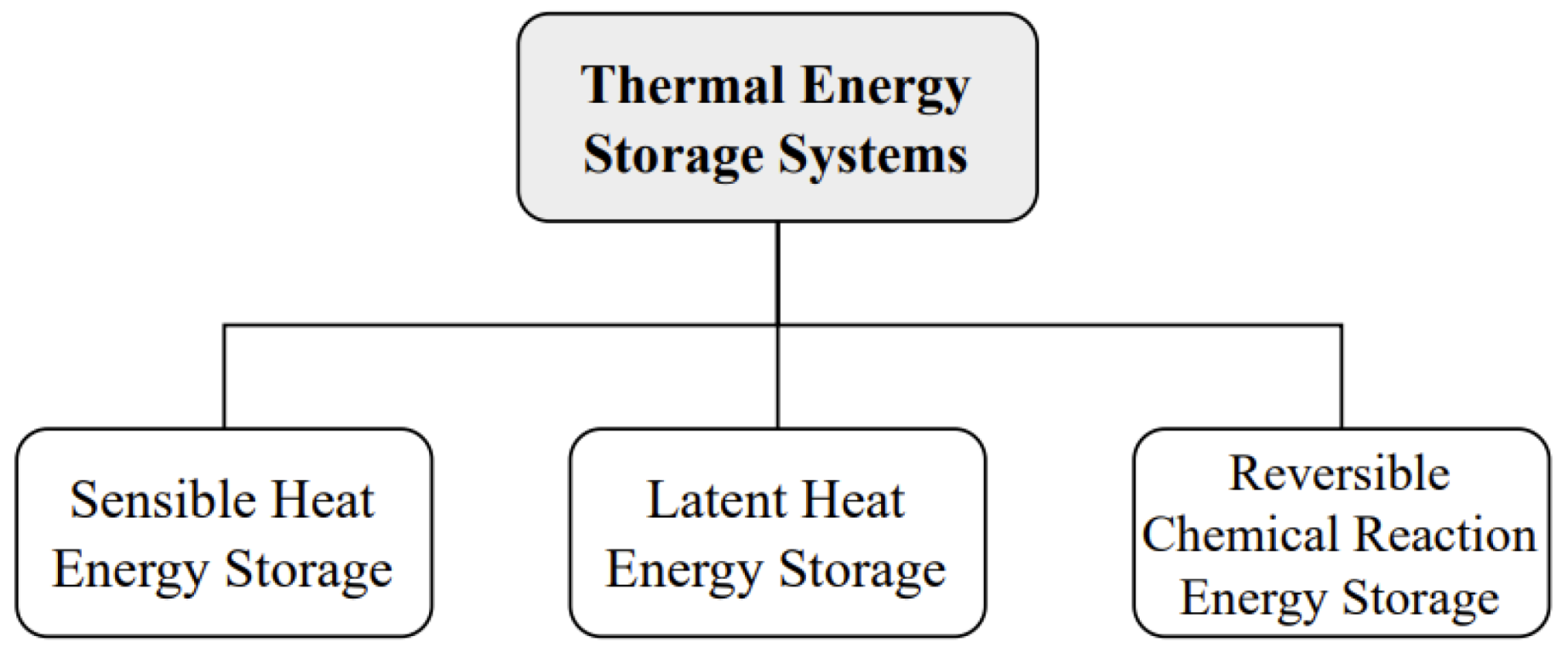
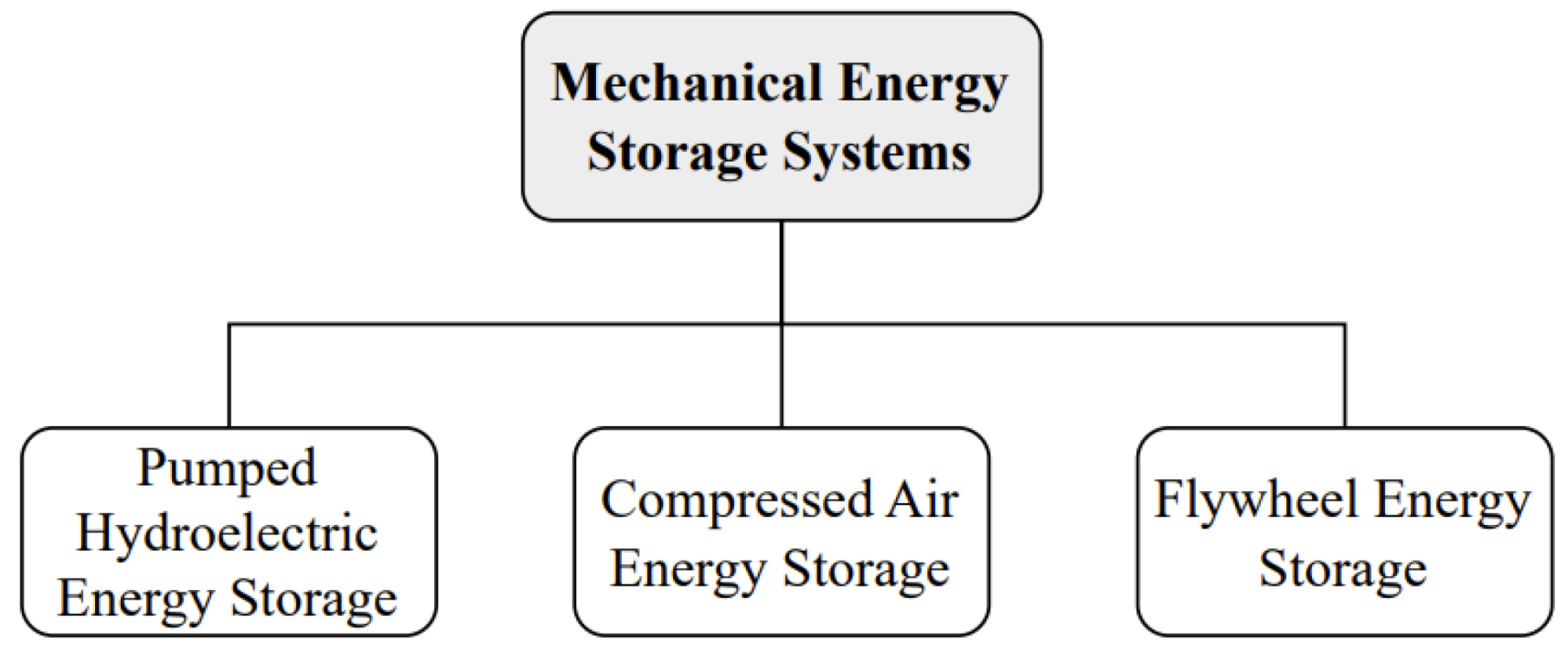
2.4.1. Conventional Batteries
The main components of a battery are the cathode, the anode, an electrolyte, and a separator. The storage capacity, discharge rate, and life cycle of these batteries depend on the type of materials used in its production. Typically, batteries are made up of nickel-related compounds like nickel metal hydrides (NiMH), nickel cadmium (NiCd), and nickel iron (NiFe), or zinc-related compounds like zinc silver oxide (ZnAg) and zinc manganese dioxide (ZnMn). Other types of batteries include lithium-ion batteries (Li-ion) and lead-acid batteries [
65]. The performance of these batteries depends on a variety of factors such as operating temperature, wattage, etc. If the batteries are non-rechargeable, they are called primary or single-use batteries, while rechargeable batteries are known as secondary batteries.
Zinc silver oxide batteries are perfect for both high- and low-current applications and have high durability along with a low environmental impact. However, the high cost of silver prevents these batteries from becoming the most popular among their counterparts. Hence, their use is mostly limited to low-current applications such as watches and hearing aids [
66]. They have also been used in many popular applications, such as in the Apollo command module. To this day, zinc silver batteries find uses in submarines and torpedoes [
67]. Recent developments have allowed for the manufacturing of rechargeable ZnAg batteries for high-current applications [
68].
Another commonly used type of battery is alkaline zinc manganese dioxide (ZnMn) batteries. These are suitable for operation in low-temperature conditions and in applications with high and low current drain. When compared with zinc silver batteries, alkaline zinc manganese batteries have a higher discharge rate than ZnAg batteries and are produced at much cheaper rates [
66]. However, their popularity and high usage has led to an increased amount of waste batteries; therefore, a better recovery and recycling strategy is needed [
69]. A lot of work has been carried out to improve these batteries. The use of nanomaterials has resulted in better and more durable batteries with longer life cycles [
70,
71]. The addition of bismuth has also resulted in better cycle life [
72]. Some studies have also focused on grid-level applications using flow-assisted alkaline batteries [
73]. In addition, 3D printing techniques have been used to produce printable zinc manganese cells [
74,
75].
Another popular type of battery is the lead-acid battery. Its high power output, low maintenance schedule and costs, greater operating temperature range, and longer life cycle before performance degradation are some of its standout qualities. It is the go-to battery for starting, lighting, and ignition (SLI) applications as well as for uninterruptable power supplies (UPS). There are a few varieties of lead-acid batteries available on the market, including valve-regulated lead-acid (VRLA) batteries and sealed lead-acid batteries. VRLA batteries are designed to be maintenance-free, while SLA batteries are more suited for rougher conditions and are resistant to shock, vibration, chemicals, and heat [
76]. However, lead-acid batteries are prone to sulfation in discharged states [
66]. Research has been conducted into making thin-film variations of lead-acid batteries, which would lead to lower manufacturing costs. The use of titanium oxide has led to improved specific energy in bipolar batteries [
77,
78].
2.4.2. Lithium Ion Battery
Li-ion batteries are known for their long life cycles, low self-discharge rates and shelf life, and lower costs compared to other rechargeable batteries. Due to their high specific energy, energy densities, and good performance at low temperatures, they are mostly used in portable electronics and sometimes in space applications [
66]. Despite all the pros mentioned in the preceding text, lithium-ion batteries are susceptible to thermal runaway, which can cause venting, explosion, or fire. It is imperative that a well-designed charging and monitoring schedule is implemented to prevent this. Lithium-ion batteries are difficult to recycle and are not suitable for large-scale applications [
79,
80].
When it comes to lithium-ion batteries, improvements have been made in a number of areas, with constant experiments and studies being conducted all over the world. The main areas for improvement have been better materials for anodes and cathode as well as better electrolytes. Anodes are generally made of graphite and are known for their low performance. Titanium niobium oxide (TNO) has been suggested as an alternative due to its high working voltage, safety, cycling stability, and pseudocapacitive behavior [
81]. However, its conductivity is low, and the use of carbon nanotubes and graphene have been proposed to improve this limitation [
82]. Other improvement methods that have been used include forming composites, doping, and size refinement [
83,
84,
85,
86,
87].
Another material that has been has extensively researched for use in anodes is transition metal oxide [
88,
89]. Ni(OH)
2 has been tested and researched as a viable option for anodes in lithium-ion batteries, but pure Ni(OH)
2 exhibits only 30% capacity after 50 cycles [
90,
91]. Cu(OH)
2 is another metal oxide that has been considered as a potential anode candidate in lithium-ion batteries [
92,
93]. Cu(OH)
2 nanoflower arrays consisting of 50% Cu(OH)
2 and 50% carbon nanotubes have shown a capacity retention of 95% after 50 cycles [
90].
2.4.3. Nickel Batteries
Several different elements have been tested with nickel to form a suitable and cost-effective energy storage system. The most popular among these have been the nickel metal hydride (NiMH) batteries. They are commercially available as sealed and maintenance-free batteries. They are in direct competition with nickel cadmium (NiCd) batteries. When compared with NiCd batteries, NiMH offers higher energy densities, higher charge/discharge cycles before degradation, and a longer shelf life. However, when compared with lithium-ion batteries, NiMH tends to have a lower charge retention and specific power, higher costs, and a limited operating temperature range. These negative aspects are the driving force behind NiMH’s replacement by lithium-ion batteries [
66]. There has been significant research on improving nickel metal hydride batteries. Some studies have focused on improving its low temperature performance and developing better electrodes [
94]. The use of cobalt, zinc, and manganese in the electrodes has improved its resistance to corrosion as well as its operating temperature range [
94,
95].
Another nickel-based battery is the nickel-cadmium battery. In terms of their construction, they are like NiMH, but instead of a metal electrode, they use cadmium. NiCd batteries are suitable for high power output, low-temperature operations, and long life cycles [
66]. Nickel cadmium batteries are best suited to applications with moderate discharge rates because they tend to exhibit a memory effect at low discharge rates. However, due to the toxic nature of cadmium, regulating authorities have favored NiMH over NiCd. Their use is now mostly restricted to aerospace applications.
Nickel iron (NiFe) batteries were once very popular due to their rugged construction and having the longest life cycle compared to contemporary batteries. They tend to have deep discharge capabilities and flexible charging schedules without any significant memory effect. However, their high cost of construction, high self-discharge rate, and internal resistance were the main factors for their phasing out after the advent of lead-acid batteries. Despite recent developments and improvements, their cost tends to be higher than that of lead-acid batteries but lower than nickel cadmium batteries [
66]. Further research has focused on the use of potassium-, lithium-, and sulfide-additive porous polymer separators and metal fiber electrodes. Heating during charge/discharge cycles, high self-discharge rates, and hydrogen venting issues are still the main concerns relating to nickel iron batteries [
96].
Another type of nickel battery is the nickel zinc (NiZn) battery. These have the best charge/discharge rates and low environmental impacts. Their commercial utilization is marred by their higher cost of construction and very short cycle life. Most of the recent research has focused on increasing the cycle life of nickel zinc batteries. The stability of the zinc electrode is the main cause of this shorter cycle life. Use of heavy metals such as mercury and hydroxides have improved the stability of zinc electrodes and hence the cycle life. Electrolytes with reduced alkalinity also tend to have a positive effect on life cycles [
96]. The use of Bi
2O
3 coating on zinc anodes decreases the dissolution of discharge residues and protects the zinc anode from dendrites formation and shape changes [
97]. The use of polyethylene glycol (PEG-300) as a protective layer in the electrolyte has also been demonstrated to prevent the self-corrosion of zinc electrodes [
98].
In
Table 6, common batteries, lithium-ion batteries, and nickel batteries are compared based on their parametric performance.
Lead-acid batteries are the main contender in many industrial and residential backup power systems due to their higher number of charging cycles and longer lifespan. These batteries can provide high power and withstand higher operating temperatures than their counterparts.
Table 6 and
Table 7 compare the eight mostly widely used batteries around the world. Nickel batteries are often used at the industrial level. However, for residential consumers, lithium batteries have been the go-to in many electronic products for many years now due to increasing battery capacities and moderate power capabilities.
2.4.4. Molten Salt Batteries
In recent years, several non-traditional or emerging batteries have been made, tested, and improved. Since they are still in the early stages of their development, their applications are limited. One such technology uses molten salts, which simultaneously act as electrodes and electrolytes [
102]. Sodium sulfur (NaS) and sodium nickel chloride (NaNiCl) are the two examples of such batteries. The most common application of sodium sulfur batteries is load leveling [
103,
104]. The operating temperatures of NaS batteries are within the range of 300–350 °C, which is much higher than traditional chemical batteries. They are cheaper than their counterparts and have seen a slower but steadier rise in commercial applications. Other applications include use in electric vehicles and grid voltage regulation [
105,
106], which could resolve the challenges behind electric vehicles’ [
106] slow penetration into the market [
107]. These batteries have also been proposed for a mission to Venus [
108]. NaS batteries are known for their high power and energy density and are tolerant to external temperature variations. They have very low self-discharge rates and require little maintenance. They are also cheap to manufacture. However, thermal management is one of the main challenges associated with NaS batteries [
66]. A heating mechanism is required to continuously maintain the operating temperature of the batteries. The heat produced during charging/discharging cycles is also used for this purpose. The efficiency of the batteries is reduced if the temperature falls below the operating range of 300–350 °C [
104]. These batteries require not only a heating system but also a distribution system to evenly distribute the produced heat [
109]. Well-designed insulation also reduces heating costs and helps retain heat for longer durations. Micro-porous materials and fiber boards are the most used insulators for this purpose. Another method involves the use of variable conductance insulators [
110].
When it comes to NaS batteries, safety issues have always been a prime concern, and several tests have been proposed to evaluate them [
111]. These batteries have also been linked to the infamous incident at the Tsukuba plant [
112]. One of the methods for mitigating risks is to build a well-designed spill-free enclosure by lining the vessels with chromium and molybdenum. Lowering the operating temperature of batteries below 100 °C also reduces the corrosion of electrodes, which, in turn, prolongs the battery’s life. However, this step results in a slight amount of polysulfide reactions with electrodes [
113].
NaNiCl batteries have primarily been designed for mobile applications and have thus undergone rigorous testing regimes including crash tests, fire exposure, water immersion, and short-circuiting [
66]. NaNiCl batteries operate at a lower temperature (270 °C) compared to NaS batteries and thus have a lower negative environmental impact [
113]. A battery named ZEBRA has been developed for electric vehicles [
114]. This battery takes its name from the project under which it was developed, namely the Zeolite Battery Research Africa Project. These batteries are typically of 38 Ah and operate within 234–339 °C. NaS and NaNiCl batteries are some of the viable alternatives to traditional lithium-ion batteries because of their energy storage capacities and ruggedness.
2.4.5. Metal-Air Batteries
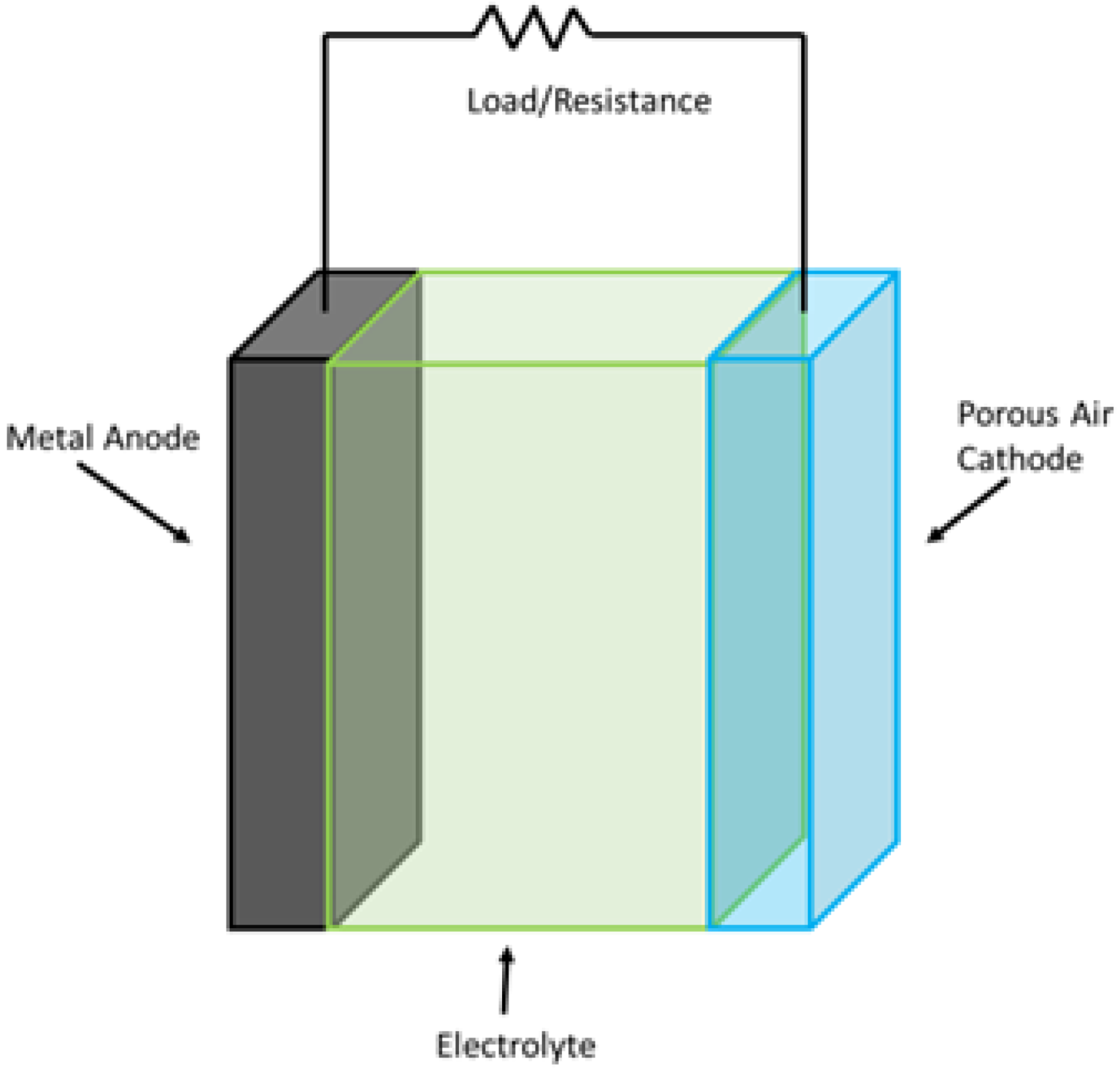
This class of batteries is composed of a metal electrode and an oxygen electrode. The metal electrode acts as an anode and is usually made up of lithium, zinc, iron, or aluminum. A specially designed separator separates an air- and carbon-based cathode, which acts as a catalyst to produce hydroxyl ions. A layout diagram of the battery is shown in
Figure 9. When compared to other chemical-based energy storage systems, these batteries tend to offer higher energy densities. Moreover, lithium-air batteries offer energy densities that are comparable to those of fossil fuels.
One of the primary factors limiting the commercialization of these batteries is the hydrogen evolution reaction of the anode, due to which the anode’s life is severely shortened. Different methods have been proposed and tested to overcome this problem. One such solution involves the introduction of a bifunctional membrane between the metal anode and the electrolyte. This membrane performs two functions: allowing the entry of excess water and the transport of hydroxide ions. In the case of aluminum-air (Al-air) batteries, this membrane is made up of Al
2O
3@PAN. By the introduction of this membrane, a utilization rate of 61.2% of the aluminum anode can be achieved [
115].
Another variation of metal-air batteries is the zinc air (Zn-air) battery. Its pros compared to other metal-air batteries include flat discharge curves at low current drains, lower costs compared to its counterparts, and a long shelf life when inactivated [
66]. However, Zn-air batteries are sensitive to humidity and temperature variations. Zinc air batteries are suited for electric vehicle applications, and with the introduction of mechanical recharging by replacing the anode, they are capable of providing high energy densities [
116,
117]. They can be further improved by utilizing them with a mixture of nickel cadmium and manganese dioxide. Zn-air batteries are commonly utilized in electronics such as hearing aids.
Iron-air (Fe-air) batteries are yet another type of liquid metal-air battery. Fe-air batteries do not suffer from the corrosive effects of hydroxides as much as other metals like zinc or aluminum. This results in a longer service life and makes them suitable for large-scale applications. Despite these advantages, Fe-air batteries suffer from poor charging/discharging efficiencies. Some improvements have been observed using bismuth sulfide, carbonyl iron, and magnetite additions [
118]. Recent improvements have increased the cycle life up to 5000 cycles and made the battery about 80% more efficient. Despite these improvements, these batteries currently seem to be a long way from commercialization. Two varieties of metal-air and molten salt batteries are compared in
Table 8.
Molten salt batteries may not yet be the best option for many applications due to their short life cycles, high discharge rate, and higher costs. However, with more research and better products, they have the potential to replace many conventional batteries. On the other hand, metal-air batteries offer very low or negligible discharge rates along with long lifespans.
2.4.6. Fuel Cells
Fuel cells can be classified as both generation and storage devices. Fuel cell devices are made by stacking several single cells together to make high-voltage assemblies. Each cell is composed of three parts: electrodes, electrolyte, and the catalyst. The electrolyte is sandwiched between the two electrodes. Fuel can take the form of hydrogen, methanol, natural gas, ammonia, or other hydrocarbon gases. Other systems such as fuel and waste management and power conversion are also part of the complete fuel cell systems [
66]. Although these cells use hydrocarbons for generation, the efficiencies of fuel cells are not limited by the Carnot cycle. They are also more environmentally friendly when compared to other similar generation and storage devices.
The major hurdles to the commercialization of fuel cells are related to its short lifespan and high costs. These cells have better energy densities compared to other chemical batteries, except for metal-air batteries. Impurities found in fuel cells can significantly reduce their performance. These impurities can produce corrosive gases which have a long-term negative impact on the environment and ecology [
120].
In the subsequent sections, only four of the many existing fuel cell topologies are discussed: proton exchange membrane fuel cells (PEMFCs), molten carbonate fuel cells (MCFCs), direct methanol fuel cells (DMFCs), and solid oxide fuel cells (SOFCs).
PEMFCs work by directly converting hydrogen and oxygen for energy production. They require an operating temperature of about 70–85°C, and for this purpose, they are coupled with NiMH batteries to provide the optimal temperature. When compared to Li-ion batteries, PEMFCs have a better energy density but are expensive due to the higher costs of catalysts and membranes. As mentioned earlier, these cells are highly sensitive to impurities and thus have shorter lifespans. Cells come in different sizes, and each has its own pros and cons. Small cells can utilize replaceable storage cartridges. For this purpose, metal hydrides, chemical hydrides, compressed gas, and carbon hydrides have been considered. Hydrides require either high or very low cryogenic temperatures for hydrogen retention and release. However, there is a long way to go before these fuel cells can be commercialized.
DMFCs can operate at low temperatures by directly converting methanol into electricity and are perfect for small applications. However, their performance is marred by a slow startup time, low power density, and issues related to toxicity [
121]. Catalysts can significantly increase the total cost of these fuel cells and thus remain a focus of research. Catalysts like rhodium, plutonium, and palladium can be expensive, while cheaper alternatives include zirconium alloys and nickel [
122]. Modern research has focused on the biogenic synthesis of platinum from plant extracts to lower costs and improve catalytic activity [
123]. Another study has focused on the use of TiCN coatings for use in DMFCs. The coatings were made at an average thickness of 15µm and have been recognized for their toughness and high electrical conductivity [
124]. Nanocrystalline cellulose (NCC) membranes have also been studied for use in DMFCs [
125]. Moreover, maintaining an optimal methanol concentration in DMFCs is of paramount importance, and the use of graphene aerogel (GA) on the membrane electrode assembly has been suggested to achieve this [
126].
MCFCs are known for large grid-scale applications and generally have an operational temperature within the range of 600 °C. This allows hydrocarbon fuels to be converted into rich hydrogen gas. Of the three fuel cells mentioned so far, MCFCs are preferred for their high resistance to impurities and lower costs of production and operation. Special emphasis is given to electrode development, as it must have high porosity, mechanical resistance, and electrical conductivity, and low solubility [
127]. The electrodes used in MCFCs are a constant area of research and improvement. The effect of pore size distribution inside the cathode has also been observed and studied, as it governs the electrolyte–gas interaction area [
128]. The cathode is often made up of NiO and thus has high internal resistance and low power density in MCFCs. A three-layered cathode made up of porous silver tape cast on a standard NiO cathode with the addition of nickel foam as a support layer results in decreased resistance and high power density [
129].
SOFCs are yet another type of fuel cell that operates at around 1000 °C with very high efficiency. In terms of their applications, these cells are used more often than MCFCs. The high temperature requirements of these cells result in additional heating costs and increased startup and shutdown times. Therefore, lowering the operational temperature of SOFCs has been an area of active research and experiments over the past few years. However, reducing the operational temperature results in a reduction in the conductivity of the cells. Therefore, newer electrolytes and membranes have been suggested for SOFCs [
130]. Recent research has focused on the search for better electrolytes and electrodes. LiNi-oxide has been proposed for symmetrical electrolytes and electrodes in low-temperature SOFCs [
131]. It has also been observed that the addition of reduced graphene oxide to the cathode functional layer improves the peak performance of cells by roughly 26%. This is due to the high electrical properties of graphene [
132]. Ni-free anodes for SOFCs have also been suggested that can directly utilize anhydrous ethanol with the help of copper-based anodes [
133].
Table 9 provides a comparison of their parametric performance values.
The numbers representing fuel cells may not be as impressive as those for other chemical energy storage systems, but with recent progress in industry, and thanks to initiatives taken by a few major industries, there are now hybrid vehicles on the road and many other applications are under development. However, fuel cell technology still requires a lot of innovation and research to make it a household name.
2.4.7. Flow Batteries
In flow batteries, active materials dissolved in the electrolyte traverse the cell to generate electricity. Flow batteries can be categorized into two types—redox flow batteries and metal/halide batteries—each having its own pros and cons. Redox flow batteries are made up of several bipolar cell stacks connected in parallel or series, a couple of tanks to hold the anolyte and catholyte, and a pumping system that is responsible for the flow of these active materials. These batteries are often used for grid-scale storage applications and their state of charge can be estimated by the color of the electrolyte. They are also suitable for applications involving the repeated deep discharge of batteries and are thus suitable for many applications [
134]. The overall cost of this type of battery could be estimated by the cost of electrolyte in them [
135].
In this section, three different types of flow batteries will be discussed: vanadium redox batteries (VRBs), zinc bromine batteries (ZBBs), and polysulphide bromine batteries (PBBs). VRBs are, without question, the best flow battery at the moment and also the most widely researched. They are known for their long service life along with constant deep discharge cycles and non-toxic materials. These batteries contain two variants of vanadium dissolved in the electrolyte [
134]. Comparatively, they are currently more expensive than lead-acid batteries, but trends in the research have shown that they will soon be more economical.
The flexibility in the design of VRBs is astonishing. Large tanks for holding the electrolytes—one for the catholyte and one for the anolyte—can be built underground, which further prolongs the system’s lifespan by protecting it from the environment. These tanks can be made from various polymers. VRBs are also safe from the risk of short-circuiting [
135]. Recent research on the development of VRBs has been multifaceted. Finding, testing, and developing new electrodes for flow batteries is often challenging and time-consuming. A novel technique for quickly scanning and testing carbon-based nanostructured materials as potential electrode candidates has been proposed [
136]. The addition of chloride ions to the electrolytes can help increase the vanadium utilization ratio to 86.3% and the energy efficiency to 82.5%. These values are higher than those for electrolytes without additive chloride ions [
137]. Other areas of research have focused on overcoming water and electrolyte imbalances and finding an optimal electrolyte flow rate control [
138,
139].
There have also been studies that have focused on finding cheaper alternatives to VRBs. One such variant is PBBs. Despite being cheap, they suffer from problems like corrosion, cross-contamination, and electrolyte imbalance. Another bromine variant, ZBB, is also available as a potential candidate for future promising technologies for large scale energy storage. They have high specific energy and efficiency, a long lifespan with repeated deep discharge cycles, and low manufacturing costs, thus remaining a topic of research and development. One of the challenges faced by zinc batteries is their low operating voltages—below 1.8 V. An alkaline–acid hybrid electrolyte has been suggested to improve voltages up to 3V [
140]. One study has focused on carbonized Pomelo peel as an electrode substitute; the battery was observed for 100 cycles and no electrode degradation was observed [
141]. Some researchers have focused on incorporating manganese into variants of flow batteries to achieve better performance [
142,
143].
Table 10 provides comparisons of their parametric values.
Flow batteries offer higher efficiencies compared to other chemistries, but their low energy and power density are among their biggest disadvantages. Flow batteries can be widely commercialized if they have higher energy and power densities along with corrosion-resistant materials. Another limitation is the requirement of larger electrolyte tanks to increase battery capacity.