A Whole-Stand Model for Estimating the Productivity of Uneven-Aged Temperate Pine-Oak Forests in Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Modeling Approach
2.2.1. Transition Function for Dominant Height (H)
2.2.2. Transition Function for the Number of Trees per Unit Area (N)
| Author | Initial Condition | Mathematical Expression of the Transition Function |
|---|---|---|
| Clutter and Jones (1980) [44] | β ≠ 0 | |
| Pienaar et al. (1990) [46] | β ≠ 0 | |
| Woollons (1998) [48] | β = 0.5 | |
| García (2013) [49] | β ≠ 0 | |
| Pienaar and Shiver (1981) [45] | β = 0 | |
| Tomé et al. (1997) [47] | β = 0 | |
| Zunino and Ferrando (1997) [51] | β = 0 | |
| Bailey et al. (1985) [50] | β = 0 |
2.2.3. Transition Function for Basal Area (BA)
2.2.4. Transition Function for Stand Volume and Above-Ground Biomass
3. Results and Discussion
3.1. Transition Function for Dominant Height (H)
3.2. Transition Function for Stand Density (N)
3.2.1. Models for Estimating Natural Mortality
3.2.2. Models for Estimating Recruitment
3.3. Transition Functions for Stand Basal Area (BA), Stand Volume (V), and Above-Ground Biomass (AGB)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Niinemets, U.; Sheffield, J.; Lichstein, J.W. Shifts in Tree Functional Composition Amplify the Response of Forest Biomass to Climate. Nature 2018, 556, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Blundo, C.; Carilla, J.; Grau, R.; Malizia, A.; Malizia, L.; Osinaga-Acosta, O.; Bird, M.; Bradford, M.; Catchpole, D.; Ford, A.; et al. Taking the Pulse of Earth’s Tropical Forests Using Networks of Highly Distributed Plots. Biol. Conserv. 2021, 260, 108849. [Google Scholar] [CrossRef]
- Geijzendorffer, I.R.; Cohen-Shacham, E.; Cord, A.F.; Cramer, W.; Guerra, C.; Martín-López, B. Ecosystem Services in Global Sustainability Policies. Environ. Sci. Policy 2017, 74, 40–48. [Google Scholar] [CrossRef]
- Feurer, M.; Rueff, H.; Celio, E.; Heinimann, A.; Blaser, J.; Htun, A.M.; Zaehringer, J.G. Regional scale mapping of ecosystem services supply, demand, flow and mismatches in southern Myanmar. Ecosyst. Serv. 2021, 52, 101363. [Google Scholar] [CrossRef]
- Díaz, S.; Pascual, U.; Stenseke, M.; Martín-López, B.; Watson, R.T.; Molnár, Z.; Hill, R.; Chan, K.M.; Baste, I.A.; Brauman, K.A.; et al. Assessing nature’s contributions to people. Science 2018, 359, 270–272. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Rifai, S.; Shenkin, A.; Oliveras, I.; Bentley, L.P.; Svátek, M.; Girardin, C.A.; Both, S.; Riutta, T.; Berenguer, E.; et al. Pantropical Modelling of Canopy Functional Traits Using Sentinel-2 Remote Sensing Data. Remote Sens. Environ. 2021, 252, 112122. [Google Scholar] [CrossRef]
- Davies, S.J.; Abiem, I.; Salim, K.A.; Aguilar, S.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.; Andrade, A.; Arellano, G.; Ashton, P.S.; et al. ForestGEO: Understanding Forest Diversity and Dynamics through a Global Observatory Network. Biol. Conserv. 2021, 253, 108907. [Google Scholar] [CrossRef]
- Vanclay, J. Modelling Forest Growth and Yield: Applications to Mixed Tropical Forests; CAB International: Wallingford, UK, 1994; 330p. [Google Scholar]
- Randin, C.F.; Ashcroft, M.B.; Bolliger, J.; Cavender-Bares, J.; Coops, N.C.; Dullinger, S.; Dirnböck, T.; Eckert, S.; Ellis, E.; Fernández, N.; et al. Monitoring Biodiversity in the Anthropocene Using Remote Sensing in Species Distribution Models. Remote Sens. Environ. 2020, 239, 111626. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación de México, 1st ed.; Limusa: Mexico City, Mexico, 1978. [Google Scholar]
- Rzedowski, J. Diversidad y orígenes de la flora fanerogámica de México. Acta Bot. Mex. 1991, 14, 3–21. [Google Scholar] [CrossRef]
- Rzedowski, J.; Reyna-Trujillo, T. Provincias Florísticas; Instituto de Geografía, Universidad Nacional Autónoma de México: Mexico City, Mexico, 1990. [Google Scholar]
- Sarukhán, J.; Dirzo, R. México Ante los Retos de la Biodiversidad; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Tlalpan, Mexico, 1992.
- Feeley, K.J.; Davies, S.J.; Pérez, R.; Hubbell, S.P.; Foster, R.B. Directional changes in the species composition of a tropical forest. Ecology 2011, 92, 871–882. [Google Scholar] [CrossRef]
- Harfoot, M.B.; Johnston, A.; Balmford, A.; Burgess, N.D.; Butchart, S.H.; Dias, M.P.; Hazin, C.; Hilton-Taylor, C.; Hoffmann, M.; Isaac, N.J.B.; et al. Using the IUCN Red List to map threats to terrestrial vertebrates at global scale. Nat. Ecol. Evol. 2021, 5, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- González-Elizondo, M.S.; González Elizondo, M.; Márquez Linares, M. Vegetación y Ecorregiones de Durango; Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional (CIIDIR), Instituto Politécnico Nacional: Durango, Mexico, 2007. [Google Scholar]
- INEGI-CONABIO. Ecorregiones Terrestres de México. 2008. Available online: https://geoportal.conabio.gob.mx/metadatos/doc/html/ecort08gw.html (accessed on 27 February 2025).
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar] [CrossRef]
- Ellis, T.; Bowman, D.; Williamson, G. Humans have substantially extended fire seasons in all biomes on Earth. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Change 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Daskalova, G.N.; Myers-Smith, I.H.; Bjorkman, A.D.; Blowes, S.A.; Supp, S.R.; Magurran, A.E.; Dornelas, M. Landscape-scale forest loss as a catalyst of population and biodiversity change. Science 2020, 368, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Blowes, S.A.; Knight, T.M.; Gerstner, K.; May, F. Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 2020, 584, 238–243. [Google Scholar] [CrossRef]
- Burkhart, H.E.; Tomé, M. Modeling Forest Trees and Stands; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Gadow, K.v.; Álvarez-González, J.G.; Zhang, C.; Pukkala, T.; Zhao, X. Sustaining Forest Ecosystems; Springer Nature: Cham, Switzerland, 2021. [Google Scholar]
- Weiskittel, A.R.; Hann, D.W.; Kershaw, J.A.; Vanclay, J.K. Forest Growth and Yield Modeling; John Wiley & Sons: Oxford, UK, 2011. [Google Scholar]
- da Rocha, S.J.; Torres, C.M.; Villanova, P.H.; Júnior, I.D.; Rufino, M.P.; Romero, F.M.; Jacovine, L.A.; de Morais Junior, V.T.; de Jesus França, L.C.; Schettini, B.L.; et al. Machine learning methods: Modeling net growth in the Atlantic Forest of Brazil. Ecol. Inform. 2024, 81, 102564. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, S.; Li, H.; Liang, J.; Liu, W.; Piao, S.; Tian, H.; Zhou, G.; Lu, C.; You, W.; et al. Maximizing carbon sequestration potential in Chinese forests through optimal management. Nat. Commun. 2024, 15, 3154. [Google Scholar] [CrossRef]
- Gadow, K.v.; Zhao, X.H.; Tewari, V.P.; Zhang, C.Y.; Kumar, A.; Corral Rivas, J.J.; Kumar, R. Forest Observational Studies: An Alternative to Designed Experiments. Eur. J. For. Res. 2016, 135, 417–431. [Google Scholar] [CrossRef]
- Fernández-Eguiarte, A.; Romero-Centeno, R.; Zavala-Hidalgo, J. Atlas Climático de México y Áreas Adyacentes. Vol. 1; Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México: Mexico City, Mexico; Servicio Meteorológico Nacional, Comisión Nacional del Agua: Mexico City, Mexico, 2012; Available online: https://atlasclimatico.unam.mx/ACM/#14/z (accessed on 27 February 2025).
- Corral-Rivas, J.J.; Vargas-Larreta, B.; Wehenkel, C.; Aguirre-Calderon, O.A.; Álvarez-González, J.G.; Rojo Alboreca, A. Guía Para el Establecimiento de Sitios de Investigación Forestal y de Suelos en Bosques del Estado de Durango; Universidad Juárez del Estado de Durango: Durango, Mexico, 2009. [Google Scholar]
- Vargas-Larreta, B.J.; Corral-Rivas, J.; Aguirre-Calderón, O.A.; López-Martínez, J.O.; De los Santos-Posadas, H.M.; Zamudio-Sánchez, F.J.; Treviño-Garza, E.J.; Martínez-Salvador, M.; Aguirre-Calderón, C.G. SiBiFor: Forest Biometric System for Forest Management in Mexico. Rev. Chapingo Ser. Cienc. For. Y Del Ambient. 2017, 23, 437–455. [Google Scholar] [CrossRef]
- Vargas-Larreta, B.; López-Sánchez, C.A.; Corral-Rivas, J.J.; López-Martínez, J.O.; Aguirre-Calderón, C.G.; Álvarez-González, J.G. Allometric Equations for Estimating Biomass and Carbon Stocks in the Temperate Forests of North-Western Mexico. Forests 2017, 8, 269. [Google Scholar] [CrossRef]
- García, O. The state-space approach in growth modelling. Can. J. For. Res. 1994, 24, 1894–1903. [Google Scholar] [CrossRef]
- Stout, B.B.; Shumway, D.L. Site quality estimation using height and diameter. For. Sci. 1982, 28, 639–645. [Google Scholar]
- Huang, S.; Titus, S.J. An index of site productivity for uneven-aged or mixed-species stands. Can. J. For. Res. 1993, 23, 558–562. [Google Scholar] [CrossRef]
- Padilla-Martínez, J.R.; Paul, C.; Corral-Rivas, J.J.; Husmann, K.; Diéguez-Aranda, U.; Gadow, K.v. Evaluation of the Site Form as a Site Productive Indicator in Temperate Uneven-Aged Multispecies Forests in Durango, Mexico. Plants 2022, 11, 2764. [Google Scholar] [CrossRef]
- Clutter, J.L.; Fortson, J.C.; Pienaar, L.V.; Brister, G.H.; Bailey, R.L. Timber Management. A Quantitative Approach; John Wiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- Bertalanffy, L.v. Problems of Organic Growth. Nature 1949, 163, 156–158. [Google Scholar] [CrossRef]
- Bertalanffy, L.v. Quantitative Laws in Metabolism and Growth. Q. Rev. Biol. 1957, 32, 217–231. [Google Scholar] [CrossRef]
- Hossfeld, J.W. Mathematik für Forstmänner, Ökonomen und Cameralisten. Praktische Geometrie. Bierter Band: Gotha, Thüringen, Germany, 1822; Available online: https://www.digitale-sammlungen.de/de/details/bsb10295229 (accessed on 27 February 2025).
- Korf, V. Pfispevek k matematicke definici vzrustoveho zakona lesnich porostii. Lesnicka Prace 1939, 18, 339–356. [Google Scholar]
- Monserud, R.A. Simulation of forest tree mortality. For. Sci. 1976, 22, 438–444. [Google Scholar]
- Clutter, J.L.; Jones, E.P. Prediction of Growth After Thinning in Oldfield Slash Pine Plantations; USDA For. Serv. Pap. SE-217; Department of Agriculture, Forest Service, Southeastern Forest Experiment Station: Washington, DC, USA, 1980.
- Pienaar, L.V.; Shiver, B.D. Survival Functions for Site-Prepared Slash Pine Plantations in the Flatwoods of Georgia and Northern Florida. South. J. Appl. For. 1981, 5, 59–62. [Google Scholar] [CrossRef]
- Pienaar, L.V.; Page, H.H.; Rheney, J.W. Yield Prediction for Mechanically Site-Prepared Slash Pine Plantations. South. J. Appl. For. 1990, 14, 104–109. [Google Scholar] [CrossRef]
- Tomé, M.; Falcao, A.; Amaro, A. Globulus V1.0.0: A regionalised growth model for eucalypt plantations in Portugal. In Proceedings of the IUFRO Conference: Modelling Growth of Fast-Grown Tree Species, Valdivia, Chile, 5–7 September 1997; Ortega, A., Gezan, S., Eds.; Universidad Austral de Chile: Valdivia, Chile, 1997; pp. 138–145. [Google Scholar]
- Woollons, R.C. Even-aged stand mortality estimation through a two-step regression process. For. Ecol. Manag. 1998, 105, 189–195. [Google Scholar] [CrossRef]
- García, O. Building a dynamic growth model for trembling aspen in western Canada without age data. Can. J. For. Res. 2013, 43, 256–265. [Google Scholar] [CrossRef]
- Bailey, R.L.; Borders, B.E.; Ware, K.D.; Jones, E.P. A Compatible Model Relating Slash Pine Plantation Survival to Density, Age, Site Index, and Type and Intensity of Thinning. For. Sci. 1985, 31, 180–189. [Google Scholar] [CrossRef]
- Zunino, C.A.; Ferrando, M.T. Modelación del crecimiento y rendimiento de plantaciones de Eucalyptus en Chile. Una primera etapa. In Proceedings of the IUFRO Conference: Modelling Growth of Fast-Grown Tree Species, Valdivia, Chile, 5–7 September 1997; Ortega, A., Gezan, S., Eds.; Universidad Austral de Chile: Valdivia, Chile, 1997; pp. 155–164. [Google Scholar]
- Álvarez-González, J.G.; Cañellas, I.; Alberdi, I.; Gadow, K.v.; Ruiz-González, A.D. National Forest Inventory and Forest Observational Studies in Spain: Applications to Forest Modeling. For. Ecol. Manag. 2014, 316, 54–64. [Google Scholar] [CrossRef]
- Borders, B.E.; Bailey, R.L. A compatible system of growth and yield equations for slash pine fitted with restricted three-stage least squares. For. Sci. 1986, 32, 185–201. [Google Scholar] [CrossRef]
- Forss, E. Das Wachstum der Baumart Acacia mangium in Südkalimantan, Indonesien. Master’s Thesis, Fac. of Forestry, University of Göttingen, Göttingen, Germany, 1994. [Google Scholar]
- SAS Institute Inc. SAS OnDemand for Academics [Software]; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Ahmadi, K.; Alavi, S.J.; Kouchaksaraei, M.T. Constructing site quality curves and productivity assessment for uneven-aged and mixed stands of oriental beech (Fagus orientalis Lipsky) in Hyrcanian forest, Iran. For. Sci. Technol. 2017, 13, 41–46. [Google Scholar] [CrossRef]
- Molina-Valero, J.A.; Camarero, J.; Álvarez-González, J.G.; Cerioni, M.; Hevia, A.; Sánchez-Salguero, R.; Martin-Benito, D.; Pérez-Cruzado, C. Mature forests hold maximum live biomass stocks. For. Ecol. Manag. 2021, 480, 118635. [Google Scholar] [CrossRef]
- Aguirre, A.; Moreno-Fernández, D.; Alberdi, I.; Hernández, L.; Adame, P.; Cañellas, I.; Montes, F. Mapping forest site quality at national level. For. Ecol. Manag. 2022, 508, 120043. [Google Scholar] [CrossRef]
- Bailey, R.L.; Clutter, J.L. Base-Age Invariant Polymorphic Site Curves. For. Sci. 1974, 20, 155–159. [Google Scholar] [CrossRef]
- Palahí, M.; Tomé, M.; Pukkala, T.; Trasobares, A.; Montero, G. Site index model for Pinus sylvestris in north-east Spain. For. Ecol. Manag. 2004, 187, 35–47. [Google Scholar] [CrossRef]
- Wu, R.; Dong, C.; Zhang, C.; Gao, W.; Zheng, X.; Lou, X. Classification Model of Site Quality for Mixed Forests Based on the TWINSPAN Method and Site Form in Southwestern Zhejiang. Forests 2024, 15, 2247. [Google Scholar] [CrossRef]
- Mohamed, A.; Reich, R.M.; Khosla, R.; Aguirre-Bravo, C.; Mendoza, B.M. Influence of climatic, topography and soil attributes on the spatial distribution of site productivity index of the species rich forests of Jalisco, Mexico. J. For. Res. 2014, 25, 87–95. [Google Scholar] [CrossRef]
- Do, H.T.T.; Zimmer, H.C.; Vanclay, J.K.; Grant, J.C.; Trinh, B.N.; Nguyen, H.H.; Nichols, J.D. Site form classification– a practical tool for guiding site-specific tropical forest landscape restoration and management. Forestry 2021, 95, 261–273. [Google Scholar] [CrossRef]
- Zhao, D.; Borders, B.; Wang, M.; Kane, M. Modeling mortality of second-rotation loblolly pine plantations in the Piedmont/Upper Coastal Plain and Lower Coastal Plain of the southern United States. For. Ecol. Manag. 2007, 252, 132–143. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.A.; Gezan, S.A.; Samuelson, L.J.; Cropper, W.P.; Leduc, D.J.; Martin, T.A. Modeling survival, yield, volume partitioning and their response to thinning for longleaf pine plantations. Forests 2012, 3, 1104–1132. [Google Scholar] [CrossRef]
- Thapa, R.; Burkhart, H.E. Modeling stand-level mortality of Loblolly pine (Pinus taeda L.) using stand, climate, and soil variables. For. Sci. 2015, 61, 834–846. [Google Scholar] [CrossRef]
- Maleki, K.; Astrup, R.; Kuehne, C.; McLean, J.P.; Antón-Fernández, C. Stand-level growth models for long-term projections of the main species groups in Norway. Scand. J. For. Res. 2022, 37, 130–143. [Google Scholar]
- Lexerød, N.S. Recruitment models for different tree species in Norway. For. Ecol. Manag. 2005, 206, 91–108. [Google Scholar] [CrossRef]
- Fortin, M.; DeBlois, J. Modeling tree recruitment with zero-inflated models: The example of hardwood stands in southern Quebec, Canada. For. Sci. 2007, 53, 529–539. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Y.; Cai, D.; Liu, F. Predicting tree recruitment with negative binomial mixture models. For. Ecol. Manag. 2012, 270, 209–215. [Google Scholar] [CrossRef]
- García, O.; Burkhart, H.E.; Amateis, R.L. A biologically-consistent stand growth model for loblolly pine in the Piedmont physiographic region, USA. For. Ecol. Manag. 2011, 262, 2035–2041. [Google Scholar] [CrossRef]
- Tewari, V.P.; Álvarez-González, J.G.; García, O. Developing a dynamic growth model for teak plantations in India. For. Ecosyst. 2014, 1, 9. [Google Scholar] [CrossRef]
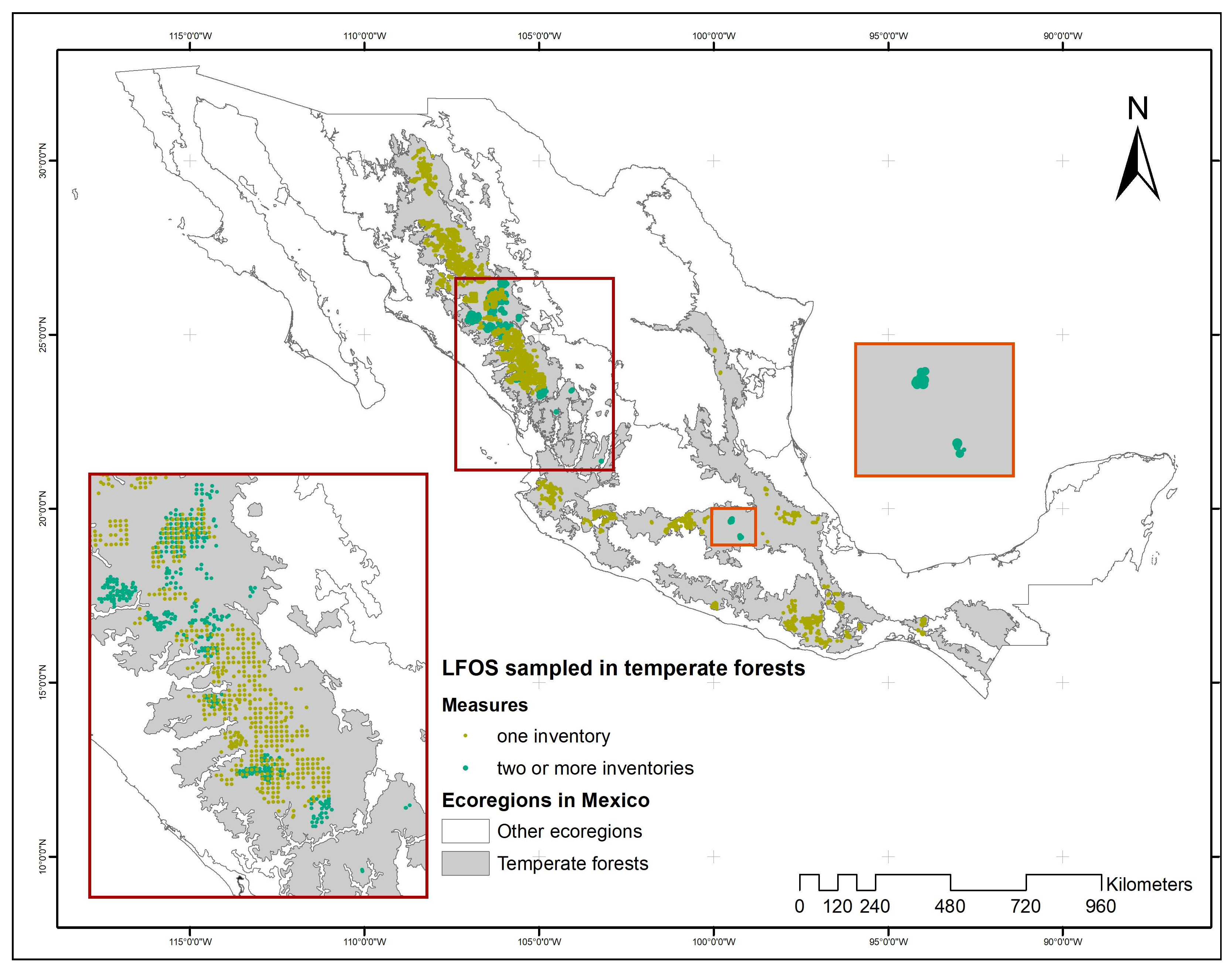
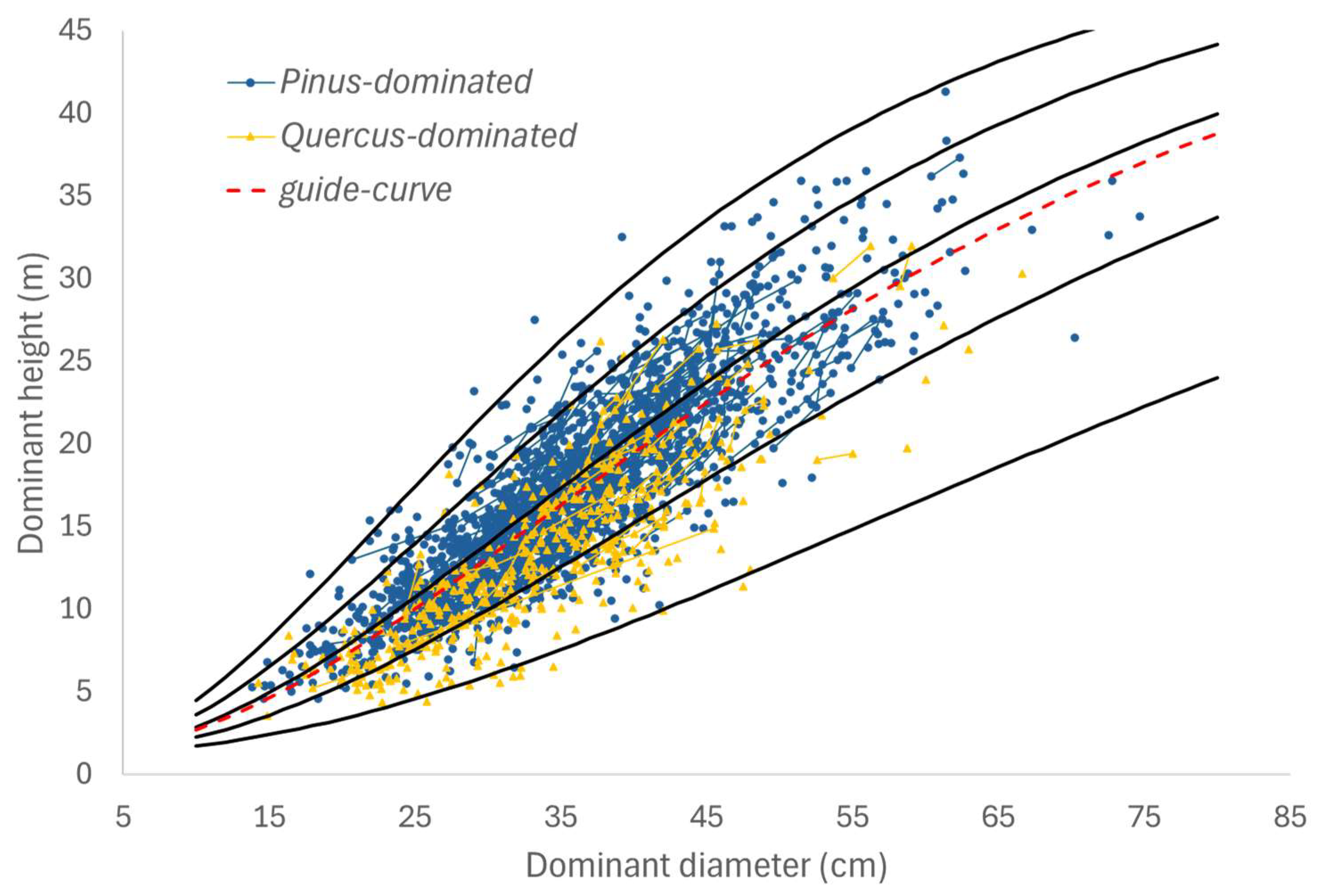
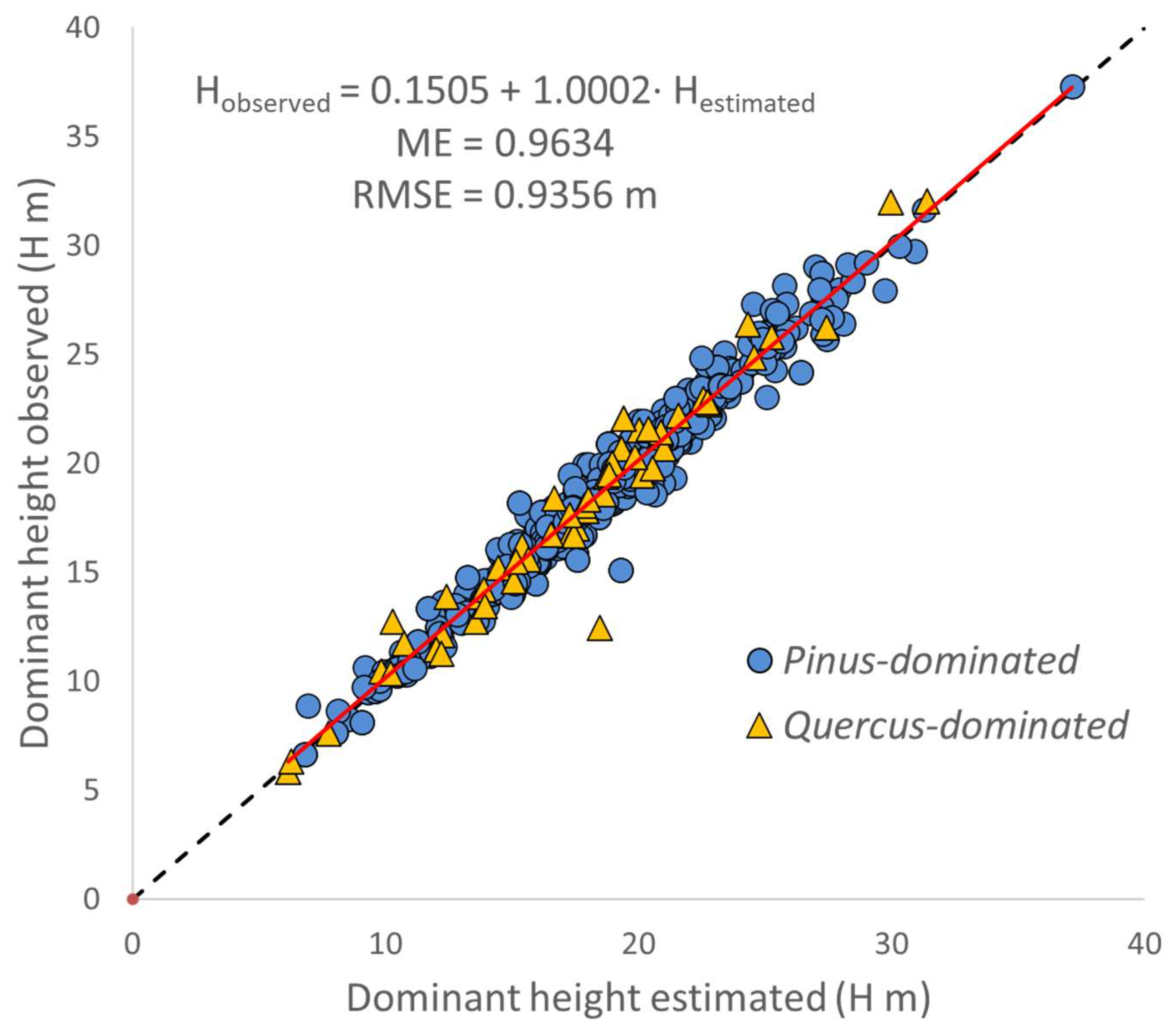
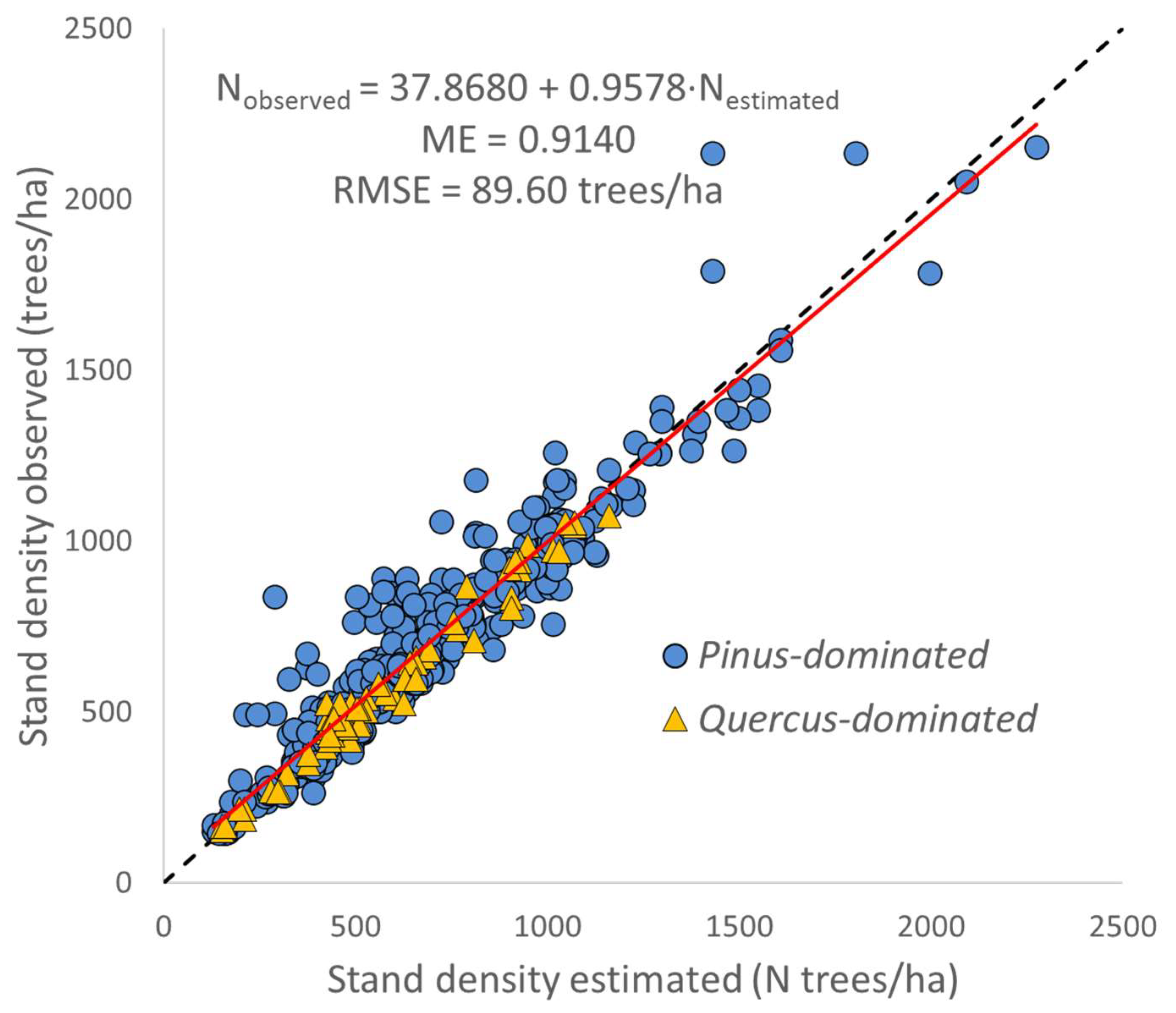


| Variable | All Data (2444 Field Inventories) | Sample Plots with Remeasurements | |
|---|---|---|---|
| First Remeasurement (457 Field Inventories) | Second Remeasurement (105 Field Inventories) | ||
| N (trees/ha) | 474.19 ± 253.99 (20–2264) | 646.89 ± 291.08 (120–2264) | 651.04 ± 294.44 (144–2152) |
| BA (m2/ha) | 18.67 ± 10.48 (0.37–75.09) | 20.59 ± 7.21 (3.1–43.34) | 23.14 ± 8.09 (3.92–51.65) |
| dg (cm) | 23.14 ± 6.87 (8.42–54.42) | 20.78 ± 4.03 (12.37–34.43) | 21.97 ± 4.12 (13.02–37.88) |
| H (m) | 15.9 ± 6.63 (3.36–41.3) | 16.25 ± 4.05 (5.26–24.89) | 17.85 ± 4.4 (5.83–28) |
| D (cm) | 34.71 ± 9.6 (8.66–74.7) | 35.1 ± 6.28 (17.99–54.89) | 36.88 ± 6.39 (20–57.09) |
| RSI | 0.36 ± 0.25 (0.08–3.29) | 0.28 ± 0.16 (0.12–1.63) | 0.28 ± 0.17 (0.12–1.39) |
| SDI (trees/ha) | 423.70 ± 196.27 (9.10–1560.52) | 459.40 ± 162.67 (73.57–976.68) | 477.71 ± 177.93 (90.66–1047.89) |
| V (m3/ha) | 172.36 ± 141.6 (2.04–927.93) | 180.16 ± 89.63 (11.68–485.91) | 218.41 ± 107.59 (16–594.9) |
| AGB (Mg/ha) | 109.99 ± 77.87 (1.82–510.26) | 113.31 ± 46.45 (11.76–256.35) | 130.61 ± 52.69 (15.7–304.72) |
| %BAPinus | 63.32 ± 23.63 (0–100) | 67.56 ± 15.78 (32.24–100) | 67.42 ± 15.26 (30.19–100) |
| Base Equation | Local Parameter | Transition Function |
|---|---|---|
| Bertalanffy [39,40] | ||
| Hossfeld [41] | ||
| Korf [42] | ||
| Model | a | b | c | ME | RMSE (m) | RMSE (%) |
|---|---|---|---|---|---|---|
| Bertalanffy | 50.9920 | 0.0268 | 2.4630 | 0.7493 | 3.0453 | 18.49 |
| Hossfeld | 6.4397 | 0.0747 | --- | 0.7474 | 3.0564 | 18.56 |
| Korf | 5.3065 | −19.1039 | 0.5615 | 0.7484 | 3.0509 | 18.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Miranda, M.G.; Álvarez-González, J.G.; Corral-Rivas, J.J.; Vega-Nieva, D.J.; Briseño-Reyes, J.; Aguirre-Gutiérrez, J.; von Gadow, K. A Whole-Stand Model for Estimating the Productivity of Uneven-Aged Temperate Pine-Oak Forests in Mexico. Sustainability 2025, 17, 3393. https://doi.org/10.3390/su17083393
Nava-Miranda MG, Álvarez-González JG, Corral-Rivas JJ, Vega-Nieva DJ, Briseño-Reyes J, Aguirre-Gutiérrez J, von Gadow K. A Whole-Stand Model for Estimating the Productivity of Uneven-Aged Temperate Pine-Oak Forests in Mexico. Sustainability. 2025; 17(8):3393. https://doi.org/10.3390/su17083393
Chicago/Turabian StyleNava-Miranda, María Guadalupe, Juan Gabriel Álvarez-González, José Javier Corral-Rivas, Daniel José Vega-Nieva, Jaime Briseño-Reyes, Jesús Aguirre-Gutiérrez, and Klaus von Gadow. 2025. "A Whole-Stand Model for Estimating the Productivity of Uneven-Aged Temperate Pine-Oak Forests in Mexico" Sustainability 17, no. 8: 3393. https://doi.org/10.3390/su17083393
APA StyleNava-Miranda, M. G., Álvarez-González, J. G., Corral-Rivas, J. J., Vega-Nieva, D. J., Briseño-Reyes, J., Aguirre-Gutiérrez, J., & von Gadow, K. (2025). A Whole-Stand Model for Estimating the Productivity of Uneven-Aged Temperate Pine-Oak Forests in Mexico. Sustainability, 17(8), 3393. https://doi.org/10.3390/su17083393









