Impact of Three Decades of Conservation Management Systems on Carbon Management Index and Aggregate Stability
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental and Treatment Characterization
2.2. Soil Sampling
2.3. Determining the State of Soil Aggregation
2.4. Fractionation of SOM, C Analysis, and C Stock Calculation
2.5. Calculation of Carbon Management Index
2.6. Statistical Analysis
PSCjkl + Error d(ijkl).
3. Results
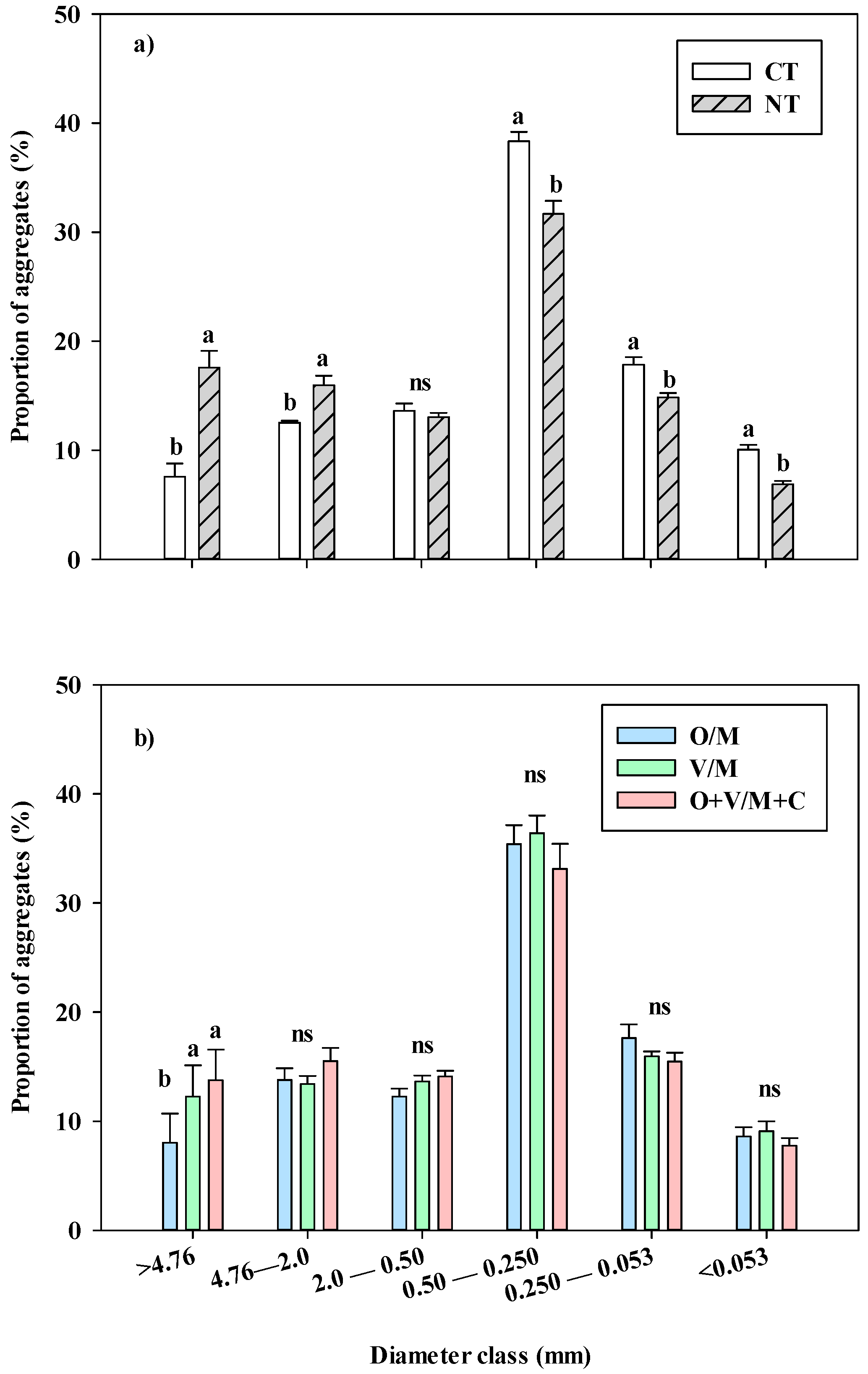
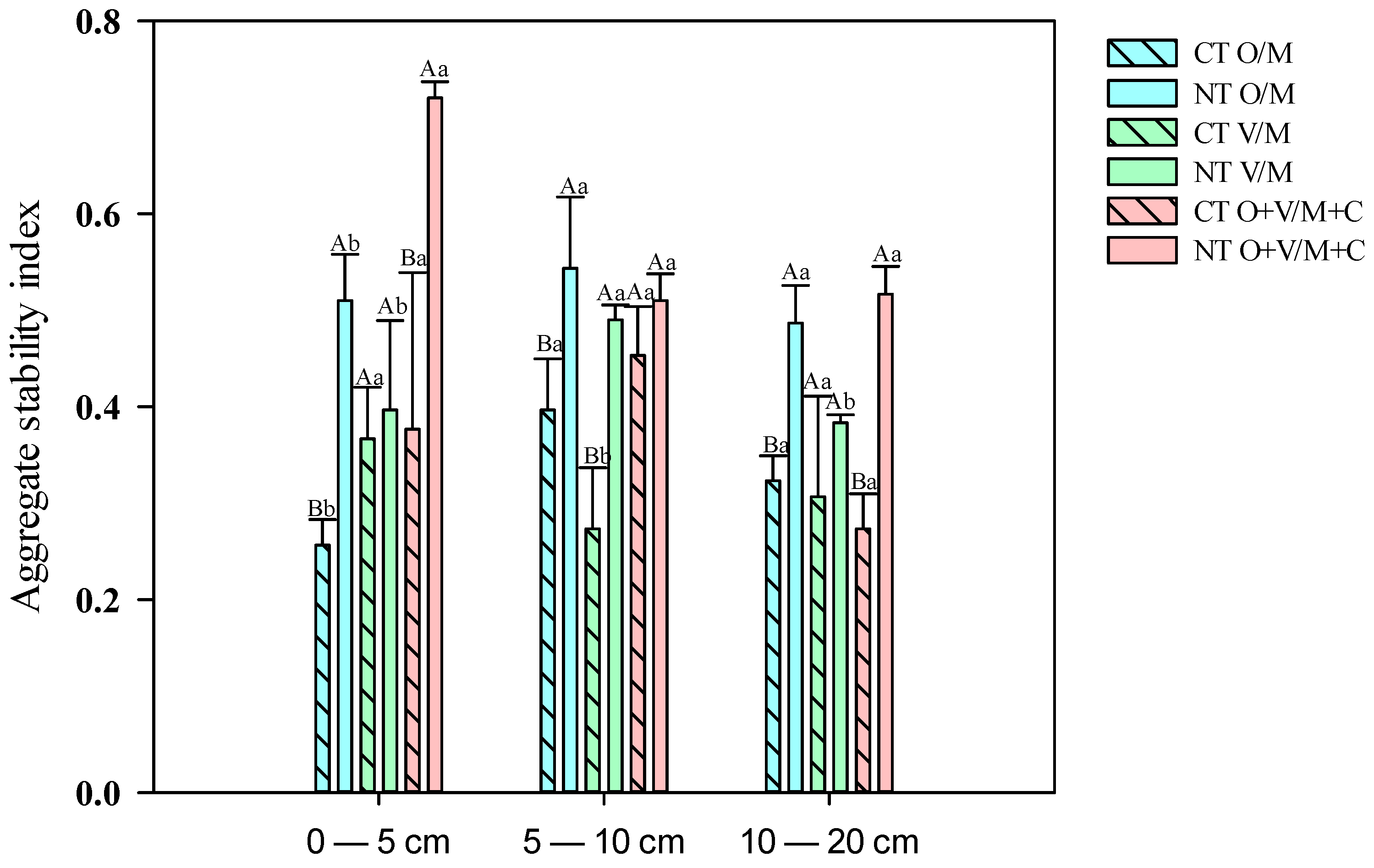
3.1. State of Soil Aggregation
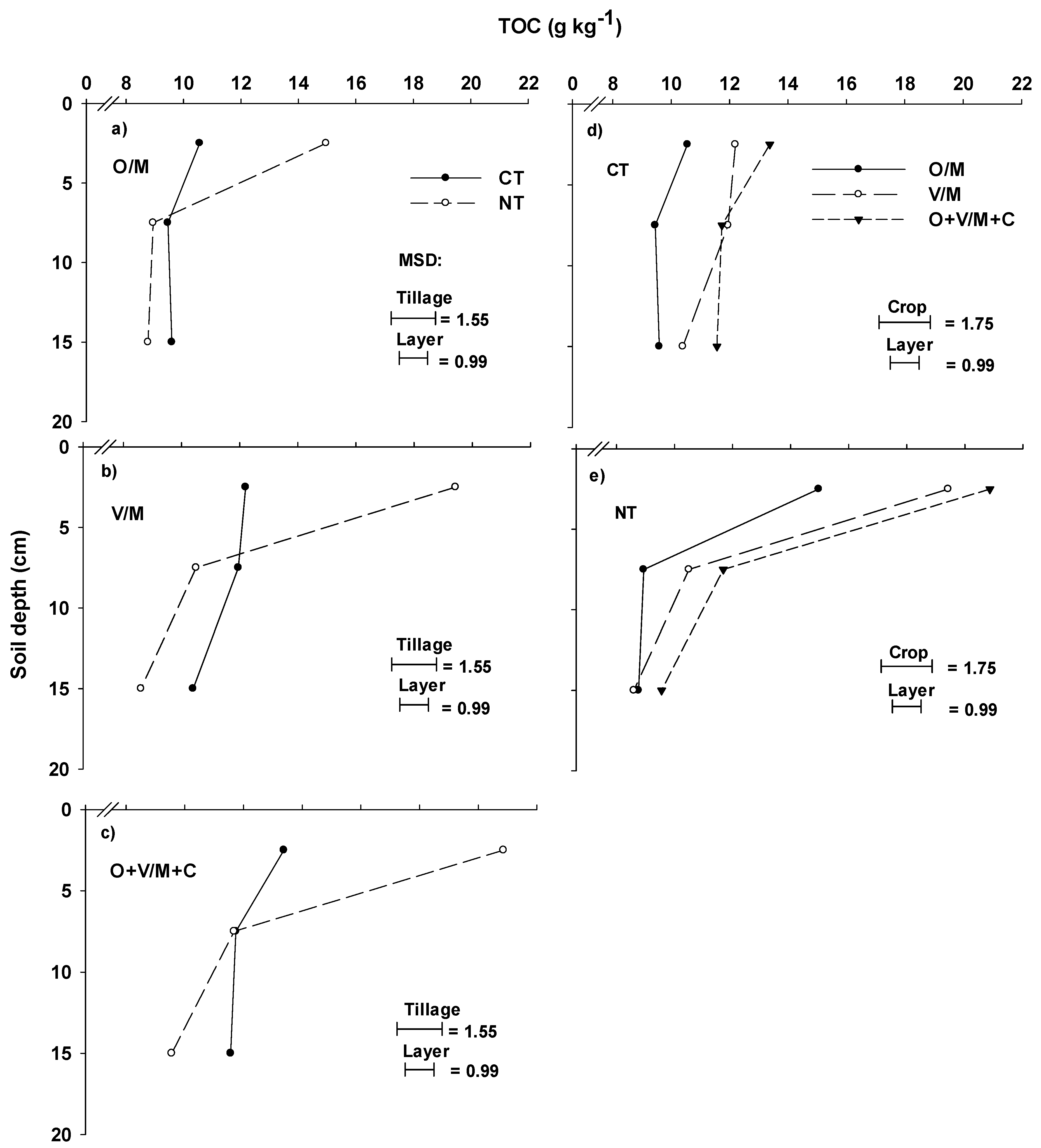
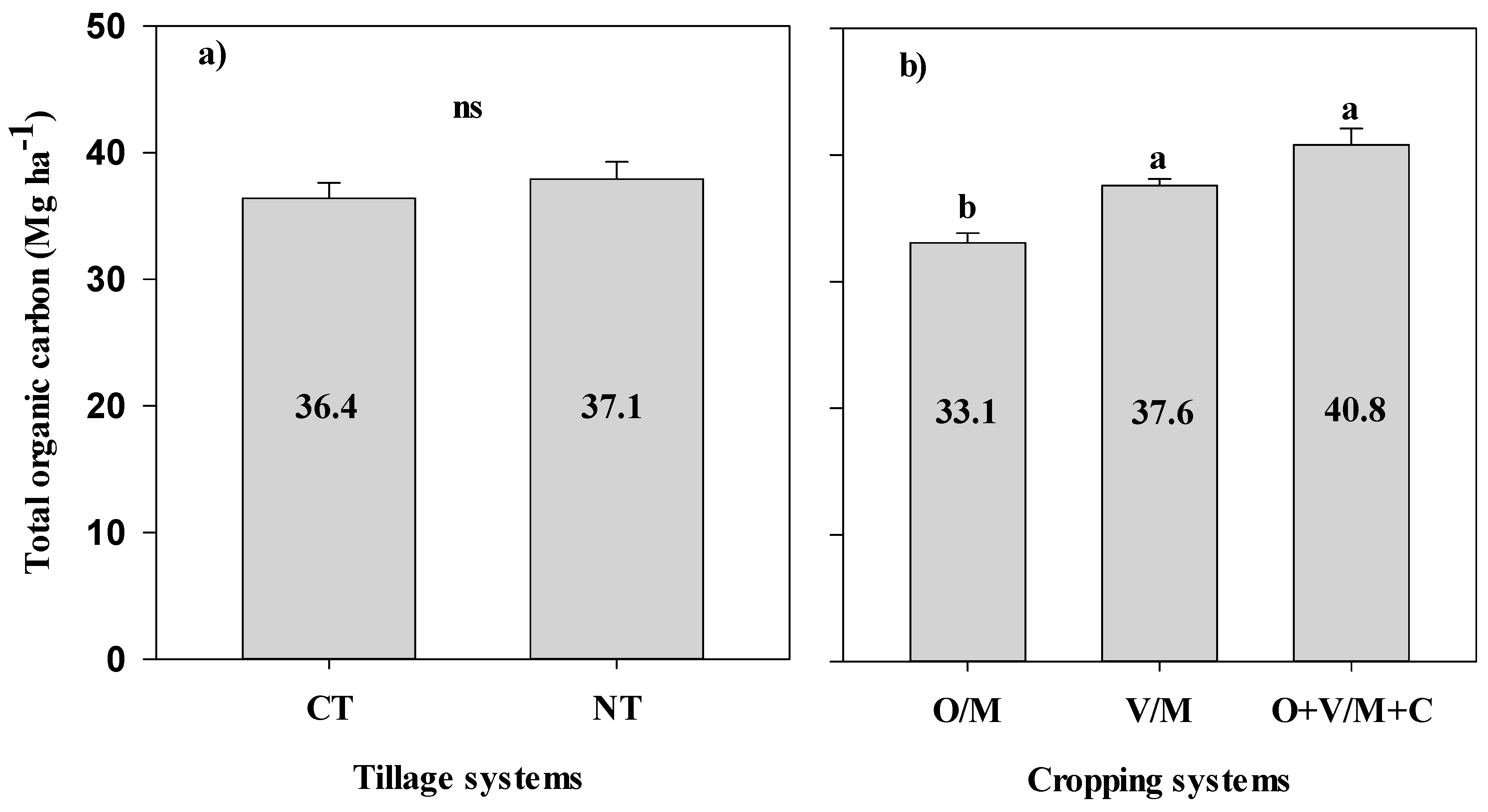
3.2. Concentration, Stock, and Accumulation Rate of Total Organic Carbon in the Soil
3.3. Soil Carbon Stock in the Granulometric Fractions of Soil Organic Matter
3.4. Carbon Management Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global Meta-Analysis of the Relationship between Soil Organic Matter and Crop Yields. Soil 2019, 5, 15–32. [Google Scholar] [CrossRef]
- Mahmood, S.; Nunes, M.R.; Kane, D.A.; Lin, Y. Soil Health Explains the Yield-Stabilizing Effects of Soil Organic Matter under Drought. Soil Environ. Health 2023, 1, 100048. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Clim. 2019, 1, 482133. [Google Scholar] [CrossRef]
- Bieluczyk, W.; Asselta, F.O.; Navroski, D.; Gontijo, J.B.; Venturini, A.M.; Mendes, L.W.; Simon, C.P.; de Camargo, P.B.; Tadini, A.M.; Martin-Neto, L.; et al. Linking above and Belowground Carbon Sequestration, Soil Organic Matter Properties, and Soil Health in Brazilian Atlantic Forest Restoration. J. Environ. Manag. 2023, 344, 118573. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.d.S.; Tavares, R.L.M.; Filho, S.V.d.P.; Cantão, V.C.G.; Simon, G.A.; Alves, J.M.; Nascimento, H.L.B.D.; Bilego, U.O. Stock of Carbon and Soil Organic Fractions in No-Tillage and Crop–Livestock Integration Systems. Sustainability 2024, 16, 3025. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V. Tillage, cover crop and crop rotation effects on selected soil chemical properties. Sustainability 2019, 11, 2770. [Google Scholar] [CrossRef]
- Freitas, V.S.; Babos, D.V.; Guedes, W.N.; Silva, F.P.; Tozo, M.L.d.L.; Andrade, C.A.; Villas-Boas, P.R.; Milori, D.M.B.P.; Martin-Neto, L. Soil Organic Matter Dynamics and Soil Carbon Stocks from Tropical and Sub-Tropical Areas under No-till: An on-Farm Research. Catena 2024, 247, 108491. [Google Scholar] [CrossRef]
- Veloso, M.G.; Cecagno, D.; Bayer, C. Legume Cover Crops under No-Tillage Favor Organomineral Association in Microaggregates and Soil C Accumulation. Soil Tillage Res. 2019, 190, 139–146. [Google Scholar] [CrossRef]
- Hewelke, E.; Mielnik, L.; Weber, J.; Perzanowska, A.; Jamroz, E.; Gozdowski, D.; Szacki, P. Chemical and Physical Aspects of Soil Health Resulting from Long-Term No-Till Management. Sustainability 2024, 16, 9682. [Google Scholar] [CrossRef]
- Six, J.; Schultz, P.A.; Jastrow, J.D.; Merckx, R. Recycling of Sodium Polytungstate Used in Soil Organic Matter Studies. Soil Biol. Biochem. 1999, 31, 1193–1196. [Google Scholar] [CrossRef]
- Balesdent, J.; Balabane, M. Maize Root-Derived Soil Organic Carbon Estimated by Natural 15c Abundance. Soil Biol. Biochem. 1992, 24, 97–101. [Google Scholar] [CrossRef]
- Conceição, P.C.; Dieckow, J.; Bayer, C. Combined Role of No-Tillage and Cropping Systems in Soil Carbon Stocks and Stabilization. Soil Tillage Res. 2013, 129, 40–47. [Google Scholar] [CrossRef]
- Dieckow, J.; Mielniczuk, J.; Knicker, H.; Bayer, C.; Dick, D.P.; Kögel-Knabner, I. Composition of Organic Matter in a Subtropical Acrisol as Influenced by Land Use, Cropping and N Fertilization, Assessed by CPMAS 13C NMR Spectroscopy. Eur. J. Soil Sci. 2005, 56, 705–715. [Google Scholar] [CrossRef]
- Ardenti, F.; Capra, F.; Lommi, M.; Fiorini, A.; Tabaglio, V. Long-Term C and N Sequestration under No-till Is Governed by Biomass Production of Cover Crops Rather than Differences in Grass vs. Legume Biomass Quality. Soil Tillage Res. 2023, 228, 105630. [Google Scholar] [CrossRef]
- Sá, J.C.d.M.; Séguy, L.; Tivet, F.; Lal, R.; Bouzinac, S.; Borszowskei, P.R.; Briedis, C.; dos Santos, J.B.; Hartman, D.d.C.; Bertoloni, C.G.; et al. Carbon Depletion by Plowing and its Restoration by No-Till Cropping Systems in Oxisols of Subtropical and Tropical Agro-Ecoregions in Brazil. Land Degrad. Dev. 2013, 26, 531–543. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization Mechanisms of Soil Organic Matter: Implications for C-Saturatin of Soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Winck, B.R.; Vezzani, F.M.; Dieckow, J.; Favaretto, N.; Molin, R. Carbono e Nitrogênio Nas Frações Granulométricas Da Matéria Orgânica Do Solo, Em Sistemas de Culturas Sob Plantio Direto. Rev. Bras. Cienc. Solo 2014, 38, 980–989. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Francesca Cotrufo, M.; Lavallee, J.M.; Zhang, Y.; Hansen, P.M.; Paustian, K.H.; Schipanski, M.; Wallenstein, M.D. In-N-Out: A Hierarchical Framework to Understand and Predict Soil Carbon Storage and Nitrogen Recycling. Glob. Change Biol. 2021, 27, 4465–4468. [Google Scholar] [CrossRef]
- Briedis, C.; Baldock, J.; de Moraes Sá, J.C.; dos Santos, J.B.; McGowan, J.; Milori, D.M.B.P. Organic Carbon Pools and Organic Matter Chemical Composition in Response to Different Land Uses in Southern Brazil. Eur. J. Soil Sci. 2020, 72, 1083–1100. [Google Scholar] [CrossRef]
- Creamer, C.A.; Foster, A.L.; Lawrence, C.; McFarland, J.; Schulz, M.; Waldrop, M.P. Mineralogy Dictates the Initial Mechanism of Microbial Necromass Association. Geochim. Cosmochim. Acta 2019, 260, 161–176. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.; Elsgaard, L.; Rasmussen, J.; Kuzyakov, Y.; Banfield, C.C.; Dippold, M.A.; Olesen, J.E. Decreased Rhizodeposition, but Increased Microbial Carbon Stabilization with Soil Depth down to 3.6 M. Soil Biol. Biochem. 2020, 150, 108008. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic Matter and Water Stable Aggregates in Soil. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, H.; Masters, M.D.; Luo, Y.; Zhang, X.; Liang, C. Microbial Trade-off in Soil Organic Carbon Storage in a No-till Continuous Corn Agroecosystem. Eur. J. Soil Biol. 2020, 96, 103146. [Google Scholar] [CrossRef]
- Sohi, S.P.; Mahieu, N.; Arah, J.R.M.; Powlson, D.S.; Madari, B.; Gaunt, J.L. A Procedure for Isolating Soil Organic Matter Fractions Suitable for Modeling. Soil Sci. Soc. Am. J. 2001, 65, 1121–1128. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Lavallee, J.M. Soil Organic Matter Formation, Persistence, and Functioning: A Synthesis of Current Understanding to Inform Its Conservation and Regeneration. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 172, pp. 1–66. ISBN 9780323989534. [Google Scholar]
- Samson, M.E.; Chantigny, M.H.; Vanasse, A.; Menasseri-Aubry, S.; Royer, I.; Angers, D.A. Management Practices Differently Affect Particulate and Mineral-Associated Organic Matter and Their Precursors in Arable Soils. Soil Biol. Biochem. 2020, 148, 107867. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil Organic Matter Turnover Is Governed by Accessibility Not Recalcitrance. Glob. Change Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Friedrich, T.; Derpsch, R.; Kassam, A. Sustainable Development of Organic Agriculture; Taylor & Francis: London, UK, 2012; pp. 1–7. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources; FAO: Rome, Italy, 2002. [Google Scholar]
- Veloso, M.G.; Angers, D.A.; Tiecher, T.; Giacomini, S.; Dieckow, J.; Bayer, C. High Carbon Storage in a Previously Degraded Subtropical Soil under No-Tillage with Legume Cover Crops. Agric. Ecosyst. Environ. 2018, 268, 15–23. [Google Scholar] [CrossRef]
- Zanatta, J.; Bayer, C.; Dieckow, J.; Vieira, F.; Mielniczuk, J. Soil Organic Carbon Accumulation and Carbon Costs Related to Tillage, Cropping Systems and Nitrogen Fertilization in a Subtropical Acrisol. Soil Tillage Res. 2007, 94, 510–519. [Google Scholar] [CrossRef]
- Carpenedo, V.; Mielniczuk, J. Estado de Agregação e Qualidade de Agregados de Latossolos Roxos, Submetidos a Diferentes Sistemas de Manejo. Rev. Bras. Cienc. Solo 1990, 14, 99–105. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of Organic Matter and Nutrients Stored in Soils under Contrasting Management Regimes. Can. J. Soil Sci 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover Crops for Sustainable Cropping Systems: A Review. Agriculture 2022, 12, 2076. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Management Impact and Benefit of Cover Crops on Soil Quality: A Review. Soil Tillage Res. 2020, 204, 104717. [Google Scholar] [CrossRef]
- Lovato, T.; Mielniczuk, J.; Bayer, C.; Vezzani, F. Adição de Carbono e Nitrogênio e Sua Relação Com Os Estoques No Solo e Com o Rendimento Do Milho Em Sistemas de Manejo. Rev. Bras. Cienc. Solo 2004, 28, 175–187. [Google Scholar] [CrossRef]
- Amado, T.J.C.; Bayer, C.; Conceição, P.C.; Spagnollo, E.; de Campos, B.-H.C.; da Veiga, M. Potential of Carbon Accumulation in No-Till Soils with Intensive Use and Cover Crops in Southern Brazil. J. Environ. Qual. 2006, 35, 1599. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Redin, M.; Guénon, R.; Recous, S.; Schmatz, R.; de Freitas, L.L.; Aita, C.; Giacomini, S.J. Carbon Mineralization in Soil of Roots from Twenty Crop Species, as Affected by Their Chemical Composition and Botanical Family. Plant Soil 2014, 378, 205–214. [Google Scholar] [CrossRef]
- Rodrigues, L.A.T.; Giacomini, S.J.; Dieckow, J.; Cherubin, M.R.; Sangiovo Ottonelli, A.; Bayer, C. Carbon Saturation Deficit and Litter Quality Drive the Stabilization of Litter-Derived C in Mineral-Associated Organic Matter in Long-Term No-till Soil. Catena 2022, 219, 106590. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Wu, M.; Li, C. Effects of Long-Term Conservation Tillage on Soil Aggregate Carbon Fractions and Microbial Characteristics under Rice-Oilseed Rape Rotation Systems. Appl. Soil Ecol. 2025, 206, 105848. [Google Scholar] [CrossRef]
- Liu, X.; Song, X.; Li, S.; Liang, G.; Wu, X. Understanding How Conservation Tillage Promotes Soil Carbon Accumulation: Insights into Extracellular Enzyme Activities and Carbon Flows between Aggregate Fractions. Sci. Total Environ. 2023, 897, 165408. [Google Scholar] [CrossRef]
- Li, P.; Ying, D.; Li, J.; Deng, J.; Li, C.; Tian, S.; Zhao, G.; Wu, C.; Jiao, J.; Jiang, M.; et al. Global-Scale No-Tillage Impacts on Soil Aggregates and Associated Carbon and Nitrogen Concentrations in Croplands: A Meta-Analysis. Sci. Total Environ. 2023, 881, 163570. [Google Scholar] [CrossRef] [PubMed]
- Veloso, M.G.; Angers, D.A.; Chantigny, M.H.; Bayer, C. Carbon Accumulation and Aggregation Are Mediated by Fungi in a Subtropical Soil under Conservation Agriculture. Geoderma 2020, 363, 114159. [Google Scholar] [CrossRef]
- Beare, M.; Coleman, D.; Hendrix, P.F.; Odum, E.P. A Hierarchical Approach to Evaluating the Significance of Soil Biodiversity to Biogeochemical Cycling. Plant Soil 1995, 170, 5–22. [Google Scholar] [CrossRef]
- Yu, S.; Wang, T.; Meng, Y.; Yao, S.; Wang, L.; Zheng, H.; Zhou, Y.; Song, Z.; Zhang, B. Leguminous Cover Crops and Soya Increased Soil Fungal Diversity and Suppressed Pathotrophs Caused by Continuous Cereal Cropping. Front. Microbiol. 2022, 13, 993214. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The Effects of Organic Inputs over Time on Soil Aggregate Stability—A Literature Analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Connell, R.K.; James, T.Y.; Blesh, J. A Legume-Grass Cover Crop Builds Mineral-Associated Organic Matter across Variable Agricultural Soils. Soil Biol. Biochem. 2025, 203, 109726. [Google Scholar] [CrossRef]
- Vezzani, F.M.; Mielniczuk, J. Agregação e Estoque de Carbono Em Argissolo Submetido a Diferentes Práticas de Manejo Agrícola. Rev. Bras. Ciência do Solo 2011, 35, 213–223. [Google Scholar] [CrossRef]
- Prigogine, I. As Leis Do Caos; Universidade: São Paulo, Brazil, 2002. [Google Scholar]


| Cropping System | Soil Layer (cm) | Tillage Method | |||
|---|---|---|---|---|---|
| CT | NT | CT | NT | ||
| POM (Mg ha−1) | MAOM (Mg ha−1) | ||||
| O/M | 0–5 | 0.68 ± 0.11 Bba | 1.53 ± 0.08 Aba | 7.16 ± 0.75 Bab | 9.60 ± 0.39 Aab |
| 5–10 | 0.51 ± 0.18 Aaa | 0.40 ± 0.05 Aab | 7.49 ± 2.50 Aab | 7.05 ± 0.35 Abc | |
| 10–20 | 1.00 ± 0.07 Aaa | 0.70 ± 0.16 Aab | 15.62 ± 0.57 Aba | 14.48 ± 0.79 Aaa | |
| V/M | 0–5 | 1.02 ± 0.14 Baba | 3.43 ± 0.56 Aaa | 8.04 ± 0.60 Bab | 11.01 ± 0.00 Aab |
| 5–10 | 0.91 ± 0.11 Aaa | 0.89 ± 0.16 Aab | 9.06 ± 0.07 Aab | 7.87 ± 0.00 Aac | |
| 10–20 | 1.30 ± 0.28 Aaa | 0.40 ± 0.01 Bab | 16.71 ± 0.64 Aba | 14.51 ± 0.00 Baa | |
| O+V/M+C | 0–5 | 1.51 ± 0.07 Baa | 3.60 ± 0.51 Aaa | 8.43 ± 0.74 Bab | 11.91 ± 1.53 Aab |
| 5–10 | 0.81 ± 0.09 Aab | 0.75 ± 0.11 Aab | 8.98 ± 0.22 Aab | 8.99 ± 0.41 Aac | |
| 10–20 | 0.80 ± 0.30 Aab | 0.67 ± 0.16 Aab | 19.23 ± 0.98 Aaa | 15.89 ± 0.90 Baa | |
| Tillage Method | Cropping System | CSI | L | CLI | CMI |
|---|---|---|---|---|---|
| Conventional tillage | O/M 1 | 1.00 ± 0.00 Ab | 0.073 ± 0.00 Aa | 1.00 ± 0.00 Aa | 100.0 ± 00.0 Aa |
| V/M | 1.15 ± 0.04 Aab | 0.096 ± 0.02 Ba | 1.33 ± 0.25 Ba | 151.8 ± 27.0 Ba | |
| O+V/M+C | 1.23 ± 0.03 Aa | 0.086 ± 0.01 Ba | 1.18 ± 0.17 Ba | 144.7 ± 21.7 Ba | |
| No till | O/M | 1.05 ± 0.7 Ab | 0.085 ± 0.01 Ab | 1.18 ± 0.14 Ab | 122.0 ± 7.9 Ab |
| V/M | 1.18 ± 0.03 Aab | 0.142 ± 0.02 Aa | 1.97 ± 0.30 Aa | 231.2 ± 31.6 Aa | |
| O+V/M+C | 1.30 ± 0.09 Aa | 0.141 ± 0.03 Aa | 1.96 ± 0.46 Aa | 246.3 ± 43.1 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso, M.; Amorim, F.F.; de Souza, J.P.; Bayer, C. Impact of Three Decades of Conservation Management Systems on Carbon Management Index and Aggregate Stability. Sustainability 2025, 17, 3378. https://doi.org/10.3390/su17083378
Veloso M, Amorim FF, de Souza JP, Bayer C. Impact of Three Decades of Conservation Management Systems on Carbon Management Index and Aggregate Stability. Sustainability. 2025; 17(8):3378. https://doi.org/10.3390/su17083378
Chicago/Turabian StyleVeloso, Murilo, Fábio Farias Amorim, Jéssica Pereira de Souza, and Cimélio Bayer. 2025. "Impact of Three Decades of Conservation Management Systems on Carbon Management Index and Aggregate Stability" Sustainability 17, no. 8: 3378. https://doi.org/10.3390/su17083378
APA StyleVeloso, M., Amorim, F. F., de Souza, J. P., & Bayer, C. (2025). Impact of Three Decades of Conservation Management Systems on Carbon Management Index and Aggregate Stability. Sustainability, 17(8), 3378. https://doi.org/10.3390/su17083378






