Prospects for the Industrialization of Nitride-Based Photocatalytic CO2 Reduction Research Achievements: A Net Present Value Analysis
Abstract
1. Introduction
2. Research Overview of Photocatalytic Reduction of CO2
2.1. General Trend of the Number of Published Papers
2.2. Analysis of the Number of Published Papers on Photocatalytic Reduction of CO2 Under Different Catalyst Categories
3. Cost–Benefit Analysis of Nitride-Based Photocatalytic Reduction of CO2
3.1. Selection of Catalysts
3.2. Cost and Economic Benefit Analysis
3.2.1. Analysis of Relevant Costs
3.2.2. Economic Benefit Analysis
- NPV represents the net present value;
- CI_t represents the cash inflow in the t-th year;
- CO_t represents the cash outflow in the t-th year;
- i represents the discount rate, that is, the expected minimum rate of return;
- n represents the expected lifespan of the investment project.
- Decision-making principles:
4. Influence of Key Factors on Economic Benefits
4.1. Analysis of Influencing Factors
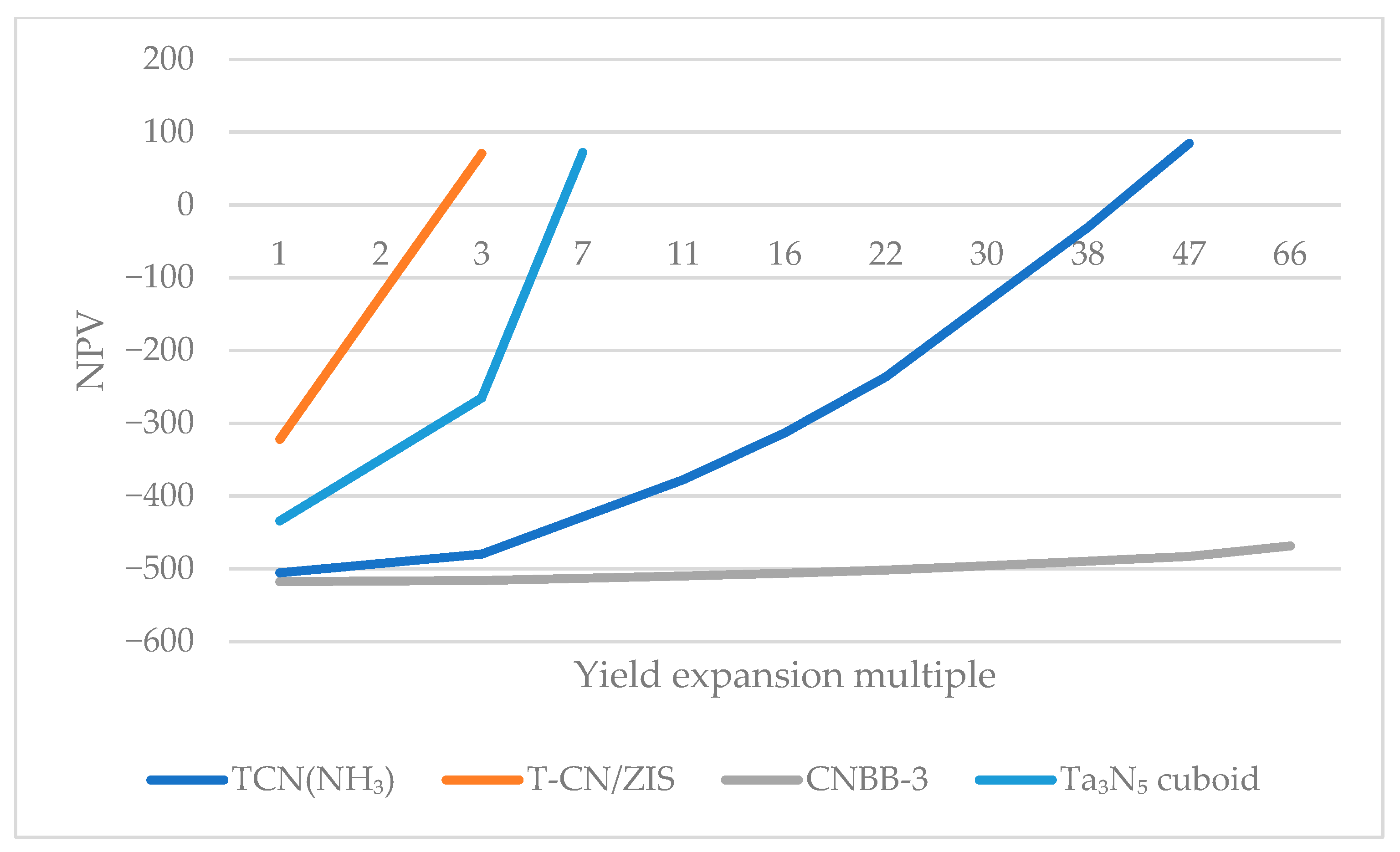
4.1.1. Catalytic Efficiency
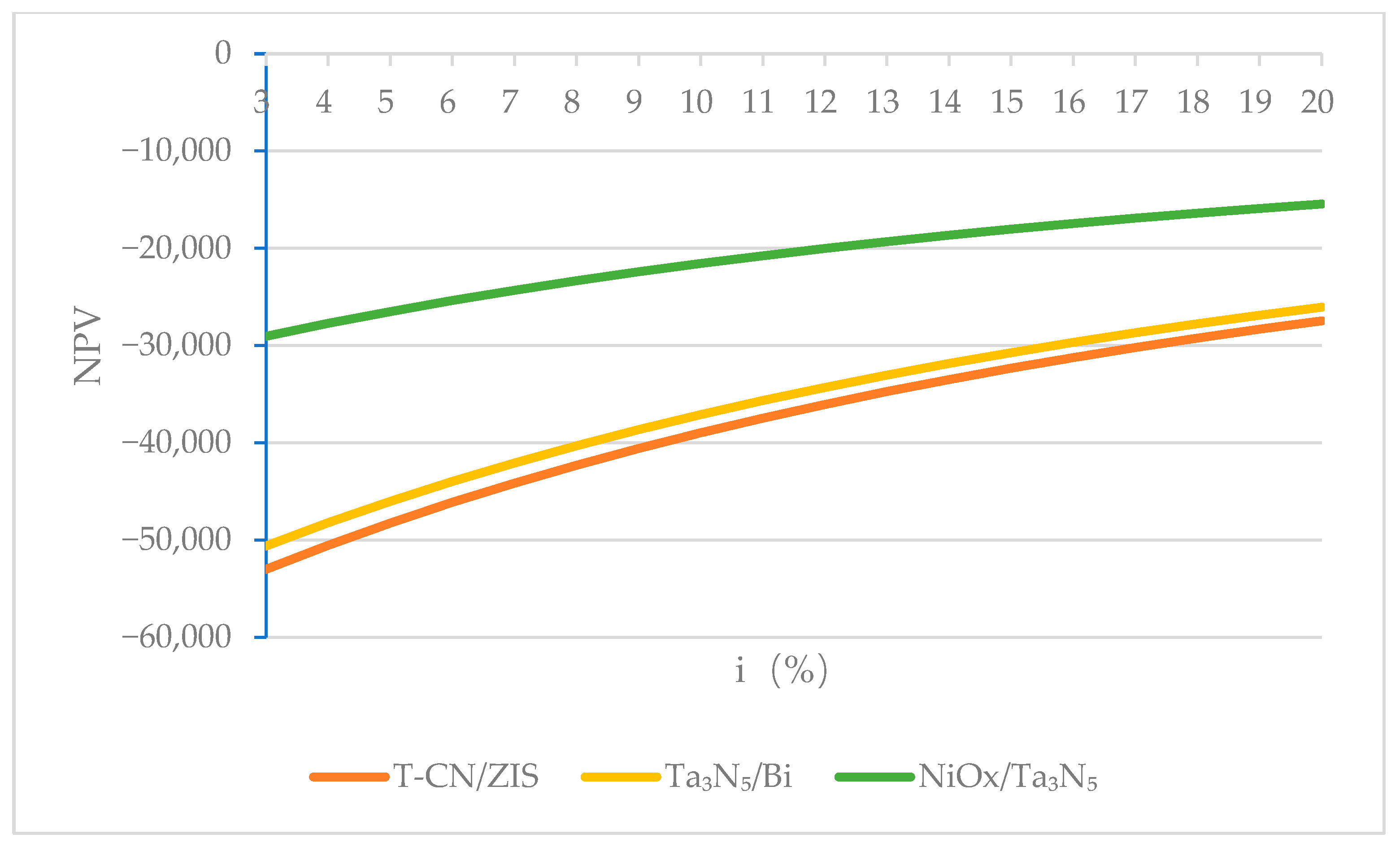
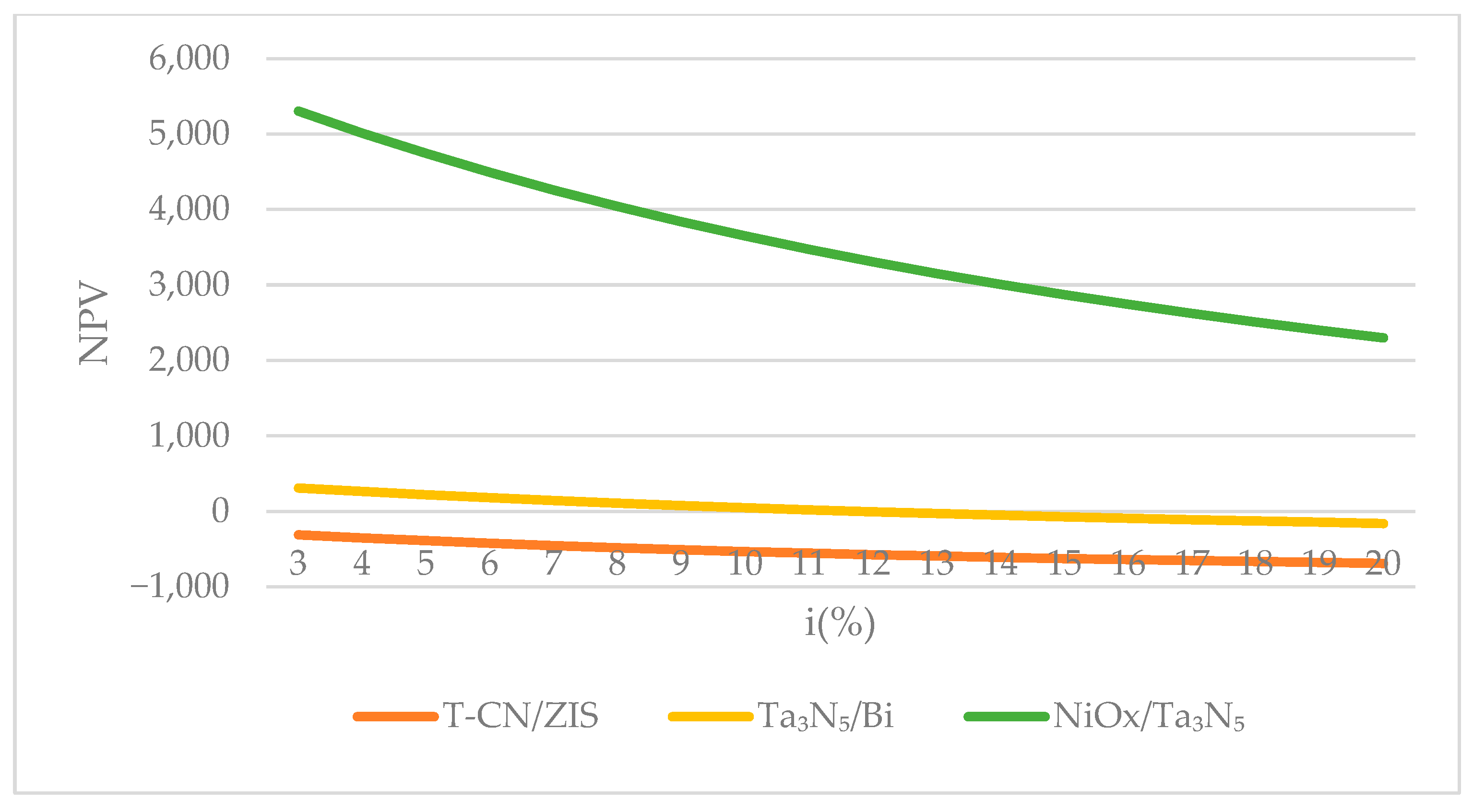
4.1.2. Discount Rate
4.1.3. Depreciation Period
4.1.4. Lighting Conditions
4.2. Analysis of Changing Key Factors Under Different Lighting Conditions
4.2.1. Changing the Performance of the Catalyst
4.2.2. Changing the Discount Rate
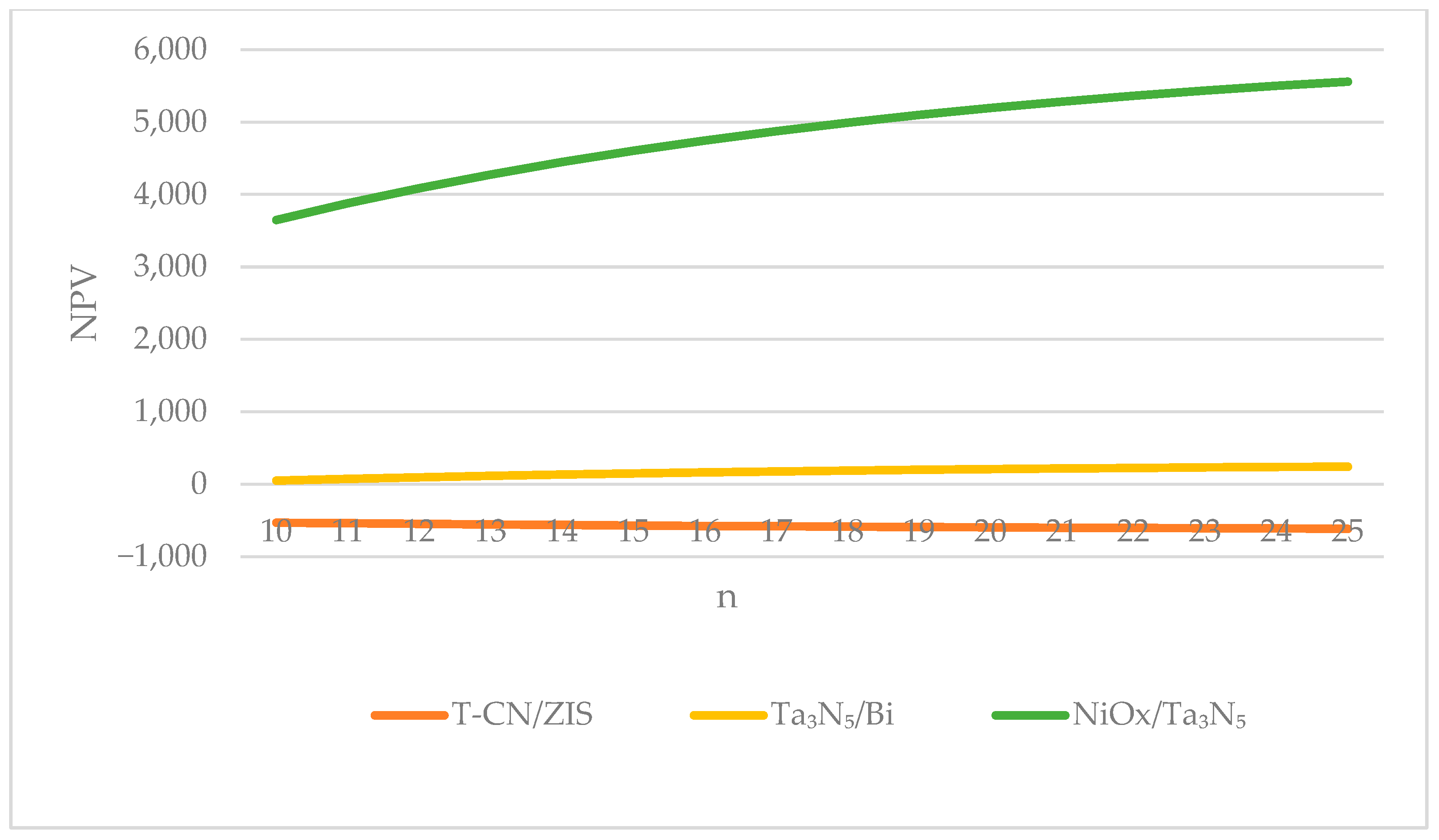
4.2.3. Changing the Depreciation Period
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Xiang, Z.; Ruan, J.; Ren, K.; Zhang, M.; Zhou, J. Research Progress and Considerations on Ultra-thick Bed Sintering for Extreme Energy Efficiency and Carbon Reduction. Sinter. Pelletizing 2025, 1–10. [Google Scholar]
- Yi, F.H.; Liao, G.S.; Cai, L.R.; Chen, G.; Chen, L. Research progress on heterogeneous photocatalytic reduction of carbon dioxide by polypyridyl cobalt molecular catalysts. J. Mol. Catal. Chin. Engl. 2024, 38, 467–482. [Google Scholar]
- Wang, Y.; Arandiyan, H.; Scott, J.; Aguey-Zinsou, K.F.; Amal, R. Single Atom and Nanoclustered Pt Catalysts for Selective CO2 Reduction. ACS Appl. Energy Mater. 2018, 1, 6781–6789. [Google Scholar] [CrossRef]
- Bie, C.; Zhu, B.; Xu, F.; Zhang, L.; Yu, J. In Situ Grown Monolayer N-Doped Graphene on CdS Hollow Spheres with Seamless Contact for Photocatalytic CO2 Reduction. Adv. Mater. 2019, 31, 1902868. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Ali, A.; Cho, K.Y.; Oh, W.C. Novel synthesis of WSe2—Graphene—TiO2 ternary nano—Composite via ultrasonic technics for high photocatalytic reduction of CO2 into CH3OH. Ultrason. Sonochemistry 2018, 42, 738–746. [Google Scholar] [CrossRef]
- Patial, S.; Kumar, R.; Raizada, P.; Singh, P.; Van Le, Q.; Lichtfouse, E.; Nguyen, D.L.; Nguyen, V.H. Boosting light—Driven CO2 reduction into solar fuels: Mainstream avenues for engineering ZnO—Based photocatalysts. Environ. Res. 2021, 197, 111134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z—Scheme α—Fe2O3/g—C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A Hierarchical Z—Scheme CdS—WO3 Photocatalyst with Enhanced CO2 Reduction Activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, W.; Miao, W.; Yuan, Z.; Yang, G.; Kong, F.; Yan, T.; Chen, J.; Huang, B.; An, C.; et al. Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Ponnamma, D.; Wang, J.; Prasad, S.; Ahamed, M.; Cheng, C.; Byrappa, K. CeO2 Nanostructures Enriched with Oxygen Vacancies for Photocatalytic CO2 Reduction. ACS Appl. Nano Mater. 2020, 3, 138–148. [Google Scholar] [CrossRef]
- Khan, J.; Sun, Y.; Han, L. A Comprehensive Review on Graphitic Carbon Nitride for Carbon Dioxide Photoreduction. Small Methods 2022, 6, 2201013. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Wijaya, D.T.; Na, J.; Lee, C.W. Towards the Large—Scale Electrochemical Reduction of Carbon Dioxide. Catalysts 2021, 11, 253. [Google Scholar] [CrossRef]
- Huang, Z.; Grim, R.G.; Schaidle, J.A.; Tao, L. The Economic Outlook for Converting CO2 and Electrons to Molecules. Energy Environ. Sci. 2021, 14, 3664–3678. [Google Scholar] [CrossRef]
- He, J.; Janáky, C. Recent Advances in Solar—Driven Carbon Dioxide Conversion: Expectations versus Reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H. Discussion on Low—Carbon Management from the Perspective of Policy. Manag. Obs. 2013, 8–10. [Google Scholar]
- Zhu, J.R. (Ed.) East Asian Energy Cooperation: The Road to Common Prosperity; Shanghai People’s Publishing House: Shanghai, China, 2012; Volume 12. [Google Scholar]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.; Liu, D.; Li, H.; Yang, X.; She, Y.; Lei, Y.; Yuan, S.; et al. Porous nitrogen—Rich g—C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 256, 117854. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Li, Q.; Feng, Y.N.; Chen, F.F.; Yu, Y. Spatial distribution of ZnIn2S4 nanosheets on g—C3N4 microtubes promotes photocatalytic CO2 reduction. Chem. Eng. J. 2021, 418, 129476. [Google Scholar] [CrossRef]
- Guo, L.; You, Y.; Huang, H.; Tian, N.; Ma, T.; Zhang, Y. Z—Scheme g—C3N4/Bi2O2 [BO2 (OH)] heterojunction for enhanced photocatalytic CO2 reduction. J. Colloid Interface Sci. 2020, 568, 139–147. [Google Scholar] [CrossRef]
- Wang, S.; Guan, Y.; Lu, L.; Shi, Z.; Yan, S.; Zou, Z. Effective separation and transfer of carriers into the redox sites on Ta3N5/Bi photocatalyst for promoting conversion of CO2 into CH4. Appl. Catal. B Environ. 2018, 224, 10–16. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, J.; Lu, L.; Wang, J.; Shi, Z.; Wang, B.; Pei, L.; Yan, S.; Zhentao, Y.; Zou, Z. Surface electric field driven directional charge separation on Ta3N5 cuboids enhancing photocatalytic solar energy conversion. Appl. Catal. B Environ. 2018, 237, 742–752. [Google Scholar] [CrossRef]
- Pei, L.; Wang, X.; Zhu, H.; Yu, H.; Bandaru, S.; Yan, S.; Zou, Z. Photothermal Effect—And Interfacial Chemical Bond—Modulated NiOx/Ta3N5 Heterojunction for Efficient CO2 Photoreduction. ACS Appl. Mater. Interfaces 2023, 15, 51300–51308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Research on Enterprise Investment Project Decision—Making and Methods. China Bus. Mark. 2020, 79–80. [Google Scholar]


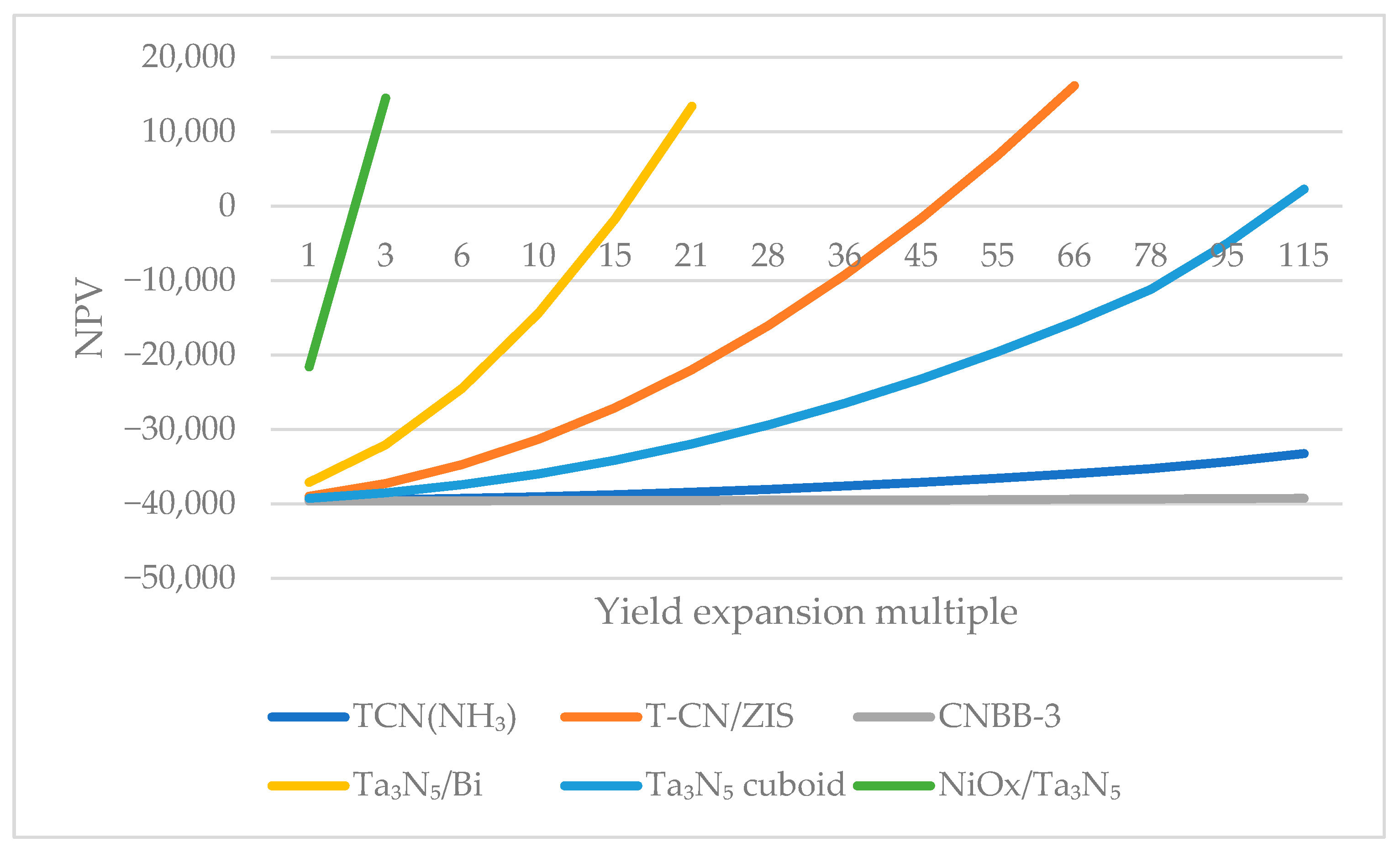

| CO | CH4 | C2H5OH | References | |
|---|---|---|---|---|
| TCN(NH3) | 103.6 | [17] | ||
| T-CN/ZIS | 1453 | 863 | [18] | |
| CNBB-3 | 6.09 | [19] | ||
| Ta3N5/Bi | 4.52 | [20] | ||
| Ta3N5 cuboid | 0.652 | [21] | ||
| NiOx/Ta3N5 | 32.3 | [22] |
| Catalyst Cost | Equipment Investment | Working Capital Advances | Annual Xenon Lamp Cost | Annual Electricity Cost | Other Annual Expenses | |
|---|---|---|---|---|---|---|
| TCN(NH3) | 0.01 | 2000 | 200.00 | 7590 | 598.75 | 14 |
| T-CN/ZIS | 310.95 | 2000 | 2542.05 | 7590 | 598.75 | 14 |
| CNBB-3 | 7.88 | 2000 | 2208.66 | 7590 | 598.75 | 14 |
| Ta3N5/Bi | 26.90 | 2000 | 2229.59 | 7590 | 598.75 | 14 |
| Ta3N5 cuboid | 19.88 | 2000 | 2221.86 | 7590 | 598.75 | 14 |
| NiOx/Ta3N5 | 20.03 | 2000 | 2222.03 | 7590 | 598.75 | 14 |
| Initial Cash Flow | Operating Cash Flow | Terminal Cash Flow | NPV | |
|---|---|---|---|---|
| TCN(NH3) | −2200.01 | −6093.04 | 200.01 | −39,562.00 |
| T-CN/ZIS | −2542.05 | −5963.94 | 542.05 | −38,981.25 |
| CNBB-3 | −2208.66 | −6101.53 | 208.66 | −39,622.04 |
| Ta3N5/Bi | −2229.59 | −5690.84 | 229.59 | −37,111.17 |
| Ta3N5 cuboid | −2221.86 | −6042.75 | 221.86 | −39,268.90 |
| NiOx/Ta3N5 | −2222.03 | −3163.44 | 222.03 | −21,575.64 |
| Initial Cash Flow | Operating Cash Flow | Terminal Cash Flow | NPV | |
|---|---|---|---|---|
| TCN(NH3) | −550.01 | 4.09 | 50.01 | −505.61 |
| T-CN/ZIS | −892.05 | 33.97 | 392.05 | −532.15 |
| CNBB-3 | −558.66 | 2.12 | 58.66 | −532.00 |
| Ta3N5/Bi | −579.59 | 97.19 | 79.59 | 48.29 |
| Ta3N5 cuboid | −571.86 | 15.73 | 71.86 | −447.50 |
| NiOx/Ta3N5 | −572.03 | 682.24 | 72.03 | 3647.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fang, H.; Ren, Q.; Li, H.; Zhang, X.; Ye, M.; Zhang, F. Prospects for the Industrialization of Nitride-Based Photocatalytic CO2 Reduction Research Achievements: A Net Present Value Analysis. Sustainability 2025, 17, 3902. https://doi.org/10.3390/su17093902
Wang Y, Fang H, Ren Q, Li H, Zhang X, Ye M, Zhang F. Prospects for the Industrialization of Nitride-Based Photocatalytic CO2 Reduction Research Achievements: A Net Present Value Analysis. Sustainability. 2025; 17(9):3902. https://doi.org/10.3390/su17093902
Chicago/Turabian StyleWang, Yingrui, Haiyan Fang, Qianqian Ren, Hengji Li, Xingyu Zhang, Minhong Ye, and Fengjun Zhang. 2025. "Prospects for the Industrialization of Nitride-Based Photocatalytic CO2 Reduction Research Achievements: A Net Present Value Analysis" Sustainability 17, no. 9: 3902. https://doi.org/10.3390/su17093902
APA StyleWang, Y., Fang, H., Ren, Q., Li, H., Zhang, X., Ye, M., & Zhang, F. (2025). Prospects for the Industrialization of Nitride-Based Photocatalytic CO2 Reduction Research Achievements: A Net Present Value Analysis. Sustainability, 17(9), 3902. https://doi.org/10.3390/su17093902






