Successional Allelopathic Interactions of Amaranthus palmeri S. Wats. and Cereals
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection and Supply
2.2. Petri Experiments
2.3. Pot Experiments
2.3.1. The Effects of Cereal Residues on Germination and Development of AMAPA
2.3.2. The Effects of AMAPA Residues on Germination and Development of Cereals
2.4. Statistical Analysis
3. Results
3.1. Petri Experiments
3.1.1. Effects of Cereal’s Aqueous Extracts on Relative Germination and Delay in MGT of AMAPA
3.1.2. Effects of AMAPA Aqueous Extracts on Relative Germination and Delay in MGT of Cereals
3.2. Pot Experiments
3.2.1. The Effects of Cereal Residues on AMAPA Germination and Growth
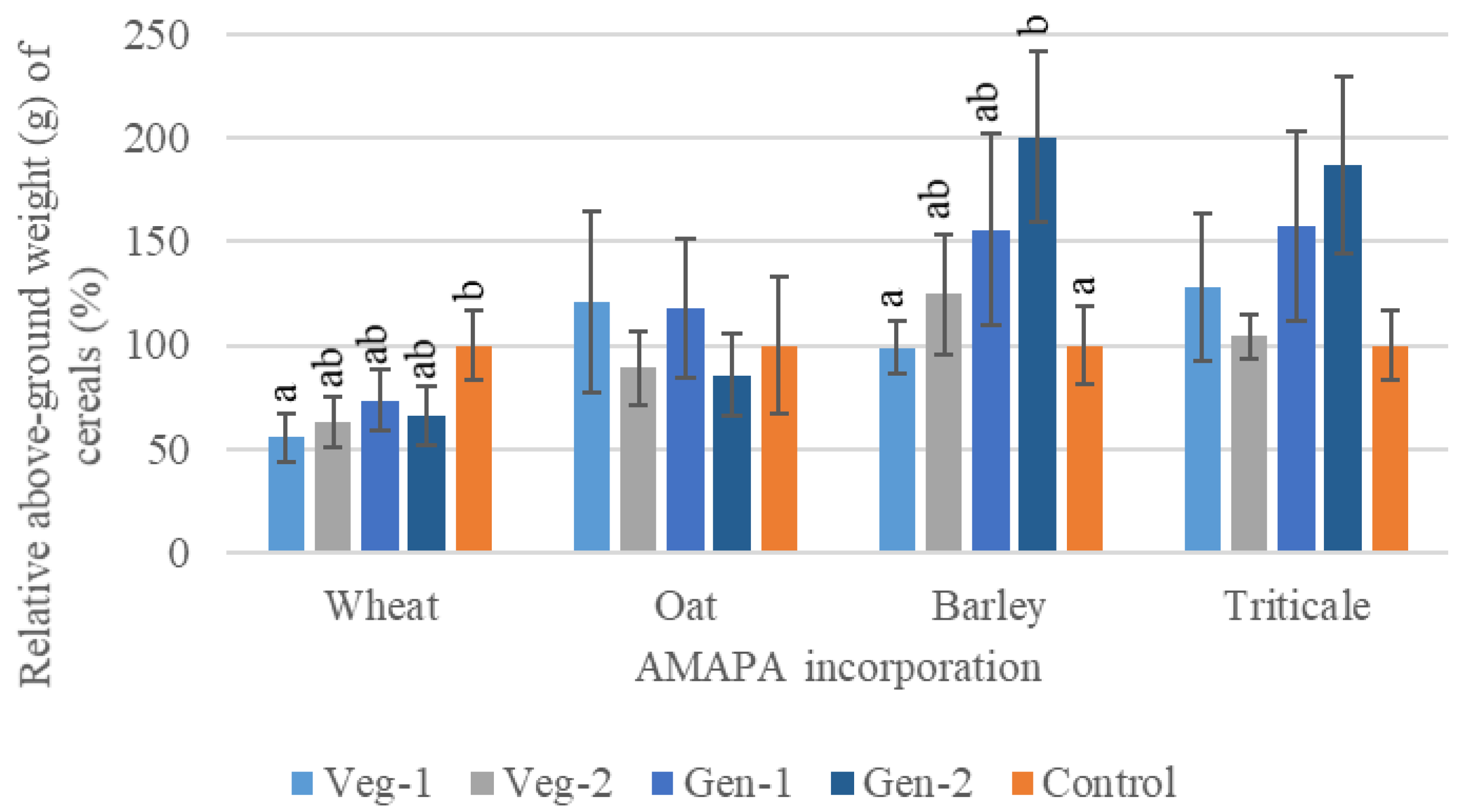
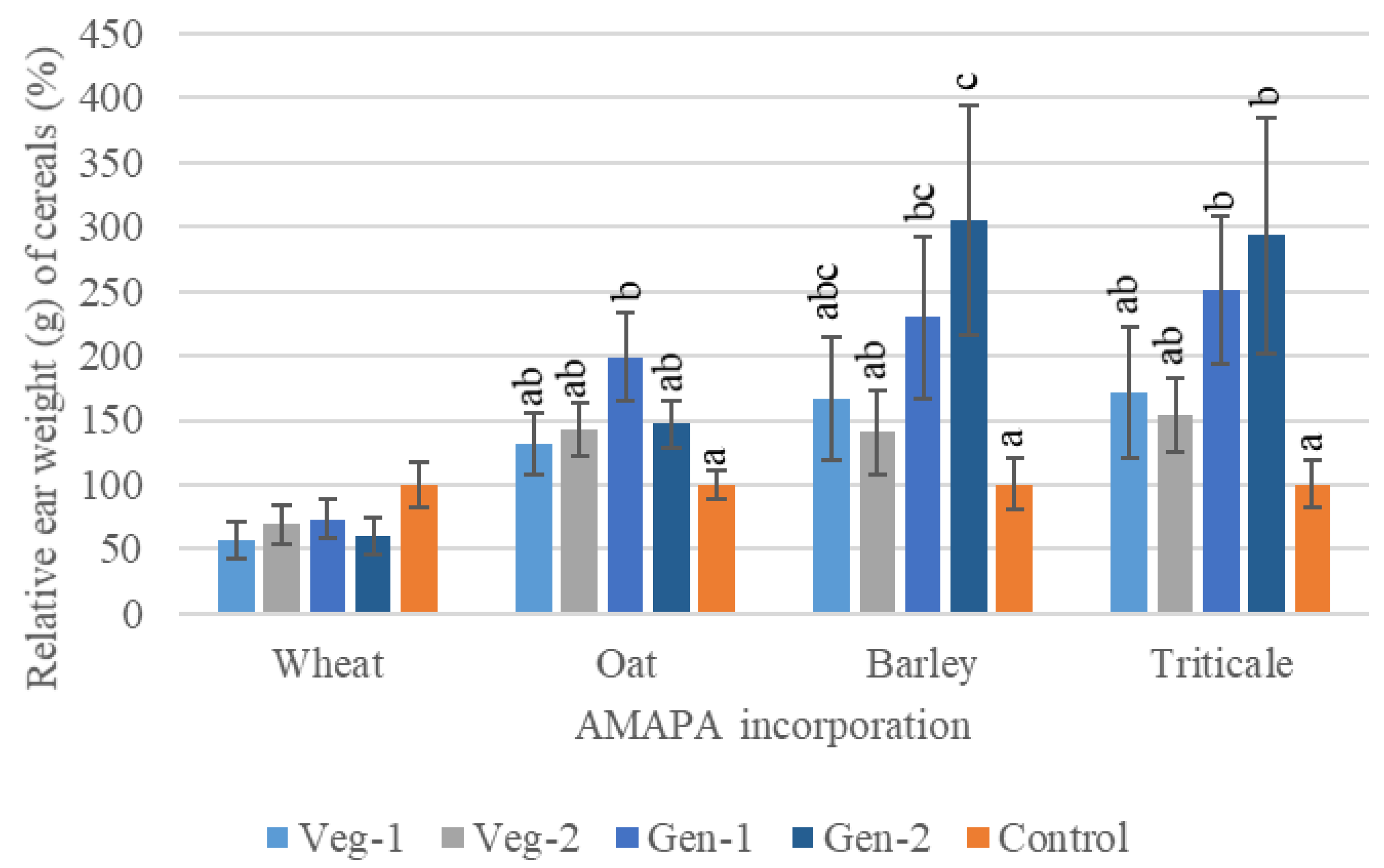
3.2.2. The Effects of A. palmeri Residues on Cereal Germination and Growth
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMAPA | Amaranthus palmeri |
| LN | Natural logarithm |
| MGT | Mean germination time |
| NaOCl | Sodium hypochlorite |
| NPK | Nitrogen, phosphorus, potassium |
| RPM | Round per minute |
| w/v | Weight/volume |
References
- International Allelopathy Society. What Is Allelopathy? Available online: https://allelopathy-society.osupytheas.fr/about/ (accessed on 25 December 2023).
- Weston, L.A. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Chou, C.H. Role of allelopathy in plant biodiversity and sustainable agriculture. Crit. Rev. Plant Sci. 1999, 18, 609–636. [Google Scholar] [CrossRef]
- Vivanco, J.M.; Bais, H.P.; Stermitz, T.R.; Thelen, G.C.; Callaway, R.M. Biogeochemical variation in community response to root allelochemistry: Novel weapons and exotic invasion. Ecol. Lett. 2004, 7, 285–292. [Google Scholar] [CrossRef]
- Bertholdsson, N.O.; Andersson, S.C.; Merker, A. Allelopathic potential of Triticum spp., Secale spp. and Triticosecale spp. and use of chromosome substitutions and translocations to improve weed suppression ability in winter wheat. Plant Breed. 2012, 131, 75–80. [Google Scholar] [CrossRef]
- Jabran, K. Manipulation of Allelopathic Crops for Weed Control; Springer Briefs in Plant Science; Springer: Cham, Switzerland, 2017; pp. 13–20, 57–63. [Google Scholar]
- Carvalho, M.D.; Halecki, W.; Mozdzen, K.; Synowiec, A. Autoallelopathic and allelopathic influence of aqueous winter-cereal extracts. Acta Agrobot. 2022, 75, 753. [Google Scholar] [CrossRef]
- Ma, Y. Allelopathic studies of common wheat (Triticum aestivum L.). Weed Biol. Manag. 2005, 5, 93–104. [Google Scholar] [CrossRef]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in wheat (Triticum aestivum L.): Variation of phenolic acids in shoot tissues. Chem. Ecol. 2001, 27, 125–135. [Google Scholar] [CrossRef]
- Kremer, R.J.; Ben-Hammouda, M. Allelopathic Plants. 19. Barley (Hordeum vulgare L.). Allelopath. J. 2009, 24, 225–242. [Google Scholar]
- Reiss, A.; Fomsgaard, I.S.; Mathiassen, S.K.; Kudsk, P. Weed suppressive trait of winter cereals: Allelopathy and competition. Biochem. Syst. Ecol. 2018, 76, 35–41. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Eleftherohorinos, I.G.; Lithourgidis, A.S. Allelopathic potential of winter cereal cover crop mulches on grass weed suppression and sugarbeet development. Crop Sci. 2006, 46, 345–352. [Google Scholar] [CrossRef]
- Mathiassen, S.K.; Kudsk, P.; Mogensen, B.B. Herbicidal effects of soil-incorporated wheat. J. Agric. Food Chem. 2006, 54, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, M.A.; Marshall, D.R.; Copeland, L. Hydroxamic acids in cereal roots inhibit the growth of take-all. Soil Biol. Biochem. 1999, 31, 1831–1836. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic acids (4-hydroxy-1, 4-benzoxazin-3-ones), defence chemicals in the gramineae. Phytochemistry 1988, 27, 3349–3358. [Google Scholar] [CrossRef]
- Grimmer, O.P.; Masiunas, J.B. The weed control potential of oat cultivars. HortTechnology 2015, 15, 140–144. [Google Scholar] [CrossRef]
- Chovancova, S.; Neugschwandtner, R.W.; Ebrahimi, E.; Kaul, H.P. Effects of aqueous above-ground biomass extracts of cover crops on germination and seedlings of maize. Die Bodenkult. 2015, 66, 17–23. [Google Scholar]
- Carraro-Lemes, C.F.; Scheffer-Basso, S.M.; Deuner, C.C.; Berghahn, S.C.T. Analysis of genotypic variability in Avena spp. regarding allelopathic potentiality. Planta Daninha 2019, 37, e019191107. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kosemura, S.; Yamamura, S.; Mizutani, J.; Hasegawa, K. Allelopathy of oats. I. Assessment of allelopathic potential of extract of oat shoots and identification of an allelochemical. J. Chem. Ecol. 1994, 20, 309–314. [Google Scholar] [CrossRef]
- Ward, S.M.; Webster, T.M.; Steckel, L.E. Palmer amaranth (Amaranthus palmeri): A review. Weed Technol. 2013, 27, 12–27. [Google Scholar] [CrossRef]
- EPPO. Amaranthus palmeri (AMAPA). Available online: https://gd.eppo.int/taxon/AMAPA/distribution (accessed on 4 May 2024).
- Eren, Ö.; Dogan, M.N.; Boz, Ö.; Türkseven, S.; Özcan, R. Amaranthus palmeri S. In Euro Med-Checklist Notulae, 6; Wats. Raab-Straube, E.V., Raus, T., Eds.; Willdenowia 2016, 46, 423–442. Available online: https://bioone.org/journals/willdenowia/volume-46/issue-3/wi.46.46310/Euro-Med-Checklist-Notulae-6/10.3372/wi.46.46310.full (accessed on 10 November 2024).
- Connick, W.J.; Bradow, J.M.; Legendre, M.G. Identification and bioactivity of volatile allechemicals from amaranth residues. J. Agric. Food Chem. 1989, 37, 792–796. [Google Scholar] [CrossRef]
- Broster, K.; Mattews, J.L.; Gage, K.L. The allelopathic effects of Palmer amaranth (Amaranthus palmeri) on the growth and phenology of crop plants. In Proceedings of the Annual Meeting of Managing Global Resources for A Secure Future, Tampa, FL, USA, 22–25 October 2017. Available online: https://scisoc.confex.com/crops/2017am/webprogram/Paper107472.html (accessed on 10 November 2024).
- Efil, F. Investigations of the Allelopathic Effects of Palmer Amaranth (Amaranthus palmeri S. Watson) Between Some Weed Species and Crops. Ph.D. Thesis, Aydin Adnan Menderes University, Aydin, Turkiye, 2023. [Google Scholar]
- Zeng, R.S.; Mallik, A.U.; Luo, S.M. Allelopathy in Sustainable Agriculture and Forestry; Springer Science + Business Media, LLC: New York, NY, USA, 2008; pp. 255–270. [Google Scholar]
- Matloob, A.; Khaliq, A.; Farooq, M.; Cheema, Z.A. Quantification of allelopathic potential of different crop residues for the purple nutsedge suppression. Pak. J. Weed Sci. Res. 2010, 16, 1–12. [Google Scholar]
- Norsworthy, J.K.; Malik, M.S.; Jha, P.; Riley, M.B. Suppression of Digitaria sanguinalis and Amaranthus palmeri using autumn-sown glucosinolate-producing cover crops in organically grown bell pepper. Weed Res. 2007, 47, 425–432. [Google Scholar] [CrossRef]
- Wang, X.; Gu, M.; Niu, G.; Baumann, P.A. Herbicidal activitiy of mustard seed meal (Sinapis alba ‘IdaGold’ and Brassica juncea ‘Pacific Gold’) on weed emergence. Ind. Crops Prod. 2015, 77, 1004–1013. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Farooq, M.; Khaliq, A. Allelopathy Current Trends and Future Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 113–144. [Google Scholar]
- Ellis, R.A.; Roberts, E.H. The Quantification of Ageing and Survival in Orthodox Seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Ben-Hammouda, M.; Ghorbal, H.; Kremer, R.; Queslati, O. Allelopathic effects of barley extracts on germination and seedlings growth of bread and durum wheats. Agron. Rev. 2001, 21, 65–71. [Google Scholar] [CrossRef]
- Chon, S.U.; Kim, Y.M. Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J. Agron. Crop Sci. 2004, 190, 145–150. [Google Scholar] [CrossRef]
- Yu, J.; Vasanthan, T.; Temelli, F. Analysis of phenolic acids in barley by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 4352–4358. [Google Scholar] [CrossRef]
- Liu, D.L.; Lovett, J.V. Biologically active secondary metabolites of barley, II. Phytotoxicity of barley allelochemicals. J. Chem. Ecol. 1993, 19, 2231–2244. [Google Scholar] [CrossRef]
- Maver, M.; Miras-Moreno, B.; Lucini, L.; Trevisan, M.; Pii, Y.; Cesco, S.; Mimmo, T. New insights in the allelopathic traits of different barley genotypes: Middle Eastern and Tibetan wild-relative accessions vs. cultivated modern barley. PLoS ONE 2020, 15, e0231976. [Google Scholar] [CrossRef]
- Steinsiek, J.W.; Oliver, L.R.; Collins, F.C. Allelopathic potential of wheat (Triticum aestivum) straw on selected weed species. Weed Sci. 1982, 30, 495–497. [Google Scholar] [CrossRef]
- Alghamdi, S.A.; Al-Nehmi, A.A.; Ibrahim, O.H.M. Potential allelopathic effect of wheat straw aqueous extract on bermudagrass noxious weed. Sustainability 2022, 14, 15989. [Google Scholar] [CrossRef]
- Rambakudzibga, A.M. Allelopathic effects of aqueous wheat (Triticum aestivum L.) straw extracts on the germination of eight arable weeds commonly found in Zimbabwe. Zimb. J. Agric. Res. 1991, 29, 77–79. [Google Scholar]
- Aslam, F.; Khaliq, A.; Matloob, A.; Tanveer, A.; Hussain, S.; Zahir, Z. Allelopathy in agro-ecosystems: A critical review of wheat allelopathy-concepts and implications. Chemoecology 2017, 27, 1–24. [Google Scholar] [CrossRef]
- Price, A.J.; Stoll, M.E.; Bergtold, J.S.; Arriaga, F.J.; Balkcom, K.S.; Kornecki, T.S.; Raper, R.L. Effect of cover crop extracts on cotton and radish radicle elongation. Commun. Biometry Crop Sci. 2008, 3, 60–66. [Google Scholar]
- Khaliq, A.; Matloob, A.; Hussain, A.; Hussain, S.; Aslam, F.; Zamir, S.I.; Chattha, M.U. Wheat residue management options affect crop productivity, weed growth, and soil properties in direct-seeded fine aromatic rice. Clean-Soil Air Water 2015, 43, 1259–1265. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; McClelland, M.; Griffith, G.; Bangarwa, S.K.; Still, J. Evaluation of cereal and Brassicaceae cover crops in conservation-tillage, enhanced, glyphosate-resistant cotton. Weed Tech. 2011, 25, 6–13. [Google Scholar] [CrossRef]
- Li, S.L.; You, Z.G.; Li, S.R.; Zhang, L. Allelopathy of wheat extraction to the growth of two weeds. Chin. J. Biol. Control 1996, 12, 168–170. [Google Scholar]
- Petcu, E.; Babeanu, N.; Popa, O. Screening methods for evaluating the allelopathic potential of wheat and triticale genotypes. Sci. Pap. Ser. A Agron. 2017, 60, 370–374. [Google Scholar]
- Thilsted, E.; Murray, D.S. Effect of Wheat Straw on Weed Control in No-Tillage Soybeans; Southern Weed Science Society: Hot Springs, AR, USA, 15–17 January 1980. [Google Scholar]
- Burgos, N.R.; Talbert, R.E. Differential allelochemicals from Secale cereale in seedling bioassays. Weed Sci. 2000, 48, 302–310. [Google Scholar] [CrossRef]
- Timper, P.; Davis, R.F.; Webster, T.M.; Brenneman, T.B.; Meyer, S.L.F.; Zasada, I.A.; Cai, G.; Rice, C.P. Response of root-knot nematodes and Palmer amaranth to tillage and rye green manure. Agron. J. 2011, 103, 813–821. [Google Scholar] [CrossRef]
- Bertholdsson, N.O. Allelopathy—A tool to improve the weed competitive ability of wheat with herbicide-resistant black-grass (Alopecurus myosuroides Huds.). Agronomy 2012, 2, 284–294. [Google Scholar] [CrossRef]
- Tabaglio, V.; Gavazzi, C.; Schulz, M.; Marocco, A. Alternative weed control using the allelopathic effect of natural benzoxazinoids from rye mulch. Agron. Sustain. Dev. 2008, 28, 397–401. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Hou, X. Allelopathic effects of wheat, soybean and oat residues on cucumber and Fusarium oxysporum f.sp. cucumerinum Owen. Allelopath. J. 2010, 25, 107–114. [Google Scholar]
- Schulz, M.; Wieland, I. Variations in metabolism of BOA among species in various field communities—Biochemical evidence for co-evolutionary processes in plant communities? Chemoecology 1999, 9, 133–141. [Google Scholar] [CrossRef]
- Joseph, D.D. The Below-Ground Competitive Ability and Allelopathic Potential of Palmer Amaranth (Amaranthus palmeri) in Soybean. Ph.D. Dissertation, Clemson University, Clemson, SC, USA, 2019. Available online: https://tigerprints.clemson.edu/all_dissertations/2469 (accessed on 25 November 2024).
- Shekoofa, A.; Safikhan, S.; Raper, T.B.; Butler, S.A. Allelopathic impacts of cover crop species and termination timing on cotton germination and seedling growth. Agronomy 2020, 10, 638. [Google Scholar] [CrossRef]
- Cereal Rye Cover Crops, Allelopathy and Corn. Available online: https://dr.lib.iastate.edu/server/api/core/bitstreams/13252cb1-47a2-4781-b5bb-278923997991/content (accessed on 4 October 2024).
- Bertholdsson, N.O. Variation in allelopathic activity over 100 years of barley selection and breeding. Weed Res. 2004, 44, 78–86. [Google Scholar] [CrossRef]
- Shah, A.N.; Iqbal, J.; Ullah, A.; Yang, G.; Yousaf, M.; Fahad, S.; Tanveer, M.; Hassan, W.; Tung, S.A.; Wang, L.; et al. Allelopathic potential of oil seed crops in production of crops: A review. Environ. Sci. Pollut. Res. 2016, 23, 14854–14867. [Google Scholar] [CrossRef]
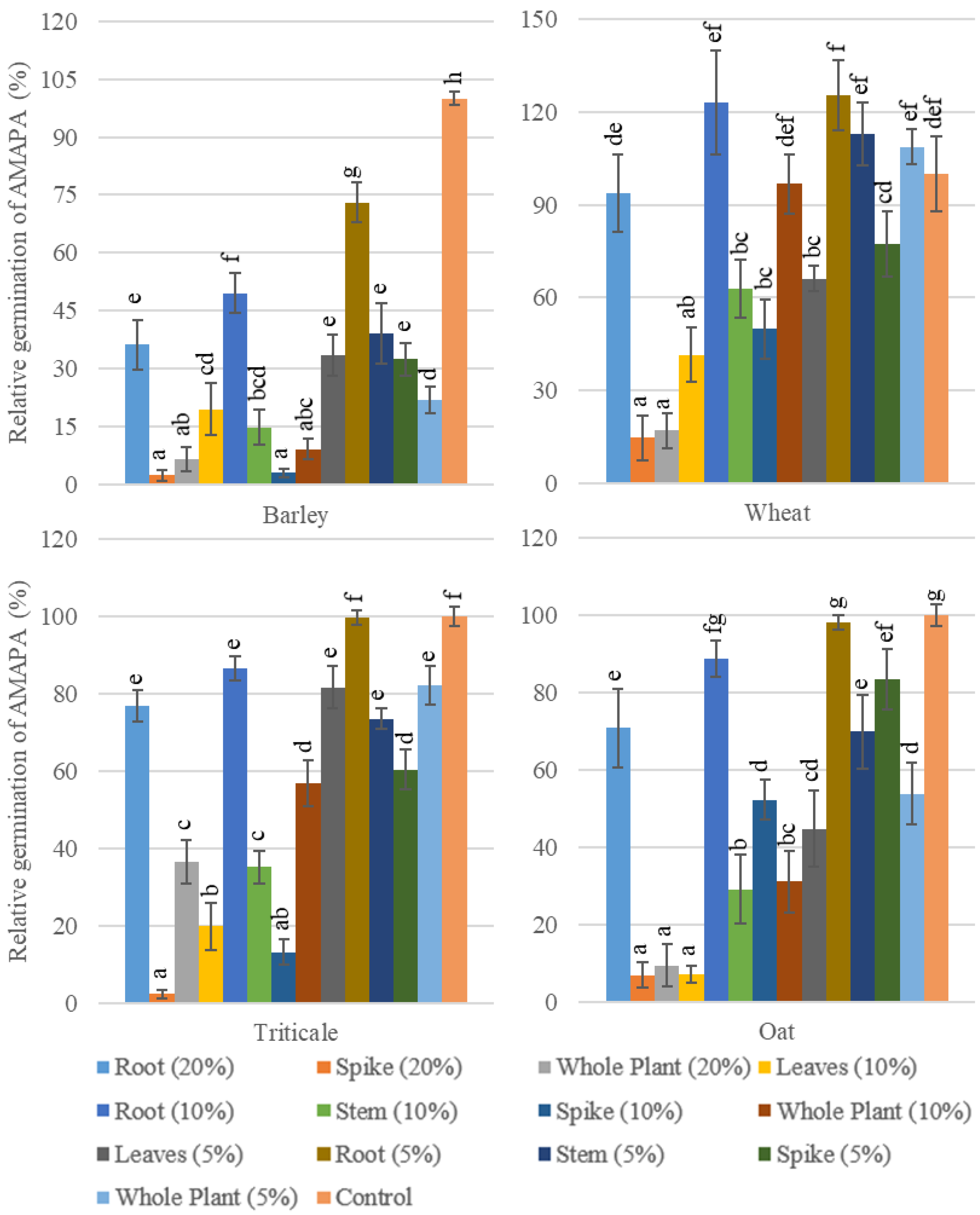


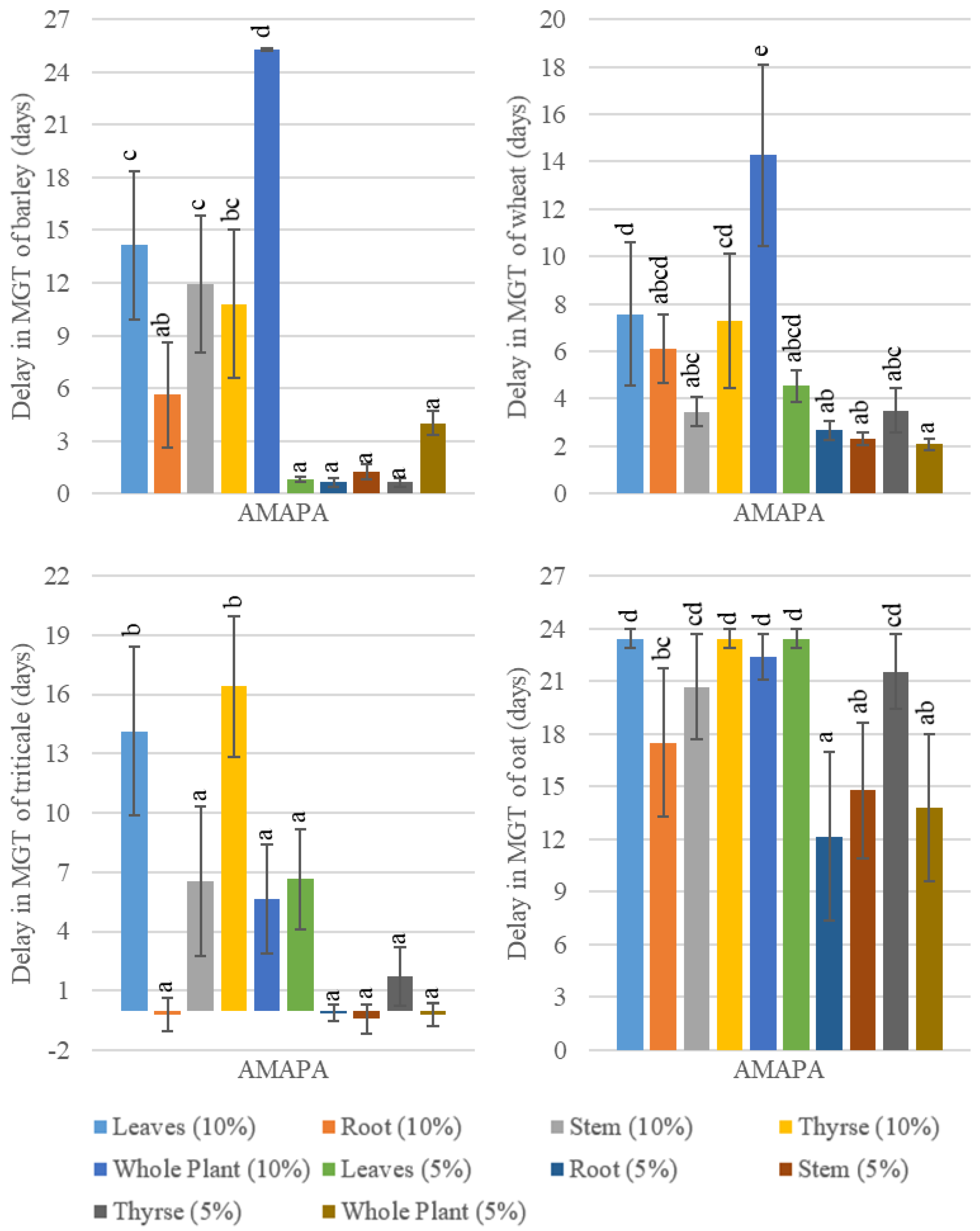
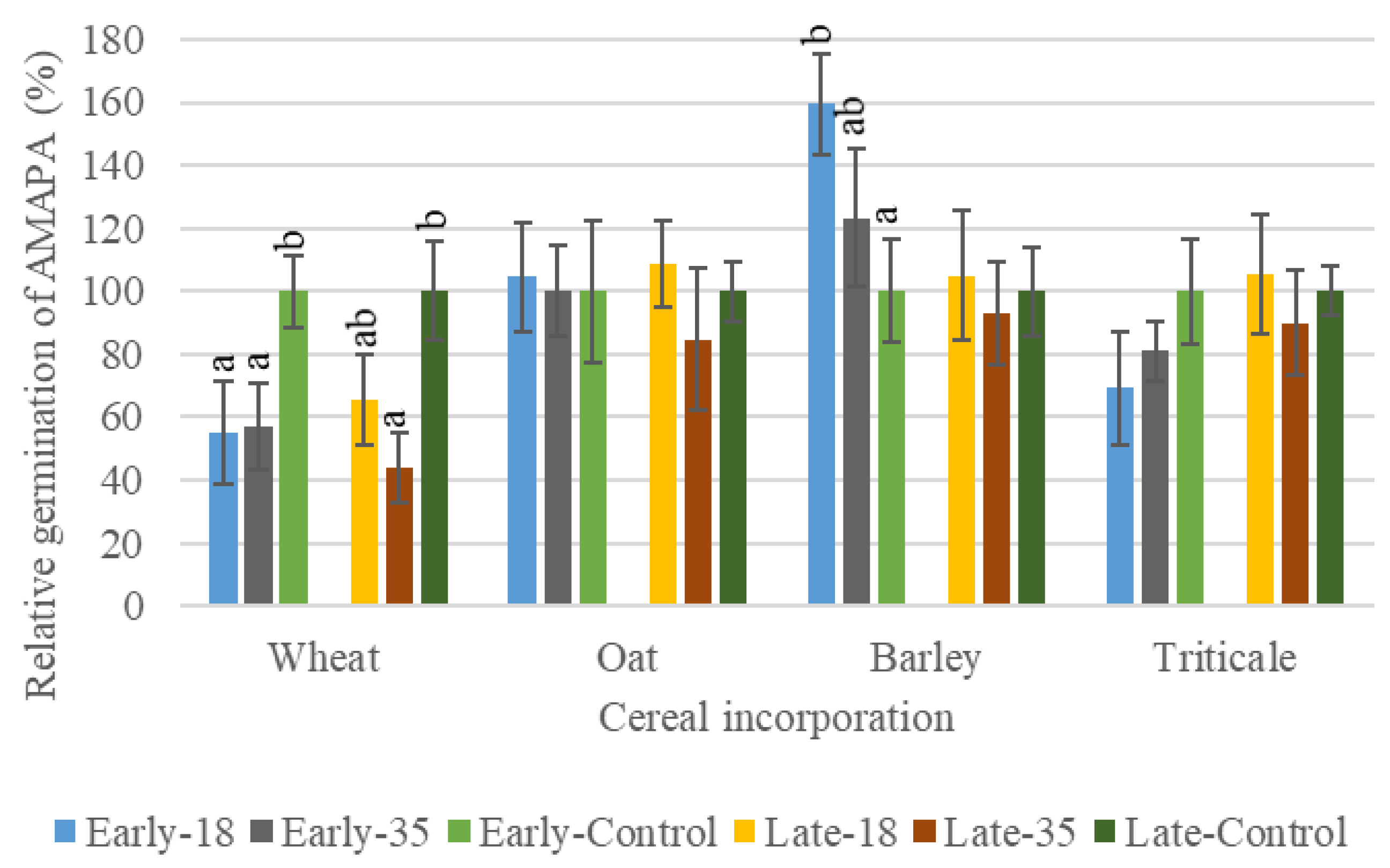



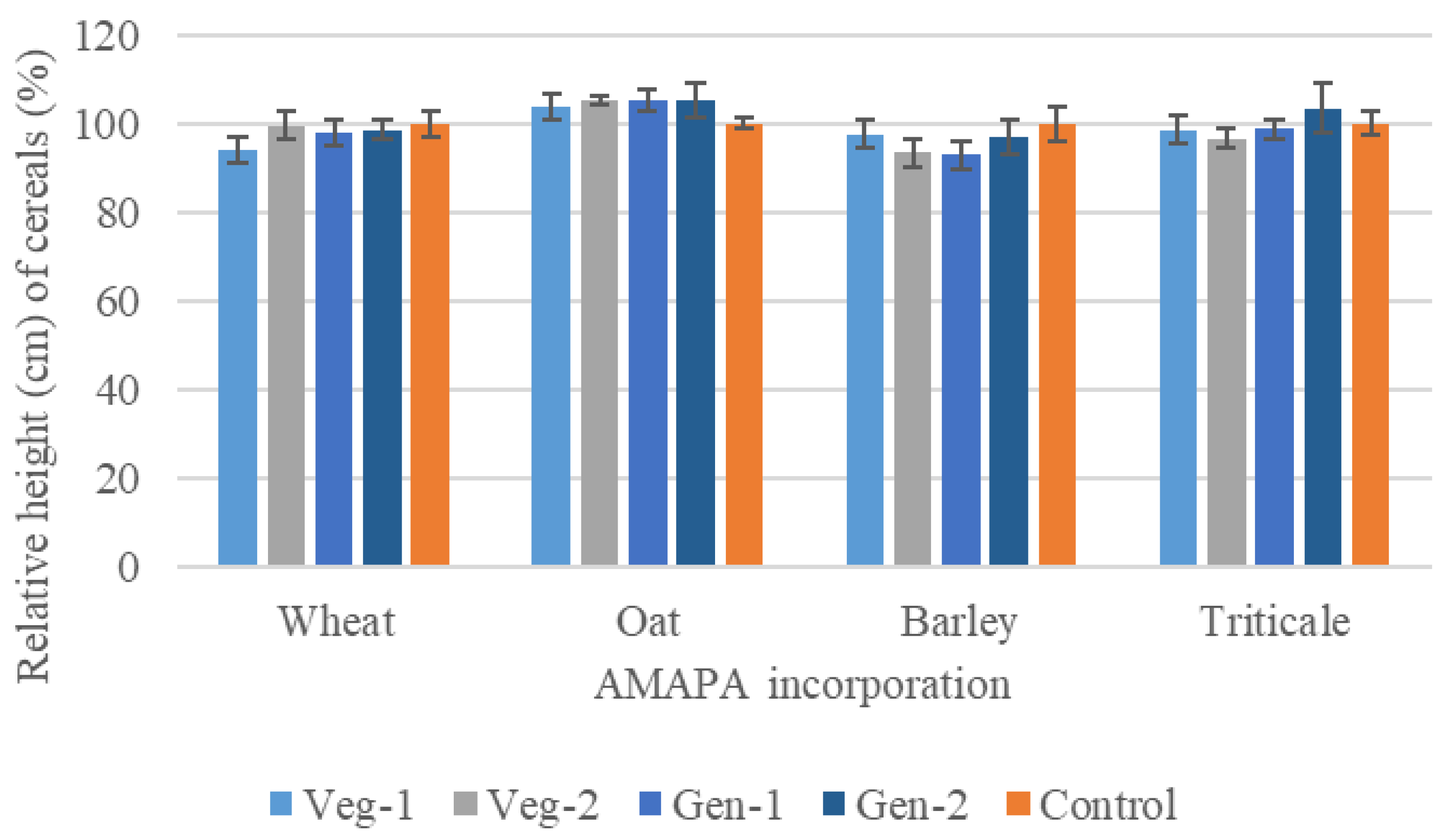


| AMAPA Extracts and Concentrations | Cereal Extracts and Concentrations |
|---|---|
| Leaf/10 and 5% | Leaf/10 and 5% |
| Root/10 and 5% | Root/20, 10 and 5% |
| Thyrse/10 and 5% | Spike/20, 10 and 5% |
| Stem/10 and 5% | Stem/10 and 5% |
| Whole Plant/10 and 5% | Whole Plant/20, 10 and 5% |
| Applications | First Experiment | Second Experiment |
|---|---|---|
| Cereal seeds were sown | 16 November 2020 | 2 November 2021 |
| Cereals were incorporated into the potting mix at the end of the tillering stage (early stage) | 22 March 2021 | 7 March 2022 |
| AMAPA seeds were sown in the early-stage pots | 14 April 2021 | 28 March 2022 |
| Germinated AMAPA seeds were counted (Early stage) | 24 May 2021 7 June 2021 | 28 April 2022 21 June 2022 |
| Cereals were incorporated into the potting mix at harvest time (late stage) | 24 May 2021 | 2 June 2022 |
| AMAPA seeds were sown in the late-stage pots | 14 June 2021 | 14 June 2022 |
| Germinated AMAPA seeds were counted (late stage) | 28 June 2021 12 July 2021 | 21 June 2022 5 July 2022 |
| Height and dry weight of AMAPA were measured (early stage) | 13 July 2021 16 July 2021 | 27 July 2022 30 July 2022 |
| Height and dry weights of AMAPA were measured (late stage) | 12 September 2021 15 September 2021 | 31 August 2022 3 September 2022 |
| Applications | First Experiment | Second Experiment |
|---|---|---|
| AMAPA seeds were sown | 17 August 2021 | 10 August 2022 |
| AMAPA were incorporated into the potting mix at the vegetative stage | 22 September 2021 | 13 August 2022 |
| AMAPA were incorporated into the potting mix at the generative stage | 7 October 2021 | 10 October 2022 |
| Cereal seeds were sown | 1 November 2021 | 1 November 2022 |
| Germinated cereals were counted | 8 November 2021 29 November 2021 | 8 November 2022 25 November 2022 |
| Height, total spike weight, total grain weight per plant, and thousand-grain weight of cereals were measured and calculated | 8 June 2022 | 9 June 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erbas, F. Successional Allelopathic Interactions of Amaranthus palmeri S. Wats. and Cereals. Sustainability 2025, 17, 3871. https://doi.org/10.3390/su17093871
Erbas F. Successional Allelopathic Interactions of Amaranthus palmeri S. Wats. and Cereals. Sustainability. 2025; 17(9):3871. https://doi.org/10.3390/su17093871
Chicago/Turabian StyleErbas, Filiz. 2025. "Successional Allelopathic Interactions of Amaranthus palmeri S. Wats. and Cereals" Sustainability 17, no. 9: 3871. https://doi.org/10.3390/su17093871
APA StyleErbas, F. (2025). Successional Allelopathic Interactions of Amaranthus palmeri S. Wats. and Cereals. Sustainability, 17(9), 3871. https://doi.org/10.3390/su17093871






