Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review
Abstract
1. Introduction
2. AOPs
2.1. Hydroxyl Radicals Oxidation
2.2. Ozone-Based AOPs
2.3. UV Radiation
2.4. Fenton Reactions
2.5. Other AOPs
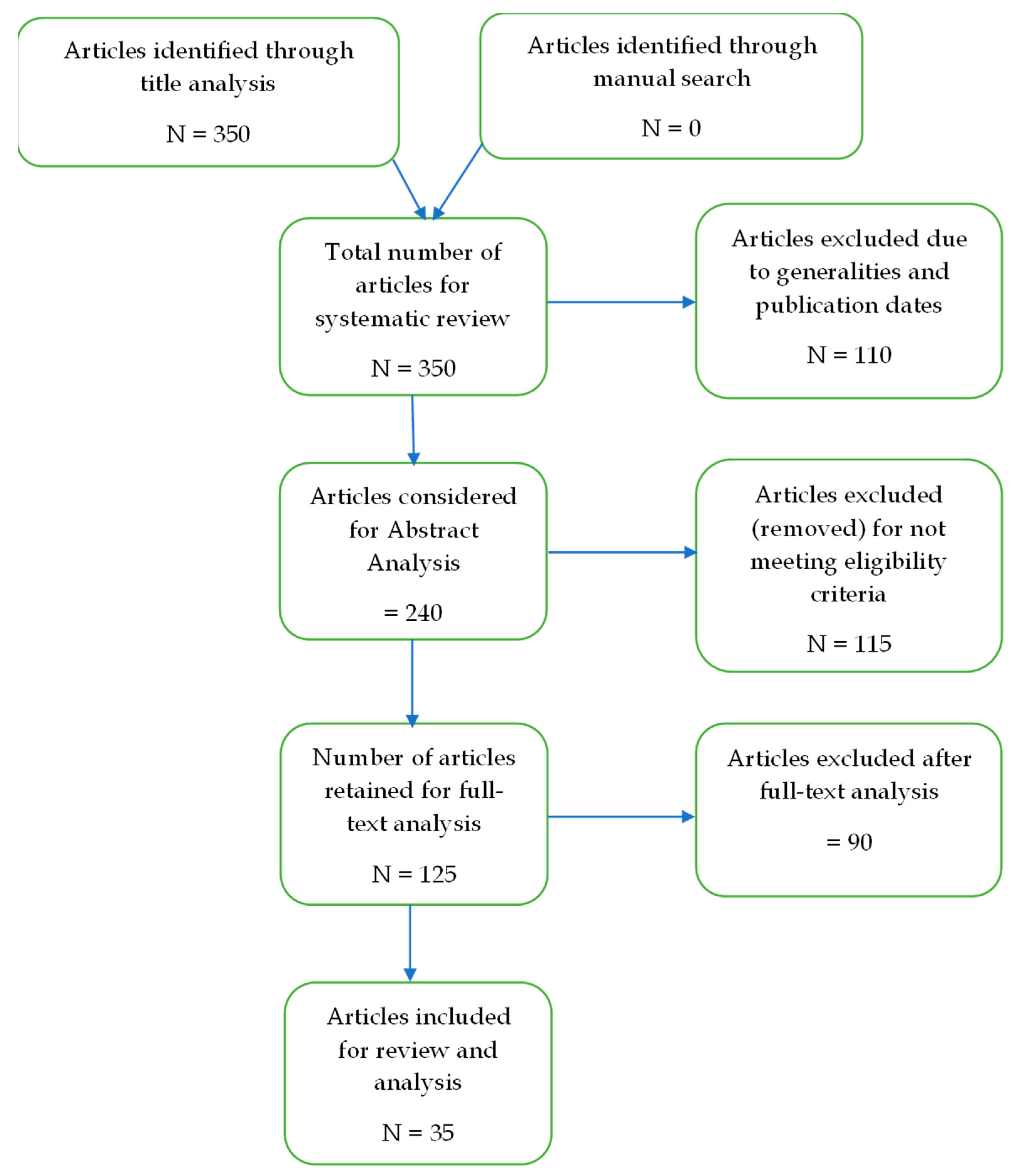
3. Methods
- How a Research Question is Framed:
- 2.
- Identifying Relevant Sources:
- 3.
- Usefulness of Studies:
- 4.
- Summarizing the Evidence:
- 5.
- Interpreting the Findings
3.1. Framing the Question
3.2. Identifying Sources
3.3. Selecting and Evaluating Sources
- Is the article relevant to the review question?
- Is the article published between 2010 and 2025?
- Does the article explore sustainability advantages associated with the application of AOPs in treating refractory wastewater?
- Is the source a government document, journal, website, policy paper, or book?
- Does the article’s affiliation or funding indicate any significant bias regarding the findings?
3.4. Analyzing Data
3.5. Data Synthesis Methods
3.6. Potential Limitations
4. Results
5. Discussion
5.1. Mechanisms of AOPs
5.2. Applications of AOPs
5.3. Comparative Analysis of AOPs
5.4. Standardization and Quality Controls
5.5. Comparison with Other AOPs: Efficiency, Cost, and Energy Consumption
5.6. Barriers to Large-Scale Implementation and Policy Implications
5.7. Critical Considerations
5.8. Applications and Policy Recommendations
6. Conclusions
- Demonstration approaches at pilot scale to validate the efficiencies in real wastewater environment and fluctuating contaminant loads.
- Assessments of economic feasibility, including adjustments for measuring life-cycle costs and affordability for operators, both municipal and industrial.
- The design of cheaper, less toxic catalysts with greater stability and lower energy demands.
- Enhancing sustainability and reducing operational expenses through renewable energy source integration.
- The integration of intelligent monitoring systems like real-time feedback control to maintain compliance, streamline processes, and save energy.
- Policy incentives and regulatory structures to facilitate investment in sustainable treatment technologies as well as mechanisms to support knowledge transfer between regions.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNEP. Global Water Shortages Are Looming. Here Is What Can Be Done About Them. United Nations Environmental Program. 2024. Available online: https://www.unep.org/news-and-stories/story/global-water-shortages-are-looming-here-what-can-be-done-about-them (accessed on 9 March 2025).
- Jamil, T. Role of Advance Oxidation Processes (AOPs) in Textile Wastewater Treatment: A Critical Review. Desalination Water Treat. 2024, 318, 100387. [Google Scholar] [CrossRef]
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of Advanced Oxidation Processes for the Treatment of Recalcitrant Agro-Industrial Wastewater: A Review. Water 2019, 11, 205. [Google Scholar] [CrossRef]
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost comparison of advanced oxidation processes for wastewater treatment using accumulated oxygen equivalent-criteria. Water Res. 2021, 200, 117234. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of Industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Merrylin, J.; Kavitha, S.; Yukesh Kannah, R.; Selvakumar, P.; Gopikumar, S.; Kumar, G. Trends in Biological Nutrient Removal for the Treatment of Low Strength Organic Wastewaters. Curr. Pollut. Rep. 2021, 7, 1–30. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Shan, C.; Li, H.; Yin, Y.; Pan, B. Toward Selective Oxidation of Contaminants in Aqueous Systems. Environ. Sci. Technol. 2021, 55, 14494–14514. [Google Scholar] [CrossRef]
- Ponnusami, A.B.; Sinha, S.; Ashokan, H.; Paul, M.V.; Hariharan, S.P.; Arun, J.; Pugazhendhi, A. Advanced Oxidation Process (AOP) Combined Biological Process for Wastewater Treatment: A Review on Advancements, Feasibility and Practicability of Combined Techniques. Environ. Res. 2023, 237, 116944. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, R.; Kumar, A.; Shankar, R.; Khan, N.A.; Gupta, K.N.; Arya, R.K. Pharmaceutical Waste-Water Treatment via Advanced Oxidation Based Integrated Processes: An Engineering and Economic Perspective. J. Water Process Eng. 2023, 54, 103977. [Google Scholar] [CrossRef]
- United Nations. Goal 6: Ensure Availability and Sustainable Management of Water and Sanitation for All. United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 4 April 2025).
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A Comprehensive Review on Reactive Oxygen Species (ROS) in Advanced Oxidation Processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Li, J.Y.; Hu, R.; Shan, L.; Liu, Z.Q.; Yang, S.Q.; Yang, J.; Cui, Y.H. Effect of Operating Conditions and Water Matrix on the Performance of UV Combined Electrochemical Process for Treating Chloride-Containing Solution and Its Reaction Mechanism. Sep. Purif. Technol. 2022, 286, 120465. [Google Scholar] [CrossRef]
- Spiniello, I.; De Carluccio, M.; Castiglione, S.; Amineva, E.; Kostryyukova, N.; Cicatelli, A.; Rizzo, L.; Guarino, F. Landfill leachate treatment by a combination of a multiple plant hybrid constructed wetland system with a solar photo-Fenton process in a raceway pond reactor. J. Environ. Manag. 2023, 331, 117211. [Google Scholar] [CrossRef] [PubMed]
- Mirza, N.R.; Huang, R.; Du, E.; Peng, M.; Pan, Z.; Ding, H.; Shan, G.; Ling, L.; Xie, Z. A review of the textile wastewater treatment techniques with special focus on advanced oxidation processes (AOPs), membrane separation and integrated AOP-membrane processes. Desalin. Water Treat. 2020, 206, 83–107. [Google Scholar] [CrossRef]
- Selvabharathi, G.; Kumar, A.S.; Jenefa, S.; Ginni, G.; Banu, R.J.; Yeom, I.T. Combined homogeneous and heterogeneous advanced oxidation process for the treatment of tannery wastewaters. J. Water Reuse Desal. 2016, 6, 59–71. [Google Scholar] [CrossRef]
- Moravvej, Z.; Soroush, E.; Rahimpour, M.R. Chapter 9-Achievements in hybrid processes for wastewater and water treatment. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–262. [Google Scholar]
- Meng, T.; Sun, W.; Su, X.; Sun, P. The optimal dose of oxidants in UV-based advanced oxidation processes with respect to primary radical concentrations. Water Res. 2021, 206, 117738. [Google Scholar] [CrossRef]
- Della-Flora, A.; Wilde, M.L.; Lima, D.; Lima, E.C.; Sirtori, C. Combination of tertiary solar photo-Fenton and adsorption processes in the treatment of hospital wastewater: The removal of pharmaceuticals and their transformation products. J. Environ. Chem. Eng. 2021, 9, 105666. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Keen, O.S.; McKay, G.; Mezyk, S.P.; Linden, K.G.; Rosario-Ortiz, F.L. Identifying the factors that influence the reactivity of effluent organic matter with hydroxyl radicals. Water Res. 2014, 50, 408–419. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 206, p. 012089. [Google Scholar]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Tian, J.; Wu, C.; Yu, H.; Gao, S.; Li, G.; Cui, F.; Qu, F. Applying ultraviolet/persulfate (UV/PS) pre-oxidation for controlling ultra-filtration membrane fouling by natural organic matter (NOM) in surface water. Water Res. 2018, 132, 190–199. [Google Scholar] [CrossRef]
- Gil, L.A.G.A.; Vicente, M.Á. The Handbook of Environmental Chemistry—Applications of Advanced Oxidation Processes (AOPs) in Drinking Water; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Snyder, H. Literature Review as a Research Methodology: An Overview and Guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Paré, G.; Kitsiou, S. Chapter 9 Methods for Literature Reviews. In Handbook of eHealth Evaluation: An Evidence-Based Approach; Lau, F., Kuziemsky, C., Eds.; University of Victoria: Victoria, BC, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK481583/ (accessed on 27 February 2025).
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Macías-Quiroga, I.F.; Henao-Aguirre, P.A.; Marín-Flórez, A.; Arredondo-López, S.M.; Sanabria-González, N.R. Bibliometric analysis of advanced oxidation processes (AOPs) in wastewater treatment: Global and Ibero-American research trends. Environ. Sci. Pollut. Res. Int. 2021, 28, 23791–23811. [Google Scholar] [CrossRef] [PubMed]
- Zoschke, K.; Dietrich, N.; Bornick, H.; Worch, E. UV-based advanced oxidation processes for the treatment of odour compounds: Efficiency and by-product formation. Water Res. 2012, 46, 5365–5373. [Google Scholar] [CrossRef]
- Barcelo, M.A.; Lopez, M.I.P.; Lucena, F.; Jofre, J.; Ibanez, P.F. Solar Advanced Oxidation Processes as disinfection tertiary treatments for real wastewater: Implications for water reclamation. Appl. Catal. B Environ. 2013, 136–137, 341–350. [Google Scholar] [CrossRef]
- Rodriguez, L.P.; Oller, I.; Klamerth, N.; Aguera, A.; Rodriguez, E.M.; Malato, S. Application of AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. Water Res. Nano Energy 2013, 47, 1521–1528. [Google Scholar]
- Covinich, L.G.; Bengoechea, D.I.; Fenoglio, R.J.; Area, M.C. Advanced Oxidation Processes for Wastewater Treatment in the Pulp and Paper Industry: A Review. Am. J. Environ. Eng. 2014, 4, 56–70. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Removal of Endocrine Disruptors from Urban Wastewater by Advanced Oxidation Processes (AOPs): A Review. Open Biotechnol. J. 2016, 10, 151–172. [Google Scholar] [CrossRef]
- Davarnejad, R.; Nasiri, S. Slaughterhouse wastewater treatment using an advanced oxidation process: An optimization study. Environ. Pollut. 2016, 223, 1–10. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Kumar, A.; Khraisheh, M. Potential use of solar photocatalytic oxidation in removing emerging pharmaceuticals from wastewater: A pilot plant study. Sol. Energy 2018, 172, 128–140. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2018, 376, 120597. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef]
- Garrido-Cradenas, J.A.; Esteban-Garcia, B.; Aguera, A.; Sanchez-Perez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef]
- Popat, A.; Nidheesh, P.V.; Anantha Singh, T.S.A.; Suresh Kumar, M. Mixed industrial wastewater treatment by combined electrochemical advanced oxidation and biological process. Chemosphere 2019, 237, 124419. [Google Scholar] [CrossRef]
- Sathya, U.; Keerthi, N.M.; Balasubramanian, N. Evaluation of advanced oxidation processes (AOPs) integrated membrane bioreactor (MBR) for the real textile wastewater treatment. J. Environ. Manag. 2019, 246, 768–775. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Z.; Yu, G.; Pan, X.; Wang, J.; Zhu, W.; Han, X.; Wang, Y. Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated particle electrodes. Sep. Purif. Technol. 2019, 208, 12–18. [Google Scholar] [CrossRef]
- Chanikya, P.; Nidheesh, P.V.; Babu, D.S.; Gopinath, A.; Suresh Kumar, M. Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep. Purif. Technol. 2020, 254, 117570. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Pandey, S.; Ray, S.S. Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Hutagalung, S.S.; Muchlis, I.; Khotimah, K. Textile wastewater treatment using Advanced Oxidation Process. Mater. Sci. Eng. 2020, 722, 012032. [Google Scholar] [CrossRef]
- Brillas, E. A critical review on ibuprofen removal from synthetic waters, natural waters, and real wastewaters by advanced oxidation processes. Chemosphere 2021, 286, 131849. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced Oxidation Processes Coupled with Nanomaterials for Water Treatment. Nanomaterial 2021, 11, 2045. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Qian, J. Degradation of roxarsone in UV-based advanced oxidation processes: A comparative study. J. Hazard. Mater. 2021, 410, 124558. [Google Scholar] [CrossRef] [PubMed]
- García, A.B.E.; Szymanski, K.; Mozia, S.; Perez, J.A.S. Treatment of laundry wastewater by solar photo-Fenton process at pilot plant scale. Environ. Sci. Pollut. Res. 2021, 28, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, T.D.; Jyothi, M.S.V.N.; Rao, C.P.; Maliyekkal, S.M. Advanced Oxidation Processes: A Promising Route for Abatement of Emerging Contaminants in Water. In Nanomaterials and Nanocomposites for Environmental Remediation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 275–305. [Google Scholar]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Couras, C.; Karim, A.V.; Nadais, H. A review of integrated advanced oxidation processes for organic pollutant removal. Chem. Eng. Commun. 2021, 209, 390–432. [Google Scholar] [CrossRef]
- Tang, Y.; Lee, C.S.; Walker, H.; Gobler, C.; Apul, O.; Venkatesan, A.K.; Mao, X. Effect of residual H2O2 on the removal of advanced oxidation byproducts by two types of granular activated carbon. J. Environ. Chem. Eng. 2021, 9, 106838. [Google Scholar] [CrossRef]
- Wang, H.; Hasani, M.; Wu, F.; Warriner, K. Pre-oxidation of spent lettuce wash water by continuous Advanced Oxidation Process to reduce chlorine demand and cross-contamination of pathogens during post-harvest washing. Food Microbiol. 2021, 103, 103937. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Toxicity changes of wastewater during various advanced oxidation processes treatment: An overview. J. Clean. Prod. 2021, 315, 128202. [Google Scholar] [CrossRef]
- An, S.A.; Lee, J.; Sim, J.; Park, C.G.; Lee, J.S.; Rho, H.; Park, K.D.; Kim, H.S.; Woo, Y.C. Evaluation of the advanced oxidation process integrated with microfiltration for reverse osmosis to treat semiconductor wastewater. Process Saf. Environ. Prot. 2022, 162, 1057–1066. [Google Scholar] [CrossRef]
- Gautam, P.; Popat, A.; Lokhandwala, S. Advances & Trends in Advance Oxidation Processes and Their Applications. In Advanced Industrial Wastewater Treatment and Reclamation of Water; Springer: Berlin/Heidelberg, Germany, 2022; pp. 45–69. [Google Scholar]
- Maifadi, S.; Mhlanga, S.D.; Nxumalo, E.N.; Motsa, M.M.; Kuvarega, A.T. Treatment of salon wastewater by peroxydisulfate based advanced oxidation process (PDS-AOP) under solar light. Synergy through integrated technologies. J. Water Process Eng. 2022, 49, 103062. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment—Unexpected nitration side reactions—A serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Ali, S.S.; Sabry, R.; Ali, H.M.; Gadallah, H.; Mansor, E.S.; Abdallah, H.; Shalaby, M.; Shaban, A.M. Integrated system of anoxic/activated sludge and ultrafiltration membrane for zero liquid discharge of pharmaceutical industrial wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109068. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Liu, L.; Lee, K.; Miao, J. Review of Biological Processes in a Membrane Bioreactor (MBR): Effects of Wastewater Characteristics and Operational Parameters on Biodegradation Efficiency When Treating Industrial Oily Wastewater. J. Mar. Sci. Eng. 2022, 10, 1229. [Google Scholar] [CrossRef]
- Pamuła, J.; Karnas, M.; Paluch, A.; Styszko, K. Comparative Study on Classical and Modified UV/H₂O₂ and Fenton Reaction Based Methods for the Removal of Chemical Pollutants in Water Treatment. Desalin. Water Treat. 2022, 275, 92–102. [Google Scholar] [CrossRef]
- Lin, J.Y.; Zhang, Y.; Bian, Y.; Zhang, Y.X.; Du, R.Z.; Li, M.; Feng, X.S. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in the Environment: Recent Updates on the Occurrence, Fate, Hazards and Removal Technologies. Sci. Total Environ. 2023, 904, 166897. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of Advanced Oxidation Processes (AOPs) in Industrial Applications: A Review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef]
- Giannakis, S.; Vives, F.A.G.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of Advanced Oxidation Processes on the Micropollutants and the Effluent Organic Matter Contained in Municipal Wastewater Previously Treated by Three Different Secondary Methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Alves, A.; Madeira, L.M.; Santos, M.S. Oxidation processes for cytostatic drugs elimination in aqueous phase: A critical review. J. Environ. Chem. Eng. 2021, 9, 104709. [Google Scholar] [CrossRef]
- Bobu, M.; Yediler, A.; Siminiceanu, I.; Zhang, F.; Schulte-Hostede, S. Comparison of different advanced oxidation processes for the degradation of two fluoroquinolone antibiotics in aqueous solutions. J. Environ. Sci. Heal Part A Toxic Hazard. Subst. Environ. Eng. 2013, 48, 251–262. [Google Scholar] [CrossRef]
- Jamil, T.S.; Roland, H.; Michael, H.; Jens-Uwe, R. Homogeneous photocatalytic processes for degradation of some endocrine disturbing chemicals under UV irradiation. J. Water Process. Eng. 2017, 18, 159–168. [Google Scholar] [CrossRef]
- Machado, F.; Teixeira, A.C.S.C.; Ruotolo, L.A.M. Critical Review of Fenton and Photo-Fenton Wastewater Treatment Processes over the Last Two Decades. Int. J. Environ. Sci. Technol. 2023, 20, 13995–14032. [Google Scholar] [CrossRef]
- Thongsai, A.; Phuttaro, C.; Saritpongteeraka, K.; Charnnok, B.; Bae, J.; Noophan, P.L.; Chaiprapat, S. Efficacy of Anaerobic Membrane Bioreactor under Intermittent Liquid Circulation and Its Potential Energy Saving against a Conventional Activated Sludge for Industrial Wastewater Treatment. Energy 2022, 244, 122556. [Google Scholar] [CrossRef]
- Verma, S.; Sillanpää, M. Degradation of anatoxin-a by UV-C LED and UV-C LED/H2O2 advanced oxidation processes. Chem. Eng. J. 2015, 274, 274–281. [Google Scholar] [CrossRef]
- Bagheri, M.; Mohseni, M. Impact of hydrodynamics on pollutant degradation and energy efficiency of VUV/UV and H2O2/UV oxidation processes. J. Environ. Manag. 2015, 164, 114–120. [Google Scholar] [CrossRef]
- Ike, I.A.; Karanfil, T.; Cho, J.; Hur, J. Oxidation byproducts from the degradation of dissolved organic matter by advanced oxidation processes—A critical review. Water Res. 2019, 164, 114929. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Pintado-Herrera, M.G.; Martín-Díaz, M.L.; Acevedo-Merino, A.; Manzano, M.A. Combined AOPs for potential wastewater reuse or safe discharge based on multi-barrier treatment (microfiltration-H2O2/UV-catalytic wet per-oxide oxidation). Chem. Eng. J. 2015, 270, 80–90. [Google Scholar] [CrossRef]
- Crapulli, F.; Santoro, D.; Sasges, M.R.; Ray, A.K. Mechanistic modeling of vacuum UV advanced oxidation process in an annular photoreactor. Water Res. 2014, 64, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, E.; Boal, A.K.; Springer, J.; Stanford, B.; Rivera, S.; Kashinkunti, R.D.; Metz, D.H. Comparison of UV-mediated Advanced Oxidation. J.-Am. Water Work. Assoc. 2013, 105, 29–33. [Google Scholar] [CrossRef]
- Ali, F.; Khan, J.A.; Shah, N.S.; Sayed, M.; Khan, H.M. Carbamazepine degradation by UV and UV-assisted AOPs: Kinetics, mechanism and toxicity investigations. Process. Saf. Environ. Prot. 2018, 117, 307–314. [Google Scholar] [CrossRef]
- Blanco, J.; Torrades, F.; de la Varga, M.; García-Montaño, J. Fenton and biological-Fenton coupled processes for textile wastewater treatment and reuse. Desalination 2012, 286, 394–399. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. The feasibility of using combined Fenton-SBR for antibiotic wastewater treatment. Desalination 2012, 285, 14–21. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Evaluation of Fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments. J. Environ. Manag. 2011, 92, 749–755. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Costa, C.N. Combination of Fenton oxidation and composting for the treatment of the olive solid residue and the olive mile wastewater from the olive oil industry in Cyprus. Bioresour. Technol. 2010, 101, 7984–7987. [Google Scholar] [CrossRef]
- Zorpas, A.A. Alternative treatment of urban wastewater using electrochemical oxidation. Desalination Water Treat. 2011, 27, 268–276. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 2005, 58, 1409–1414. [Google Scholar] [CrossRef]
- Yang, C.; Wang, D.; Tang, Q. The synthesis of NdFeB magnetic activated carbon and its application in degradation of azo dye methyl orange by Fenton-like process. J. Taiwan Inst. Chem. Eng. 2014, 45, 2584–2589. [Google Scholar] [CrossRef]
- Mahdad, F.; Younesi, H.; Bahramifar, N.; Hadavifar, M. Optimization of Fenton and photo-Fenton-based advanced oxidation processes for post-treatment of composting leachate of municipal solid waste by an activated sludge process. KSCE J. Civ. Eng. 2016, 20, 2177–2188. [Google Scholar] [CrossRef]
- Punzi, M.; Anbalagan, A.; Börner, R.A.; Svensson, B.-M.; Jonstrup, M.; Mattiasson, B. Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar] [CrossRef]
- Fiorentino, A.; Esteban, B.; Garrido-Cardenas, J.A.; Kowalska, K.; Rizzo, L.; Aguera, A.; Pérez, J.A.S. Effect of solar photo-Fenton process in raceway pond reactors at neutral pH on antibiotic resistance determinants in secondary treated urban wastewater. J. Hazard. Mater. 2019, 378, 120737. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qu, Y.; Yang, B.; Liu, X.; Su, W. Lactate oxidation in pyrite suspension: A Fenton-like process in situ generating H2O. Chemosphere 2012, 86, 376–382. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Yao, B.; Yang, J.; Zhi, D. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique. J. Hazard. Mater. 2021, 418, 126313. [Google Scholar] [CrossRef]
- Chang, X.; Meyer, M.; Liu, X.; Zhao, Q.; Chen, H.; Chen, J.-A.; Qiu, Z.; Yang, L.; Cao, J.; Shu, W. Determination of antibiotics in sewage from hospitals, nursery and slaughterhouse, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ. Pollut. 2010, 158, 1444–1450. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wu, J.; Niu, J. Insights into the electrochemical degradation of sulfamethoxazole and its metabolite by Ti/SnO2-Sb/Er-PbO2 anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Ciríaco, L.; Anjo, C.; Correia, J.; Pacheco, M.; Lopes, A. Electrochemical degradation of Ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochim. Acta 2009, 54, 1464–1472. [Google Scholar] [CrossRef]
- Krasner, S.W.; Mitch, W.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef]
- Almomani, F.A.; Shawaqfah, M.; Bhosale, R.R.; Kumar, A. Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes. Environ. Prog. Sustain. Energy 2016, 35, 982–995. [Google Scholar] [CrossRef]
- Patel, S.; Mondal, S.; Majumder, S.K.; Das, P.; Ghosh, P. Treatment of a Pharmaceutical Industrial Effluent by a Hybrid Process of Advanced Oxidation and Adsorption. ACS Omega 2020, 5, 32305–32317. [Google Scholar] [CrossRef]
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.; Nan, J.; Li, G. Advanced Oxidation Processes (AOPs)-Based Sludge Conditioning for Enhanced Sludge Dewatering and Micropollutants Removal: A Critical Review. J. Water Process Eng. 2022, 45, 102468. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-Synthesized Nanocatalysts and Nanomaterials for Water Treatment: Current Challenges and Future Perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef]
- Mariño, M.A.; Fulaz, S.; Tasic, L. Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications. Magnetochemistry 2021, 7, 133. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination 2010, 256, 43–47. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.B.D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Karunakaran, C.; Senthilvelan, S. Photocatalysis with ZrO2: Oxidation of aniline. J. Mol. Catal. A Chem. 2005, 233, 1–8. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Tichagwa, L.; Mamphweli, S.; Petrik, L. Silver/Carbon Codoped Titanium Dioxide Photocatalyst for Improved Dye Degradation under Visible Light. Int. J. Photoenergy 2017, 2017, 3079276. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.; Pirola, C.; Vitali, S.; Cerrato, G.; Morandi, S.; Argirusis, C.; Sourkouni, G.; Sakkas, P.; Capucci, V. Surface decoration of commercial micro-sized TiO2 by means of high energy ultrasound: A way to enhance its photocatalytic activity under visible light. Appl. Catal. B Environ. 2015, 178, 124–132. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.; Argirusis, C.; Pifferi, V.; Neppolian, B.; Cerrato, G.; Boffito, D. Ultrasound assisted synthesis of Ag-decorated TiO2 active in visible light. Ultrason. Sonochem. 2018, 40, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Stucchi, M.; Bianchi, C.L.; Pirola, C.; Cerrato, G.; Morandi, S.; Argirusis, C.; Sourkouni, G.; Naldoni, A.; Capucci, V. Copper NPs decorated titania: A novel synthesis by high energy US with a study of the photocatalytic activity under visible light. Ultrason. Sonochem. 2016, 31, 295–301. [Google Scholar] [CrossRef]
- Pekakis, P.A.; Xekoukoulotakis, N.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Ameta, S.C.; Ameta, R. Advance Oxidation Processes for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lucas, M.S. Application of Advanced Oxidation Processes to Wastewater Treatment. Ph.D. Thesis, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal, 2009. [Google Scholar]
- Souza, B.S.; Moreira, F.C.; Dezotti, M.W.C.; Vilar, V.J.P.; Boaventura, R.A.R. Application of biological oxidation and solar driven advanced oxidation processes to remediation of winery wastewater. Catal. Today 2013, 209, 201–208. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen per-oxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Fei, H.; Leng, W.; Li, X.; Cheng, X.; Xu, Y.; Zhang, J.; Cao, C. Photocatalytic Oxidation of Arsenite over TiO2: Is Superoxide the Main Oxidant in Normal Air-Saturated Aqueous Solutions? Environ. Sci. Technol. 2011, 45, 4532–4539. [Google Scholar] [CrossRef]
- Stepnowski, P.; Zaleska, A. Comparison of different advanced oxidation processes for the degradation of room temperature ionic liquids. J. Photochem. Photobiol. A Chem. 2005, 170, 45–50. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Satayeva, A.; Abdirova, P.; Inglezakis, V.J.; Arkhangelsky, E.; Poulopoulos, S.G. Membrane Bioreactor and Advanced Oxidation Processes for Combined Treatment of Synthetic Wastewater Containing Naproxen, Bisphenol A, and Sulfamethoxazole. J. Water Process Eng. 2023, 55, 104250. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Tetteh, E.K.; Armah, E.K. Membrane Bioreactors for Produced Water Treatment: A Mini-Review. Membranes 2022, 12, 275. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhamad, M.H.; Budi Kurniawan, S.; Buhari, J.; Husain Abuzeyad, O. Managing Bisphenol A Contamination: Advances in Removal Technologies and Future Prospects. Water 2023, 15, 3573. [Google Scholar] [CrossRef]
- Kitanou, S.; Tahri, M.; Bachiri, B.; Mahi, M.; Hafsi, M.; Taky, M.; Elmidaoui, A. Comparative study of membrane bioreactor (MBR) and activated sludge processes in the treatment of Moroccan domestic wastewater. Water Sci. Technol. 2018, 78, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Shamshad, J.; Rehman, R.U. Innovative Approaches to Sustainable Wastewater Treatment: A Comprehensive Exploration of Conventional and Emerging Technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Sipma, J.; Osuna, B.; Collado, N.; Monclús, H.; Ferrero, G.; Comas, J.; Rodriguez-Roda, I. Comparison of Removal of Pharmaceuticals in MBR and Activated Sludge Systems. Desalination 2010, 250, 653–659. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef]
- Alvarino, T.; Lema, J.; Omil, F.; Suárez, S. Trends in Organic Micropollutants Removal in Secondary Treatment of Sewage. Rev. Environ. Sci. Biotechnol. 2018, 17, 447–469. [Google Scholar] [CrossRef]
- Parsa, Z.; Dhib, R.; Mehrvar, M. Dynamic Modelling, Process Control, and Monitoring of Selected Biological and Advanced Oxidation Processes for Wastewater Treatment: A Review of Recent Developments. Bioengineering 2024, 11, 189. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl Radicals Based Advanced Oxidation Processes (AOPs) for Remediation of Soils Contaminated with Organic Compounds: A Review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Hunge, Y.M. Basics and advanced developments in photocatalysis—A review (Mini review). Int. J. Hydrol. 2018, 2, 539–540. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Gagol, M.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs). Ultrason. Sonochem. 2019, 58, 104610. [Google Scholar] [CrossRef]
- Shet, A.; Shetty, V.K. Solar light mediated photocatalytic degradation of phenol using Ag-core TiO2 shell (Ag@TiO2) nanoparticles in batch and fluidized bed reactor. Sol. Energy 2016, 127, 67–78. [Google Scholar] [CrossRef]
- Abid, M.F.; Abdulla, O.N.; Kadhim, A.F. Study on removal of phenol from synthetic wastewater using solar photocatalytic reactor. J. King Saud Univ. Eng. Sci. 2017, 31, 131–139. [Google Scholar]
- Mota, A.L.N.; Albuquerque, L.F.; Beltrame, L.T.C.; Chiavone-Filho, O.; Machulek, A., Jr.; Nascimento, C.A.O. Advanced oxidation Processes and Their Application in The Petroleum Industry: A Review. Braz. J. Pet. Gas 2008, 2, 122–142. [Google Scholar]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Saritha, P.; Aparna, C.; Himabindu, V.; Anjaneyulu, Y. Comparison of various advanced oxidation processes for the degradation of 4-chloro-2 nitrophenol. J. Hazard. Mater. 2007, 149, 609–614. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef]
- Keen, O.S.; Bell, K.Y.; Cherchi, C.; Finnegan, B.J.; Mauter, M.S.; Parker, A.M.; Stretz, H.A. Emerging Pollutants–Part II: Treatment. Water Environ. Res. 2014, 86, 2036–2096. [Google Scholar] [CrossRef]
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic Titanium Dioxide Based Nanomaterials: Synthesis, Characteristics, and Photocatalytic Application in Pollutant Degradation. J. Mater. Chem. A 2015, 3, 17511–17524. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

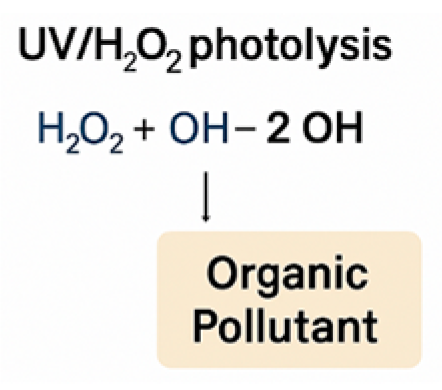
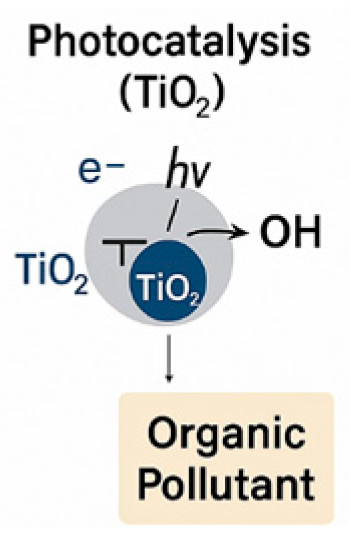
| AOP Method | Representative Reaction |
|---|---|
| Ozonation | O3 + H2O → 2 ·OH + O2 |
| Fenton Reaction | Fe2+ + H2O2 → Fe3+ + ·OH + OH− |
| Photo-Fenton Reaction | Fe3+ + H2O + hv → Fe2+ + ·OH + H+ |
| UV/H2O2 Process | H2O2 + hv → 2 ·OH |
| Photocatalysis (TiO2-based) | TiO2 + hv → e− + h+ h+ + H2O → ·OH e− + O2 → ·O2− |
| Article Groups | Rater 1 | Rater 2 | Rater 3 | Rater 4 | Agreement |
|---|---|---|---|---|---|
| 1 | 4 | 6 | 3 | 7 | 1 |
| 2 | 5 | 5 | 2 | 5 | 1 |
| 3 | 7 | 4 | 4 | 3 | 1 |
| 4 | 6 | 3 | 4 | 3 | 1 |
| 5 | 2 | 4 | 7 | 2 | 1 |
| Total | 5/5 |
| Title | Type of Document | Authors and Date | Findings |
|---|---|---|---|
| UV-based advanced oxidation processes for the treatment of odor compounds: Efficiency and by-product formation. | Journal Article | Zoschke et al., 2012 [31] | Indicates how UV-based AOPs remove bad odor compounds by oxidizing the organic compounds associated with the property. This helps in cleaning water from factories and municipal facilities by removing bad smells and colors. |
| Solar advanced oxidation processes as disinfection tertiary treatments for real wastewater: Implications for water reclamation. | Journal Article | Barcelo et al., 2013 [32] | Highlights how UV-based AOPs can be used in the tertiary treatment of wastewater as a disinfectant to kill harmful bacteria, fungi, and other contaminants. UV-based AOPs contain highly reactive species that destroy or inactivate microbes by destroying their deoxyribonucleic acid (DNA). |
| Application of AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. | Journal Article | Rodriguez et al., 2013 [33] | Indicates how municipal facilities use AOPs and ozonation to destroy micropollutants, reduce the volume of sludge, and make more organic compounds available for further microbial degradation. |
| Advanced oxidation processes for wastewater treatment in the pulp and paper industry: A review. | Journal Article | Covinich et al., 2014 [34] | Explains why AOPs are chosen for refractory wastewater from pulp and paper-making factories because they react with a wide spectrum of contaminants. These factories also use AOPs to clean their effluents and make them less toxic before discharge. |
| An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. | Journal Article | Ribeiro et al., 2015 [35] | Recommends the use of AOPs in treating micropollutants that cannot be destroyed using microbial processes and other conventional mechanisms. |
| Removal of endocrine disruptors from urban wastewater by advanced oxidation processes (AOPs): A review. | Journal Article | Cesaro and Belgiorno, 2016 [36] | Indicates how urban wastewater facilities use AOPs to remove endocrine disruptors through oxidation and provide safer water for domestic or industrial use. AOPs are also more efficient and enable urban wastewater treatment facilities to minimize their operational costs. |
| Slaughterhouse wastewater treatment using an advanced oxidation process: An optimization study. | Journal Article | Davarnejad and Nasiri, 2016 [37] | Wastewater from slaughterhouses can be highly toxic and difficult to process using traditional wastewater treatment facilities. This study shows how AOPs break down complex organic compounds into smaller and less harmful compounds that can be processed further using biological mechanisms. |
| Potential use of solar photocatalytic oxidation in removing emerging pharmaceuticals from wastewater: A pilot plant study. | Journal Article | Almomani et al., 2018 [38] | Solar photocatalytic oxidation is more effective in producing more hydroxyl radicals than using standalone AOPs such as O3 or UV radiation. Photocatalytic oxidation also produces radicals that can destroy a wide spectrum of micropollutants. |
| Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. | Journal Article | Miklos et al., 2018 [39] | The researchers evaluated the effectiveness of AOPs in destroying stubborn organic compounds while comparing the outcomes with the conventional mechanisms. The results showed that AOPs are more effective, flexible, and consume relatively less energy. |
| A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. | Journal Article | Paździor et al., 2018 [40] | Indicates how combining AOPs with biological processes enhances targeting and ensures that even the most stubborn organic compounds from textile wastewater are reduced to smaller compounds for further degradation. Biological processes remove less complex organic compounds to ensure the final product is clean and safe. |
| Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. | Journal Article | Sillanpää et al., 2018 [41] | AOPs assist in removing natural organic matter through oxidation. AOPs also mineralize the by-products into carbon dioxide and water, significantly eliminating potential sludge. |
| Wastewater treatment by advanced oxidation process and their worldwide research trends. | Journal Article | Garrido-Cradenas et al., 2019 [42] | Examines worldwide research trends aimed at improving AOPs by making them more efficient and effective. The trends include catalyst development, hybrid systems (combining AOPs with other technologies), and advanced control or monitoring systems. |
| Mixed industrial wastewater treatment by combined electrochemical advanced oxidation and biological process. | Journal Article | Popat et al., 2019 [43] | Finding that electrochemical AOPs are more suitable for removing organic compounds from mixed industrial wastewater based on their stronger and more effective oxidative capacity. |
| Evaluation of advanced oxidation processes (AOPs) integrated with membrane bioreactor (MBR) for real textile wastewater treatment. | Journal Article | Sathya et al., 2019 [44] | Combining AOPs with other technologies such as MBR enhances targeting, removes all stubborn organics, and is more effective than standalone AOPs. |
| Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated particle electrodes. | Journal Article | Zhan et al., 2019 [45] | Due to its complex organic compounds that cannot be removed using conventional biological processes, this study found that AOPs are more suitable for the treatment of pharmaceutical wastewater due to their strong oxidation effects. The process becomes even more efficient when applied in a three-dimensional process, including ozonation and in situ regenerating granular activated particle electrodes. |
| Treatment of dyeing wastewater by combined sulfate radical-based electrochemical advanced oxidation and electrocoagulation processes. | Journal Article | Chanikya et al., 2020 [46] | Wastewater containing dye can be difficult to treat due to complex organic compounds. This study recommends sulfate-radical-based AOPs for removing complex organics. |
| Photocatalysts in advanced oxidation processes for wastewater treatment. | Book Chapter | Fosso-Kankeu et al., 2020 [47] | Recommends photocatalysts in AOPs due to their stronger hydroxyl radicals that can react to a wide range of organic compounds. Photocatalysts, however, may contain by-products that require further processing to enhance environmental safety. |
| Textile wastewater treatment using advanced oxidation process. | Journal Article | Hutagalung et al., 2020 [48] | Recommends using AOPs in the treatment of textile wastewater because they are more efficient, demand less energy, and produce limited sludge. |
| A critical review on ibuprofen removal from synthetic waters, natural waters, and real wastewaters by advanced oxidation processes. | Journal Article | Brillas, 2021 [49] | Recommends AOPs for removing organic compounds from pharmaceutical wastewater due to their complex organic structures. Only AOPs can reduce them to simpler compounds for further processing. |
| Advanced oxidation processes coupled with nanomaterials for water treatment. | Journal Article | Cardoso et al., 2021 [50] | Combining AOPs with nanomaterials enhances the production of hydroxyl radicals, and supports advanced monitoring and control of all parameters, both effluent and influent. |
| Degradation of roxarsone in UV-based advanced oxidation processes: A comparative study. | Journal Article | Chen, Li, and Qian, 2021 [51] | Describes the degradation of roxarsone using UV-based AOPs as highly effective and more efficient than the conventional wastewater treatment processes. UV-based AOPs also produce stronger reactive species when combined with catalysts such as TiO2. |
| Treatment of laundry wastewater by solar photo-Fenton process at pilot plant scale. | Journal Article | García et al., 2021 [52] | Recommends using AOPs such as Fenton processes to treat wastewater from laundry activities. Solar photo-Fenton is more effective in producing hydroxyl radicals and can remove a wide range of organic compounds from wastewater, making the process more efficient and economical. |
| Advanced oxidation processes: A promising route for abatement of emerging contaminants in water. | Journal Article | Kusuma et al., 2021 [53] | Recommends using AOPs to remove emerging contaminants since they can react to and oxidize nearly all organic compounds. |
| Critical review of advanced oxidation processes in organic wastewater treatment. | Journal Article | Ma et al., 2021 [54] | AOPs are the most promising and efficient oxidation technology for treating organic wastewater. Some of the key challenges found include high initial costs and changing regulatory frameworks. |
| A review of integrated advanced oxidation processes for organic pollutant removal. | Journal Article | Nidheesh et al., 2021 [55] | AOPs possess a greater capacity to remove a wide variety of pollutants and make wastewater more biodegradable. Potential drawbacks include the high cost of operations resulting from energy demand and chemicals. |
| Effect of residual H2O2 on the removal of advanced oxidation byproducts by two types of granular activated carbon | Journal Article | Tang et al., 2021 [56] | Recommends the use of H2O2 for optimal production of hydroxyl radicals needed for removing complex organic compounds. |
| Pre-oxidation of spent lettuce wash water by continuous advanced oxidation process to reduce chlorine demand and cross-contamination of pathogens during post-harvest washing. | Journal Article | Wang et al., 2021 [57] | Pre-oxidation of wastewater using AOPs generally leads to a significant decline in the demand for chlorine throughout the process. AOPs destroy organic microbes in wastewater, leading to lower demand for disinfectants such as chlorine. |
| Toxicity changes of wastewater during various advanced oxidation processes treatment: An overview. | Journal Article | Wang and Wang, 2021 [58] | By measuring toxicity across the wastewater treatment journey, the level of toxicity significantly declines as AOPs destroy organic compounds in the wastewater. However, the types of AOPs determine the overall level of toxicity. |
| Evaluation of the advanced oxidation process integrated with microfiltration for reverse osmosis to treat semiconductor wastewater. | Journal Article | An et al., 2022 [59] | AOPs are more effective in destroying and removing organic compounds than biological processes, such as microfiltration using reverse osmosis. |
| Advances and trends in advanced oxidation processes and their applications. In Advanced Industrial Wastewater Treatment and Reclamation of Water. | Journal Article | Gautam et al., 2022 [60] | Recommends using AOPs for reclaiming industrial wastewater and addressing scarcity challenges affecting urban areas. The researcher also identifies key trends such as hybrid systems and the use of catalysts and how they may shape the future applications of AOPs in wastewater management. |
| Treatment of salon wastewater by peroxydisulfate-based advanced oxidation process (PDS-AOP) under solar light. Synergy through integrated technologies. | Journal Article | Maifadi et al., 2022 [61] | Due to the complex organics found in personal care products, findings recommend using AOPs for treating wastewater from salons. Specifically, the study found that peroxydisulfate-based advanced oxidation processes (PDS-AOP) are more effective in this task than other AOPs. |
| Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini-review. | Journal Article | Pandis et al., 2022 [62] | This review found significant evidence supporting the application of AOPs in the treatment of wastewater from pharmaceutical companies or industries. |
| Advanced oxidation processes (AOPs)-based wastewater treatment—unexpected nitration side reactions—a serious environmental issue: A review. | Journal Article | Rayaroth et al., 2022 [63] | Although AOPs are highly reactive to a wide variety of organic compounds, not much was known about potential unexpected nitration side reactions until this study. The study recommends optimal controls to prevent unexpected reactions that can damage the expected outcomes. |
| Integrated system of anoxic/activated sludge and ultrafiltration membrane for zero liquid discharge of pharmaceutical industrial wastewater treatment. | Journal Article | Ali et al., 2023 [64] | AOPs are used in sludge treatment to reduce the volume and make it more available for further biodegradation. AOPs also reduce the toxicity of the sludge and make it less harmful to discharge without damaging surrounding ecosystems. |
| Challenges and emerging trends in advanced oxidation technologies and integration of advanced oxidation processes with biological processes for wastewater treatment. | Journal Article | Gopalakrishnan et al., 2023 [65] | Key challenges were identified affecting AOPs, including energy demand, higher initial costs, and chemical use. Key trends that will address some of these challenges include the development of better catalysts, advanced monitoring systems, and integration of AOPs with other technologies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.A. Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review. Sustainability 2025, 17, 3439. https://doi.org/10.3390/su17083439
Silva JA. Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review. Sustainability. 2025; 17(8):3439. https://doi.org/10.3390/su17083439
Chicago/Turabian StyleSilva, Jorge Alejandro. 2025. "Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review" Sustainability 17, no. 8: 3439. https://doi.org/10.3390/su17083439
APA StyleSilva, J. A. (2025). Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review. Sustainability, 17(8), 3439. https://doi.org/10.3390/su17083439







