Electrode Materials Comparison for Hydrogen Production from Wastewater Electrolysis of Spiked Secondary Effluent
Abstract
1. Introduction
2. Materials and Methods
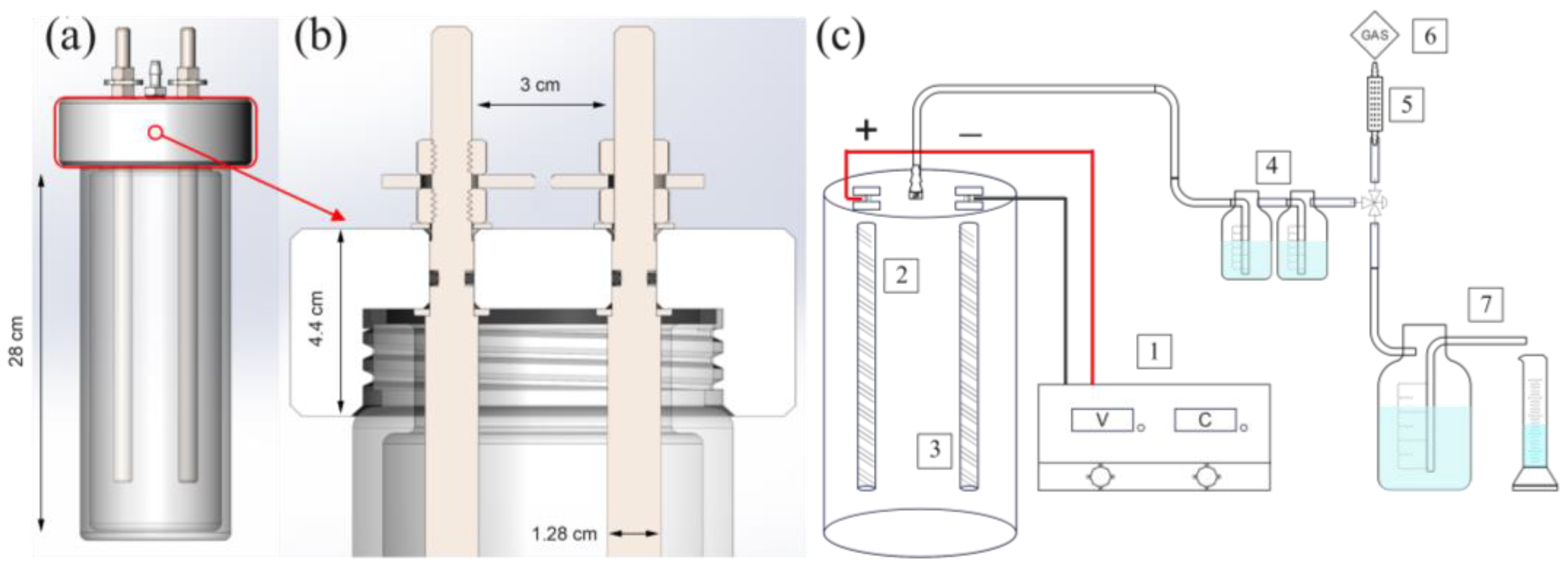
2.1. Electrochemical Cell
2.2. Experimental Procedure
2.3. Wastewater Characteristics
2.4. Analytical Methods
3. Results
3.1. Electrical Results
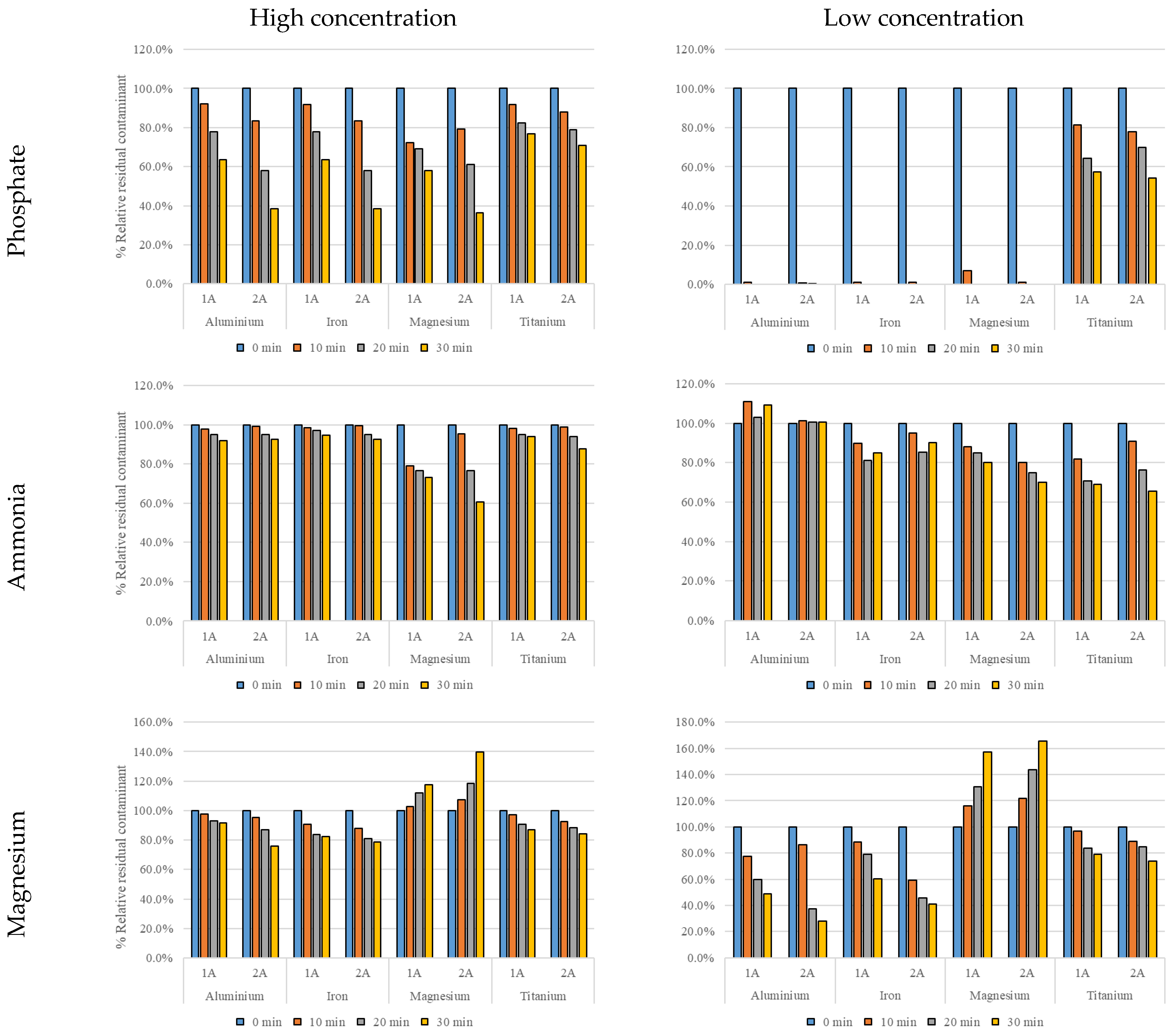
3.2. Water Quality Results
3.3. Gas Production Results
3.4. Precipitation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent Progress on Electrocoagulation Process for Wastewater Treatment: A Review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Ahmad, A.; Priyadarshini, M.; Das, S.; Ghangrekar, M.M. Electrocoagulation as an Efficacious Technology for the Treatment of Wastewater Containing Active Pharmaceutical Compounds: A Review. Sep. Sci. Technol. 2022, 57, 1234–1256. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Packiyam, M. Electrocoagulation Studies on Removal of Cadmium Using Magnesium Electrode. J. Appl. Electrochem. 2010, 40, 2023–2032. [Google Scholar] [CrossRef]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical Treatment of Wastewater: Selectivity of the Heavy Metals Removal Process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Velusamy, K.; Venkatesan, R.; Sagadevan, S.; Mahalingam, U.; Jamespandi, A.; Karazhanov, S.; Pearce, J.; Mayandi, J. Evaluation of Water Quality and Heavy Metal Contamination in Cauvery River: Tamil Nadu Region India. Z. Phys. Chem. 2024. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Nawaz, R.; Hussain, F.; Raza, M.; Ali, S.; Rizwan, M.; Oh, S.-E.; Ahmad, S. Human Health Implications, Risk Assessment and Remediation of As-Contaminated Water: A Critical Review. Sci. Total Environ. 2017, 601, 756–769. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A Review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Electroflotation Process: A Review. J. Mol. Liq. 2016, 220, 657–664. [Google Scholar] [CrossRef]
- Givirovskiy, G.; Ruuskanen, V.; Ahola, J. Electrode Material Studies and Cell Voltage Characteristics of the in Situ Water Electrolysis Performed in a pH-Neutral Electrolyte in Bioelectrochemical Systems. Heliyon 2019, 5, e01690. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical Technologies in Wastewater Treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical Oxidation of Organic Pollutants for Wastewater Treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical Hydrogen Production from Urban Wastewater on a Pilot Scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Pathak, A.K.; Kothari, R.; Tyagi, V.V.; Anand, S. Integrated Approach for Textile Industry Wastewater for Efficient Hydrogen Production and Treatment through Solar PV Electrolysis. Int. J. Hydrogen Energy 2020, 45, 25768–25782. [Google Scholar] [CrossRef]
- Nasution, M.A.; Yaakob, Z.; Ali, E.; Tasirin, S.M.; Abdullah, S.R.S. Electrocoagulation of Palm Oil Mill Effluent as Wastewater Treatment and Hydrogen Production Using Electrode Aluminum. J. Env. Qual. 2011, 40, 1332–1339. [Google Scholar] [CrossRef]
- Rahman, M.M.; Antonini, G.; Pearce, J.M. Open-Source DC-DC Converter Enabling Direct Integration of Solar Photovoltaics with Anion Exchange Membrane Electrolyzer for Green Hydrogen Production. Int. J. Hydrogen Energy 2024, 88, 333–343. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction—Scaling Up Electrolysers to Meet the 1.5 C Climate Goal; IRENA: Masdar, Arabia, 2020. [Google Scholar]
- Burton, N.A.; Padilla, R.V.; Rose, A.; Habibullah, H. Increasing the Efficiency of Hydrogen Production from Solar Powered Water Electrolysis. Renew. Sustain. Energy Rev. 2021, 135, 110255. [Google Scholar] [CrossRef]
- Pearce, J.M. Photovoltaics—a Path to Sustainable Futures. Futures 2002, 34, 663–674. [Google Scholar] [CrossRef]
- Pearce, J.; Lau, A. Net Energy Analysis for Sustainable Energy Production From Silicon Based Solar Cells. In Proceedings of the International Solar Energy Conference, Reno, NV, USA, 15–20 June 2002; pp. 181–186. [Google Scholar]
- Teresa, Z.; Mario, P.; Leonardo, S. Removal of Organic Matter from Paper Mill Effluent by Electrochemical Oxidation. J. Water Resour. Prot. 2011, 2011, 31004. [Google Scholar] [CrossRef][Green Version]
- Kim, D.; Kim, W.; Yun, C.; Son, D.; Chang, D.; Bae, H.; Lee, Y.; Sunwoo, Y.; Hong, K. Agro-Industrial Wastewater Treatment by Electrolysis Technology. Int. J. Electrochem. Sci. 2013, 8, 9835–9850. [Google Scholar] [CrossRef]
- Devlin, T.R.; Kowalski, M.S.; Pagaduan, E.; Zhang, X.; Wei, V.; Oleszkiewicz, J.A. Electrocoagulation of Wastewater Using Aluminum, Iron, and Magnesium Electrodes. J. Hazard. Mater. 2019, 368, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao-Kun, H.; Qing, Z.; Ji, M. Treatment of Secondary Effluent Using a Three-Dimensional Electrode System: COD Removal, Biotoxicity Assessment, and Disinfection Effects. Chem. Eng. J. 2014, 243, 1–6. [Google Scholar] [CrossRef]
- Antonini, G.; Rahman, M.M.; Brooks, C.; Santoro, D.; Pearce, J.M. Portable Solar-Integrated Open-Source Chemistry Lab for Water Treatment with Electrolysis. Technologies 2025, 13, 57. [Google Scholar] [CrossRef]
- American Society of Civil Engineers Benefits of Ductile Iron Pipe. Available online: https://www.asce.org/publications-and-news/civil-engineering-source/article/2022/07/11/benefits-of-ductile-iron-pipe (accessed on 17 October 2024).
- Nondestructive Evaluation Techniques: Eddy Current Testing. Available online: https://www.nde-ed.org/NDETechniques/EddyCurrent/ET_Tables/ET_matlprop_Iron-Based.xhtml (accessed on 29 October 2024).
- MatWeb Material Property Data Aluminum 6061-T6, 6061-T651. Available online: https://www.matweb.com/search/datasheet.aspx?MatGUID=b8d536e0b9b54bd7b69e4124d8f1d20a&ckck=1 (accessed on 17 October 2024).
- Corrosion Materials Titanium Grade 2—Corrosion Materials 2015. Available online: https://corrosionmaterials.com/alloys/titanium-grade-2/ (accessed on 17 October 2024).
- MatWeb Material Property Data Titanium Grade II. Available online: https://asm.matweb.com/search/specificmaterial.asp?bassnum=mtu020 (accessed on 17 October 2024).
- Yubi Steel. Titanium Grade 2 (UNS R50400); Yubi Steel: Cangzhou, China, 2024. [Google Scholar]
- Sharp, R.; Vadiveloo, E.; Fergen, R.; Moncholi, M.; Pitt, P.; Wankmuller, D.; Latimer, R. A Theoretical and Practical Evaluation of Struvite Control and Recovery. Water Environ. Res. 2013, 85, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Struvite Scale and Its Chemistry Centrifuge Centrate. Available online: https://www.thewastewaterblog.com/single-post/struvite-scale-and-its-chemistry (accessed on 25 December 2024).
- Sobotka, D.; Śniatała, B.; Mąkinia, J. Technologies for Nutrient Recovery from Municipal Wastewater. In Water in Circular Economy; Smol, M., Prasad, M.N.V., Stefanakis, A.I., Eds.; Springer International Publishing: Cham, Germany, 2023; pp. 155–166. ISBN 978-3-031-18165-8. [Google Scholar]
- Manufactures Water Quality Testing and Analytical Instruments & Reagents | Hach Canada. Available online: https://ca.hach.com/ (accessed on 17 October 2024).
- Antonini, G.; Pearce, J.M.; Loza, J.O.; Mathews, J.; Cullen, J.; Santoro, D. WastewaterElectrolysis. 2024. Available online: https://osf.io/ebdht/ (accessed on 13 February 2025).
- Zaldivar-Díaz, J.M.; Martínez-Miranda, V.; Castillo-Suárez, L.A.; Linares-Hernández, I.; Solache Ríos, M.J.; Alcántara-Valladolid, A.E. Synergistic Electrocoagulation–Precipitation Process Using Magnesium Electrodes for Denim Wastewater Treatment: Bifunctional Support Electrolyte Effect. J. Water Process Eng. 2023, 51, 103369. [Google Scholar] [CrossRef]
- Peral, A.; Youssef, A.; Dastgheib-Shirazi, A.; Akey, A.; Peters, I.M.; Hahn, G.; Buonassisi, T.; del Cañizo, C. Electrically-Inactive Phosphorus Re-Distribution during Low Temperature Annealing. J. Appl. Phys. 2017, 123, 161535. [Google Scholar] [CrossRef]
- Mehrkhah, R.; Hadavifar, M.; Mehrkhah, M.; Baghayeri, M.; Lee, B.H. Recent Advances in Titanium-Based Boron-Doped Diamond Electrodes for Enhanced Electrochemical Oxidation in Industrial Wastewater Treatment: A Review. Sep. Purif. Technol. 2024, 358, 130218. [Google Scholar] [CrossRef]
- Rangseesuriyachai, T.; Pinpatthanapong, K.; Boonnorat, J.; Jitpinit, S.; Pinpatthanapong, T.; Mueansichai, T. Optimization of COD and TDS Removal from High-Strength Hospital Wastewater by Electrocoagulation Using Aluminium and Iron Electrodes: Insights from Central Composite Design. J. Environ. Chem. Eng. 2024, 12, 111627. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Loizidou, M.; Karlis, P.K.; Zorpas, A.A.; Papaioannou, D. Electrochemical Oxidation of a Textile Dye Wastewater Using a Pt/Ti Electrode. J. Hazard. Mater. 1999, 70, 41–52. [Google Scholar] [CrossRef]
- Meng, X.; Khoso, S.A.; Jiang, F.; Zhang, Y.; Yue, T.; Gao, J.; Lin, S.; Liu, R.; Gao, Z.; Chen, P.; et al. Removal of Chemical Oxygen Demand and Ammonia Nitrogen from Lead Smelting Wastewater with High Salts Content Using Electrochemical Oxidation Combined with Coagulation–Flocculation Treatment. Sep. Purif. Technol. 2020, 235, 116233. [Google Scholar] [CrossRef]
- Ali, E.; Yaakob, Z.; Ali, E.; Yaakob, Z. Electrocoagulation for Treatment of Industrial Effluents and Hydrogen Production. In Electrolysis; IntechOpen: London, UK, 2012; ISBN 978-953-51-0793-4. [Google Scholar]
- Cho, K.; Qu, Y.; Kwon, D.; Zhang, H.; Cid, C.A.; Aryanfar, A.; Hoffmann, M.R. Effects of Anodic Potential and Chloride Ion on Overall Reactivity in Electrochemical Reactors Designed for Solar-Powered Wastewater Treatment. Environ. Sci. Technol. 2014, 48, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Kwon, D.; Hoffmann, M.R. Electrochemical Treatment of Human Waste Coupled with Molecular Hydrogen Production. RSC Adv. 2013, 4, 4596–4608. [Google Scholar] [CrossRef]
- Park, H.; Choo, K.-H.; Park, H.-S.; Choi, J.; Hoffmann, M.R. Electrochemical Oxidation and Microfiltration of Municipal Wastewater with Simultaneous Hydrogen Production: Influence of Organic and Particulate Matter. Chem. Eng. J. 2013, 215–216, 802–810. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, J.; Chen, Z.; Chen, Y.; Zheng, X.; Guo, L.; Wang, X. Efficient Removal and Recovery of Phosphorus from Industrial Wastewater in the Form of Vivianite. Environ. Res. 2023, 228, 115848. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Liu, Y.; Wei, W.; Ni, B.-J. Phosphorus Recovery from Wastewater and Sewage Sludge as Vivianite. J. Clean. Prod. 2022, 370, 133439. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Carr, P.H. Temperature Compendated Cuts of Berlinite and Beta Eucryptite. In Proceedings of the 31st Annual Frequency Control Symposium, Fort Monmouth, NJ, USA, 1–3 June 1977. [Google Scholar]
- Chang, Z.-P.; Barsch, G.R. Elastic Constants and Thermal Expansion of Berlinite. IEEE Trans. Son. Ultrason. 1976, 23, 127–135. [Google Scholar] [CrossRef]
- Muryanto, S.; Supriyo, E.; Mulyaningsih, M.; Hadi, S.; Soebiyono; Purwaningtyas, E.; Kasmiyatun, M.; Firyanto, R. Capstone Lab Project on Crystallization of Struvite. Educ. Chem. Eng. 2017, 21, 25–32. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. Assessing the Feasibility of N and P Recovery by Struvite Precipitation from Nutrient-Rich Wastewater: A Review. Env. Sci. Pollut. Res. 2015, 22, 17453–17464. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.; Hicks, A. Life Cycle Assessment Review of Struvite Precipitation in Wastewater Treatment. Resour. Conserv. Recycl. 2018, 139, 194–204. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite Formation and Decomposition Characteristics for Ammonia and Phosphorus Recovery: A Review of Magnesium-Ammonia-Phosphate Interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Banfield, J.; Waychunas, G. Atomic Structure of Nanometer-Sized Amorphous TiO2. Phys. Rev. B 2009, 78, 214106–214112. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, O.; Mousset, E.; Olvera-Vargas, H.; Lefebvre, O. Electrochemical Treatment of Highly Concentrated Wastewater: A Review of Experimental and Modeling Approaches from Lab- to Full-Scale. Crit. Rev. Environ. Sci. Technol. 2022, 52, 240–309. [Google Scholar] [CrossRef]
- Safwat, S.M. Treatment of Real Printing Wastewater Using Electrocoagulation Process with Titanium and Zinc Electrodes. J. Water Process Eng. 2020, 34, 101137. [Google Scholar] [CrossRef]




| Material | Characteristics | Anode Weight [g] | Cathode Weight [g] | Resistance [µΩ] | Refs. |
|---|---|---|---|---|---|
| Ductile iron |
| 394.2 | 399.5 | 229.24 | [23,26,27] |
| Aluminum 6061-T6 |
| 165.1 | 165.2 | 122.26 | [23,27,28] |
| Titanium grade II |
| 253.1 | 253 | 1629.22 | [29,30,31] |
| Magnesium |
| 130.1 | 129.2 | 136.54 | [1,3,23,27] |
| Liquid | Total Solids [mg/L] | NH4 [mg/L] | PO4 [mg/L] | Mg [mg/L] |
|---|---|---|---|---|
| Wastewater initial values | 0.1 | 0.3 | 1.1 | 0.05 |
| Wastewater low concentration | 0.3 | 68.5 | 279 | 102 |
| Wastewater high concentration | 0.6 | 428.75 | 2845 | 772.25 |
| Electrode Material | Low Concentration Wastewater | R2 | High Concentration Wastewater | R2 |
|---|---|---|---|---|
| Ductile iron | V = 0.07119 I–0.07797 | 0.997 | V = 0.39216 I–0.249 | 0.993 |
| Aluminum 6061 | V = 0.07534 I–0.11096 | 0.994 | V = 0.41667 I–0.633 | 0.989 |
| Titanium grade II | V = 0.07037 I–0.25185 | 0.942 | V = 0.17857 I–0.61429 | 0.939 |
| Magnesium | V = 0.07237 I–0.01579 | 0.999 | V = 0.41 I–0.52 | 0.998 |
| Material | Initial pH | Final pH | Initial Conductivity [S/m] | Final Conductivity [S/m] | |
|---|---|---|---|---|---|
| Wastewater | 7.05 | - | 0.07 | - | |
| Wastewater low concentration | Fe | 6.1 | 7.9 | 0.28 | 0.26 |
| Al | 8 | 0.25 | |||
| Ti | 6.6 | 0.27 | |||
| Mg | 8.2 | ||||
| Wastewater high concentration | Fe | 5 | 5.6 | 1.28 | 1.18 |
| Al | 6.3 | 1.15 | |||
| Ti | 5.5 | 1.21 | |||
| Mg | 6.7 | 1.15 | |||
| Electrode Material | Hydrogen [Vol %] | Oxygen [Vol %] | Nitrogen [Vol %] | Total [Vol %] |
|---|---|---|---|---|
| Ductile iron | 95.56 | 1.01 | 2.45 | 99.02 |
| Aluminum 6061 | 96.13 | 0.89 | 1.92 | 98.94 |
| Titanium grade II | 87.93 | 3.72 | 6.63 | 98.28 |
| Magnesium | 93.48 | 1.83 | 2.44 | 97.75 |
| Electrode Material | Ductile Iron | Aluminum 6061 | Titanium Grade II | Magnesium |
|---|---|---|---|---|
| Phosphate difference [mg] | 3220 | 3215 | 1866 | 3685 |
| Ammonia difference [mg] | 71 | 58 | 195 | 335 |
| Magnesium difference [mg] | 410 | 379 | 255 | −424 |
| Difference in anode weight [mg] | 540 | 390 | 150 | 270 |
| Difference in cathode weight [mg] | 11,130 | 4450 | 1800 | 4040 |
| Total solids [mg] | 15,020 | 13,020 | 3125 | 5580 |
| Electrode Material | ||||
|---|---|---|---|---|
| Element | Ductile Iron [Wt%] | Aluminum 6061 [Wt%] | Titanium Grade II [Wt%] | Magnesium [Wt%] |
| Iron | 27.4 | - | - | - |
| Aluminum | - | 13.8 | - | - |
| Titanium | - | - | 26.4 | - |
| Magnesium | 2.2 | 2.8 | 2.5 | 16.6 |
| Oxygen | 48. | 58.7 | 52.5 | 59.6 |
| Phosphorus | 13.5 | 18 | 11.3 | 19.3 |
| Potassium | 2.5 | 4.2 | 4.1 | 1.9 |
| Nitrogen | 0.2 | - | - | 2.5 |
| Chlorine | 3.4 | 1 | 2.9 | 0.2 |
| Calcium | 2.4 | 1.4 | 0.3 | - |
| Silicon | 0.1 | 0.1 | - | - |
| Electrode | Anode (Oxidation) | Cathode (Reduction) | Flocculation Reactions |
|---|---|---|---|
| Iron (Fe) | Fe → Fe2+ + 2e− (iron oxidation) | 2H2O + 2e− → H2 + 2OH− (production of OH− and H2) | Fe2+ + 2OH− → Fe(OH)2 (formation of Fe(OH)2) |
| Fe2+ → Fe3+ + e− (in presence of oxygen or oxidizing agents) | Mg2+ + 2OH− → Mg(OH)2 (formation of magnesium hydroxide flocs) | Fe3+ + 3OH− → Fe(OH)3 (formation of Fe(OH)3) | |
| 2H2O → O2 + 4H+ + 4e− (oxygen formation) | NH4+ + OH− → NH3 + H2O (ammonia formation) | 3Mg2+ + 2PO43− → Mg3(PO4)2 (magnesium phosphate formation) | |
| 2Cl− → Cl2 + 2e− (chlorine gas formation from Cl−) | |||
| Aluminum (Al) | Al → Al3+ + 3e− (aluminum oxidation) | 2H2O + 2e− → H2 + 2OH− (production of OH− and H2) | Al3+ + 3OH− → Al(OH)3 (formation of Al(OH)3) |
| 2Cl− → Cl2 + 2e− (chlorine gas formation) | NH4+ + OH− → NH3 + H2O (ammonia formation) | 3Mg2+ + 2PO43− → Mg3(PO4)2 (magnesium phosphate formation) | |
| Magnesium (Mg) | Mg → Mg2+ + 2e− (magnesium oxidation) | 2H2O + 2e− → H2 + 2OH− (production of OH− and H2) | Mg2+ + 2OH− → Mg(OH)2 (formation of Mg(OH)2) |
| 2Cl− → Cl2 + 2e− (chlorine gas formation) | NH4+ + OH− → NH3 + H2O (ammonia formation) | 3Mg2+ + 2PO43− → Mg3(PO4)2 (magnesium phosphate formation) | |
| Titanium (Ti) | Ti + 2H2O → TiO2 + 4H+ + 4e− (titanium dioxide formation) | 2H2O + 2e− → H2 + 2OH− (production of OH− and H2) | No metal flocs |
| 2Cl− → Cl2 + 2e− (chlorine gas formation) | NH4+ + OH− → NH3 + H2O (ammonia formation) | 3Mg2+ + 2PO43− → Mg3(PO4)2 (magnesium phosphate formation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonini, G.; Ordonez-Loza, J.; Mathew, J.; Cullen, J.; Muller, C.; Al-Omari, A.; Bell, K.; Santoro, D.; Pearce, J.M. Electrode Materials Comparison for Hydrogen Production from Wastewater Electrolysis of Spiked Secondary Effluent. Sustainability 2025, 17, 3988. https://doi.org/10.3390/su17093988
Antonini G, Ordonez-Loza J, Mathew J, Cullen J, Muller C, Al-Omari A, Bell K, Santoro D, Pearce JM. Electrode Materials Comparison for Hydrogen Production from Wastewater Electrolysis of Spiked Secondary Effluent. Sustainability. 2025; 17(9):3988. https://doi.org/10.3390/su17093988
Chicago/Turabian StyleAntonini, Giorgio, Javier Ordonez-Loza, Jithin Mathew, Joshua Cullen, Christopher Muller, Ahmed Al-Omari, Katherine Bell, Domenico Santoro, and Joshua M. Pearce. 2025. "Electrode Materials Comparison for Hydrogen Production from Wastewater Electrolysis of Spiked Secondary Effluent" Sustainability 17, no. 9: 3988. https://doi.org/10.3390/su17093988
APA StyleAntonini, G., Ordonez-Loza, J., Mathew, J., Cullen, J., Muller, C., Al-Omari, A., Bell, K., Santoro, D., & Pearce, J. M. (2025). Electrode Materials Comparison for Hydrogen Production from Wastewater Electrolysis of Spiked Secondary Effluent. Sustainability, 17(9), 3988. https://doi.org/10.3390/su17093988






