Sustainable Management of the Organic Fraction of Municipal Solid Waste: Microbiological Quality Control During Composting and Its Application in Agriculture on a Pilot Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Inputs: Municipal Solid Waste, Structural Material and Water
2.2. Composting Facility
2.3. Agricultural Land and Irrigation Water
2.4. Application of Compost on Agricultural Land
2.5. Sampling
2.6. Analytical Methodology
2.6.1. Microbiological Parameters
2.6.2. Physico-Chemical Parameters
- -
- Rottegrade Determination (UNE-EN 16087-2:2012 [21]): This method classifies compost maturity based on the highest temperature attained during a 10-day period. The test is performed under controlled conditions with a consistent ambient temperature of 20 °C and a sample humidity of 40%. The maximum temperature attained during this period provides insights into the degree of compost maturity.
- -
- Solvita® Test: This test evaluates the stability of compost by qualitatively measuring CO2 and ammoniacal nitrogen using a colorimetric technique. It assesses the decomposition rate and the release of ammonia, which are indicators of compost stability. This test provides results that help categorize compost according to its maturity, considering its resistance to decomposition and the lack of ammonia, organic acids, and phytotoxic elements.
3. Results and Discussion
3.1. Initial Properties of OFMSW, SM, and Water Used in Composting Process
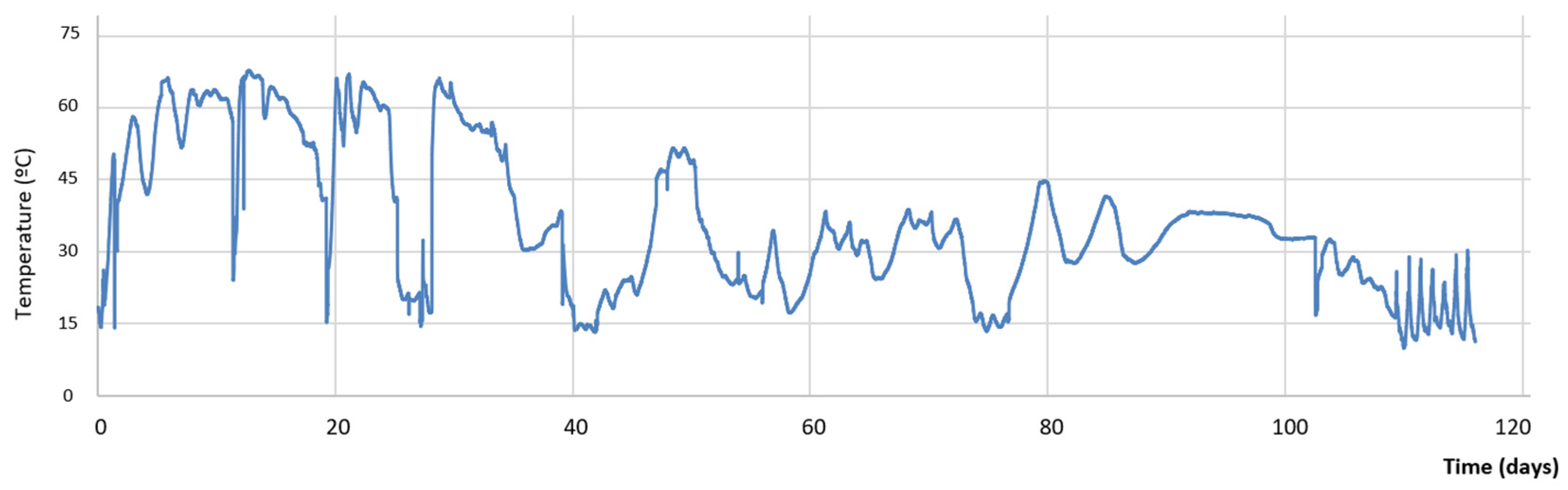
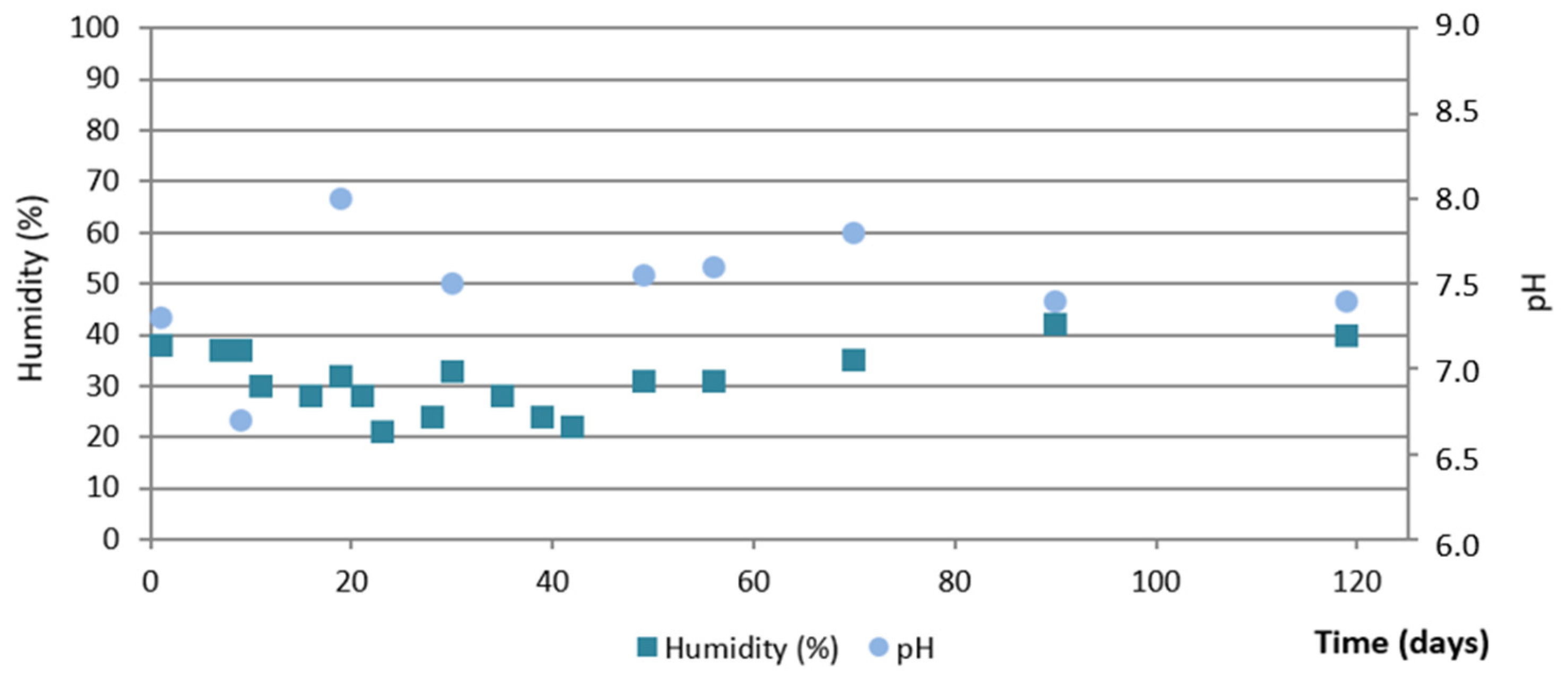
3.2. Evolution During the Composting Process
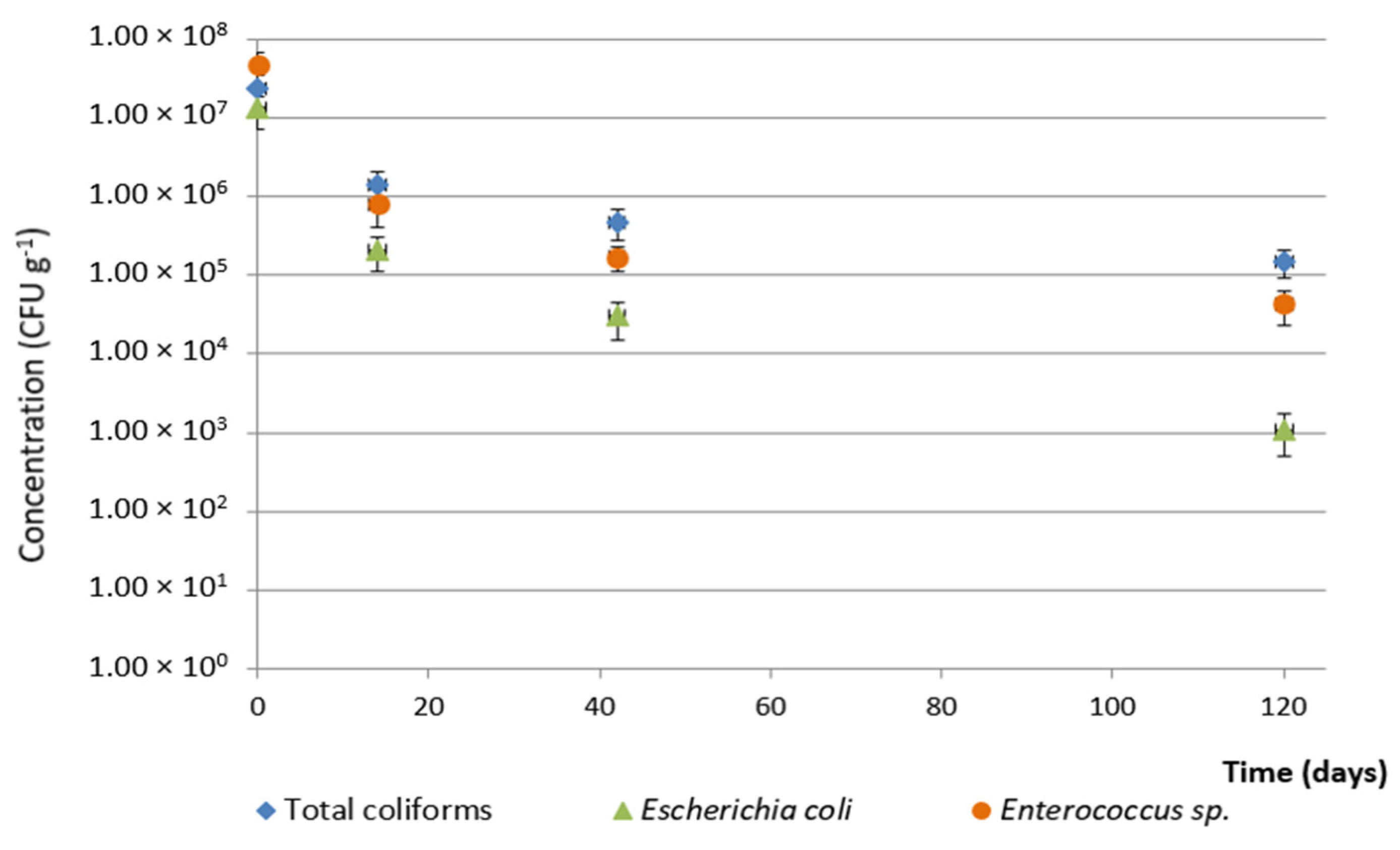
3.3. Evolution on Agricultural Land
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almendro-Candel, M.B.; Navarro-Pedreño, J.; Gómez Lucas, I.; Zorpas, A.A.; Voukkali, I.; Loizia, P. The Use of Composted Municipal Solid Waste under the Concept of Circular Economy and as a Source of Plant Nutrients and Pollutants. In Municipal Solid Waste Managemen; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Rashid, M.I.; Shahzad, K. Food waste recycling for compost production and its economic and environmental assessment as circular economy indicators of solid waste management. J. Clean. Prod. 2021, 317, 128467. [Google Scholar] [CrossRef]
- Masullo, A. Organic wastes management in a circular economy approach: Rebuilding the link between urban and rural areas. Ecol. Eng. 2017, 101, 84–90. [Google Scholar] [CrossRef]
- Brenes-Peralta, L.; Jiménez-Morales, M.F.; Campos-Rodríguez, R. Food waste valorization through composting and bio-drying for small scale fruit processing agro-industries. Ing. Ambient. Ing. Competit. 2021, 23, e9623. [Google Scholar] [CrossRef]
- Miguel, N.; López, A.; Jojoa-Sierra, S.D.; Fernández, J.; Gómez, J.; Ormad, M.P. Physico-Chemical and Microbiological Control of the Composting Process of the Organic Fraction of Municipal Solid Waste: A Pilot-Scale Experience. Int. J. Environ. Res. Public Health 2022, 19, 15449. [Google Scholar] [CrossRef]
- Ministerio para la Transición Ecológica y el Reto Demográfico (M.I.T.E.C.O.). Memoria Anual de Generación y Gestión de Residuos de Competencia Municipal 2021; M.I.T.E.C.O.: Madrid, Spain, 2021. [Google Scholar]
- Ministerio para la Transición Ecológica y el Reto Demográfico (M.I.T.E.C.O.). Plan Estatal Marco de Gestión de Residuos (PEMAR) 2023–2035; M.I.T.E.C.O.: Madrid, Spain, 2023. [Google Scholar]
- Boletín Oficial del Estado (B.O.E.). Ley 22/2011, de 28 de Julio, de Residuos y Suelos Contaminados. Boletín Oficial del Estado, 29 July 2011. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2011-13046 (accessed on 30 January 2025).
- Boletín Oficial del Estado (B.O.E.). Ley 7/2022, de Residuos y Suelos Contaminados Para una Economía Circular. Boletín Oficial del Estado, 9 April 2022. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2022-5809 (accessed on 30 January 2025).
- Boletín Oficial del Estado (B.O.E.). Real Decreto 506/2013, de 28 de Junio, Sobre Productos Fertilizantes. Boletín Oficial del Estado, 10 July 2013. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2013-7540 (accessed on 30 January 2025).
- Boletín Oficial del Estado (B.O.E.). Real Decreto 999/2017, de 24 de Noviembre, por el que se Modifica el Real Decreto 506/2013, de 28 de Junio, Sobre Productos Fertilizantes. Boletín Oficial del Estado, 6 December 2017. Available online: https://www.boe.es/diario_boe/txt.php?id=BOE-A-2017-14332 (accessed on 30 January 2025).
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: New York, NY, USA, 2016; ISBN 978-0133254488. [Google Scholar]
- U.S. Department of Agriculture; US Composting Council. Test Methods for the Examination of Composting and Compost, TMECC; Edapho International: Raleigh, NC, USA, 2001. [Google Scholar]
- Carter, M.R. Soil Sampling and Methods of Analysis; Lewis Publishers; Taylor & Francis Group: Boca Raton, FL, USA, 1993; ISBN 978-0-8493-3586-0. [Google Scholar]
- ISO 5667-3:2018; Water Quality—Sampling—Part 3: Preservation and Handling of Water Samples. ISO: Geneva, Switzerland, 2018.
- ISO 9308-1:2000; Water Quality—Detection and Enumeration of Escherichia coli and Coliform Bacteria—Part 1: Membrane Filtration Method. ISO: Geneva, Switzerland, 2000.
- American Public Health Association (A.P.H.A.). Standard Methods for the Examination of Water & Wastewater, 21st ed.; A.P.H.A./A.W.W.A./W.E.F.: Washington, DC, USA, 2005; ISBN 9780875530475. [Google Scholar]
- ISO 7899-2:2000; Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method. ISO: Geneva, Switzerland, 2000.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- ISO 11465:1993; Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method. ISO: Geneva, Switzerland, 1993.
- UNE EN 16087-2:2012; Mejoradores de Suelo y Sustratos de Cultivo. Determinación de la Actividad Biológica Aerobia. Parte 2: Ensayo de Autocalentamiento Para Compost. UNE: Madrid, Spain, 2012.
- Gurtler, J.B.; Doyle, M.P.; Erickson, M.C.; Jiang, X.; Millner, P.; Sharma, M. Composting to Inactivate Foodborne Pathogens for Crop Soil Application: A Review. J. Food Prot. 2018, 81, 1821–1837. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Broussolle, V.; Colin, P.; Nguyen-The, C.; Prieto, M. The adaptive response of bacterial food-borne pathogens in the environment, host and food: Implications for food safety. Int. J. Food Microbiol. 2015, 213, 99–109. [Google Scholar] [CrossRef]
- Mols, M.; Abee, T. Primary and secondary oxidative stress in Bacillus. Environ. Microbiol. 2011, 13, 1387–1394. [Google Scholar] [CrossRef]
- Poole, R.K. Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2002; Volume 46. [Google Scholar]
- Imlay, J.A. Pathways of Oxidative Damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Chang, S.-J.; Lee, M.-H.; Kim, W.-J.; Chae, Y.; Iwasa, M.; Han, K.-I.; Kim, W.-J.; Kim, T.-J. Effect of Heat-Killed Enterococcus faecalis, EF-2001 on C2C12 Myoblast Damage Induced by Oxidative Stress and Muscle Volume Decreased by Sciatic Denervation in C57BL/6 Mice. J. Life Sci. 2019, 29, 215–222. [Google Scholar] [CrossRef]
- Ramírez Santos, J.; Guzmán, G.S.; Eichelmann, C.G. Regulación Genética en la Respuesta al Estrés Calórico en Escherichia coli. Rev. Latinoam. Microbiol. 2001, 43, 51–63. [Google Scholar]
- Sörqvist, S. Heat Resistance in Liquids of Enterococcus spp., Listeria spp., Escherichia coli, Yersinia enterocolitica, Salmonella spp. and Campylobacter spp. Acta Vet. Scand. 2003, 44, 1. [Google Scholar] [CrossRef] [PubMed]
- De Bertoldi, M.; Vallini, G.; Pera, A. The Biology of Composting: A Review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Goyal, S.; Dhull, S.K.; Kapoor, K.K. Chemical and Biological Changes during Composting of Different Organic Wastes and Assessment of Compost Maturity. Bioresour. Technol. 2005, 96, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Ryckeboer, J.; Mergaert, J.; Coosemans, J.; Deprins, K.; Swings, J. Microbiological Aspects of Biowaste during Composting in a Monitored Compost Bin. J. Appl. Microbiol. 2003, 94, 127–137. [Google Scholar] [CrossRef]
- Lepesteur, M. Human and Livestock Pathogens and Their Control during Composting. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1639–1683. [Google Scholar] [CrossRef]
- Berry, E.D.; Millner, P.D.; Wells, J.E.; Kalchayanand, N.; Guerini, M.N. Fate of Naturally Occurring Escherichia coli O157:H7 and Other Zoonotic Pathogens during Minimally Managed Bovine Feedlot Manure Composting Processes. J. Food Prot. 2013, 76, 1308–1321. [Google Scholar] [CrossRef]
- Kuok, F.; Mimoto, H.; Nakasaki, K. Effects of Turning on the Microbial Consortia and the In Situ Temperature Preferences of Microorganisms in a Laboratory-Scale Swine Manure Composting. Bioresour. Technol. 2012, 116, 421–427. [Google Scholar] [CrossRef]
- Erickson, M.C.; Liao, J.; Boyhan, G.; Smith, C.; Ma, L.; Jiang, X.; Doyle, M.P. Fate of Manure-Borne Pathogen Surrogates in Static Composting Piles of Chicken Litter and Peanut Hulls. Bioresour. Technol. 2010, 101, 1014–1020. [Google Scholar] [CrossRef]
- Shepherd, M.W.; Liang, P.; Jiang, X.; Doyle, M.P.; Erickson, M.C. Fate of Escherichia coli O157:H7 during On-Farm Dairy Manure-Based Composting. J. Food Prot. 2007, 70, 12. [Google Scholar] [CrossRef]
- Shepherd, M.W.; Singh, R.; Kim, J.; Jiang, X. Effect of Heat-Shock Treatment on the Survival of Escherichia coli O157:H7 and Salmonella enterica Typhimurium in Dairy Manure Co-Composted with Vegetable Wastes under Field Conditions. Bioresour. Technol. 2010, 101, 5407–5413. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Mohaibes, M.; Karinen, P.; Koivunen, J. Methods to Reduce Pathogen Microorganisms in Manure. Livest. Sci. 2006, 102, 248–255. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, T.H.; Bui, X.T.; Nguyen, T.N.; Vo, T.D.H.; Lin, C.; Vu, T.M.H.; Nguyen, H.H.; Nguyen, D.D.; Senoro, D.B.; et al. Effects of C/N Ratios and Turning Frequencies on the Composting Process of Food Waste and Dry Leaves. Bioresour. Technol. Rep. 2020, 11, 100527. [Google Scholar] [CrossRef]
- Şevik, F.; Tosun, İ.; Ekinci, K. The Effect of FAS and C/N Ratios on Co-Composting of Sewage Sludge, Dairy Manure and Tomato Stalks. Waste Manag. 2018, 80, 450–456. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Gómez, J.; Sarasa, J.; Miguel, N.; Labadía, J.; Lasheras, A.; Ormad, M. Estudio de la Evolución de la Calidad Microbiológica de los Suelos Abonados con Fangos de EDAR. Available online: https://www.aguasresiduales.info/revista/articulos/estudio-de-la-evolucion-de-la-calidad-microbiologica-de-los-suelos-abonados-con-fangos-de-edar (accessed on 19 March 2025).



| Bacteria | Culture Media | Standard Methodology |
|---|---|---|
| Total coliforms | Chromogenic Coliform Agar (CCA) | ISO 9308-1 [16] 9215B-C-D [17] |
| Escherichia coli | Chromogenic Coliform Agar (CCA) Glucuronic Agar tryptone and bile (TBX) | ISO 9308-1 [16] 9215B-C-D and 9222D [17] |
| Enterococcus sp. | Slanetx and Bartley Agar | ISO 7899-2 [18] 9215B-C-D [17] |
| Salmonella sp. | XLD Agar Chromogenic Agar Salmonella Latex test | ISO 6579-1 [19] |
| Parameter | Equipment | Standard Methodology |
|---|---|---|
| pH | Multiparametric meter Orion Star A3295 | 4500H+-B [17] |
| Humidity (%) | Scale, cooker | ISO 11465:1993 [20] |
| Total solids (%) | Scale, cooker | ISO 11465:1993 [20] |
| Organic matter (% o.d.m. 1) | Carbon analyzer | 5310B [17] |
| Nitrogen (% o.d.m. 1) | System Kjeldahl | 4500-N [17] |
| Phosphorus (mg P2O5 kg−1) | Inductively Coupled Plasma Mass Spectrometer (ICP-MS) | 4500-P [17] |
| Cadmium, cobalt, nickel, lead, zinc, mercury, chromium | Atomic Emission Spectroscope with Inductively Coupled Plasma (ICP-OES) | 3120B [17] |
| Bacteria | OFMSW (CFU g−1) | SM (CFU g−1) |
|---|---|---|
| Total coliforms | 9.70 ± 5.30 × 107 | 1.24 ± 1.23 × 107 |
| Escherichia coli | 7.75 ± 5.25 × 107 | 2.04 ± 1.10 × 104 |
| Enterococcus sp. | 3.70 ± 2.60 × 108 | 3.20 ± 0.70 × 103 |
| Salmonella sp. | Not detected | Not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, N.; López, A.; Jojoa-Sierra, S.D.; Gómez, J.; Ormad, M.P. Sustainable Management of the Organic Fraction of Municipal Solid Waste: Microbiological Quality Control During Composting and Its Application in Agriculture on a Pilot Scale. Sustainability 2025, 17, 4169. https://doi.org/10.3390/su17094169
Miguel N, López A, Jojoa-Sierra SD, Gómez J, Ormad MP. Sustainable Management of the Organic Fraction of Municipal Solid Waste: Microbiological Quality Control During Composting and Its Application in Agriculture on a Pilot Scale. Sustainability. 2025; 17(9):4169. https://doi.org/10.3390/su17094169
Chicago/Turabian StyleMiguel, Natividad, Andrea López, Sindy Dayana Jojoa-Sierra, Jairo Gómez, and María P. Ormad. 2025. "Sustainable Management of the Organic Fraction of Municipal Solid Waste: Microbiological Quality Control During Composting and Its Application in Agriculture on a Pilot Scale" Sustainability 17, no. 9: 4169. https://doi.org/10.3390/su17094169
APA StyleMiguel, N., López, A., Jojoa-Sierra, S. D., Gómez, J., & Ormad, M. P. (2025). Sustainable Management of the Organic Fraction of Municipal Solid Waste: Microbiological Quality Control During Composting and Its Application in Agriculture on a Pilot Scale. Sustainability, 17(9), 4169. https://doi.org/10.3390/su17094169







