Comprehensive Ecotoxicity of the Complex System of Polycyclic Aromatic Hydrocarbon-Contaminated Sites to Wheat (Triticum aestivum L.) During Microbial Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Sample
2.1.1. Chemicals and Reagents
2.1.2. Soil Samples
2.2. Batch Experiments
2.2.1. Wheat Seed Germination and Growth
2.2.2. Wheat Physiological Properties
2.3. Soil Properties
2.3.1. BaA and BaP Concentrations
2.3.2. Soil Enzyme Activity
2.3.3. Soil DOC Content
2.4. Analysis of the Soil Ecotoxicity
2.4.1. Partial Least Squares Discriminant Analysis
2.4.2. Comprehensive Biotoxicity Analysis (IBRv2, Integrated Biomarker Response Version 2)
2.5. Statistical Analysis
3. Results and Discussion
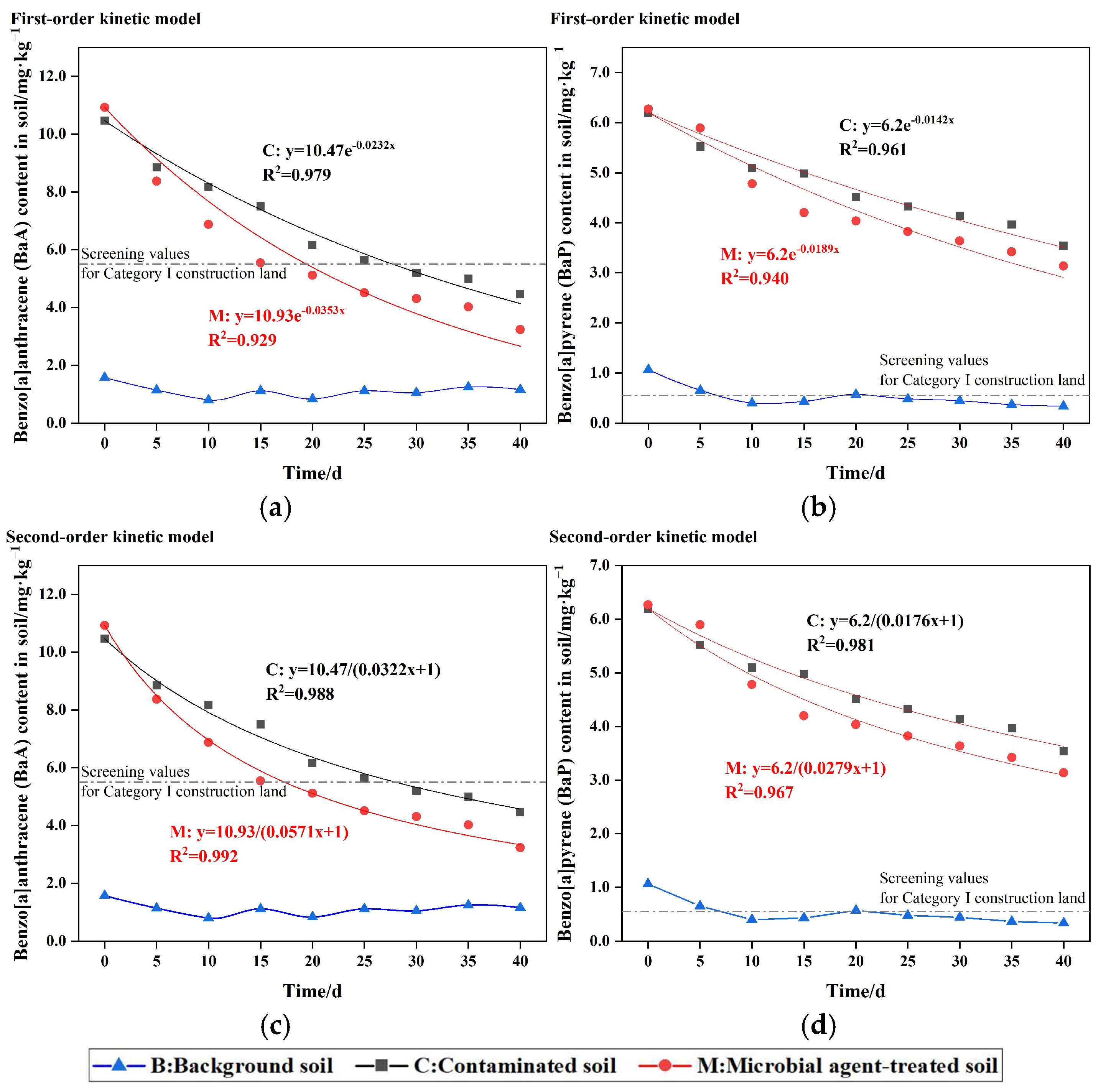
3.1. PAH Concentration During Microbial Remediation
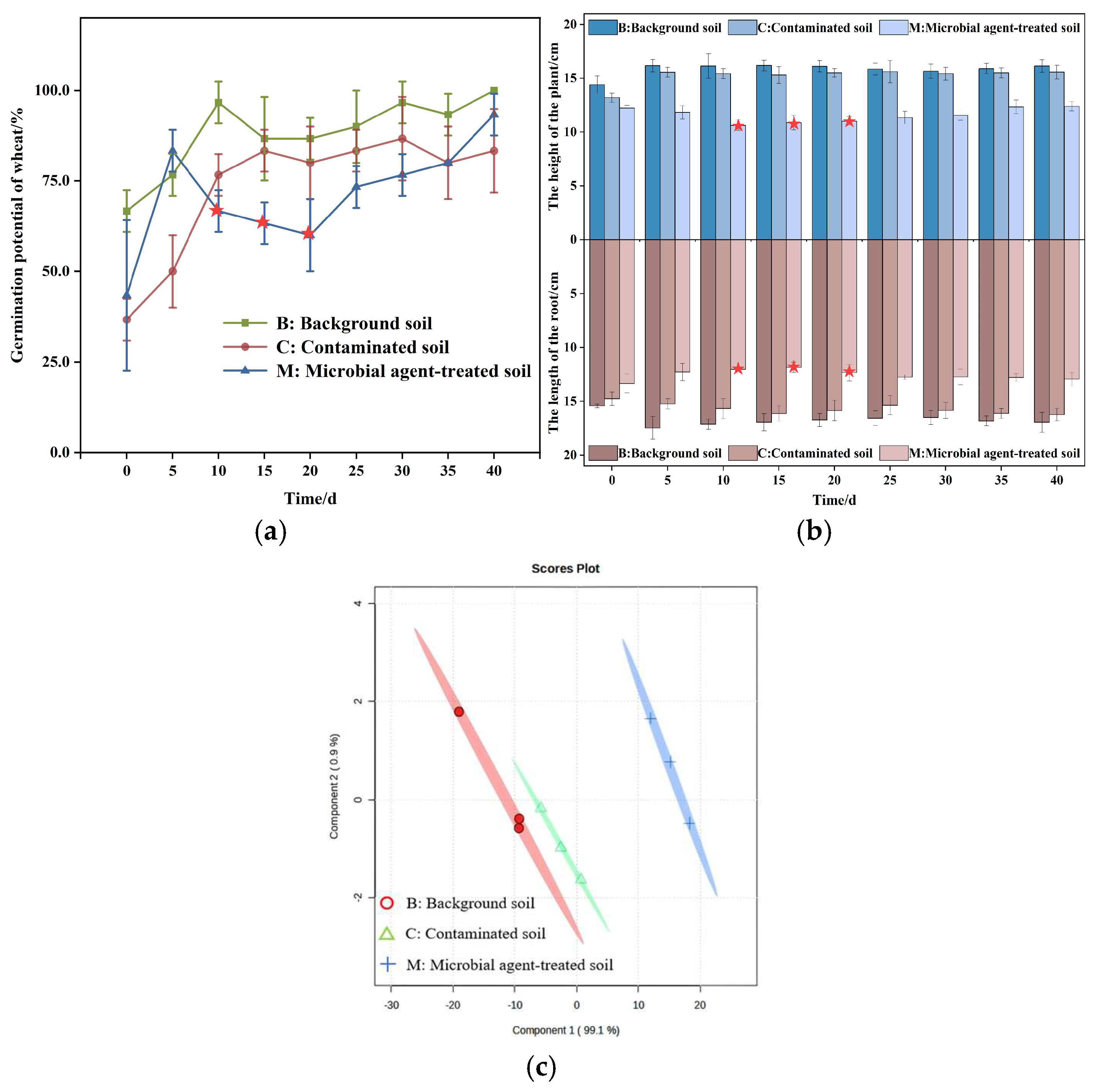
3.2. Wheat Growth Indicators
3.3. Physiological Characteristics of Wheat Planted in Soils
3.3.1. Photosynthetic Pigments and Wheat Leaf Damage
3.3.2. Wheat Antioxidant System
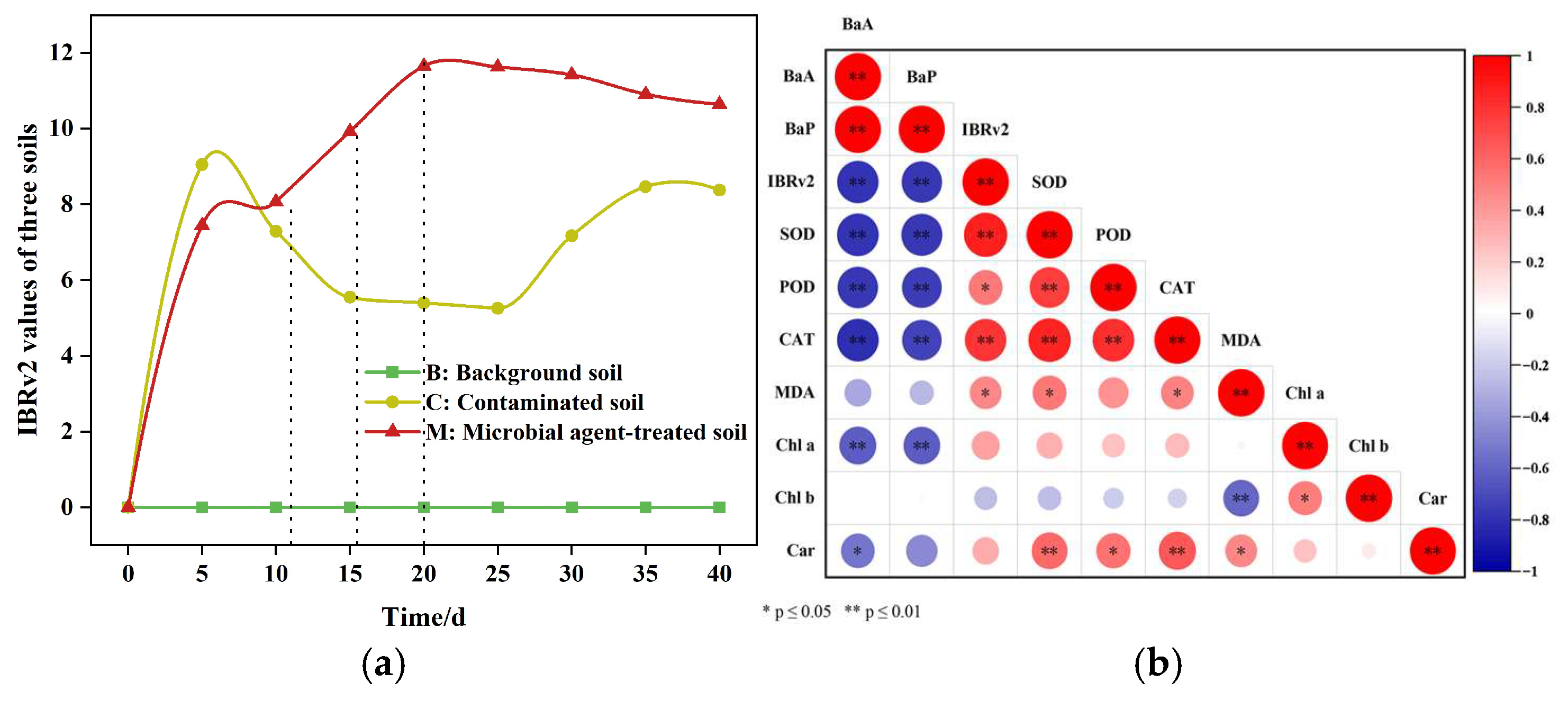
3.3.3. Evaluation of Soil Toxicity During Microbial Remediation Using the IBRv2 Index
3.4. GC-MS-Based Identification of Substances Increases Soil Ecotoxicity
3.5. Practical Applications and Policy Recommendations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpson, S.L.; Spadaro, D.A.; Batley, G.E.; Irvine, I.A.; Synnot, R.N. Remediation criteria for gasworks-impacted sediments: Assessing the effects of legacy hydrocarbons and more recent metal contamination. Sci. Total Environ. 2020, 737, 139725. [Google Scholar] [CrossRef] [PubMed]
- Jacek, G.; Rozan, A.; Desrousseaux, M.; Combroux, I. Brownfields over the years: From definition to sustainable reuse. Environ. Rev. 2021, 30, 50–60. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, Q.; Zhao, N.; Jin, B.; Zhuang, X.; Bai, Z. Distributions and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils around a Chemical Plant in Shanxi, China. Int. J. Environ. Res. Public Health 2017, 14, 1198. [Google Scholar] [CrossRef]
- Yang, M.; Wei, R.; Chen, W.; Yang, L.; Ni, J. Distribution patterns, toxic equivalence, and environmental risks of polycyclic aromatic hydrocarbons in different density fractions of long-term field-contaminated soils. J. Soils Sediments 2022, 23, 1370–1380. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Yi, S.; Ge, F. Genetically engineered microbial remediation of soils co-contaminated by heavy metals and polycyclic aromatic hydrocarbons: Advances and ecological risk assessment. J. Environ. Manag. 2021, 296, 113185. [Google Scholar] [CrossRef] [PubMed]
- Davie-Martin, C.L.; Stratton, K.G.; Teeguarden, J.G.; Waters, K.M.; Simonich, S.L.M. Implications of Bioremediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soils for Human Health and Cancer Risk. Environ. Sci. Technol. 2017, 51, 9458–9468. [Google Scholar] [CrossRef]
- Barathi, S.; Gitanjali, J.; Rathinasamy, G.; Sabapathi, N.; Aruljothi, K.N.; Lee, J.; Kandasamy, S. Recent trends in polycyclic aromatic hydrocarbons pollution distribution and counteracting bio-remediation strategies. Chemosphere 2023, 337, 139396. [Google Scholar] [CrossRef]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of Petroleum-Contaminated Soils with Microbial and Microbial Combined Methods: Advances, Mechanisms, and Challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- García Frutos, F.J.; Escolano, O.; García, S.; Babín, M.; Fernández, M.D. Bioventing remediation and ecotoxicity evaluation of phenanthrene-contaminated soil. J. Hazard. Mater. 2010, 183, 806–813. [Google Scholar] [CrossRef]
- Sleight, T.W.; Khanna, V.; Gilbertson, L.M.; Ng, C.A. Network Analysis for Prioritizing Biodegradation Metabolites of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 2020, 54, 10735–10744. [Google Scholar] [CrossRef]
- Yang, Q.; Li, G.; Jin, N.; Zhang, D. Synergistic/antagonistic toxicity characterization and source-apportionment of heavy metals and organophosphorus pesticides by the biospectroscopy-bioreporter-coupling approach. Sci. Total Environ. 2023, 905, 167057. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Fang, W.; Liang, J.; Zhang, P.; Zhang, G.; Zhang, H.; Zhang, Y.; Wang, Q. A critical review on interaction of microplastics with organic contaminants in soil and their ecological risks on soil organisms. Chemosphere 2022, 306, 135573. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.; Zhu, J.; Wang, J.; Wang, H.; Zhan, X. The joint toxicity of polyethylene microplastic and phenanthrene to wheat seedlings. Chemosphere 2021, 282, 130967. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, Z.; Zhou, J.; Liu, J.; Chen, J.; Li, A.; Wang, C.; Zhang, C.; Dai, Y.; Jia, H. Accumulation, root-shoot translocation and phytotoxicity of substituted polycyclic aromatic hydrocarbons in wheat. Environ. Chem. Lett. 2023, 21, 2509–2517. [Google Scholar] [CrossRef]
- Nikolaeva, O.; Karpukhin, M.; Streletskii, R.; Rozanova, M.; Chistova, O.; Panina, N. Linking pollution of roadside soils and ecotoxicological responses of five higher plants. Ecotoxicol. Environ. Saf. 2020, 208, 111586. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Z.; Song, Y.; Zheng, X.; Qu, L.; Qian, J.; Wu, Y.; Wu, X.; Wu, Z. Remediation of phenanthrene contaminated soil by g-C3N4/Fe3O4 composites and its phytotoxicity evaluation. Chemosphere 2019, 221, 554–562. [Google Scholar] [CrossRef]
- Zhao, W.; Teng, M.; Zhang, J.; Wang, K.; Zhang, J.; Xu, Y.; Wang, C. Insights into the mechanisms of organic pollutant toxicity to earthworms: Advances and perspectives. Environ. Pollut. 2022, 305, 119120. [Google Scholar] [CrossRef]
- Hu, J.; Adrion, A.C.; Nakamura, J.; Shea, D.; Aitken, M.D. Bioavailability of (Geno)toxic Contaminants in Polycyclic Aromatic Hydrocarbon–Contaminated Soil Before and After Biological Treatment. Environ. Eng. Sci. 2014, 31, 176–182. [Google Scholar] [CrossRef]
- Trine, L.S.D.; Davis, E.L.; Roper, C.; Truong, L.; Tanguay, R.L.; Simonich, S.L.M. Formation of PAH Derivatives and Increased Developmental Toxicity during Steam Enhanced Extraction Remediation of Creosote Contaminated Superfund Soil. Environ. Sci. Technol. 2019, 53, 4460–4469. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Cao, Z.; Wang, J. Sorption-desorption mechanisms and environmental friendliness of different surfactants in enhancing remediation of soil contaminated with polycyclic aromatic hydrocarbons. J. Soils Sediments 2020, 20, 2817–2828. [Google Scholar] [CrossRef]
- Al-Mutairi, N.; Bufarsan, A.; Al-Rukaibi, F. Ecorisk evaluation and treatability potential of soils contaminated with petroleum hydrocarbon-based fuels. Chemosphere 2008, 74, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Schrlau, J.E.; Kramer, A.L.; Chlebowski, A.; Truong, L.; Tanguay, R.L.; Simonich, S.L.M.; Semprini, L. Formation of Developmentally Toxic Phenanthrene Metabolite Mixtures by Mycobacterium sp. ELW1. Environ. Sci. Technol. 2017, 51, 8569–8578. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, Y.; Dai, Y.; Wu, Y.; Lin, X. Effects of polycyclic aromatic hydrocarbon structure on PAH mineralization and toxicity to soil microorganisms after oxidative bioremediation by laccase. Environ. Pollut. 2021, 287, 117581. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Tao, H.; Cao, H.; Liao, X.; Liu, X. Three-dimensional distribution characteristics of multiple pollutants in the soil at a steelworks mega-site based on multi-source information. J. Hazard. Mater. 2023, 448, 130934. [Google Scholar] [CrossRef]
- GB 36600-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Development Land. Ministry of Ecology and Emvironment of the People's Republic of China: Beijing, China, 2018.
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro-plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Q.; Sun, Y.; Zhang, J. Effects of oxygenated brackish water on germination and growth characteristics of wheat. Agric. Water Manag. 2021, 245, 106520. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Zhou, H.; Li, Y.; Zhang, J.; Yu, S.; Wang, M.; Hao, M.; Zhao, Q.; Liu, L.; et al. Comparison of Sample Preparation Techniques for Inspection of Leaf Epidermises Using Light Microscopy and Scanning Electronic Microscopy. Front. Plant Sci. 2020, 11, 133. [Google Scholar] [CrossRef]
- Qiu, Z.; He, Y.; Zhang, Y.; Guo, J.; Wang, L. Characterization of miRNAs and their target genes in He-Ne laser pretreated wheat seedlings exposed to drought stress. Ecotoxicol. Environ. Saf. 2018, 164, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: New York, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Pillay, V.; Moodley, B. Assessment of the impact of reforestation on soil, riparian sediment and river water quality based on polyaromatic hydrocarbon pollutants. J. Environ. Manag. 2022, 324, 116331. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Li, X.; Xing, Z. Effects of biological soil crusts on soil enzyme activities in revegetated areas of the Tengger Desert, China. Appl. Soil Ecol. 2014, 80, 6–14. [Google Scholar] [CrossRef]
- Gao, T.; Tian, H.; Niu, H.; Wang, Z.; Dai, Y.; Megharaj, M.; He, W. Soil phosphatase assay to evaluate arsenic toxicity should be performed at the soil’s actual pH. Sci. Total Environ. 2023, 859, 160184. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yi, W.; Zhou, Y.; He, S.; Tang, L.; Yin, X.; Zhao, P.; Long, G. Intercropping and N application enhance soil dissolved organic carbon concentration with complicated chemical composition. Soil Tillage Res. 2021, 210, 104979. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J.; Yi, M. Cost estimation for general aviation aircrafts using regression models and variable importance in projection analysis. J. Clean. Prod. 2020, 256, 120648. [Google Scholar] [CrossRef]
- Yao, D.; Wang, N.; Dai, W.; Liu, Y.; Tian, K.; Wang, H.; Liu, Y. Degradation of benzo [a] pyrene in the soil enhanced by soapwort: The role of soapwort and functional microbial community. J. Hazard. Mater. 2023, 485, 131993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, Y.; Leng, G.; Ling, W.; Wang, J. The addition of Glomalin-related soil protein and functional microbial consortium increased bound PAH residue degradation in soil. Appl. Soil Ecol. 2023, 193, 105158. [Google Scholar] [CrossRef]
- Baran, S.; Bielińska, J.E.; Oleszczuk, P. Enzymatic activity in an airfield soil polluted with polycyclic aromatic hydrocarbons. Geoderma 2004, 118, 221–232. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Ji, L.; Song, F.; Li, T.; Fu, X.; Li, Q.; Xing, Y.; Zhang, Q.; Wang, J. Effects of Salinity on the Biodegradation of Polycyclic Aromatic Hydrocarbons in Oilfield Soils Emphasizing Degradation Genes and Soil Enzymes. Front. Microbiol. 2022, 12, 824319. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; Tájmel, D.; Rousk, J. Can moisture affect temperature dependences of microbial growth and respiration? Soil Biol. Biochem. 2021, 156, 108223. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Aranda, B. Microbial Growth under Limiting Conditions-Future Perspectives. Microorganisms 2023, 11, 1641. [Google Scholar] [CrossRef]
- Chai, X.; Wang, M.; Fu, X.; Zhang, W.; Huang, Y.; Germaine, K.J.; Wang, J. Shift of combined ecotoxicity index in petroleum polluted soils during a bacterial remediation. Front. Environ. Sci. 2023, 11, 1141562. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, L. Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils. Chemosphere 2004, 55, 1169–1178. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Silva, C.M. What’s wrong with Lagergreen pseudo first order model for adsorption kinetics? Chem. Eng. J. 2016, 306, 1138–1142. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Wilcke, W. Analysis of polycyclic aromatic hydrocarbons and their oxygen-containing derivatives and metabolites in soils. J. Environ. Qual. 2010, 39, 1349–1358. [Google Scholar] [CrossRef]
- Song, X.; Li, C.; Chen, W. Phytoremediation potential of Bermuda grass (Cynodon dactylon (L.) pers.) in soils co-contaminated with polycyclic aromatic hydrocarbons and cadmium. Ecotoxicol. Environ. Saf. 2022, 234, 113389. [Google Scholar] [CrossRef] [PubMed]
- Płaza, G.A.; Nałecz-Jawecki, G.; Pinyakong, O.; Illmer, P.; Margesin, R. Ecotoxicological and microbiological characterization of soils from heavy-metal- and hydrocarbon-contaminated sites. Environ. Monit. Assess. 2009, 163, 477–488. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef]
- Yu, H.; Xia, Q.; Yan, J.; Herreno-Saenz, D.; Wu, Y.-S.; Tang, I.W.; Fu, P.P. Photoirradiation of polycyclic aromatic hydrocarbons with UVA light—A pathway leading to the generation of reactive oxygen species, lipid peroxidation, and dna damage. Int. J. Environ. Res. Public Health 2006, 3, 348–354. [Google Scholar] [CrossRef]
- Toyooka, T.; Ibuki, Y. New method for testing phototoxicity of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2006, 40, 3603–3608. [Google Scholar] [CrossRef]
- Guha, S.; Bhaumik, S.R. Transcription-coupled DNA double-strand break repair. DNA Repair 2021, 109, 103211. [Google Scholar] [CrossRef]
- Xue, W.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef]
- Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T. The Genotoxicity of Priority Polycyclic Aromatic Hydrocarbons (PAHs) Containing Sludge Samples. Toxicol. Mech. Methods 2007, 17, 1–12. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Li, H.; Qiu, B.; Gao, Y.; Cui, D.; Yang, Z. Antioxidant defense system in lettuces tissues upon various As species exposure. J. Hazard. Mater. 2020, 399, 123003. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2020, 11, 618835. [Google Scholar] [CrossRef]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J.I. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Tejeda-Agredano, M.C.; Jimenez-Sanchez, C.; Congiu, E.; Sungthong, R.; Niqui-Arroyo, J.L.; Cantos, M. Is it possible to increase bioavailability but not environmental risk of PAHs in bioremediation? J. Hazard. Mater. 2013, 261, 733–745. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Sheng, Y.; Gao, Y.; Zhao, Z. Phenanthrene-degrading bacteria on root surfaces: A natural defense that protects plants from phenanthrene contamination. Plant Soil 2018, 425, 335–350. [Google Scholar] [CrossRef]
- Manasa, M.; Kavitha, R.; Kumar, P.S.; Pooja, M.; Revathy, R.; Gayathri, K.V. Performance evaluation and mechanism analysis of halotolerant bacterial strains and cerium oxide nanoparticle to degrade Benzo[a]pyrene. Environ. Technol. Innov. 2021, 24, 101980. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, C.; Li, A.; Qiu, J.; Yan, W.; Dang, H. Effects of the plastic additive 2,4-di-tert-butylphenol on intestinal microbiota of zebrafish. J. Hazard. Mater. 2024, 469, 133987. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, J.; Li, J.; Zhou, L.; Zhang, H.; Sun, J.; Zhuang, S. The evaluation of endocrine disrupting effects of tert-butylphenols towards estrogenic receptor α, androgen receptor and thyroid hormone receptor β and aquatic toxicities towards freshwater organisms. Environ. Pollut. 2018, 240, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Kasai, D.; Iwasaki, T.; Nagai, K.; Araki, N.; Nishi, T.; Fukuda, M. 2,3-Dihydroxybenzoate meta-Cleavage Pathway is Involved in o-Phthalate Utilization in Pseudomonas sp. strain PTH10. Sci. Rep. 2019, 9, 1253. [Google Scholar] [CrossRef]
- Sun, H.; Lei, C.; Yuan, Y.; Xu, J.; Han, M. Nanoplastic impacts on the foliar uptake, metabolism and phytotoxicity of phthalate esters in corn (Zea mays L.) plants. Chemosphere 2022, 304, 135309. [Google Scholar] [CrossRef]
- Gao, M.; Dong, Y.; Zhang, Z.; Song, W.; Qi, Y. Growth and antioxidant defense responses of wheat seedlings to di-n-butyl phthalate and di (2-ethylhexyl) phthalate stress. Chemosphere 2017, 172, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Song, Q.; Li, X.; Zhao, Z.; Li, D.; Li, Y.; Hou, N. Insights into ecotoxicity effects of PAHs and ecosystem responses of remediation strategies under cold stress: From PAHs degradation to ecological restoration regulated by signal molecular. Chem. Eng. J. 2023, 475, 146042. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B.; Song, X.; Zeng, G.; Lee, K. Photocatalytic ozonation of offshore produced water by TiO2 nanotube arrays coupled with UV-LED irradiation. J. Hazard. Mater. 2021, 402, 123456. [Google Scholar] [CrossRef]
- Rabhi, L.; Cherifi, N.; Cecinato, A.; Lemou, A.; Balducci, C.; Romagnoli, P.; Ladji, R.; Yassaa, N. Concentration levels of anthropogenic organic compounds in suspended particulates matter and indoor settled from Algiers, Algeria. Atmos. Pollut. Res. 2024, 15, 102051. [Google Scholar] [CrossRef]
- Bao, H.; Wang, J.; Zhang, H.; Li, J.; Li, H.; Wu, F. Effects of biochar and organic substrates on biodegradation of polycyclic aromatic hydrocarbons and microbial community structure in PAHs-contaminated soils. J. Hazard. Mater. 2019, 385, 121595. [Google Scholar] [CrossRef]
- He, L.; Fan, S.; Müller, K.; Hu, G.; Huang, H.; Zhang, X.; Lin, X.; Che, L.; Wang, H. Biochar reduces the bioavailability of di-(2-ethylhexyl) phthalate in soil. Chemosphere 2016, 142, 24–27. [Google Scholar] [CrossRef]



| Soil Type | TN (G·KG−1) | TP (G·KG−1) | TK (G·KG−1) | CEC [CMOL(+)·KG−1] | Particle-Size (mm) Distribution/% | ||
|---|---|---|---|---|---|---|---|
| <0.002 | 0.002–0.02 | 0.02–2 | |||||
| BACKGROUND SOIL (B) | 0.57 | 1.27 | 17.24 | 8.20 | 0.37 | 99.63 | 0 |
| CONTAMINATED SOIL (C) | 0.55 | 1.37 | 20.07 | 4.51 | 0.11 | 99.37 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Sun, W.; Liu, X.; Chi, Z.; Sheng, H.; Liu, F. Comprehensive Ecotoxicity of the Complex System of Polycyclic Aromatic Hydrocarbon-Contaminated Sites to Wheat (Triticum aestivum L.) During Microbial Remediation. Sustainability 2025, 17, 4127. https://doi.org/10.3390/su17094127
Du X, Sun W, Liu X, Chi Z, Sheng H, Liu F. Comprehensive Ecotoxicity of the Complex System of Polycyclic Aromatic Hydrocarbon-Contaminated Sites to Wheat (Triticum aestivum L.) During Microbial Remediation. Sustainability. 2025; 17(9):4127. https://doi.org/10.3390/su17094127
Chicago/Turabian StyleDu, Xiaoli, Wenqian Sun, Xiaolu Liu, Zhongwen Chi, Huihui Sheng, and Fei Liu. 2025. "Comprehensive Ecotoxicity of the Complex System of Polycyclic Aromatic Hydrocarbon-Contaminated Sites to Wheat (Triticum aestivum L.) During Microbial Remediation" Sustainability 17, no. 9: 4127. https://doi.org/10.3390/su17094127
APA StyleDu, X., Sun, W., Liu, X., Chi, Z., Sheng, H., & Liu, F. (2025). Comprehensive Ecotoxicity of the Complex System of Polycyclic Aromatic Hydrocarbon-Contaminated Sites to Wheat (Triticum aestivum L.) During Microbial Remediation. Sustainability, 17(9), 4127. https://doi.org/10.3390/su17094127






