From River to Reservoir: The Impact of Environmental Variables on Zooplankton Assemblages in Karst Ecosystems
Abstract
1. Introduction
2. Materials and Methods
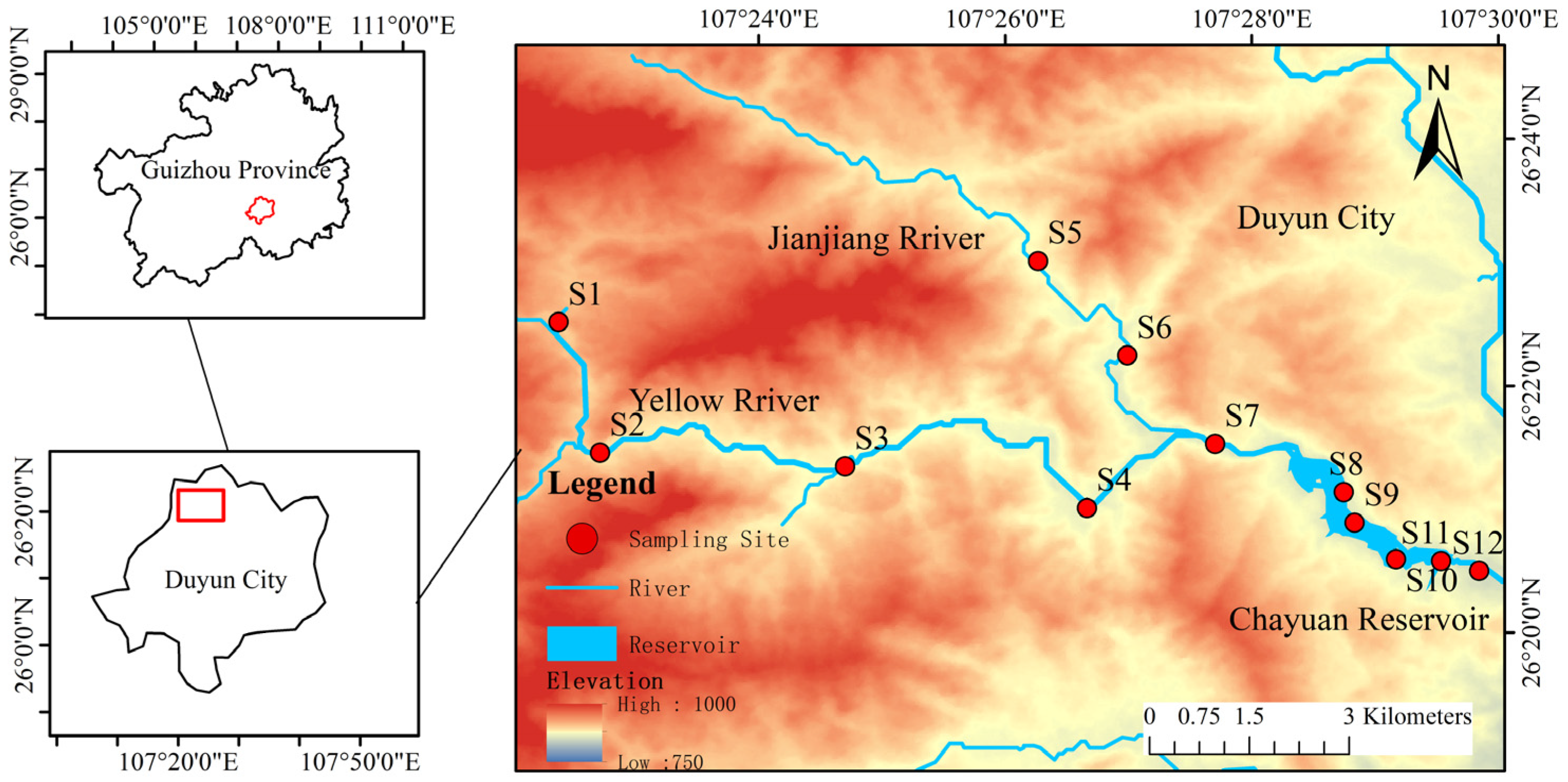
2.1. Study Area
2.2. Sampling
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Aquatic Environmental Variables
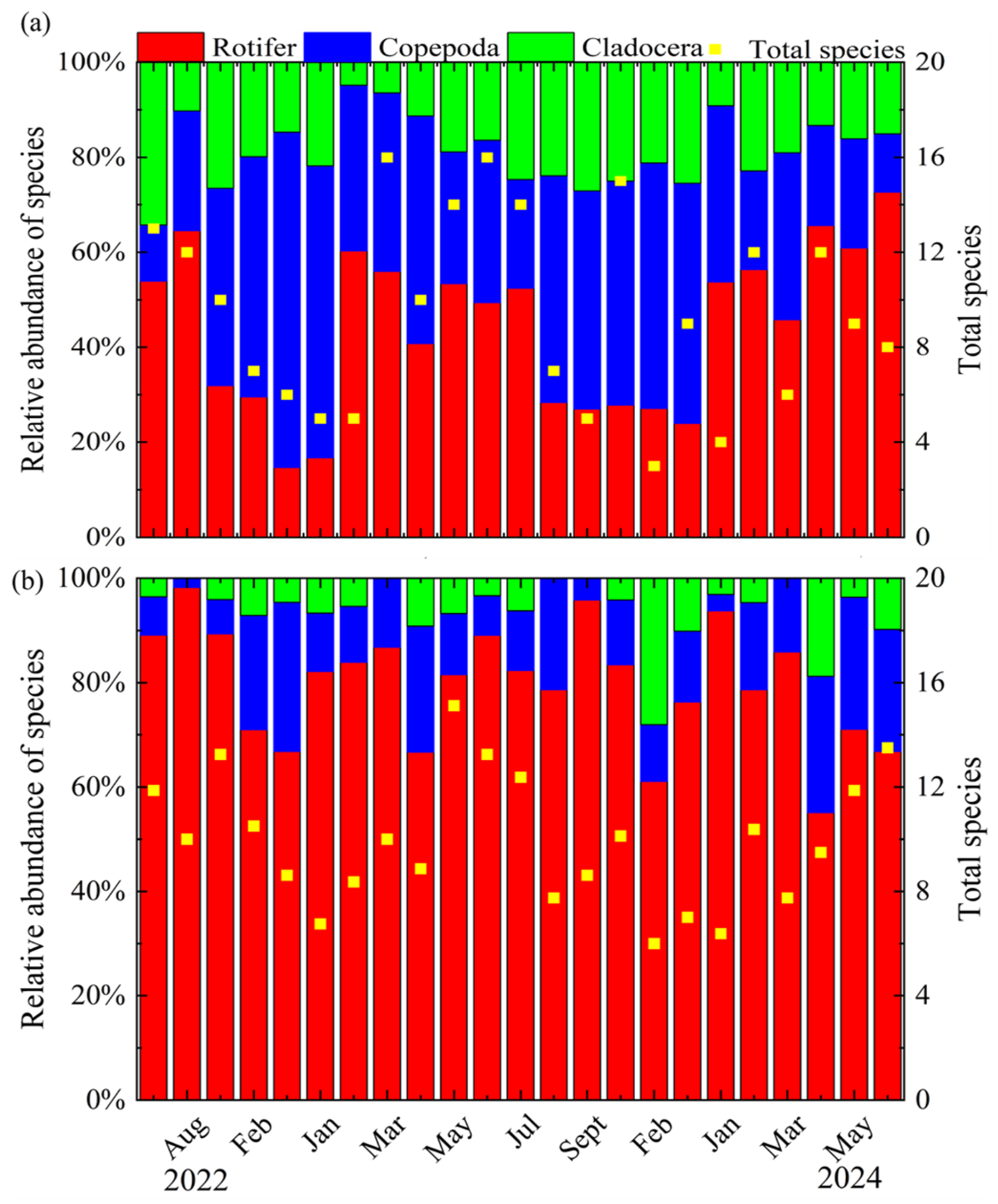
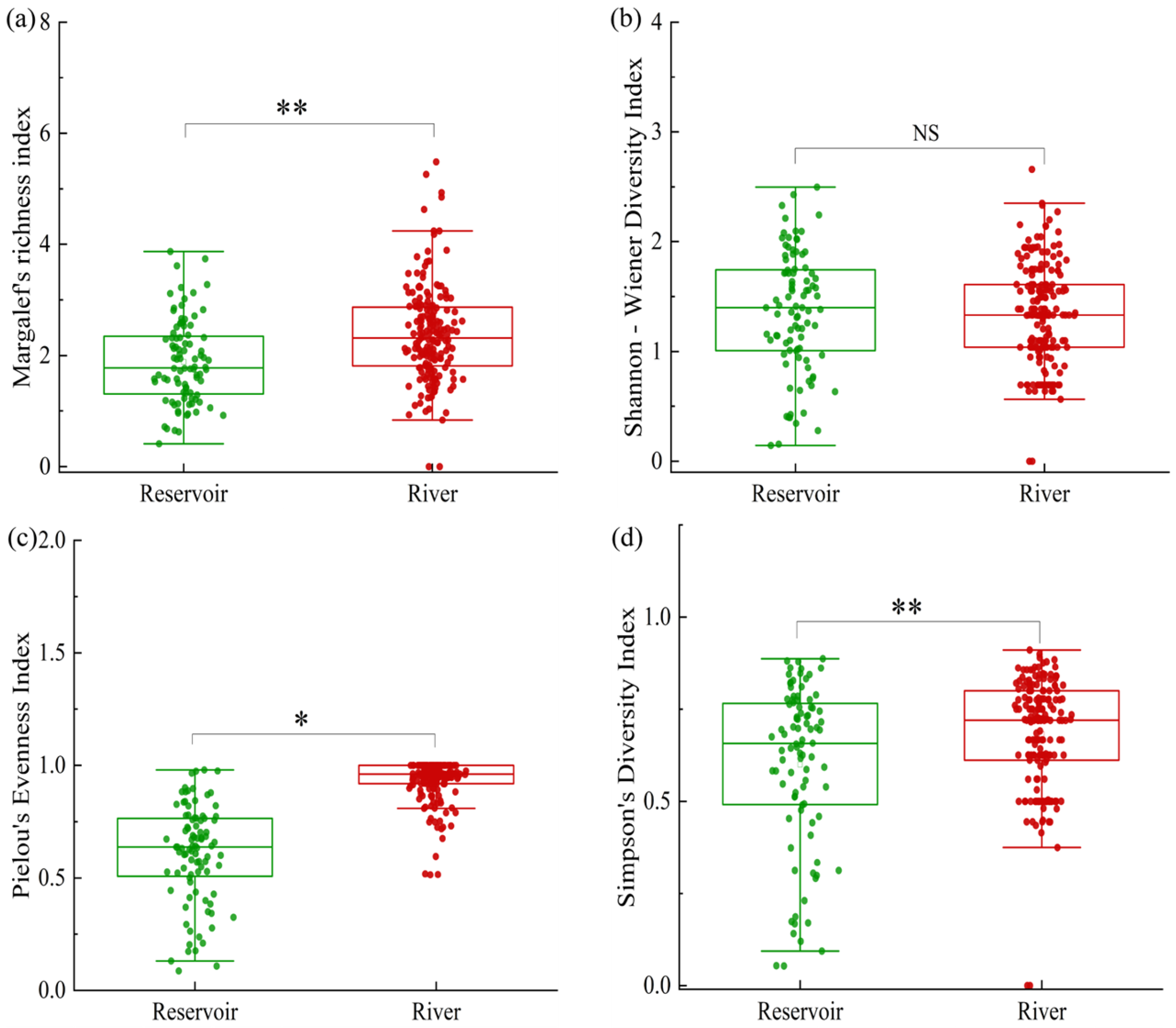
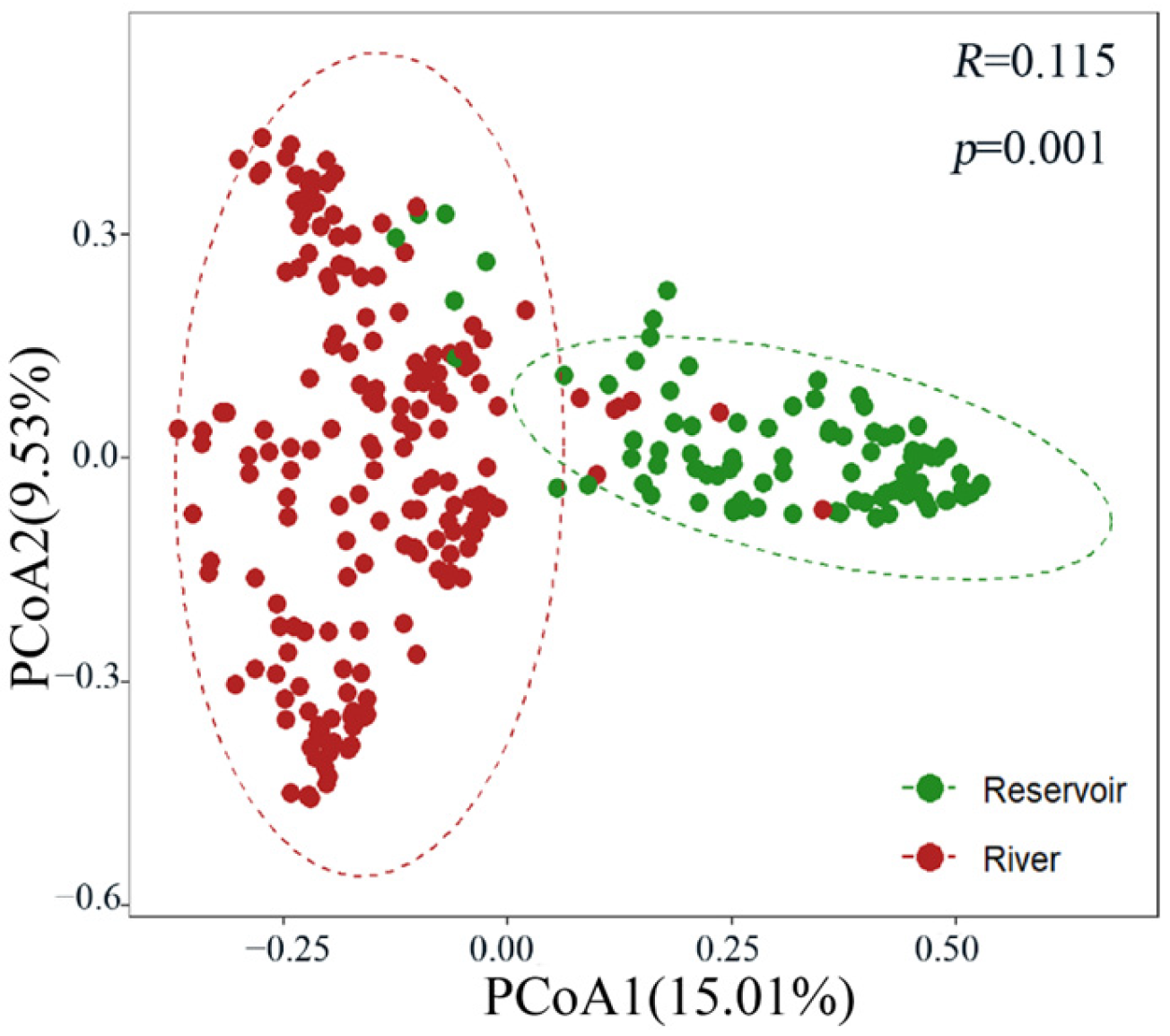
3.2. Community Structure and Diversity of the Zooplankton
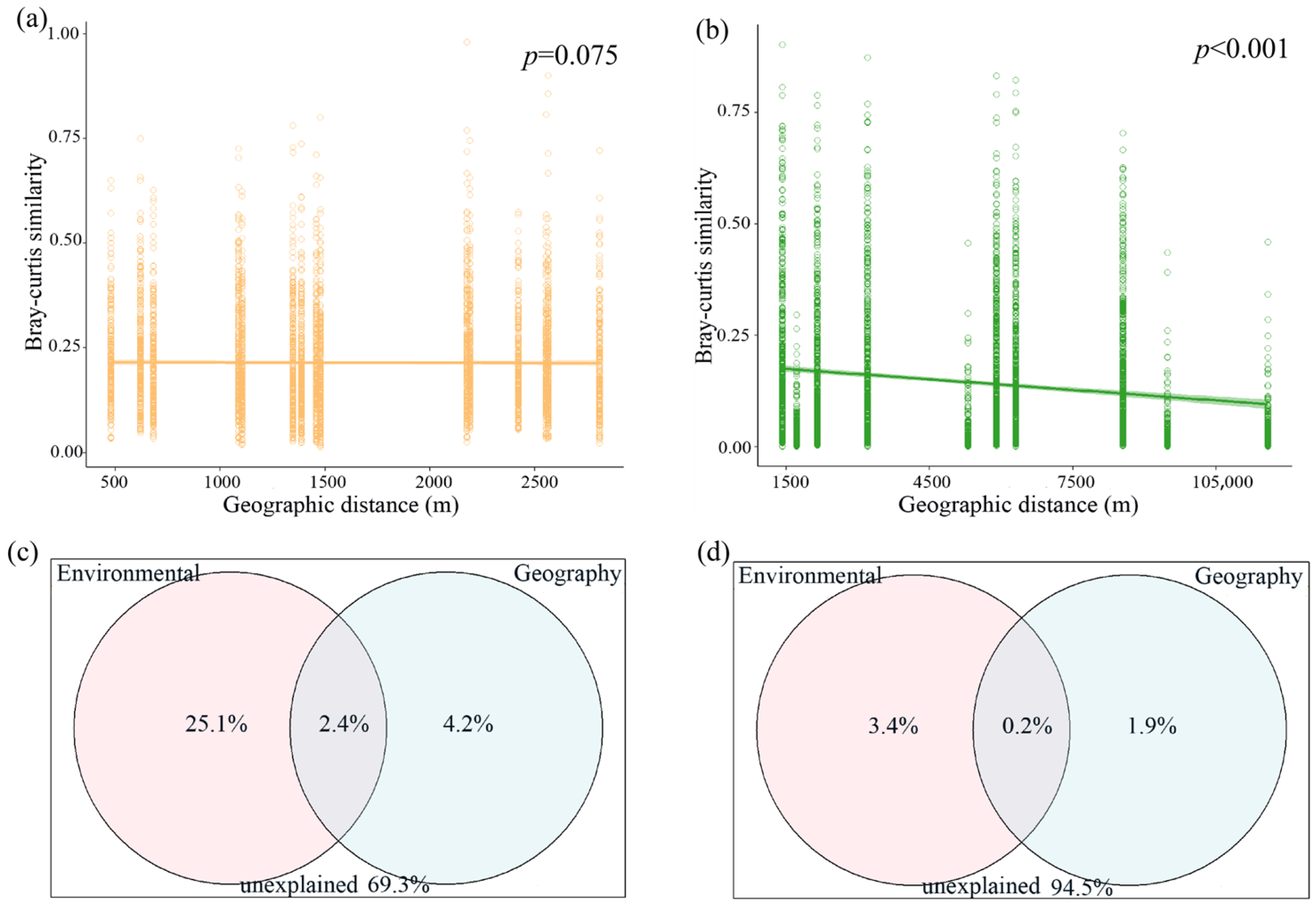
3.3. Changes in Community Assembly Process
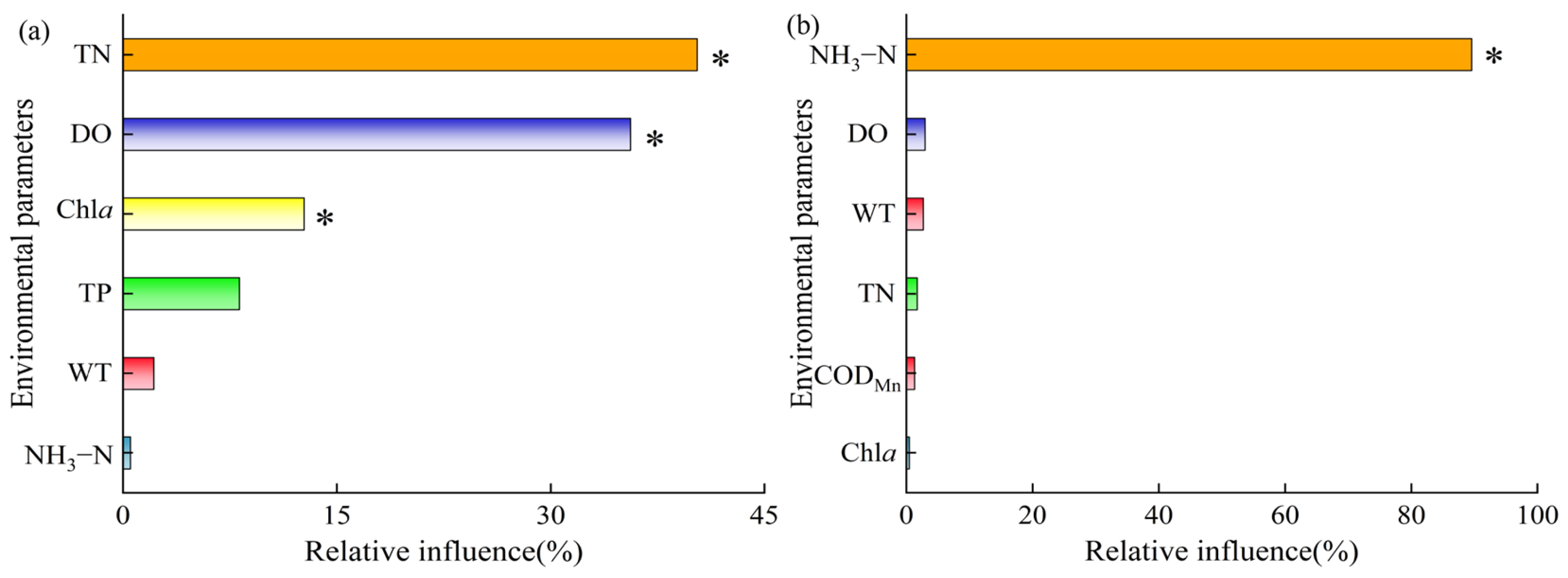
3.4. Responses of Zooplankton Community Assembly to Environmental Variables
4. Discussion
4.1. Composition and Diversity of Zooplankton Communities
4.2. Dynamics of Zooplankton Community Assembly Processes
4.3. Environmental Drivers of Zooplankton Community Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.P.; Sharma, A.; Sharma, A.K. Impact of fear effect on plankton-fish system dynamics incorporating zooplankton refuge. Chaos Solitons Fractals 2021, 143, 110563. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog. Oceanogr. 2015, 130, 205–248. [Google Scholar]
- Loick-Wilde, N.; Fernandez-Urruzola, I.; Eglite, E.; Liskow, I.; Nausch, M.; Schulz-Bull, D.; Wodarg, D.; Wasmund, N.; Mohrholz, V. Stratification, nitrogen fixation, and cyanobacterial bloom stage regulate the planktonic food web structure. Glob. Change Biol. 2019, 25, 794–810. [Google Scholar] [CrossRef]
- Kuiper, J.J.; van Altena, C.; de Ruiter, P.C.; van Gerven, L.P.A.; Janse, J.H.; Mooij, W.M. Food-web stability signals critical transitions in temperate shallow lakes. Nat. Commun. 2015, 6, 7727. [Google Scholar] [CrossRef]
- Ratnarajah, L.; Abu-Alhaija, R.; Atkinson, A.; Batten, S.; Bax, N.J.; Bernard, K.S.; Canonico, G.; Cornils, A.; Everett, J.D.; Grigoratou, M.; et al. Monitoring and modelling marine zooplankton in a changing climate. Nat. Commun. 2023, 14, 564. [Google Scholar] [CrossRef]
- Bashevkin, S.M.; Hartman, R.; Thomas, M.; Barros, A.; Burdi, C.E.; Hennessy, A.; Tempel, T.; Kayfetz, K. Five decades (1972–2020) of zooplankton monitoring in the upper San Francisco Estuary. PLoS ONE 2022, 17, e0265402. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, W.; Xiong, P.; Li, H.; Liu, Z.; Ai, J.; Yuan, D.; Wan, F.; Wan, Y.; Zou, H.; et al. Temporal variation of plankton and zoobenthos communities in a freshwater reservoir: Structure feature, construction mechanism, associated symbiosis and environmental response. Ecol. Indic. 2023, 154, 110774. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, C.; Wang, Y.; Chen, G. Advances in multiplex molecular detection technologies for harmful algae. Environ. Sci. Pollut. Res. 2022, 29, 43745–43757. [Google Scholar] [CrossRef]
- Kalwak, W.; Weihgold, V. The Relationality of Ecological Emotions: An Interdisciplinary Critique of Individual Resilience as Psychology’s Response to the Climate Crisis. Front. Psychol. 2022, 13, 823620. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, M.; Zhao, Z.; Wang, Y.; Li, T.; Wei, Y.; Li, R.; Yang, F. Insight into the role of niche concept in deciphering the ecological drivers of MPs-associated bacterial communities in mangrove forest. Water Res. 2024, 249, 120995. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Zou, X.; Qu, M.; Li, J. Groundwater depth regulates assembly processes of abundant and rare bacterial communities across arid inland river basin. J. Environ. Manag. 2022, 319, 115767. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.Y. Foundational errors in the Neutral and Nearly-Neutral theories of evolution in relation to the Synthetic Theory. Is a new evolutionary paradigm necessary? Biol. Res. 2013, 46, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar]
- Wang, W.; Ren, K.; Chen, H.; Gao, X.; Ronn, R.; Yang, J. Seven-year dynamics of testate amoeba communities driven more by stochastic than deterministic processes in two subtropical reservoirs. Water Res. 2020, 185, 116232. [Google Scholar] [CrossRef]
- Lee, H.; Bloxham, B.; Gore, J. Resource competition can explain simplicity in microbial community assembly. Proc. Natl. Acad. Sci. USA 2023, 120, 130603. [Google Scholar] [CrossRef]
- Liu, X.; Pan, B.; Liu, X.; He, H.; Zhao, X.; Huang, Z.; Li, M. Riverine microbial community assembly with watercourse distance-decay patterns in the north-south transitional zone of China. J. Hydrol. 2024, 628, 130603. [Google Scholar] [CrossRef]
- Mo, Y.; Bier, R.; Li, X.; Daniels, M.; Smith, A.; Yu, L.; Kan, J. Agricultural practices influence soil microbiome assembly and interactions at different depths identified by machine learning. Commun. Biol. 2024, 7, 1349. [Google Scholar] [CrossRef]
- Ruppert, J.L.W.; Hogg, J.; Poesch, M.S. Community assembly and the sustainability of habitat offsetting targets in the first compensation lake in the oil sands region in Alberta, Canada. Biol. Conserv. 2018, 219, 138–146. [Google Scholar] [CrossRef]
- Si, Y.-J.; Xu, Y.; Li, B.-Q.; Liu, J.; Meng, L.-P.; Li, Y.; Ji, R.-Q.; Liu, S.-Y. Ectomycorrhizospheric Microbiome Assembly Rules of Quercus mongolica in the Habitat of SongRong Tricholoma matsutake and the Effect of Neighboring Plants. Diversity 2022, 14, 810. [Google Scholar] [CrossRef]
- Yan, R.; Xu, G.; Xu, F.; Song, J.; Yuan, H.; Luo, X.; Fu, X.; Cao, Z. The multistage dissolution characteristics and their influence on mound-shoal complex reservoirs from the Sinian Dengying Formation, southeastern Sichuan Basin, China. Mar. Petrol. Geol. 2022, 139, 105596. [Google Scholar] [CrossRef]
- Mo, H.; Yang, R.; Luo, C.; Li, X.; Ji, Y.; Yang, G.; Zhou, X.; Gao, C.; Hu, X.; Zeng, Z. Effect of Karst Geomorphology on the Sedimentary Mineralization and Geochemical Distribution of Bauxite: An Example from the Xiaoyuan Area in Qingzhen, Guizhou Province. Minerals 2023, 13, 1013. [Google Scholar] [CrossRef]
- Swedberg, D.A.; Mollenhauer, R.; Brewer, S.K. The context dependency of fish-habitat associations in separated karst ecoregions. Ecol. Evol. 2023, 13, e10701. [Google Scholar] [CrossRef] [PubMed]
- Sforzi, L.; Di Camillo, A.T.; Di Lorenzo, T.; Galassi, D.M.P.; Balestra, V.; Piccini, L.; Cabigliera, S.B.; Ciattini, S.; Laurati, M.; Chelazzi, D.; et al. (Micro-)Plastics in Saturated and Unsaturated Groundwater Bodies: First Evidence of Presence in Groundwater Fauna and Habitats. Sustainability 2024, 16, 2532. [Google Scholar] [CrossRef]
- Clune, J.W.; Cravotta Iii, C.A.; Husic, A.; Dozier, H.J.; Schimdt, K.E. Complex hydrology and variability of nitrogen sources in a karst watershed. J. Environ. Qual. 2024, 53, 492–507. [Google Scholar] [CrossRef]
- Gu, X.; Jamshidi, S.; Qian, J.; Hao, Y.; Sun, H.; Niyogi, D. Quantifying the Effect of Driving Factors on Spring Discharge in an Industrialized Karst Watershed. J. Hydrol. Eng. 2023, 28, 05023013. [Google Scholar] [CrossRef]
- Shao, M.; Liu, Z.; Zeng, S.; Sun, H.; He, H.; Adnan, M.; Yan, J.; Shi, L.; Han, Y.; Lai, C.; et al. Carbon sinks associated with biological carbon pump in karst surface waters: Progress, challenges, and prospects. Environ. Res. 2025, 267, 120712. [Google Scholar] [CrossRef]
- Maznah, W.O.W.; Intan, S.; Sharifah, R.; Lim, C.C. Lentic and lotic assemblages of zooplankton in a tropical reservoir, and their association with water quality conditions. Int. J. Environ. Sci. Technol. 2018, 15, 533–542. [Google Scholar] [CrossRef]
- Smith, G.R.; Krishnamurthy, S.V.B.; Burger, A.C.; Rettig, J.E. Effects of malathion and nitrate exposure on the zooplankton community in experimental mesocosms. Environ. Sci. Pollut. Res. 2018, 25, 9992–9997. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Xie, Y.; Song, C.; Zhang, Y.; Yu, H.; Burton, G.A. Zooplankton Community Profiling in a Eutrophic Freshwater Ecosystem-Lake Tai Basin by DNA Metabarcoding. Sci. Rep. 2017, 7, 1773. [Google Scholar] [CrossRef]
- Liu, X.; Dur, G.; Ban, S.; Sakai, Y.; Ohmae, S.; Morita, T. Quasi-decadal periodicities in growth and production of the copepod Eodiaptomus japonicus in Lake Biwa, Japan, related to the Arctic Oscillation. Limnol. Oceanogr. 2021, 66, 3783–3795. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, H.; Xiao, L.; Lin, Q.; Han, B.-P.; Naselli-Flores, L. Spatial heterogeneity of spring phytoplankton in a large tropical reservoir: Could mass effect homogenize the heterogeneity by species sorting? Hydrobiologia 2018, 819, 109–122. [Google Scholar] [CrossRef]
- Dickerson, K.D.; Medley, K.A.; Havel, J.E. Spatial variation in zooplankton community structure is related to hydrologic flow units in the missouri river, USA. River Res. Appl. 2010, 26, 605–618. [Google Scholar] [CrossRef]
- Jo, H.; Jeppesen, E.; Ventura, M.; Buchaca, T.; Gim, J.-S.; Yoon, J.-D.; Kim, D.-H.; Joo, G.-J. Responses of fish assemblage structure to large-scale weir construction in riverine ecosystems. Sci. Total Environ. 2019, 657, 1334–1342. [Google Scholar] [CrossRef]
- Miller, E.C. Comparing diversification rates in lakes, rivers, and the sea. Evolution 2021, 75, 2055–2073. [Google Scholar] [CrossRef]
- Sidharthan, A.; Dahanukar, N.; Sundar, R.L.; Ranjeet, K.; Raghavan, R. Beyond waterfalls and dams: Riverscape genetics of two endemic mountain loaches in the Western Ghats biodiversity hotspot. River Res. Appl. 2022, 38, 152–159. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, D.; Jia, S.; Li, Q.; Liu, S.; Zhang, X.-X. Dynamics of bacterioplankton communities in the estuary areas of the Taihu Lake: Distinct ecological mechanisms of abundant and rare communities. Environ. Res. 2024, 242, 117782. [Google Scholar] [CrossRef]
- Kang, X.; Cui, Y.; Zeng, L.; Tian, Z.; Xu, Y.; Chen, Q.; Gadd, G.M.; Leng, X.; Yu, X. Assembly processes and ecological dynamics of root-associated bacterial communities during phytoremediation of vanadium-titanium mine tailings using Millettia pinnata. Rhizosphere 2024, 29, 100837. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.-A.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Yuan, H.; Mei, R.; Liao, J.; Liu, W.-T. Nexus of Stochastic and Deterministic Processes on Microbial Community Assembly in Biological Systems. Front. Microbiol. 2019, 10, 1536. [Google Scholar] [CrossRef]
- Liao, J.; Cao, X.; Zhao, L.; Wang, J.; Gao, Z.; Wang, M.C.; Huang, Y. The importance of neutral and niche processes for bacterial community assembly differs between habitat generalists and specialists. FEMS Microbiol. Ecol. 2016, 92, fiw174. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, K.; Wemheuer, B.; Pena, R.; Schoening, I.; Ehbrecht, M.; Schall, P.; Ammer, C.; Daniel, R.; Polle, A. Assembly processes of trophic guilds in the root mycobiome of temperate forests. Mol. Ecol. 2019, 28, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, S.; Yan, R.; Wang, R.; Gao, Y.; Kong, M.; Yi, Q.; Zhang, Y. Similar geographic patterns but distinct assembly processes of abundant and rare bacterioplankton communities in river networks of the Taihu Basin. Water Res. 2022, 211, 118057. [Google Scholar] [CrossRef]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Colas, F.; Baudoin, J.-M.; Gob, F.; Tamisier, V.; Valette, L.; Kreutzenberger, K.; Lambrigot, D.; Chauvet, E. Scale dependency in the hydromorphological control of a stream ecosystem functioning. Water Res. 2017, 115, 60–73. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Zhang, X.; Pan, C.; Zhang, S.; Zheng, B. Water Quality and Microbial Community in the Context of Ecological Restoration: A Case Study of the Yongding River, Beijing, China. Int. J. Environ. Res. Public Health 2022, 19, 13056. [Google Scholar] [CrossRef]
- Hays, C.G.; Hanley, T.C.; Hughes, A.R.; Truskey, S.B.; Zerebecki, R.A.; Sotka, E.E. Local Adaptation in Marine Foundation Species at Microgeographic Scales. Biol. Bull. 2021, 241, 16–29. [Google Scholar] [CrossRef]
- Lopez-Goldar, X.; Agrawal, A.A. Ecological Interactions, Environmental Gradients, and Gene Flow in Local Adaptation. Trends Plant Sci. 2021, 26, 796–809. [Google Scholar] [CrossRef]
- Lopez-Figueroa, N.B.; Walters, T.L.; Laureano-Rosario, A.E.; Digeronimo, S.P.; Hallock, P.; Frischer, M.E.; Rodriguez-Santiago, A.E.; Gibson, D.M. Zooplankton community variability in the South Atlantic Bight (2015–2017). J. Plankton Res. 2023, 45, 312–324. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Wang, Z.; Huang, T.; Tian, W.; Huang, H. Responses of Zooplankton Community Pattern to Environmental Factors along the Salinity Gradient in a Seagoing River in Tianjin, China. Microorganisms 2023, 11, 1638. [Google Scholar] [CrossRef]


| Species | Reservoir | River | |
|---|---|---|---|
| Rotifer | Keratella cochlearis | 0.054 | 0.154 |
| Keratella valga | 0.182 | 0.138 | |
| Trichocerca cylindrica | 0.247 | 0.029 | |
| Trichocerca longiseta | 0.156 | 0.062 | |
| Trichocerca pusilla | 0.034 | 0.025 | |
| Trichocerca similis | 0.064 | ||
| Polyarthra remata | 0.034 | ||
| Polyarthra dolichoptera | 0.208 | ||
| Asplanchna priodonta | 0.101 | ||
| Synchaeta oblonga | 0.891 | ||
| Conochilloides dossuarius | 0.102 | ||
| Conochilus unicornis | 0.073 | ||
| Ascomorpha ecaudis | 0.087 | ||
| Lepadella patella | 0.066 | ||
| Colurella adriatica | 0.079 | ||
| Philodina Ehrenberg | 0.080 | ||
| Notholon labis | 0.023 | ||
| Cephalodella gibba | 0.031 | ||
| Rotaria rotatoria | 0.068 | ||
| Euchlanis dilatata | 0.031 | ||
| Copepoda | Phyllodiaptomus tunguidus | 0.076 | 0.029 |
| Mesocyclops leuckarti | 0.048 | 0.023 | |
| nauplii | 0.235 | 0.087 | |
| Tropocyclops longiabdominalis | 0.128 | ||
| Thermocyclops hyalinus | 0.024 | ||
| Thermocyclops brevifurcatus | 0.040 | ||
| Tropocyclops prasinus | 0.033 | ||
| Cladocera | Bosmina coregoni | 0.052 | |
| Ceriodaphnia pulchella | 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, Q.; Wang, P.; Song, X.; Li, J.; Han, M.; Zhou, S. From River to Reservoir: The Impact of Environmental Variables on Zooplankton Assemblages in Karst Ecosystems. Sustainability 2025, 17, 4240. https://doi.org/10.3390/su17094240
Li B, Li Q, Wang P, Song X, Li J, Han M, Zhou S. From River to Reservoir: The Impact of Environmental Variables on Zooplankton Assemblages in Karst Ecosystems. Sustainability. 2025; 17(9):4240. https://doi.org/10.3390/su17094240
Chicago/Turabian StyleLi, Binbin, Qiuhua Li, Pengfei Wang, Xiaochuan Song, Jinjuan Li, Mengshu Han, and Si Zhou. 2025. "From River to Reservoir: The Impact of Environmental Variables on Zooplankton Assemblages in Karst Ecosystems" Sustainability 17, no. 9: 4240. https://doi.org/10.3390/su17094240
APA StyleLi, B., Li, Q., Wang, P., Song, X., Li, J., Han, M., & Zhou, S. (2025). From River to Reservoir: The Impact of Environmental Variables on Zooplankton Assemblages in Karst Ecosystems. Sustainability, 17(9), 4240. https://doi.org/10.3390/su17094240





