Driving Effects of Coal Mining Activities on Microbial Communities and Hydrochemical Characteristics in Different Zones
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Overview and Sample Collection
2.2. Hydrochemical Characterization
2.3. DNA Extraction and 16S rRNA Gene Sequencing
2.4. Tools for Processing and Statistical Analysis
3. Results and Discussion
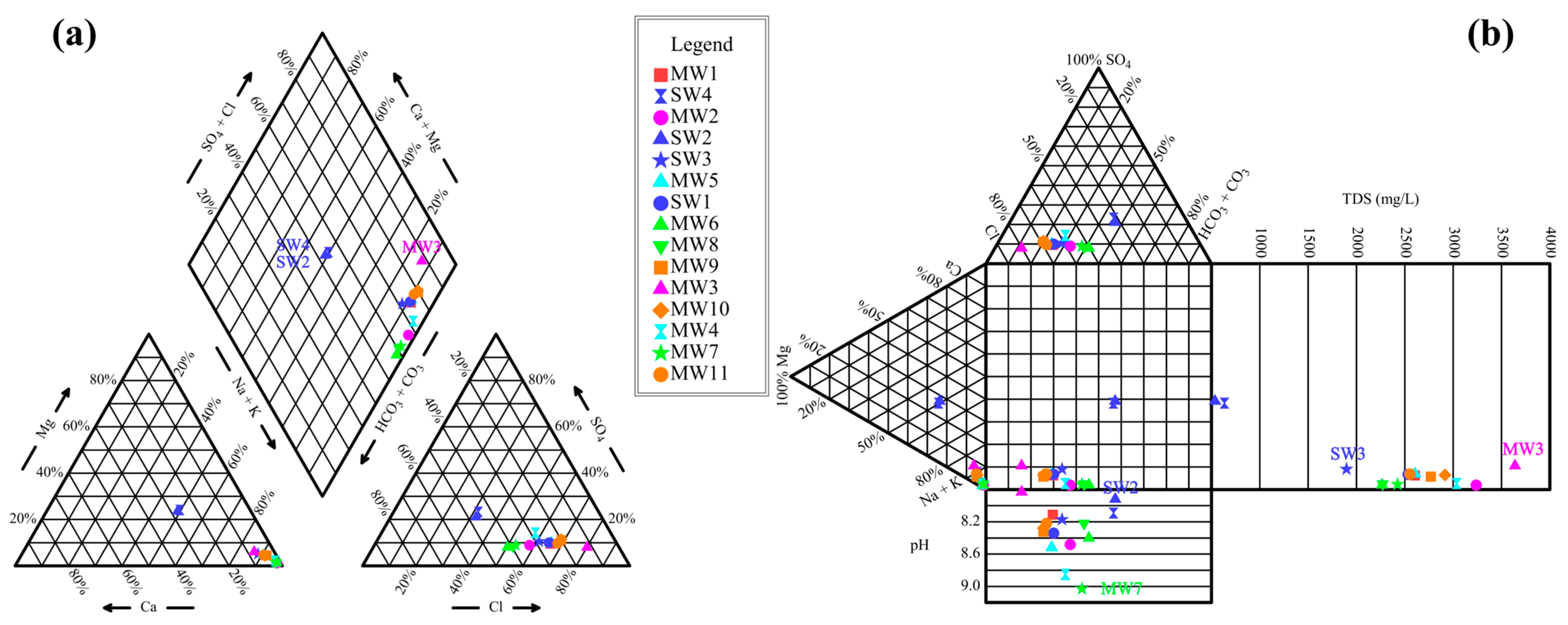
3.1. Spatial Distribution Characteristics of Hydrochemical Components
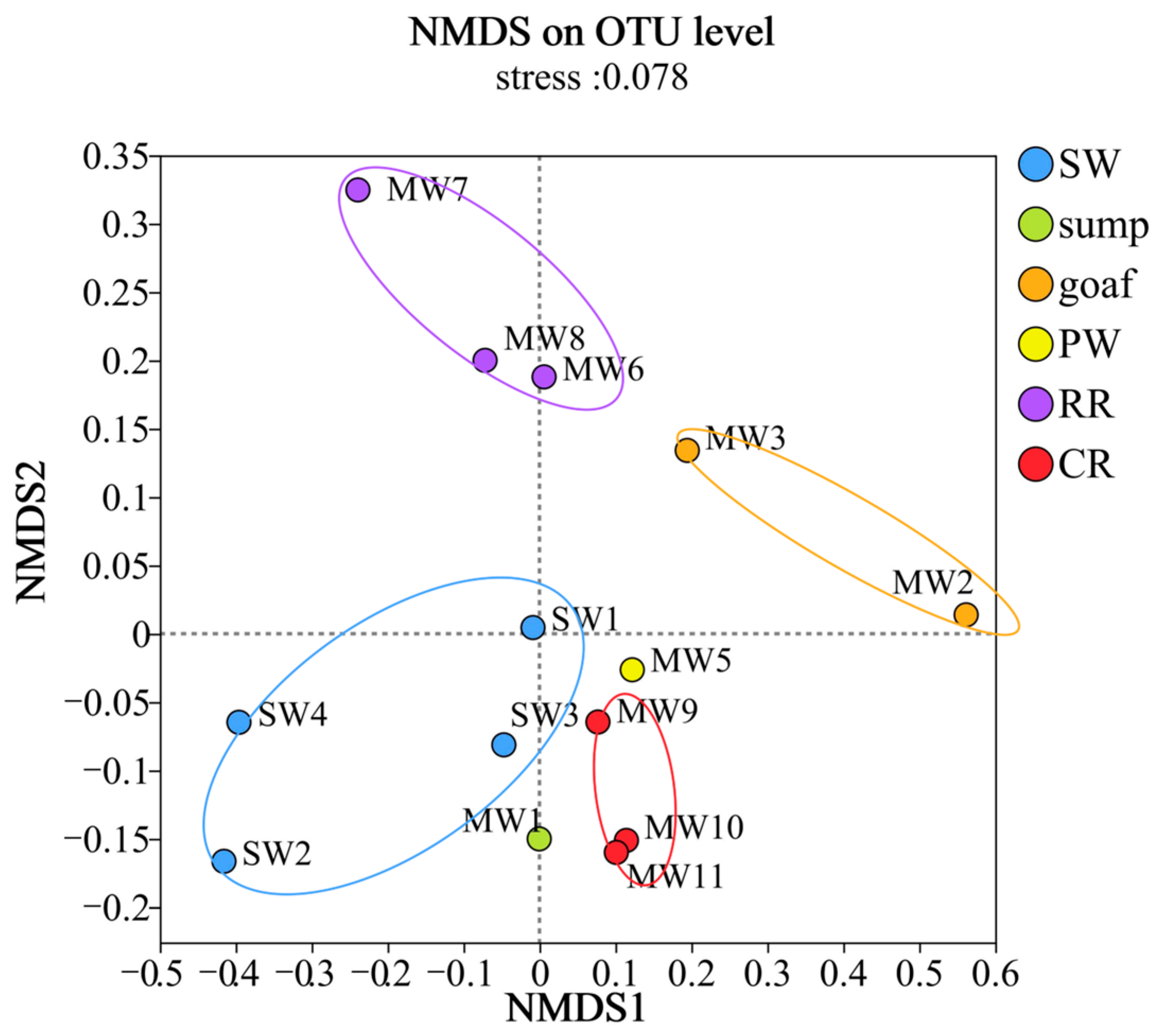
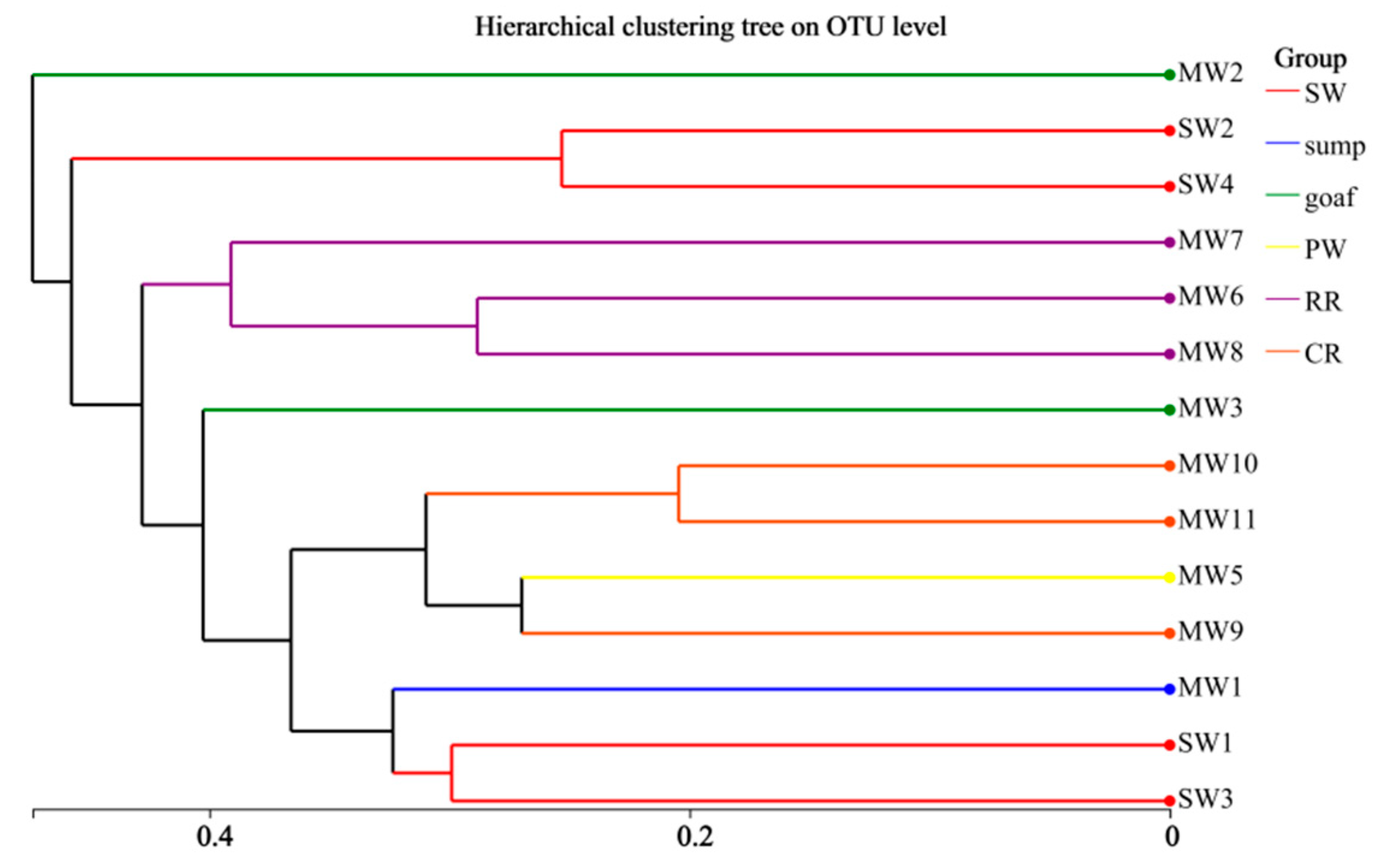
3.2. Differences in Microbial Diversity and Community Structure Across Zones
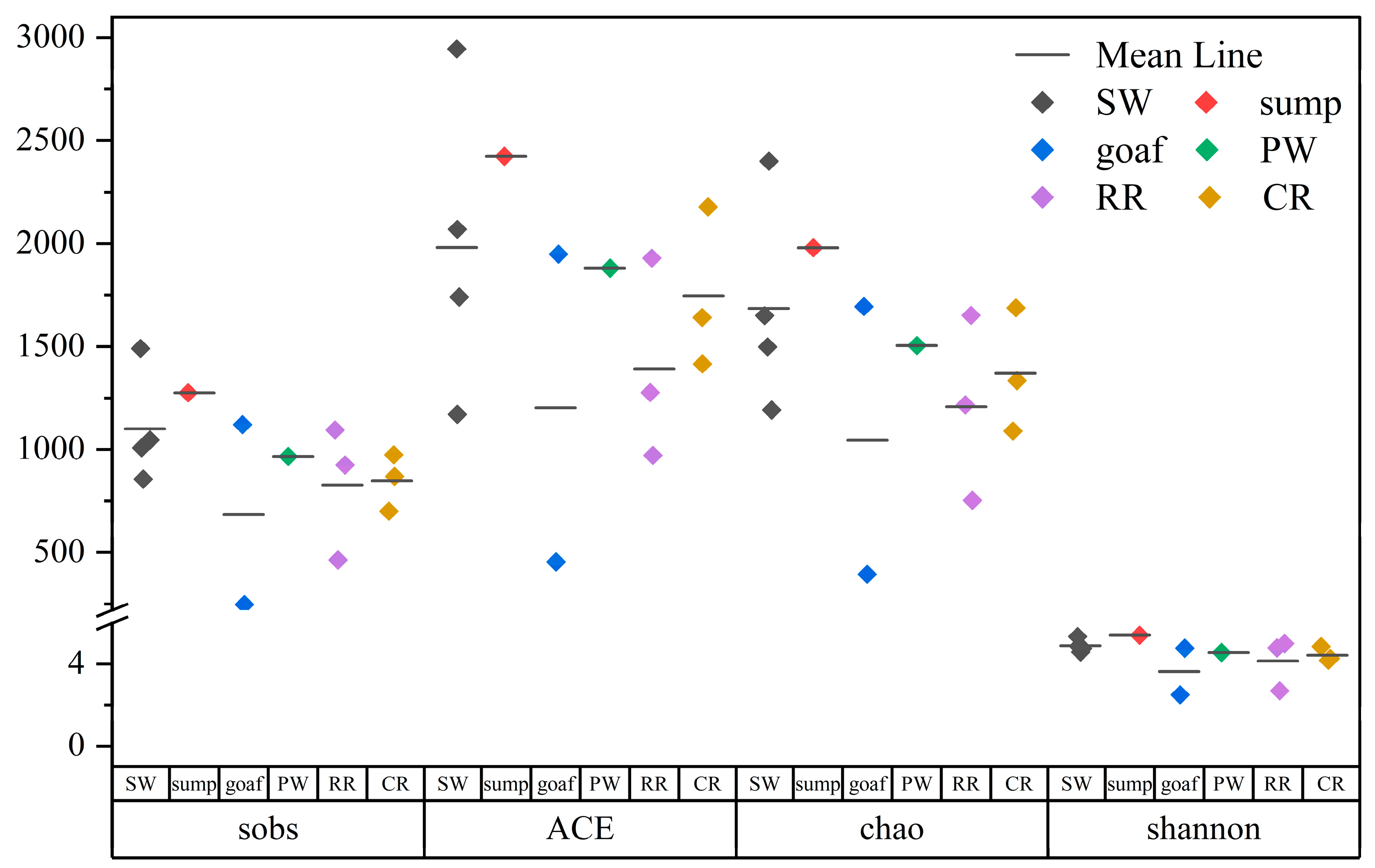
3.2.1. Analysis of Microbial Community Diversity
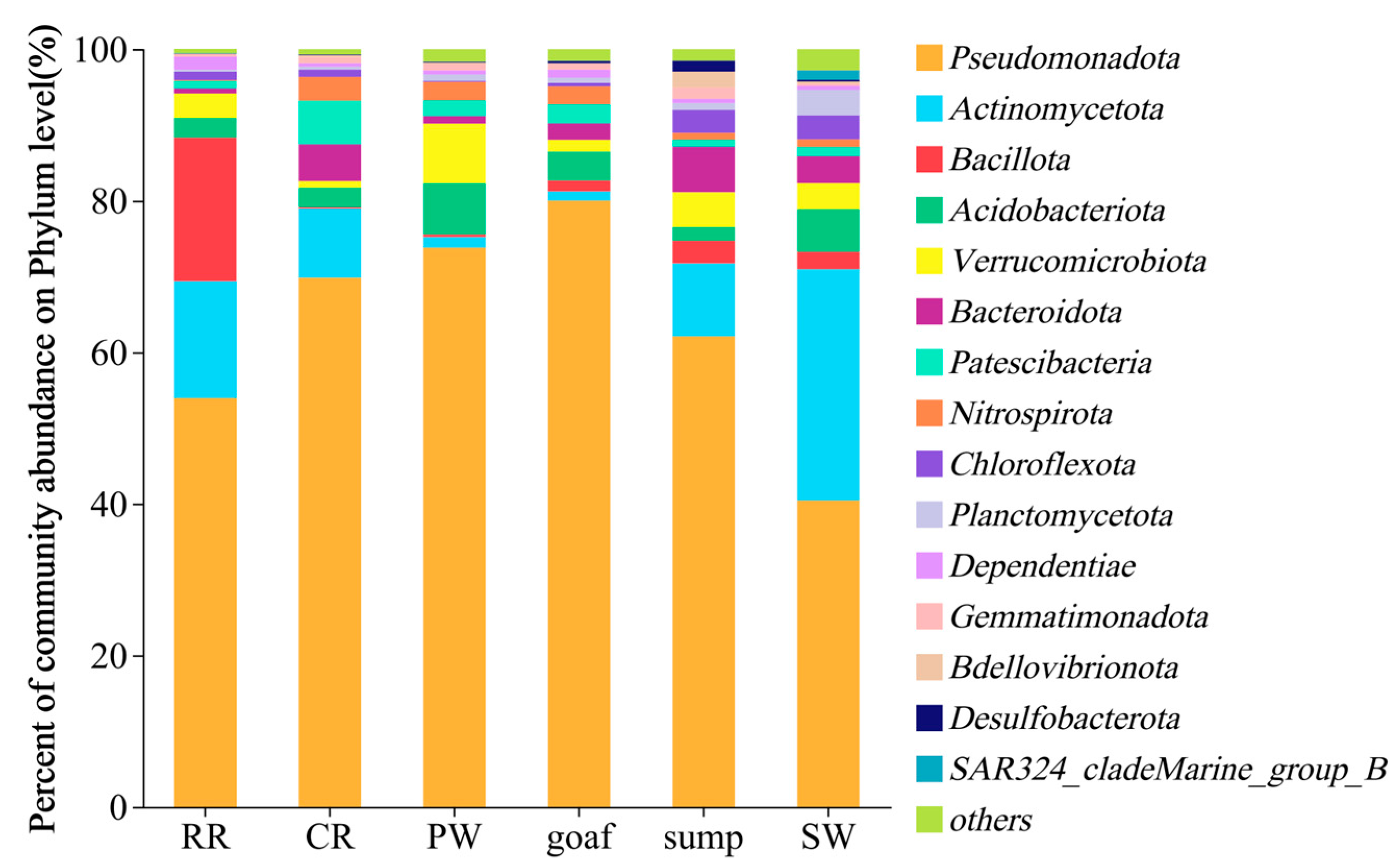
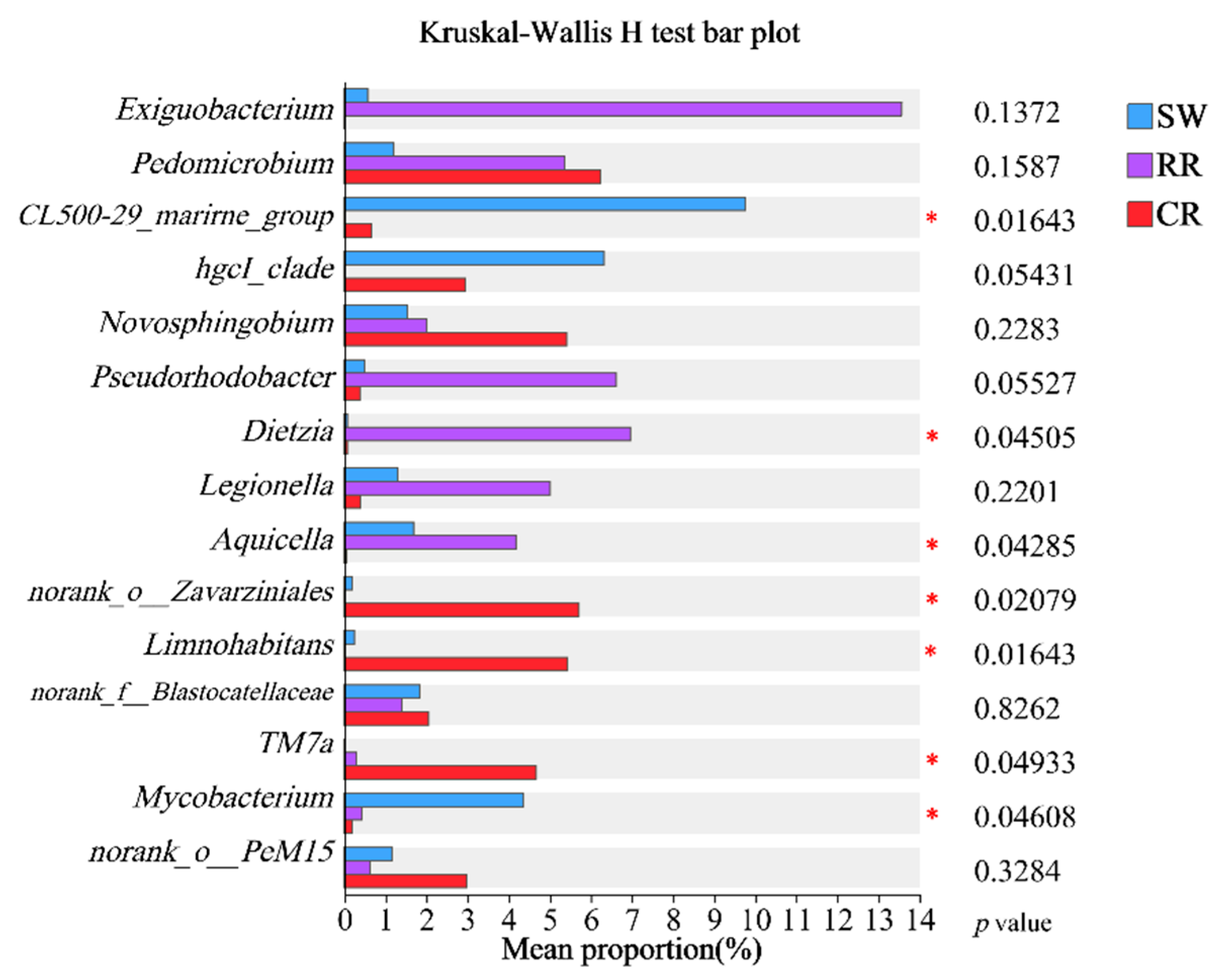
3.2.2. Composition and Differential Analysis of Microbial Community Structures
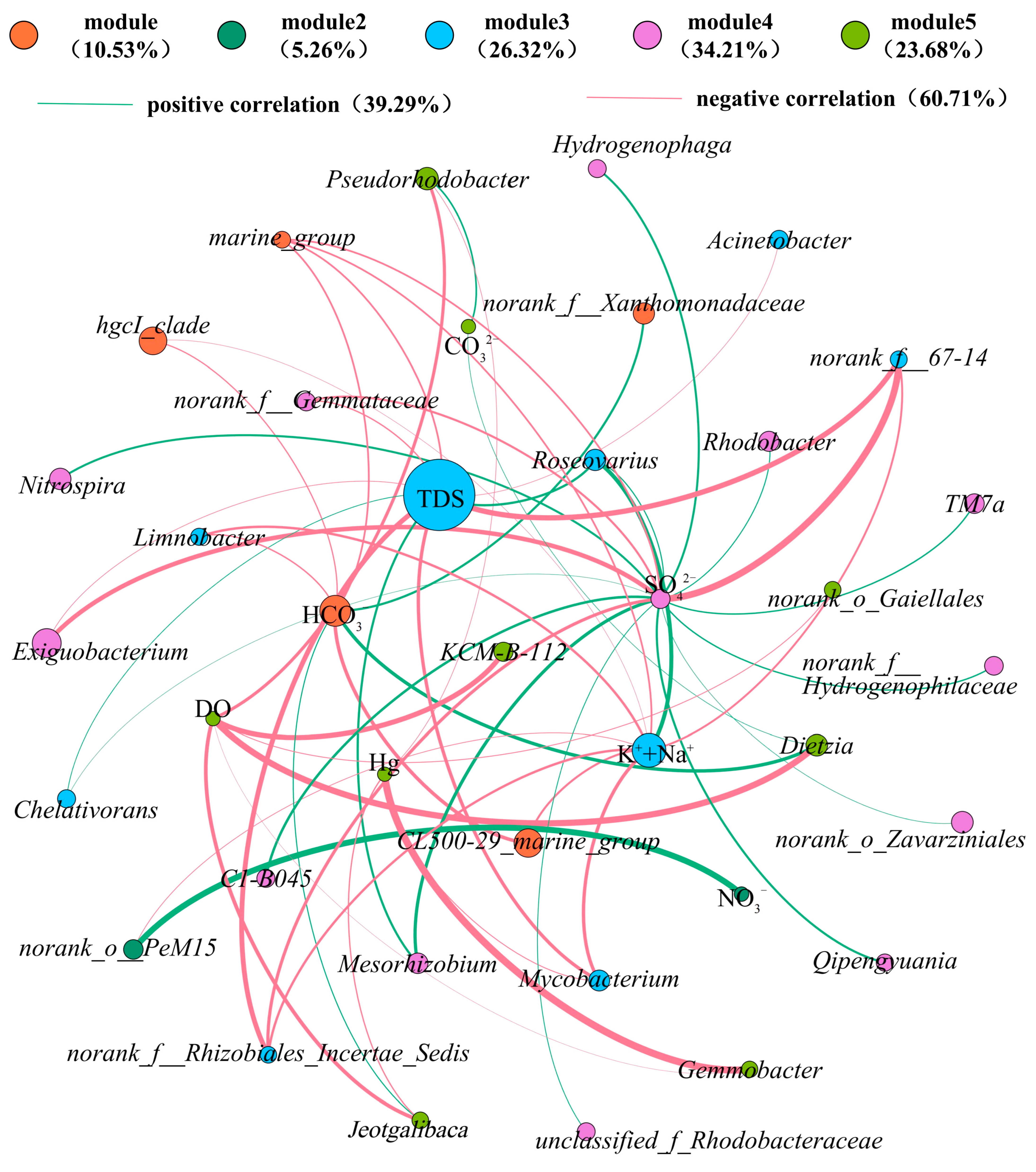
3.3. Correlation Between Microbial Communities and Environmental Characteristics
3.4. Impact of Mine Drainage on Microbial Community Structures in Surface Rivers
4. Conclusions
- (1)
- The hydrochemical type of mine water in the Xinji mining area is Cl-Na, while surface water exhibits a HCO3−Na·Ca type. This distinct difference indicates weak hydraulic connectivity between mine aquifers and surface water. The hydrochemical characteristics of goaf water are closely related to closure duration, sealing efficiency, and associated minerals in coal. Microbial community richness and diversity across six functional zones follow the order: sump water > surface water > working face drainage > coal roadway water > rock roadway water > goaf water. After sump water is discharged to the surface, changes in temperature, light exposure, and oxygenation promote the growth of specific bacterial genera, resulting in the highest richness at the surface drainage outlet (SW1).
- (2)
- At the phylum level, Pseudomonadota is the most abundant, followed by Actinomycetota. Aerobic Actinomycetota dominates surface water samples. At the genus level, dominant taxa include Erythrobacter, Pedomicrobium, and Novosphingobium. CL500-29_marine_group, previously identified as a dominant genus in surface water systems such as rivers and lakes, shows high abundance in surface water samples. The rock roadway water samples exhibit notably high abundance of Dietzia, a genus with hydrocarbon degradation capabilities.
- (3)
- Environmental factors including K+ + Na+, SO42−, HCO3−, CO32−, pH, and DO exhibit strong correlations with microbial community structures. The negative correlation between microorganisms and TDS indicates that most genera cannot adapt to high-salinity environments. Sulfur, as a critical energy-supplying element for microbial survival, is closely linked to bacterial community distribution. Microbial communities responsive to SO42− hold significant importance for groundwater pollution control in coal mining areas using microbial technologies. Low DO levels in mines, caused by poor hydraulic conditions and inadequate ventilation, hinder the formation of stable microbial communities compared to surface environments.
- (4)
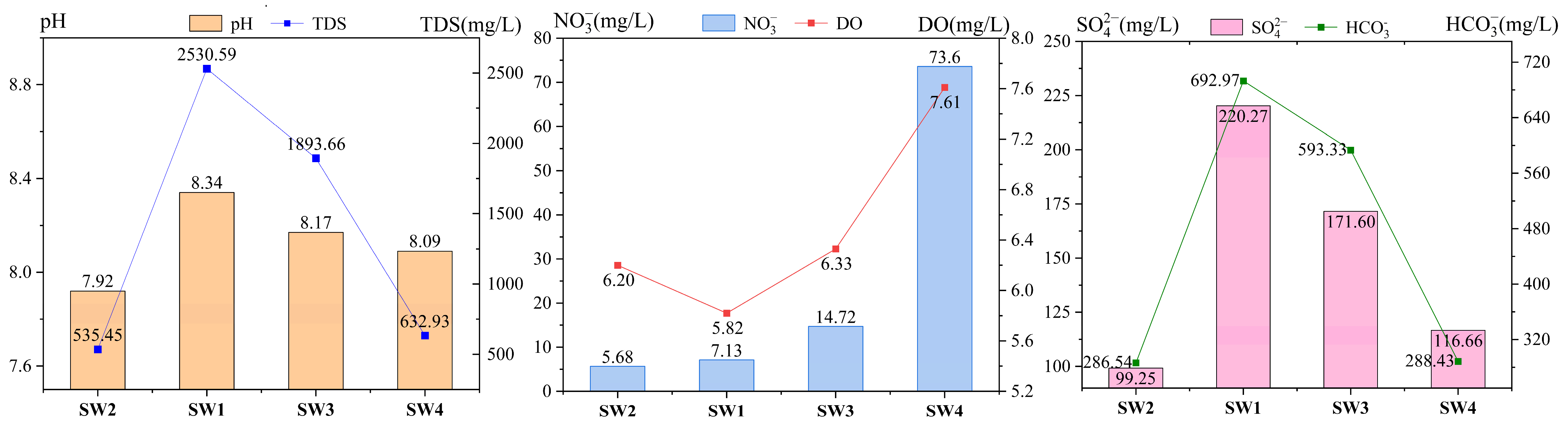
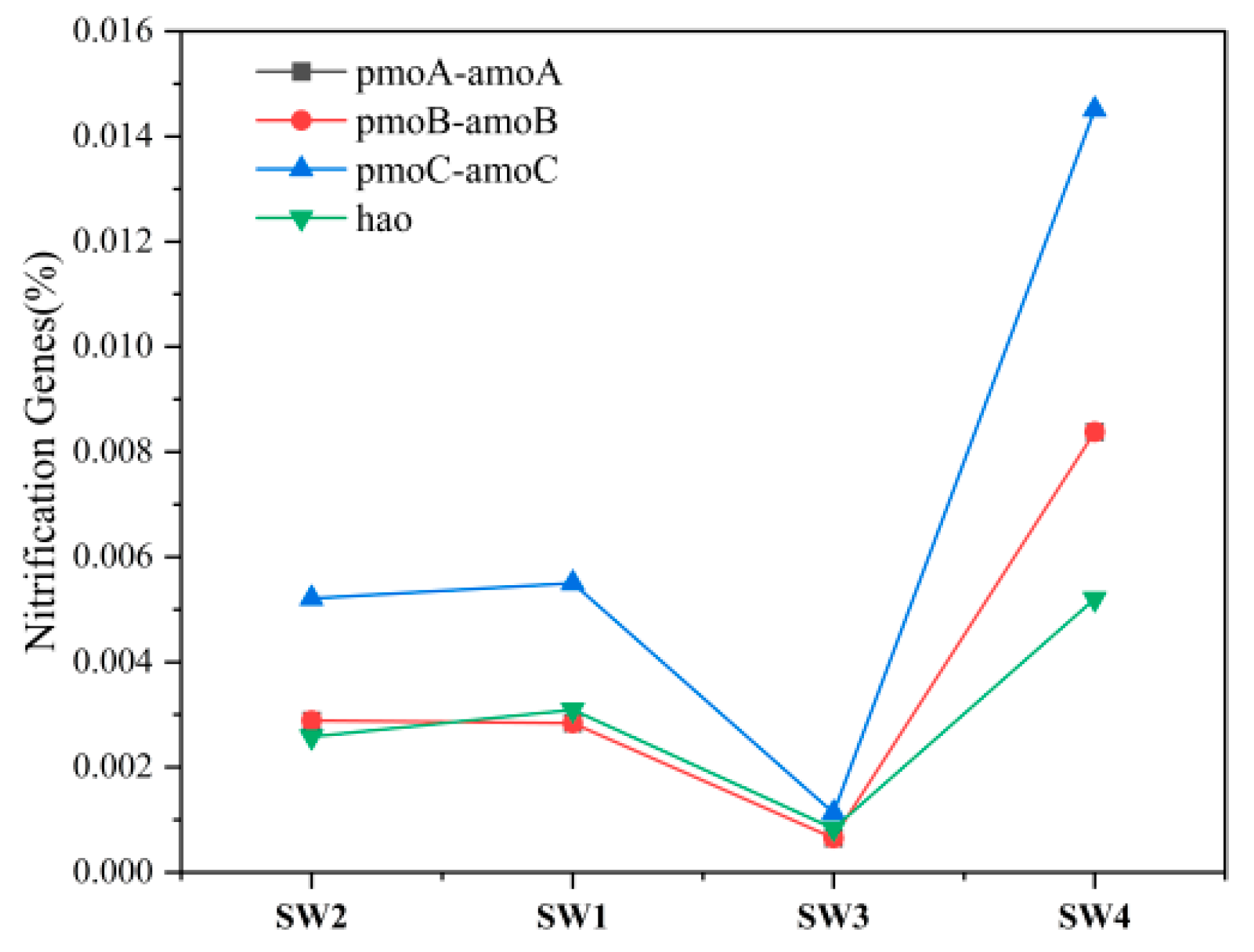
- After mine drainage enters the river, the confluence zone (SW3) shows a significant increase in HCO3− concentration—a byproduct of microbial organic degradation—due to its higher microbial richness compared to upstream. Additionally, the discharge of mine water with elevated SO42− concentrations raises SO42− levels at the confluence above upstream values. Downstream, microbial richness returns to upstream levels, accompanied by a decline in HCO3−. SO42− concentrations also decrease to upstream levels due to sulfate reduction by SRB (e.g., norank_f__Desulfuromonadaceae). However, NO3− concentrations increase more than tenfold downstream, suggesting that nitrogen cycle-related bacteria and organic matter from mine water enhance microbial activity, driving continuous nitrate accumulation in surface water.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Zhang, L.; Xu, Z.; Chen, G.; Zhao, X.; Li, X.; Gao, Y.; Zhang, S.; Zhu, L. Multi field action mechanism and research progress of coal mine water quality formation and evolution. J. China Coal Soc. 2022, 47, 423–437. [Google Scholar]
- Sun, W.J.; Ren, S.L.; Wu, Q.; Dong, D.; Gan, X. Water pollution’s prevention and comprehensive utilization of abandoned coal mines in China under the new normal life. J. China Coal Soc. 2022, 47, 2161–2169. [Google Scholar]
- Price, P.; Wright, I.A. Water Quality Impact from the Discharge of Coal Mine Wastes to Receiving Streams: Comparison of Impacts from an Active Mine with a Closed Mine. Water Air Soil Pollut. 2016, 227, 155. [Google Scholar] [CrossRef]
- Cao, Q.; Yan, R.; Yang, L.; Takaoka, M. Effects of water-coal interactions on coal mine water quality in China: A lixiviation experiment and actual water quality investigation. Environ. Sci. Pollut. Res. 2023, 30, 107350–107364. [Google Scholar] [CrossRef]
- Gu, D.; Li, T.; Li, J.; Guo, Q.; Jiang, B.; Bian, W.; Bao, Y. Current status and prospects of coal mine water treatment technology in China. Coal Sci. Technol. 2021, 49, 11–18. [Google Scholar]
- Yang, F.; Liu, S.; Jia, C.; Wang, Y. Identification of groundwater microbial communities and their connection to the hydrochemical environment in southern Laizhou Bay, China. Environ. Sci. Pollut. Res. 2022, 29, 14263–14278. [Google Scholar] [CrossRef]
- Méheust, R.; Castelle, C.J.; Carnevali, P.B.M.; Farag, I.F.; He, C.; Chen, L.-X.; Amano, Y.; Hug, L.A.; Banfield, J.F. Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog. Isme J. 2020, 14, 2907–2922. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Zhong, M.; Qi, P.; Xu, Z. Mutual Effects of Temperature and Microorganisms on the Chemical Characteristics of Coal Mine Drainage. Geomicrobiol. J. 2023, 40, 676–687. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, C.; Wang, H.; Lu, G.; Yang, C.; Chen, M.; Dang, Z. Fe- and S-metabolizing microbial communities dominate an AMD-contaminated river ecosystem and play important roles in Fe and S cycling. Geomicrobiol. J. 2017, 34, 695–705. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Wang, M.; Sun, W.; Xie, Y.; Peng, H.; Zhong, A.; Liu, H.; Zhang, X.; Yu, H.; et al. Acid mine drainage affects the diversity and metal resistance gene profile of sediment bacterial community along a river. Chemosphere 2019, 217, 790–799. [Google Scholar] [CrossRef]
- Jin, L.; Gerson, J.R.; Rocca, J.D.; Bernhardt, E.S.; Simonin, M. Alkaline mine drainage drives stream sediment microbial community structure and function. Sci. Total Environ. 2022, 805, 150189. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Chen, X.; Li, X.; Li, Z.; Zhang, Y.; Zhang, G.; He, J. Microbial community characteristics and environmental response of water bodies in different environmental areas underground coal mine. J. China Coal Soc. 2025, 50, 1276–1293. [Google Scholar]
- Hu, G.Q. Environmental Geochemistry and Cleanliness Grade Evaluation of Typical Hazardous Elements at Huainan Coalfield. Ph.D. Dissertation, University of Science and Technology of China, Hefei, China, 2019. [Google Scholar]
- Chen, J.; Gui, H.; Guo, Y.; Li, J. Microbial community characteristics of deep groundwater in Huaibei coalfield and its significance in water source tracing. J. China Coal Soc. 2022, 47, 3503–3512. [Google Scholar]
- Raudsepp, M.J.; Gagen, E.J.; Evans, P.; Tyson, G.W.; Golding, S.D.; Southam, G. The influence of hydrogeological disturbance and mining on coal seam microbial communities. Geobiology 2016, 14, 163–175. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Sun, Y.; Gao, Y.; Zhu, L. Coal Mining Activities Driving the Changes in Microbial Community and Hydrochemical Characteristics of Underground Mine Water. Int. J. Environ. Res. Public Health 2022, 19, 13359. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the Third International Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2: An improved and extensible approach for metagenome inference. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Gao, Y.; Tan, X.; Sun, Y.; Chen, W. Effects of Coal Mining Activities on the Changes in Microbial Community and Geochemical Characteristics in Different Functional Zones of a Deep Underground Coal Mine. Water 2024, 16, 1836. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Sun, Y.; Zhu, L.; Chen, G.; Gao, Y.; Zhao, X. Hydrochemistry and microbial community composition characteristics and their environmental response in different zones of a coal mine in Ordos. Coal Sci. Technol. 2023, 51, 180–196. [Google Scholar]
- Tourova, T.; Sokolova, D.; Nazina, T.; Grouzdev, D.; Kurshev, E.; Laptev, A. Biodiversity of microorganisms colonizing the surface of polystyrene samples exposed to different aqueous environments. Sustainability 2020, 12, 3624. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Chen, C.L.; Li, H.R.; Li, L.H.; Yu, X. The variation of microbiological characteristics in surface waters during persistent precipitation. Front. Environ. Sci. Eng. 2024, 18, 111. [Google Scholar] [CrossRef]
- Lindh, M.V.; Lefébure, R.; Degerman, R.; Lundin, D.; Andersson, A.; Pinhassi, J. Consequences of increased terrestrial dissolved organic matter and temperature on bacterioplankton community composition during a Baltic Sea mesocosm experiment. Ambio 2015, 44, 402–412. [Google Scholar] [CrossRef]
- Frisk, J.H.; Pejler, G.; Eriksson, S.; Wang, L. Heavy metal tolerance of Mesorhizobium delmotii thymidylate kinase. Nucleosides Nucleotides Nucleic Acids 2022, 41, 1305–1317. [Google Scholar] [CrossRef]
- Liu, Z.; Iqbal, M.; Zeng, Z.; Lian, Y.; Zheng, A.; Zhao, M.; Li, Z.; Wang, G.; Li, Z.; Xie, J. Comparative analysis of microbial community structure in the ponds with different aquaculture model and fish by high-throughput sequencing. Microb. Pathog. 2020, 142, 104101. [Google Scholar] [CrossRef]
- Ruprecht, J.; Birrer, S.; Dafforn, K.; Mitrovic, S.; Crane, S.; Johnston, E.; Wemheuer, F.; Navarro, A.; Harrison, A.; Turner, I.; et al. Wastewater effluents cause microbial community shifts and change trophic status. Water Res. 2021, 200, 117206. [Google Scholar] [CrossRef]






| Zones | Sample ID | Sampling Location | Zones | Sample ID | Sampling Location |
|---|---|---|---|---|---|
| Surface Water (SW) | SW1 | Surface drainage outlet | Goaf Water (goaf) | MW2 | West Main Roadway Sealed Wall (−650 m) |
| SW2 | Upstream of Xi Fei River | ||||
| SW3 | Confluence of surface drainage and river | MW3 | West Main Roadway Sealed Wall (−550 m) | ||
| SW4 | Downstream of Xi Fei River | ||||
| Sump Water (sump) | MW1 | Mine-bottom sump (−750 m) | Working Face Drainage (PW) | MW4 | 230106 Working Face Drainage (−750 m) |
| MW5 | 230106 Working Face Drainage (−750 m) | ||||
| Rock Roadway Water (RR) | MW6 | East Wing Track Gate (−750 m) | Coal Roadway Water (CR) | MW9 | 2201 East District Coal Transport Rise (−650 m) |
| MW7 | MW10 | ||||
| MW8 | MW11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Gao, Y.; Zhang, L.; Sun, Y. Driving Effects of Coal Mining Activities on Microbial Communities and Hydrochemical Characteristics in Different Zones. Sustainability 2025, 17, 4000. https://doi.org/10.3390/su17094000
Zhu Z, Gao Y, Zhang L, Sun Y. Driving Effects of Coal Mining Activities on Microbial Communities and Hydrochemical Characteristics in Different Zones. Sustainability. 2025; 17(9):4000. https://doi.org/10.3390/su17094000
Chicago/Turabian StyleZhu, Zongkui, Yating Gao, Li Zhang, and Yajun Sun. 2025. "Driving Effects of Coal Mining Activities on Microbial Communities and Hydrochemical Characteristics in Different Zones" Sustainability 17, no. 9: 4000. https://doi.org/10.3390/su17094000
APA StyleZhu, Z., Gao, Y., Zhang, L., & Sun, Y. (2025). Driving Effects of Coal Mining Activities on Microbial Communities and Hydrochemical Characteristics in Different Zones. Sustainability, 17(9), 4000. https://doi.org/10.3390/su17094000







