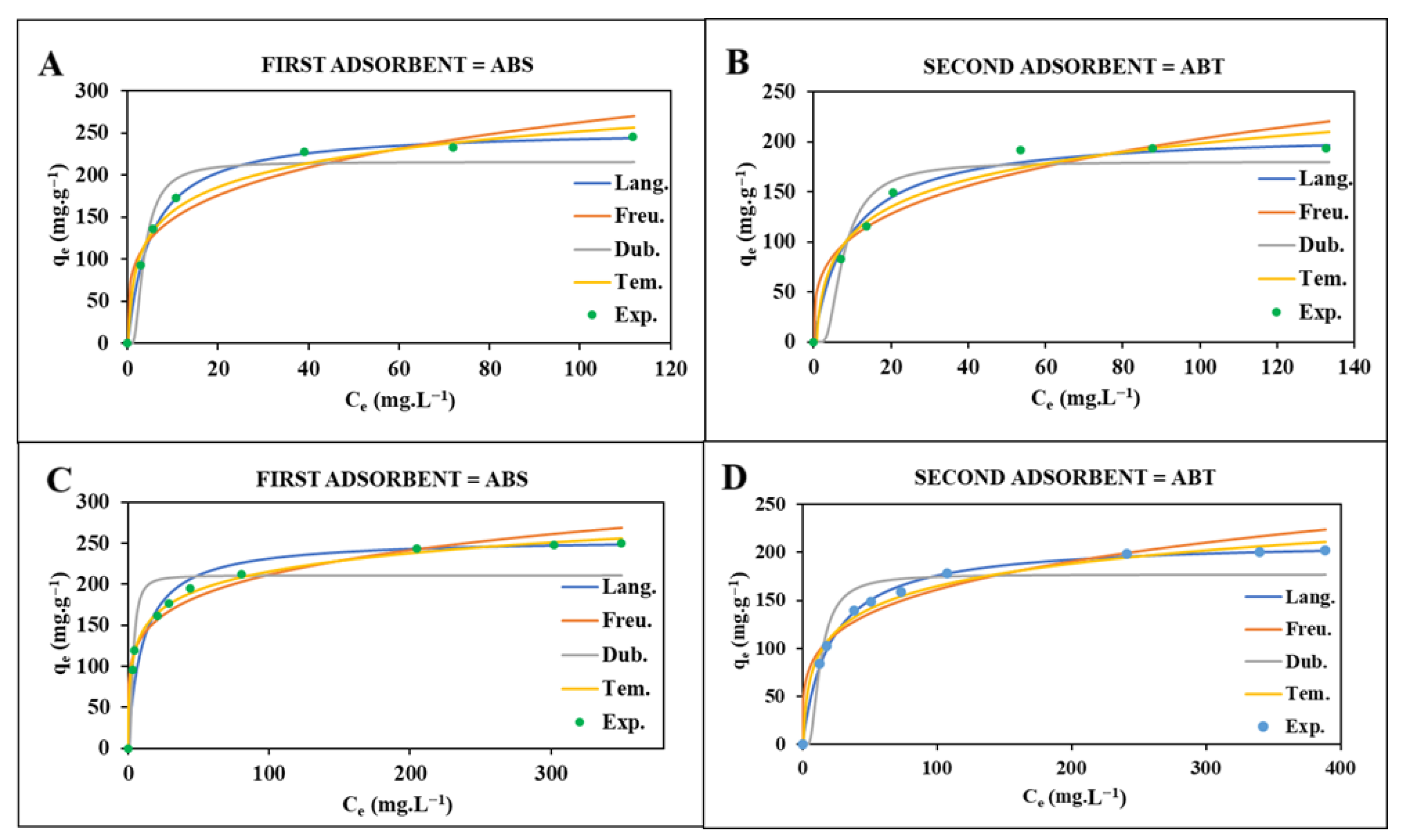
3.2.1. Adsorption Isotherm
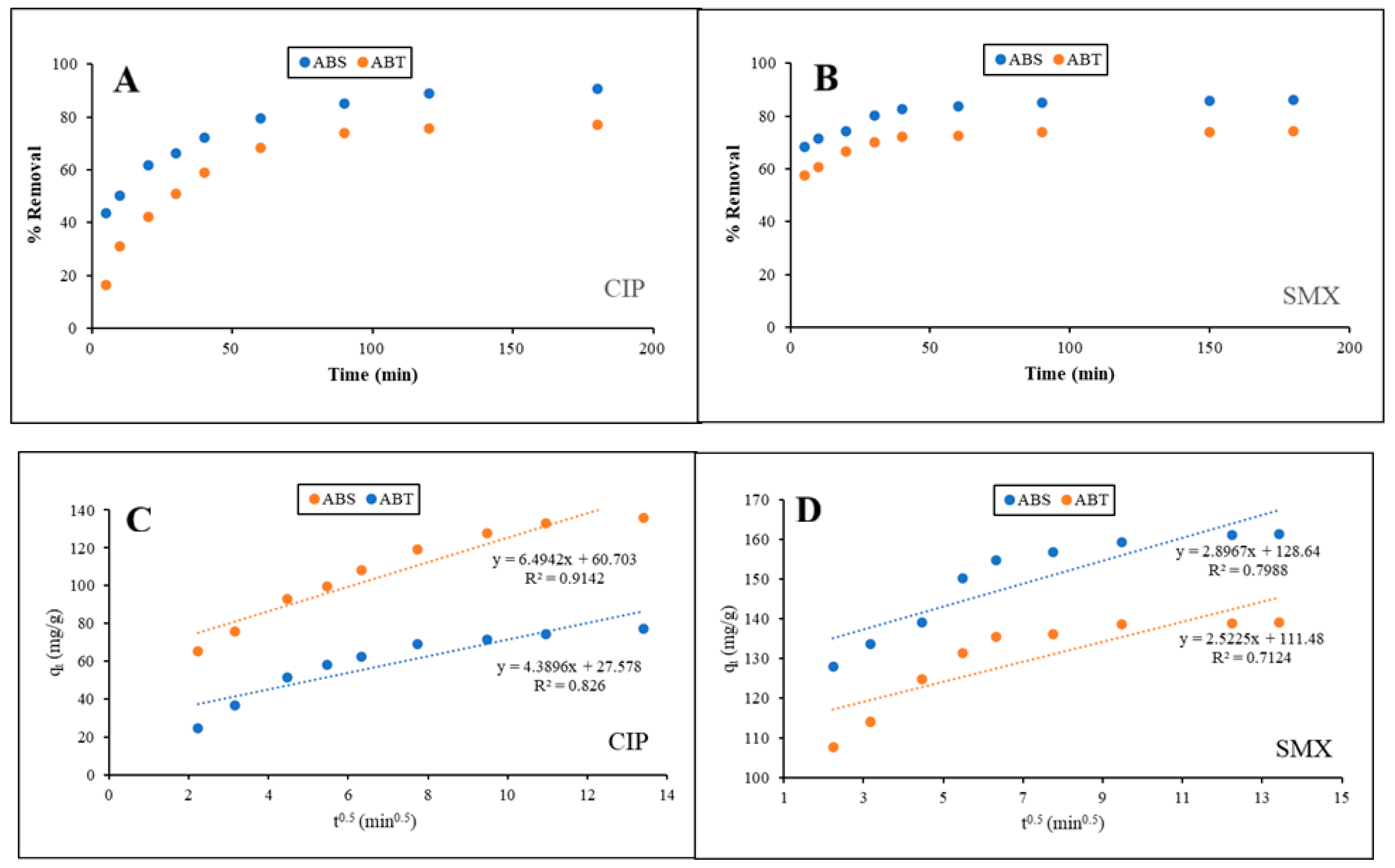
3.2.2. Adsorption Kinetics
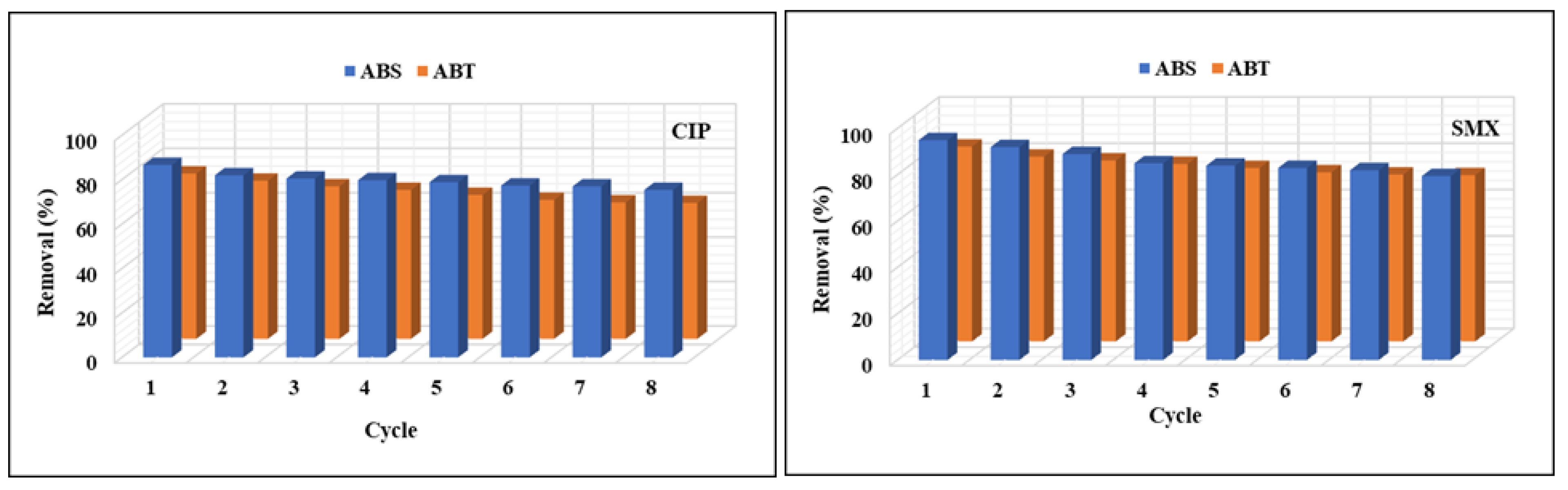
3.2.3. Regeneration/Life Span of Activated Biochar Adsorbent
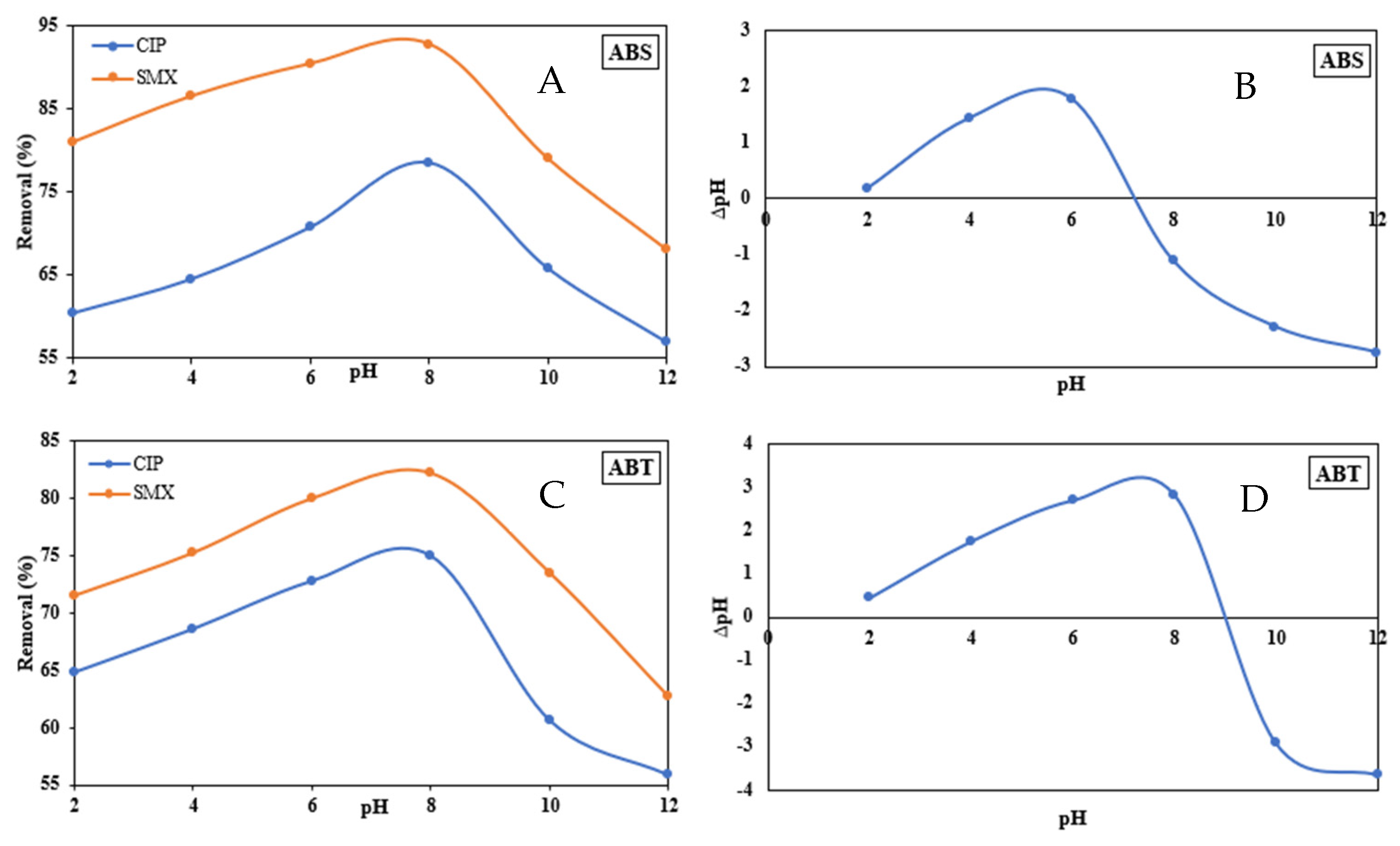
3.2.4. Effect of pH on Adsorption Capacity
3.2.5. Mechanistic Insights into the Superior Performance of ABS over ABT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, F.; Zhang, Q.; Jian, H.; Wang, C.; Xing, B.; Sun, H.; Hao, Y. Effect of Biochar-Derived Dissolved Organic Matter on Adsorption of Sulfamethoxazole and Chloramphenicol. J. Hazard. Mater. 2020, 396, 122598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, C.; Yang, Y.; Li, Z.; Qiu, X.; Gao, J.; Ji, M. Adsorption of Sulfamethoxazole on Polypyrrole Decorated Volcanics over a Wide PH Range: Mechanisms and Site Energy Distribution Consideration. Sep. Purif. Technol. 2022, 283, 120165. [Google Scholar] [CrossRef]
- Amir, M.; Fazal, T.; Iqbal, J.; Din, A.A.; Ahmed, A.; Ali, A.; Razzaq, A.; Ali, Z.; Rehman, M.S.U.; Park, Y.K. Integrated Adsorptive and Photocatalytic Degradation of Pharmaceutical Micropollutant, Ciprofloxacin Employing Biochar-ZnO Composite Photocatalysts. J. Ind. Eng. Chem. 2022, 115, 171–182. [Google Scholar] [CrossRef]
- Managaki, S.; Murata, A.; Takada, H.; Bui, C.T.; Chiem, N.H. Distribution of Macrolides, Sulfonamides, and Trimethoprim in Tropical Waters: Ubiquitous Occurrence of Veterinary Antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007, 41, 8004–8010. [Google Scholar] [CrossRef]
- Kafaei, R.; Papari, F.; Seyedabadi, M.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.A.; Asgari, G.; Ramavandi, B. Occurrence, Distribution, and Potential Sources of Antibiotics Pollution in the Water-Sediment of the Northern Coastline of the Persian Gulf, Iran. Sci. Total Environ. 2018, 627, 703–712. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mesdaghinia, A.; Hoseini, S.S.; Yunesian, M. Antibiotics in Urban Wastewater and Rivers of Tehran, Iran: Consumption, Mass Load, Occurrence, and Ecological Risk. Chemosphere 2019, 221, 55–66. [Google Scholar] [CrossRef]
- Dutta, J.; Mala, A.A. Removal of Antibiotic from the Water Environment by the Adsorption Technologies: A Review. Water Sci. Technol. 2020, 82, 401–426. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, S.; Xi, C.; Li, X.; Zhang, L.; Wang, G.; Chen, Z. A Novel Fe3O4/Graphene Oxide/Citrus Peel-Derived Bio-Char Based Nanocomposite with Enhanced Adsorption Affinity and Sensitivity of Ciprofloxacin and Sparfloxacin. Bioresour. Technol. 2019, 292, 121951. [Google Scholar] [CrossRef]
- Diao, Z.H.; Xu, X.R.; Jiang, D.; Liu, J.J.; Kong, L.J.; Li, G.; Zuo, L.Z.; Wu, Q.H. Simultaneous Photocatalytic Cr(VI) Reduction and Ciprofloxacin Oxidation over TiO2/Fe0 Composite under Aerobic Conditions: Performance, Durability, Pathway and Mechanism. J. Chem. Eng. 2017, 315, 167–176. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, D.; Hu, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Hu, Y. Fates of Hemicellulose, Lignin and Cellulose in Concentrated Phosphoric Acid with Hydrogen Peroxide (PHP) Pretreatment. RSC Adv. 2018, 8, 12714–12723. [Google Scholar] [CrossRef]
- Zhang, C.L.; Qiao, G.L.; Zhao, F.; Wang, Y. Thermodynamic and Kinetic Parameters of Ciprofloxacin Adsorption onto Modified Coal Fly Ash from Aqueous Solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- Alonso, J.J.S.; El Kori, N.; Melián-Martel, N.; Del Río-Gamero, B. Removal of Ciprofloxacin from Seawater by Reverse Osmosis. J. Environ. Manag. 2018, 217, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of Pharmaceutical from Water with an Electrocoagulation Process; Effect of Various Parameters and Studies of Isotherm and Kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; McArdell, C.S. Hospital Wastewater Treatment by Membrane Bioreactor: Performance and Efficiency for Organic Micropollutant Elimination. Environ. Sci. Technol. 2012, 46, 1536–1545. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial Wastes as Low-Cost Potential Adsorbents for the Treatment of Wastewater Laden with Heavy Metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The Utilization of Leaf-Based Adsorbents for Dyes Removal: A Review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Pamphile, N.; Xuejiao, L.; Guangwei, Y.; Yin, W. Synthesis of a Novel Core-Shell-Structure Activated Carbon Material and Its Application in Sulfamethoxazole Adsorption. J. Hazard. Mater. 2019, 368, 602–612. [Google Scholar] [CrossRef]
- Anjum, H.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Investigation of Green Functionalization of Multiwall Carbon Nanotubes and Its Application in Adsorption of Benzene, Toluene & p-Xylene from Aqueous Solution. J. Clean. Prod. 2019, 221, 323–338. [Google Scholar] [CrossRef]
- Weng, X.; Lin, Z.; Xiao, X.; Li, C.; Chen, Z. One-Step Biosynthesis of Hybrid Reduced Graphene Oxide/Iron-Based Nanoparticles by Eucalyptus Extract and Its Removal of Dye. J. Clean. Prod. 2018, 203, 22–29. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Wang, Y. Ciprofloxacin Adsorption by Biochar Derived from Co-Pyrolysis of Sewage Sludge and Bamboo Waste. Environ. Sci. Pollut. Res. 2020, 27, 22806–22817. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-Modified Biochar for Simultaneous Removal of Ammonium, Nitrate, and Phosphate from Eutrophic Water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, N.; Fang, Z. In Situ Remediation of Hexavalent Chromium Contaminated Soil by CMC-Stabilized Nanoscale Zero-Valent Iron Composited with Biochar. Water Sci. Technol. 2018, 77, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar Farming: Defining Economically Perspective Applications. Clean Technol. Environ. Policy 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The Mechanism of Cadmium Sorption by Sulphur-Modified Wheat Straw Biochar and Its Application Cadmium-Contaminated Soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Taghavi, S.; Norouzi, O.; Tavasoli, A.; Di Maria, F.; Signoretto, M.; Menegazzo, F.; Di Michele, A. Catalytic Conversion of Venice Lagoon Brown Marine Algae for Producing Hydrogen-Rich Gas and Valuable Biochemical Using Algal Biochar and Ni/SBA-15 Catalyst. Int. J. Hydrogen Energy 2018, 43, 19918–19929. [Google Scholar] [CrossRef]
- Jafarian, S.; Bolouk, A.M.L.; Norouzian, R.S.; Taghavi, S.; Mousavi, F.; Kianpour, E.; Signoretto, M. Sargassum Macro-Algae-Derived Activated Bio-Char as a Sustainable and Cost-Effective Adsorbent for Cationic Dyes: A Joint Experimental and DFT Study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132397. [Google Scholar] [CrossRef]
- Salimi, P.; Tieuli, S.; Taghavi, S.; Venezia, E.; Fugattini, S.; Lauciello, S.; Prato, M.; Marras, S.; Li, T.; Signoretto, M.; et al. Sustainable Lithium-Ion Batteries Based on Metal-Free Tannery Waste Biochar. Green Chem. 2022, 24, 4119–4129. [Google Scholar] [CrossRef]
- Bagheri, A.; Taghavi, S.; Bellani, S.; Salimi, P.; Beydaghi, H.; Panda, J.K.; Isabella Zappia, M.; Mastronardi, V.; Gamberini, A.; Balkrishna Thorat, S.; et al. Venice’s Macroalgae-Derived Active Material for Aqueous, Organic, and Solid-State Supercapacitors. J. Chem. Eng. 2024, 496, 153529. [Google Scholar] [CrossRef]
- Taghavi, S.; Ghedini, E.; Peurla, M.; Cruciani, G.; Menegazzo, F.; Murzin, D.Y.; Signoretto, M. Activated Biochars as Sustainable and Effective Supports for Hydrogenations. Carbon Trends 2023, 13, 100316. [Google Scholar] [CrossRef]
- Longo, L.; Taghavi, S.; Ghedini, E.; Menegazzo, F.; Di Michele, A.; Cruciani, G.; Signoretto, M. Selective Hydrogenation of 5-Hydroxymethylfurfural to 1-Hydroxy-2,5-Hexanedione by Biochar-Supported Ru Catalysts. ChemSusChem 2022, 15, e202200437. [Google Scholar] [CrossRef]
- Longo, L.; Taghavi, S.; Riello, M.; Ghedini, E.; Menegazzo, F.; Di Michele, A.; Cruciani, G.; Signoretto, M. Waste Biomasses as Precursors of Catalytic Supports in Benzaldehyde Hydrogenation. Catal. Today 2023, 420, 114038. [Google Scholar] [CrossRef]
- Ashebir, H.; Nure, J.F.; Worku, A.; Msagati, T.A.M. Prosopis Juliflora Biochar for Adsorption of Sulfamethoxazole and Ciprofloxacin from Pharmaceutical Wastewater. Desalination Water Treat. 2024, 320, 100691. [Google Scholar] [CrossRef]
- Hou, J.; Bao, W.; Zhang, J.; Yu, J.; Chen, L.; Di, G.; Zhou, Q.; Li, X. Characteristics and Mechanisms of Sulfamethoxazole Adsorption onto Modified Biochars with Hierarchical Pore Structures: Batch, Predictions Using Artificial Neural Network and Fixed Bed Column Studies. J. Water Process Eng. 2023, 54, 103975. [Google Scholar] [CrossRef]
- Wang, C.; Kong, L.; Wang, Y.; Cui, X.; Li, N.; Yan, B.; Chen, G. New Insight into the Synergy of Nitrogen-Related Sites on Biochar Surface for Sulfamethoxazole Adsorption from Water. Chin. Chem. Lett. 2023, 34, 108159. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Truong, Q.M.; Chen, C.W.; Chen, W.H.; Dong, C.D. Pyrolysis of Marine Algae for Biochar Production for Adsorption of Ciprofloxacin from Aqueous Solutions. Bioresour. Technol. 2022, 351, 127043. [Google Scholar] [CrossRef]
- ASTM D7582-15; Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM: West Conshohocken, PA, USA, 2015. [CrossRef]
- Angin, D. Effect of Pyrolysis Temperature and Heating Rate on Biochar Obtained from Pyrolysis of Safflower Seed Press Cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Thue, P.S.; Lima, D.R.; Lima, E.C.; Teixeira, R.A.; Dos Reis, G.S.; Dias, S.L.P.; Machado, F.M. Comparative Studies of Physicochemical and Adsorptive Properties of Biochar Materials from Biomass Using Different Zinc Salts as Activating Agents. J. Environ. Chem. Eng. 2022, 10, 107632. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Wang, W.; Wang, Y.; Li, G.; Hu, C. Pyrolysis of High-Ash Natural Microalgae from Water Blooms: Effects of Acid Pretreatment. Toxins 2021, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ji, L.; Cai, L.; Lu, S.; Li, R.; He, Q.; Liu, J.; Yang, H. Multiple Defects Algal Biochar Derived from Ulva Lactuca with Enhanced Adsorption Performance for Ciprofloxacin. J. Mol. Liq. 2025, 421, 126857. [Google Scholar] [CrossRef]
- Che, H.; Wei, G.; Fan, Z.; Zhu, Y.; Zhang, L.; Wei, Z.; Huang, X.; Wei, L. Super Facile One-Step Synthesis of Sugarcane Bagasse Derived N-Doped Porous Biochar for Adsorption of Ciprofloxacin. J. Environ. Manag. 2023, 335, 117566. [Google Scholar] [CrossRef]
- de la Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating Physical and Chemical Properties of Four Different Biochars and Their Application Rate to Biomass Production of Lolium Perenne on a Calcic Cambisol during a Pot Experiment of 79 Days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef]
- Ma, B.; Huang, Y.; Nie, Z.; Qiu, X.; Su, D.; Wang, G.; Yuan, J.; Xie, X.; Wu, Z. Facile Synthesis of Camellia Oleifera Shell-Derived Hard Carbon as an Anode Material for Lithium-Ion Batteries. RSC Adv. 2019, 9, 20424–20431. [Google Scholar] [CrossRef]
- Zhong, Y.; Deng, Q.; Zhang, P.; Wang, J.; Wang, R.; Zeng, Z.; Deng, S. Sulfonic Acid Functionalized Hydrophobic Mesoporous Biochar: Design, Preparation and Acid-Catalytic Properties. Fuel 2019, 240, 270–277. [Google Scholar] [CrossRef]
- da Luz Corrêa, A.P.; Bastos, R.R.C.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Preparation of Sulfonated Carbon-Based Catalysts from Murumuru Kernel Shell and Their Performance in the Esterification Reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of Biochar Formation from Various Agricultural By-Products Using FTIR Spectroscopy. Mod. Appl. Sci. 2015, 9, 246. [Google Scholar] [CrossRef]
- Alfattani, R.; Shah, M.A.; Siddiqui, M.I.H.; Ali, M.A.; Alnaser, I.A. Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel. Energies 2021, 15, 1. [Google Scholar] [CrossRef]
- Kaya, A.N.; Yildiz, Z.; Ceylan, S. Preparation and Characterisation of Biochar from Hazelnut Shell and Its Adsorption Properties for Methylene Blue Dye. Politek. Derg. 2018, 21, 765–776. [Google Scholar] [CrossRef]
- Koutenaei, S.S.; Vatankhah, G.; Esmaeili, H. Ziziphus Spina-Christi Leaves Biochar Decorated with Fe3O4 and SDS for Sorption of Chromium (III) from Aqueous Solution. Biomass Convers. Biorefin. 2024, 14, 10251–10264. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and Equilibrium Models of Adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Carbon Nanostructures; Springer: Cham, Switzerland, 2015; pp. 33–69. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball Milled Biochar Effectively Removes Sulfamethoxazole and Sulfapyridine Antibiotics from Water and Wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Liu, Y.; Liu, W.; Shen, Y. Generation of Microbial Protein Feed (MPF) from Waste and Its Application in Aquaculture in China. J. Environ. Chem. Eng. 2023, 11, 109297. [Google Scholar] [CrossRef]
- Puccia, V.; Avena, M.J. On the Use of the Dubinin-Radushkevich Equation to Distinguish between Physical and Chemical Adsorption at the Solid-Water Interface. Colloid Interface Sci. Commun. 2021, 41, 100376. [Google Scholar] [CrossRef]
- Hamadeen, H.M.; Elkhatib, E.A. New Nanostructured Activated Biochar for Effective Removal of Antibiotic Ciprofloxacin from Wastewater: Adsorption Dynamics and Mechanisms. Environ. Res. 2022, 210, 112929. [Google Scholar] [CrossRef]
- Arif, M.; Liu, G.; ur Rehman, M.Z.; Yousaf, B.; Ahmed, R.; Mian, M.M.; Ashraf, A.; Mujtaba Munir, M.A.; Rashid, M.S.; Naeem, A. Carbon Dioxide Activated Biochar-Clay Mineral Composite Efficiently Removes Ciprofloxacin from Contaminated Water—Reveals an Incubation Study. J. Clean. Prod. 2022, 332, 130079. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, F.; Liu, W.; Ma, L. Adsorption Behavior of Graphite-like Walnut Shell Biochar Modified with Ammonia for Ciprofloxacin in Aqueous Solution. J. Chem. Technol. Biotechnol. 2025, 100, 90–103. [Google Scholar] [CrossRef]
- Zhao, T.; Ali, A.; Su, J.; Liu, S.; Yan, H.; Xu, L. Removal of Sulfamethoxazole from Water by Biosurfactant-Modified Sludge Biochar: Properties and Mechanism. J. Environ. Chem. Eng. 2024, 12, 114200. [Google Scholar] [CrossRef]
- Fakhri, A.; Latifi, H.; Samani, K.M.; Fassnacht, F.E. Introducing a Computationally Light Approach to Estimate Forest Height and Fractional Canopy Cover from Sentinel-2 Data. J. Arid Environ. 2025, 228, 105343. [Google Scholar] [CrossRef]
- Le Na, P.T.; Tuyen, N.D.K.; Dang, B.T. Sorption of Four Antibiotics onto Pristine Biochar Derived from Macadamia Nutshell. Bioresour. Technol. 2024, 394, 130281. [Google Scholar] [CrossRef]
- Huang, B.; Huang, D.; Zheng, Q.; Yan, C.; Feng, J.; Gao, H.; Fu, H.; Liao, Y. Enhanced Adsorption Capacity of Tetracycline on Porous Graphitic Biochar with an Ultra-Large Surface Area. RSC Adv. 2023, 13, 10397–10407. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Zanella, R.; Kowenje, C.O.; Donato, F.F.; Bandeira, N.M.G.; Prestes, O.D. Single and Binary Adsorption of Sulfonamide Antibiotics onto Iron-Modified Clay: Linear and Nonlinear Isotherms, Kinetics, Thermodynamics, and Mechanistic Studies. Appl. Water Sci. 2018, 8, 175. [Google Scholar] [CrossRef]
- Yong, J.Y.; Xie, R.Y.; Huang, Q.; Zhang, X.J.; Li, B.; Xie, P.F.; Wu, C.F.; Jiang, L. Diamine-Appended Metal-Organic Framework for Carbon Capture from Wet Flue Gas: Characteristics and Mechanism. Sep. Purif. Technol. 2024, 328, 125018. [Google Scholar] [CrossRef]
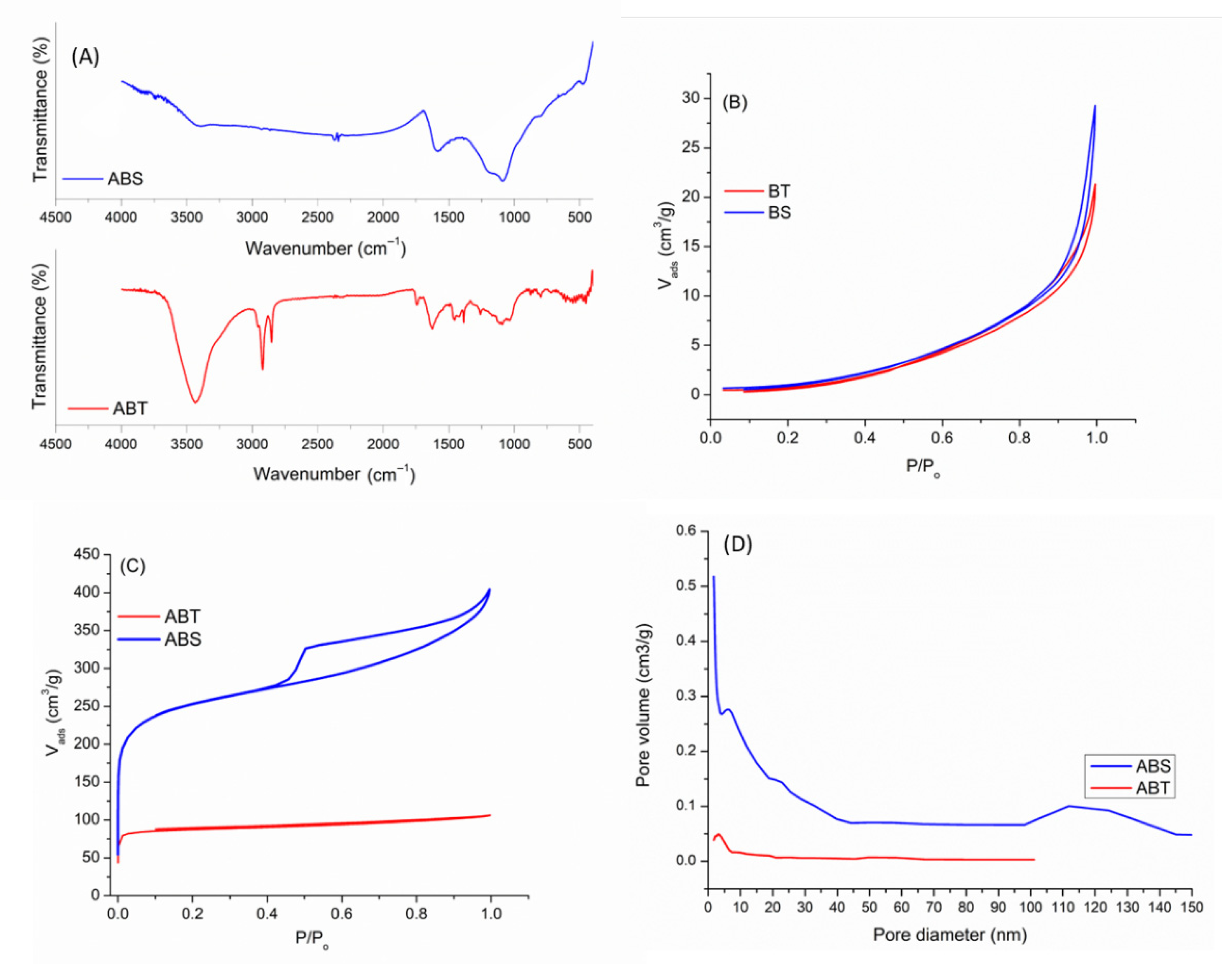
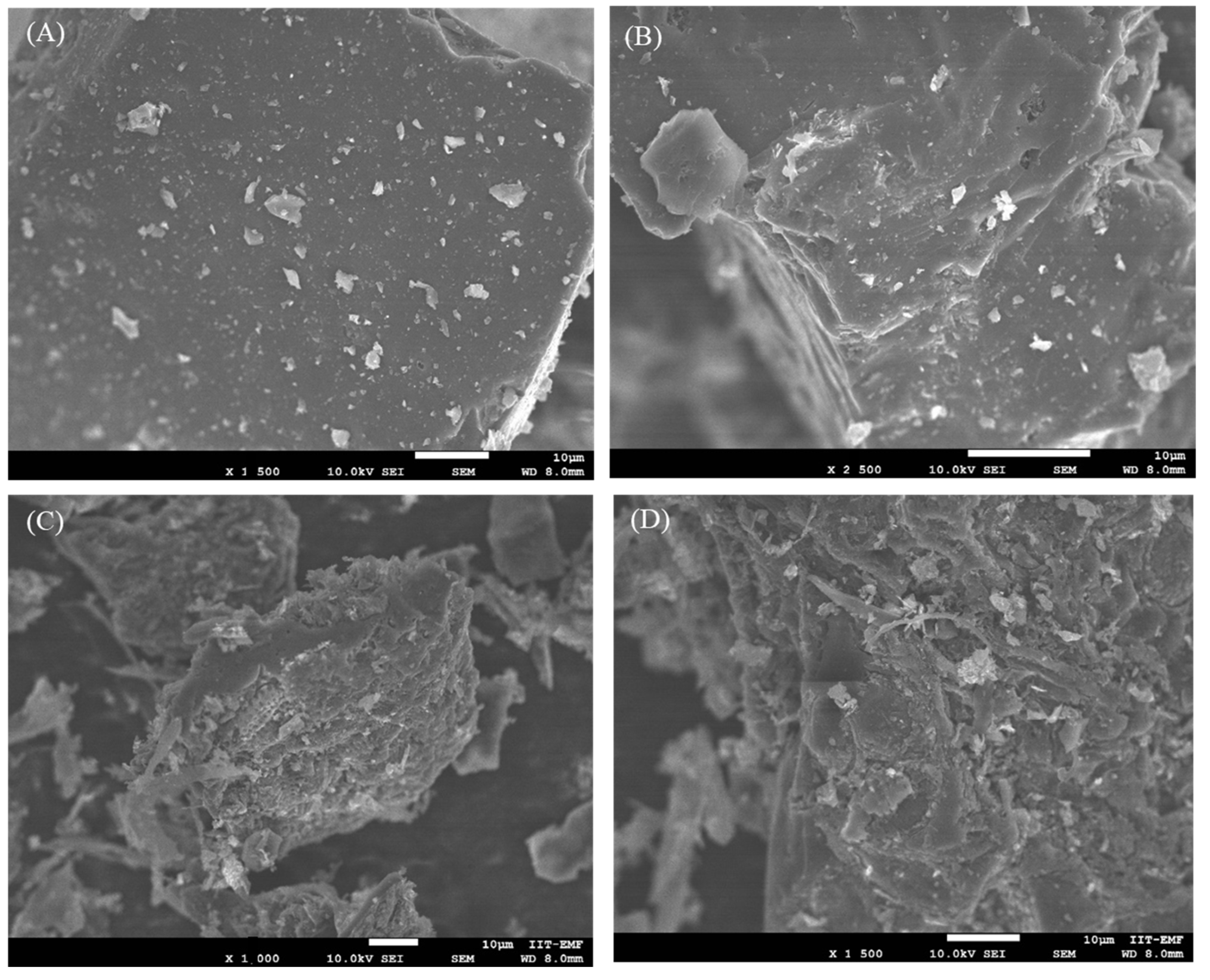
| Sample | C [%] (±0.03) | H [%] (±0.01) | N [%] (±0.02) | S [%] (±0.01) | O [%] | Ash [%] | H/C |
|---|---|---|---|---|---|---|---|
| T | 44.6 | 6.3 | 11.5 | 2.5 | N.A a | N.A | 0.14 |
| BT | 73.6 | 2.7 | 12.4 | 0.7 | 6.1 | 4.5 | 0.04 |
| ABT | 76.6 | 1.1 | 9.4 | 0.6 | 9.8 | 2.5 | 0.01 |
| S | 37.1 | 5.3 | 4.8 | 1.3 | N.A | N.A | 0.14 |
| BS | 43.4 | 1.2 | 3.6 | 2.0 | N.A | N.A | 0.03 |
| ABS | 67.1 | 1.2 | 6.8 | 2.8 | 7.9 | 14.2 | 0.02 |
| Sample | SLangmuir a (m2g−1) (±5) | Smicro b (m2g−1) (±5) | Vtot c (cm3g−1) | Vmicro d (cm3g−1) |
|---|---|---|---|---|
| ABT | 412 | 356 | 0.16 | 0.10 |
| ABS | 1305 | 905 | 0.56 | 0.17 |
| Drug | Isotherm Models | |||||||
|---|---|---|---|---|---|---|---|---|
| CIP | Lang. | Freu. | Dubin. | Tem. | ||||
| ABS | ABT | ABS | ABT | ABS | ABT | ABS | ABT | |
| qm = 256.41 | qm = 210.13 | Kf = 83.56 | Kf = 54.20 | qm = 216.37 | qm = 216.37 | A = 4.63 | A = 1.52 | |
| KL = 0.1940 | KL = 0.1096 | n = 4.02 | n = 3.49 | Kd = 2 × 10−6 | Kd = 8 × 10−6 | B = 60.43 | B = 62.82 | |
| R2 = 0.9996 | R2 = 0.9977 | R2 = 0.9088 | R2 = 0.8835 | E = 0.5 | E = 0.25 | R2 = 0.9621 | R2 = 0.9621 | |
| R2 = 0.8834 | R2 = 0.8851 | |||||||
| SMX | Lang. | Freu. | Dubin. | Tem. | ||||
| ABS | ABT | ABS | ABT | ABS | ABT | ABS | ABT | |
| qm = 256.46 | qm = 213.00 | Kf = 88.50 | Kf = 53.09 | qm = 211.03 | qm = 177.1506 | A = 7.84 | A = 1.27 | |
| KL = 0.0938 | KL = 0.0479 | n = 5.22 | n = 4.17 | Kd = 2 × 10−6 | Kd = 2.3 × 10−6 | B = 76.51 | B = 72.68 | |
| R2 = 0.9992 | R2 = 0.9998 | R2 = 0.9557 | R2 = 0.9160 | E = 0.5 | E = 0.46 | R2 = 0.9896 | R2 = 0.9666 | |
| R2 = 0.8135 | R2 = 0.8507 | |||||||
| Adsorbent | Feedstock | Activation Method | qm (CIP, mg/g) | qm (SMX, mg/g) | Reference |
|---|---|---|---|---|---|
| ABT | Leather tannery waste | CO2 physical | 210.13 | 213.00 | This work |
| ABS | Sargassum algae | CO2 physical | 256.41 | 256.46 | This work |
| PPAB | Palm kernel shell | NaOH chemical | 142.86 | - | [61] |
| AMBC-350 | Sewage sludge-clay | CO2 physical | 50.32 | - | [62] |
| Graphite-like walnut shell BC | Walnut shell | KOH chemical | 158.14 | - | [63] |
| Ball-milled BC | Hickory wood | Ball milling (physical) | - | 100.30 | [58] |
| Biosurfactant-modified sludge BC | Sludge | Biosurfactant amendment | - | 43.61 | [64] |
| Steam-activated BCA850 | Bamboo | Steam physical | - | 204.07 | [65] |
| Drug | Kinetic Model | |||||
|---|---|---|---|---|---|---|
| CIP | Pseudo-first order | Pseudo-second order | Intraparticle diffusion model | |||
| ABS | ABT | ABS | ABT | ABS | ABT | |
| Kad = 0.0604 | Kad = 0.0377 | K2 = 0.0007 | K2 = 0.0004 | Kp = 6.494 | Kp = 4.39 | |
| qe cal = 267.73 | qe cal = 122.24 | qe cal = 143.08 | qe cal = 130.22 | C = 60.7 | C = 27.58 | |
| R2 = 0.8176 | R2 = 0.9808 | R2 = 0.9985 | R2 = 0.9983 | R2 = 0.9142 | R2 = 0.8260 | |
| SMX | Pseudo-first order | Pseudo-second order | Intraparticle diffusion model | |||
| ABS | ABT | ABS | ABT | ABS | ABT | |
| Kad = 0.0479 | Kad = 0.0479 | K2 = 0.0025 | K2 = 0.0036 | Kp = 2.8970 | Kp = 2.5220 | |
| qe cal = 60.22 | qe cal = 39.05 | qe cal = 163.61 | qe cal = 140.80 | C = 128.60 | C = 111.50 | |
| R2 = 0.8658 | R2 = 0.8989 | R2 = 0.9999 | R2 = 1.0000 | R2 = 0.7988 | R2 = 0.7124 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Jafarian, S.; Taghavi, S.; Lashkar Bolouk, A.M.; Signoretto, M. Sustainable Adsorption of Antibiotics in Water: The Role of Biochar from Leather Tannery Waste and Sargassum Algae in Removing Ciprofloxacin and Sulfamethoxazole. Sustainability 2026, 18, 280. https://doi.org/10.3390/su18010280
Jafarian S, Taghavi S, Lashkar Bolouk AM, Signoretto M. Sustainable Adsorption of Antibiotics in Water: The Role of Biochar from Leather Tannery Waste and Sargassum Algae in Removing Ciprofloxacin and Sulfamethoxazole. Sustainability. 2026; 18(1):280. https://doi.org/10.3390/su18010280
Chicago/Turabian StyleJafarian, Sajedeh, Somayeh Taghavi, Amir Mohammad Lashkar Bolouk, and Michela Signoretto. 2026. "Sustainable Adsorption of Antibiotics in Water: The Role of Biochar from Leather Tannery Waste and Sargassum Algae in Removing Ciprofloxacin and Sulfamethoxazole" Sustainability 18, no. 1: 280. https://doi.org/10.3390/su18010280
APA StyleJafarian, S., Taghavi, S., Lashkar Bolouk, A. M., & Signoretto, M. (2026). Sustainable Adsorption of Antibiotics in Water: The Role of Biochar from Leather Tannery Waste and Sargassum Algae in Removing Ciprofloxacin and Sulfamethoxazole. Sustainability, 18(1), 280. https://doi.org/10.3390/su18010280









