Research on the Modification of the Coal Pore Structure by Indigenous Microbial Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Samples
2.1.1. Coal Sample Preparation
2.1.2. Experimental Procedure of Microbial Degradation
2.2. Experimental Method
2.2.1. Low-Temperature CO2 Adsorption Experiment
2.2.2. Low-Temperature N2 Adsorption Experiment
2.2.3. Isothermal Adsorption Test
2.2.4. Pore Fractal Characterization
3. Results and Discussion
3.1. Pore Changes Based on Low-Temperature CO2 Adsorption Experiments
3.2. Pore Changes Based on the Low-Temperature N2 Adsorption Experiment
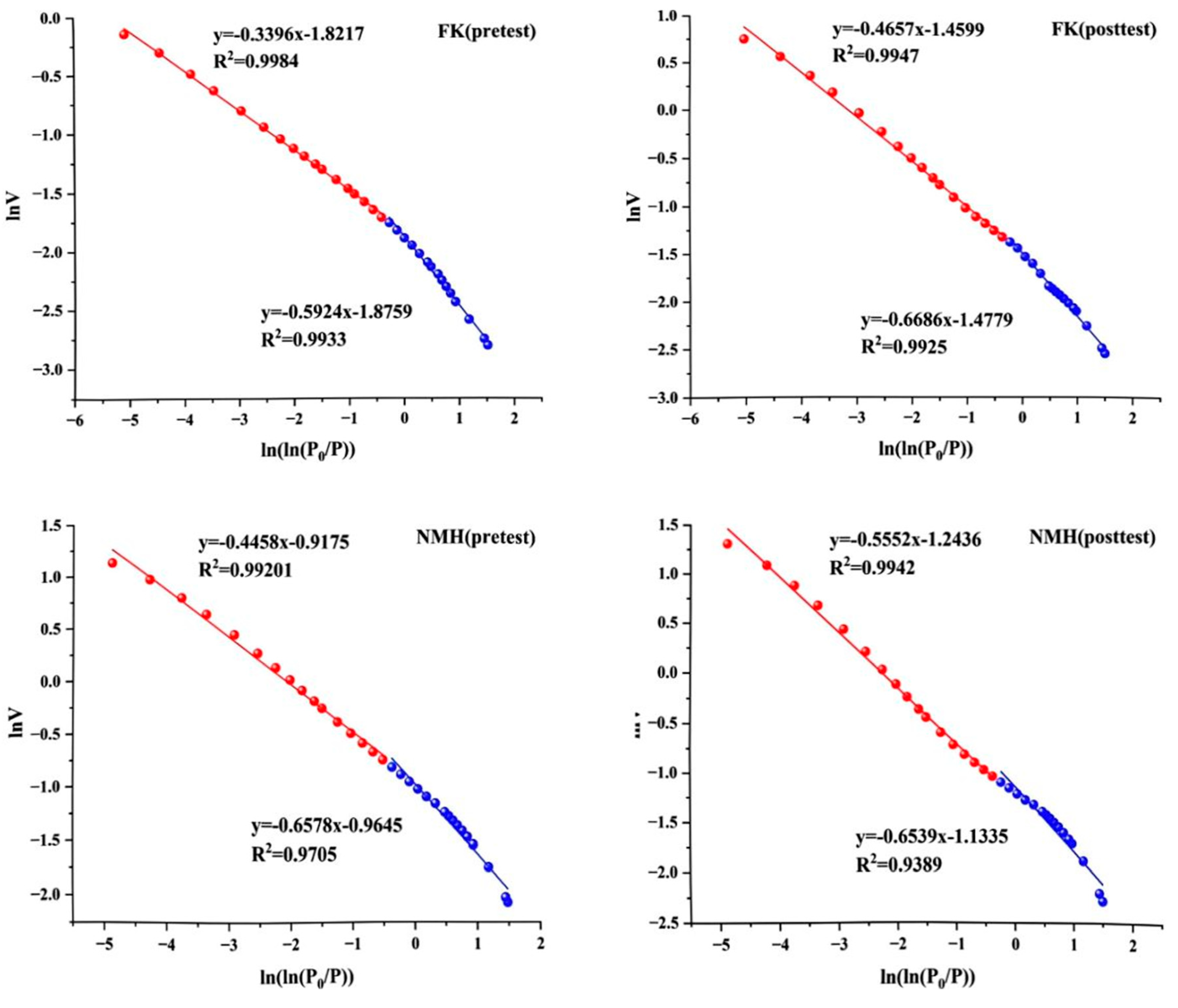
3.3. Pore Changes Based on Fractal Dimension
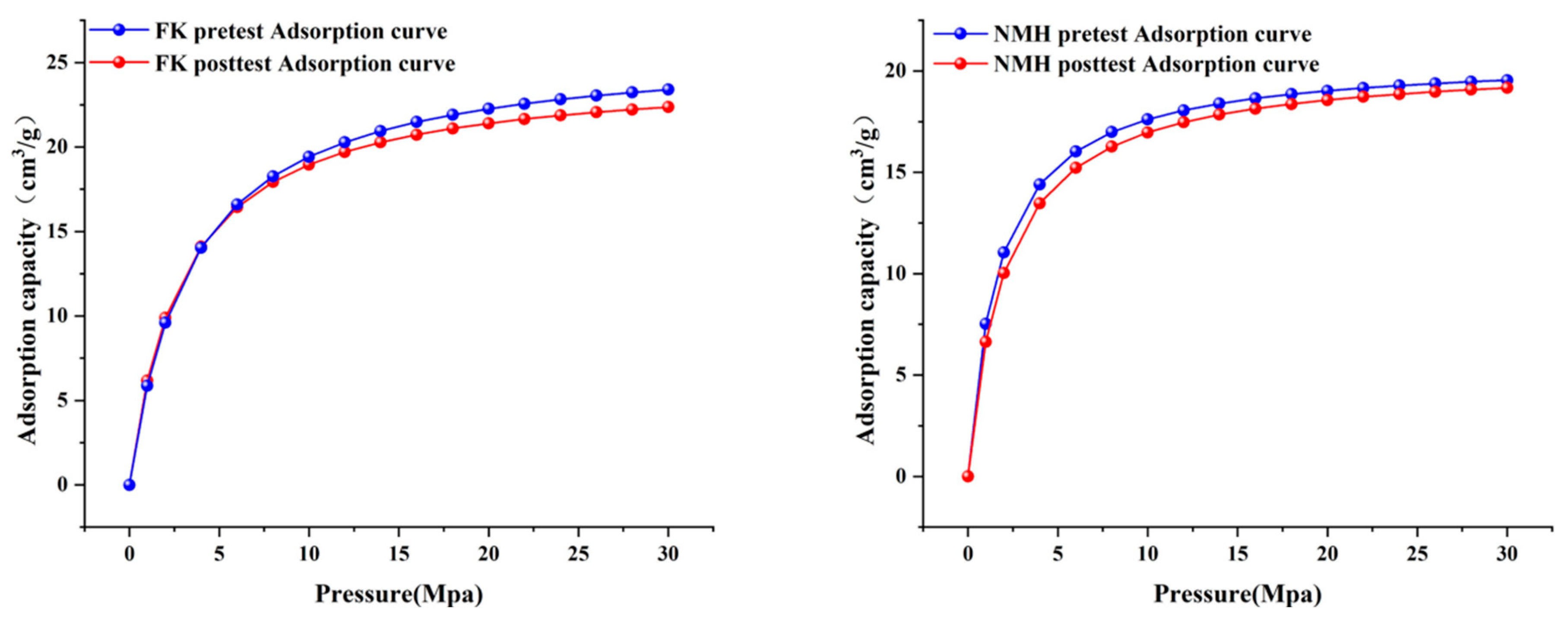
3.4. Changes in the Adsorption Characteristics of Coal Rock Based on the Isothermal Adsorption Experiment
3.5. Characterizing the Changes in the Pore Structure of Coal Rock by Combining Multiple Experimental Methods
4. Conclusions
- Microbial action can significantly optimize the pore structure of low-rank coal reservoirs. After degradation, the volume and specific surface area of mesopores and macropores increase significantly, the proportion of micropores decreases, the average pore size increases, and efficient gas migration channels are formed.
- Microbial action reduces the fractal dimension of coal pores. The pore heterogeneity weakens and the surface roughness decreases, making the pore distribution tend to be concentrated, reducing the proportion of isolated pores and enhancing the connectivity of the micro–mesopore system. The simplification of the pore structure by microbial technology promotes the adsorption and migration of coalbed methane.
- Microbial degradation reduces adsorption sites, decreases the maximum methane adsorption capacity, optimizes the Langmuir pressure to enhance desorption efficiency, strengthens the development potential of low-rank coal reservoirs, and contributes to their green and sustainable development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.Y.; Liang, Y. Biogenic methane production from coal: A review on recent research and development on microbially enhanced coalbed methane (MECBM). Fuel 2016, 166, 258–267. [Google Scholar]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar]
- Li, G.; Zhang, S.; He, H.; He, X.; Zhao, Z.; Niu, X.; Xiong, X.; Zhao, Q.; Guo, X.; Hou, Y.; et al. Coal-rock gas: Concept, connotation and classification criteria. Pet. Explor. Dev. 2024, 51, 897–911. [Google Scholar]
- Li, G.; Jia, C.; Zhao, Q.; Zhou, T.; Gao, J. Coal-rock gas accumulation mechanism and the whole petroleum system of coal measures. Pet. Explor. Dev. 2025, 52, 29–43. [Google Scholar] [CrossRef]
- Palmer, I. Permeability changes in coal: Analytical modeling. Int. J. Coal Geol. 2009, 77, 119–126. [Google Scholar]
- Wang, H.; Cheng, Y.; Wang, W.; Xu, R. Research on comprehensive CBM extraction technology and its applications in China’s coal mines. J. Nat. Gas. Sci. Eng. 2014, 20, 200–207. [Google Scholar]
- Clarkson, C.R.; Bustin, R.M.; Seidle, J.P. Production-data analysis of single-phase (gas) coalbed-methane wells. SPE Reserv. Eval. Eng. 2007, 10, 312–331. [Google Scholar]
- Liu, X.; Sun, Y.; Guo, T.; Rabiei, M.; Qu, Z.; Hou, J. Numerical simulations of hydraulic fracturing in methane hydrate reservoirs based on the coupled thermo-hydrologic-mechanical-damage (THMD) model. Energy 2022, 238, 122054. [Google Scholar]
- Duplyakov, V.M.; Morozov, A.D.; Popkov, D.O.; Shel, E.V.; Vainshtein, A.L.; Burnaev, E.V.; Osiptsov, A.A.; Paderin, G.V. Data-driven model for hydraulic fracturing design optimization. Part II: Inverse problem. J. Petrol. Sci. Eng. 2022, 208, 109303. [Google Scholar]
- Liu, Y.; Tang, D.; Xu, H.; Zhao, T.; Hou, W. Effect of interlayer mechanical properties on initiation and propagation of hydraulic fracturing in laminated coal reservoirs. J. Petrol. Sci. Eng. 2022, 208, 109381. [Google Scholar]
- Ritter, D.; Vinson, D.; Barnhart, E.; Akob, D.M.; Fields, M.W.; Cunningham, A.B.; Orem, W.; McIntosh, J.C. Enhanced microbial coalbed methane generation: A review of research, commercial activity, and remaining challenges. Int. J. Coal Geol. 2015, 146, 28–41. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Y.; Hu, Y. Recent progress in improving the yield of microbially enhanced coalbed methane production. Energy Rep. 2023, 9, 2810–2819. [Google Scholar] [CrossRef]
- Fakoussa, R.Á.; Hofrichter, M. Biotechnology and microbiology of coal degradation. Appl. Microbiol. Biot. 1999, 52, 25–40. [Google Scholar] [CrossRef]
- Welte, C.U. A microbial route from coal to gas. Science 2016, 354, 184. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Bahadur, J.; Elsworth, D.; Wang, Y.; Hu, G.; Liang, Y. Changes in pore structure of coal caused by coal-to-gas bioconversion. Sci. Rep. 2017, 7, 3840. [Google Scholar] [CrossRef]
- Kei, A.; Atsushi, K.; Gakuzo, T. Surfactin a crystalline peptidelipid surfactant produced by bacillus subtilis isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31, 488–496. [Google Scholar]
- Mara, K.; Decorosi, F.; Viti, C.; Giovannetti, L.; Papaleo, M.C.; Maida, I.; Perrin, E.; Fondi, M.; Vaneechoutte, M.; Nemec, A. Molecular and phenotypic characterization of Acinetobacter strains able to degrade diesel fuel. Res. Microbiol. 2012, 163, 161–172. [Google Scholar] [CrossRef]
- Harms, C.; Schleicher, A.; Collins, M.D.; Andreesen, J.R. Tissierella creatinophila sp. nov., a Gram-positive, anaerobic, non-spore-forming, creatinine-fermenting organism. Int. J. Syst. Evol. Microbiol. 1998, 48, 983–993. [Google Scholar] [CrossRef]
- Bao, Y.; Li, Z.; Meng, J.; Chen, X.; Liu, X. Reformation of coal reservoirs by microorganisms and its significance in CBM exploitation. Fuel 2024, 360, 130642. [Google Scholar] [CrossRef]
- Xia, D.; Gu, P.; Chen, Z.; Chen, L.; Wei, G.; Wang, Z.; Cheng, S.; Zhang, Y. Control mechanism of microbial degradation on the physical properties of a coal reservoir. Processes 2023, 11, 1347. [Google Scholar] [CrossRef]
- Guo, G.; Luo, Y.; Ma, J.; Xia, D.; Ji, C.; Su, X. Analysis of mechanism and permeability enhancing effect via microbial treatment on different-rank coals. J. China Coal Soc. 2014, 39, 1886–1891. [Google Scholar]
- Faiz, M.; Stalker, L.; Sherwood, N.; Saghafi, A.; Wold, M.; Barclay, S.; Choudhury, J.; Barker, W.; Wang, I. Bio-enhancement of coal bed methane resources in the southern Sydney Basin. Appea J. 2003, 43, 595–610. [Google Scholar]
- Faiz, M.; Hendry, P. Significance of microbial activity in Australian coal bed methane reservoirs—A review. B Can. Petrol. Geol. 2006, 54, 261–272. [Google Scholar] [CrossRef]
- Xin, F.; Xu, H.; Tang, D.; Yang, J.; Chen, Y.; Cao, L.; Qu, H. Pore structure evolution of low-rank coal in China. Int. J. Coal Geol. 2019, 205, 126–139. [Google Scholar]
- Tao, S.; Chen, S.; Tang, D.; Zhao, X.; Xu, H.; Li, S. Material composition, pore structure and adsorption capacity of low-rank coals around the first coalification jump: A case of eastern Junggar Basin, China. Fuel 2018, 211, 804–815. [Google Scholar] [CrossRef]
- Fu, H.; Yan, D.; Su, X.; Wang, J.; Li, Q.; Li, X.; Zhao, W.; Zhang, L.; Wang, X.; Li, Y. Biodegradation of early thermogenic gas and generation of secondary microbial gas in the Tieliekedong region of the northern Tarim Basin, NW China. Int. J. Coal Geol. 2022, 261, 104075. [Google Scholar]
- Li, G.; Zhang, B.; Wu, K.; Wu, S.; Wang, X.; Zhang, J.; Qi, X.; Zhang, N.; Xing, H.; Xian, C.; et al. Low organic matter abundance and highly efficient hydrocarbon generation of saline source rock in the Qaidam Basin, NW China. Pet. Explor. Dev. 2023, 50, 1030–1044. [Google Scholar]
- Sun, S.; Zhang, B.; Wang, X.; Xiao, W.; Tian, H.; Hou, G.; Zhang, S. High-resolution geochemistry in the Lucaogou Formation, Junggar Basin: Climate fluctuation and organic matter enrichment. Mar. Petrol. Geol. 2024, 162, 106734. [Google Scholar]
- Li, Y.; Chen, J.; Tang, S.; Xi, Z. Microbial Communities Affected by Hydraulic Fracturing and Environmental Factors within an In Situ Coal Reservoir. Microorganisms 2023, 11, 1657. [Google Scholar] [CrossRef]
- Su, X.; Zhao, W.; Xia, D. The diversity of hydrogen-producing bacteria and methanogens within an in situ coal seam. Biotechnol. Biofuels 2018, 11, 245. [Google Scholar]
- Li, D.; Hendry, P.; Faiz, M. A survey of the microbial populations in some Australian coalbed methane reservoirs. Int. J. Coal Geol. 2008, 76, 14–24. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, J.; Hou, Q.; Yu, B.; Li, M.; Niu, Q. Anisotropic characteristics of low-rank coal fractures in the Fukang mining area, China. Fuel 2018, 211, 182–193. [Google Scholar] [CrossRef]
- Gil, A.; Grange, P. Application of the Dubinin-Radushkevich and Dubinin-Astakhov equations in the characterization of microporous solids. Colloids Surf. A Physicochem. Eng. Asp. 1996, 113, 39–50. [Google Scholar] [CrossRef]
- Burke, K. Perspective on density functional theory. J. Chem. Phys. 2012, 136, 150901. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Barrera, D.; Sapag, K. Introducing a self-consistent test and the corresponding modification in the Barrett, Joyner and Halenda method for pore-size determination. Microporous Mesoporous Mater. 2014, 200, 68–78. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Yi, M.; Cheng, Y.; Wang, Z.; Wang, C.; Hu, B.; He, X. Effect of particle size and adsorption equilibrium time on pore structure characterization in low pressure N2 adsorption of coal: An experimental study. Adv. Powder Technol. 2020, 31, 4275–4281. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Wang, C.; Ferko, B.T.; Shen, K.; Winey, K.I.; Vohs, J.M.; Gorte, R.J. Determination of film thicknesses of metal oxides prepared by atomic layer deposition on SBA-15. Microporous Mesoporous Mater. 2024, 366, 112945. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.; Wang, X.; Yu, B.; Ding, W.; Xiong, J.; Yang, Y.; Wang, L.; Yang, C. Shale characteristics in the southeastern Ordos Basin, China: Implications for hydrocarbon accumulation conditions and the potential of continental shales. Int. J. Coal Geol. 2014, 128, 32–46. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Tang, D.; Huang, W.; Pan, Z. Determining fractal dimensions of coal pores by FHH model: Problems and effects. J. Nat. Gas. Sci. Eng. 2014, 21, 929–939. [Google Scholar]
- Zhang, C.; Jia, S.; Ren, Z.; Bai, Q.; Wang, L.; Han, P. Strength evolution characteristics of coal with different pore structures and mineral inclusions based on CT scanning reconstruction. Nat. Resour. Res. 2024, 33, 2725–2742. [Google Scholar]
- Zhang, X.; Cheng, J.; Zhang, L.; Zhou, T.; Kang, T.; Li, L. Pore Fractal Characteristics of Suancigou Long-Flame Coal after Electrochemical Treatment: An Experimental Study through the Implementation of N2 Adsorption and Mercury Intrusion Prosimetry Techniques. ACS Omega 2021, 6, 27358–27367. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, W.; Gao, D.; Liu, W. Critical pore size for micropore filling in coal samples with different rank coals. PLoS ONE 2022, 17, e264225. [Google Scholar]
- Orio, M.; Pantazis, D.A.; Neese, F. Density functional theory. Photosynth. Res. 2009, 102, 443–453. [Google Scholar]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar]
- Ravikovitch, P.I.; Neimark, A.V. Density functional theory of adsorption in spherical cavities and pore size characterization of templated nanoporous silicas with cubic and three-dimensional hexagonal structures. Langmuir 2002, 18, 1550–1560. [Google Scholar]
- Rahman, M.M.; Muttakin, M.; Pal, A.; Shafiullah, A.Z.; Saha, B.B. A statistical approach to determine optimal models for IUPAC-classified adsorption isotherms. Energies 2019, 12, 4565. [Google Scholar] [CrossRef]
- He, J.; Huang, C.; Lai, C.; Huang, C.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yong, Q. The effect of lignin degradation products on the generation of pseudo-lignin during dilute acid pretreatment. Ind. Crop Prod. 2020, 146, 112205. [Google Scholar] [CrossRef]
- Sáez-Jiménez, V.; Baratto, M.C.; Pogni, R.; Rencoret, J.; Gutiérrez, A.; Santos, J.I.; Martínez, A.T.; Ruiz-Dueñas, F.J. Demonstration of lignin-to-peroxidase direct electron transfer: A transient-state kinetics, directed mutagenesis, EPR, and NMR study. J. Biol. Chem. 2015, 290, 23201–23213. [Google Scholar]
- Avnir, D.; Jaroniec, M. An isotherm equation for adsorption on fractal surfaces of heterogeneous porous materials. Langmuir 1989, 5, 1431–1433. [Google Scholar]
- Hou, S.; Wang, X.; Wang, X.; Yuan, Y.; Pan, S.; Wang, X. Pore structure characterization of low volatile bituminous coals with different particle size and tectonic deformation using low pressure gas adsorption. Int. J. Coal Geol. 2017, 183, 1–13. [Google Scholar]
- Altowilib, A.; AlSaihati, A.; Alhamood, H.; Alafnan, S.; Alarifi, S. Reserves estimation for coalbed methane reservoirs: A review. Sustainability 2020, 12, 10621. [Google Scholar] [CrossRef]
- Alafnan, S.; Awotunde, A.; Glatz, G.; Adjei, S.; Alrumaih, I.; Gowida, A. Langmuir adsorption isotherm in unconventional resources: Applicability and limitations. J. Petrol. Sci. Eng. 2021, 207, 109172. [Google Scholar]
- Zhang, Z.; Qin, Y.; Wang, G.X.; Fu, X. Numerical description of coalbed methane desorption stages based on isothermal adsorption experiment. Sci. China Earth Sci. 2013, 56, 1029–1036. [Google Scholar]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar]
- Xu, S.; Hu, E.; Li, X.; Xu, Y. Quantitative analysis of pore structure and its impact on methane adsorption capacity of coal. Nat. Resour. Res. 2021, 30, 605–620. [Google Scholar]





| Scheme | Mad (%) | Aad (%) | Vad (%) | Vitrinite (%) | Inertinite (%) | Exinite (%) | R° max (%) |
|---|---|---|---|---|---|---|---|
| FK | 1.25 | 2.77 | 15.07 | 83 | 12 | 5 | 0.8 |
| NMH | 9.14 | 10.49 | 50.53 | 85.3 | 14.3 | 0.8 | 0.35 |
| Sample ID | DFT PV (cm3/100 g) | D-A PV (cm3/100 g) | DFT SSA (m²/g) | DFT SSA (m²/g) (0.37~1.1 nm) | D-A SSA (m²/g) |
|---|---|---|---|---|---|
| FK (pretest) | 2.09 | 3.77 | 111.92 | 64.09 | 99.29 |
| FK (posttest) | 1.43 | 3.56 | 99.50 | 42.07 | 91.16 |
| NMH (pretest) | 2.36 | 3.67 | 115.27 | 74.54 | 98.17 |
| NMH (posttest) | 2.41 | 3.99 | 125.27 | 80.10 | 106.66 |
| Sample ID | BJH PV (cm³/100 g) | BET SSA (m²/g) | APW (nm) |
|---|---|---|---|
| FK (pretest) | 0.14 | 0.44 | 9.77 |
| FK (posttest) | 0.33 | 0.71 | 14.69 |
| NMH (pretest) | 0.48 | 1.10 | 9.94 |
| NMH (posttest) | 0.57 | 0.92 | 14.43 |
| Sample ID | P/P0 < 0.5 | P/P0 > 0.5 | ||||
|---|---|---|---|---|---|---|
| A1 | D1 | R2 | A2 | D2 | R2 | |
| FK (pretest) | −0.5924 | 2.4076 | 0.9933 | −0.3396 | 2.6604 | 0.9984 |
| FK (posttest) | −0.6686 | 2.3314 | 0.9925 | −0.4657 | 2.5343 | 0.9947 |
| NMH (pretest) | −0.6578 | 2.3422 | 0.9705 | −0.4458 | 2.5542 | 0.9920 |
| NMH (posttest) | −0.6539 | 2.3461 | 0.9389 | −0.5552 | 2.4448 | 0.9942 |
| Sample ID | Langmuir Maximum Adsorption Capacity VL (cm3/g) | Rate of Change | Langmuir Pressure PL (MPa) | Adsorbed Phase Density ρad (g/cm3) |
|---|---|---|---|---|
| FK (pretest) | 26.074 | −5.06% | 3.423 | 0.41 |
| FK (posttest) | 24.755 | 2.964 | 0.411 | |
| NMH (pretest) | 20.675 | −0.85% | 1.736 | 0.383 |
| NMH (posttest) | 20.5 | 2.082 | 0.37 |
| Sample ID | Micropore PV (cm³/100 g) | Micropore SSA (m²/g) | Mesopore PV (cm³/100 g) | Mesopore SSA (m²/g) | Macropore PV (cm³/100 g) | Macropore SSA (m²/g) |
|---|---|---|---|---|---|---|
| FK (pretest) | 2.09 | 64.09 | 0.08 | 0.47 | 0.05 | 0.03 |
| FK (posttest) | 1.43 | 42.07 | 0.20 | 0.84 | 0.13 | 0.07 |
| NMH (pretest) | 2.36 | 74.54 | 0.32 | 1.88 | 0.12 | 0.06 |
| NMH (posttest) | 2.41 | 80.10 | 0.41 | 1.52 | 0.16 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Q.; Zhang, B.; Ma, X.; Zhao, S.; Fan, J.; Fan, Y.; Tang, X. Research on the Modification of the Coal Pore Structure by Indigenous Microbial Degradation. Sustainability 2025, 17, 3337. https://doi.org/10.3390/su17083337
Bai Q, Zhang B, Ma X, Zhao S, Fan J, Fan Y, Tang X. Research on the Modification of the Coal Pore Structure by Indigenous Microbial Degradation. Sustainability. 2025; 17(8):3337. https://doi.org/10.3390/su17083337
Chicago/Turabian StyleBai, Qiyuan, Bin Zhang, Xingzhi Ma, Shufeng Zhao, Jialin Fan, Yvbo Fan, and Xuan Tang. 2025. "Research on the Modification of the Coal Pore Structure by Indigenous Microbial Degradation" Sustainability 17, no. 8: 3337. https://doi.org/10.3390/su17083337
APA StyleBai, Q., Zhang, B., Ma, X., Zhao, S., Fan, J., Fan, Y., & Tang, X. (2025). Research on the Modification of the Coal Pore Structure by Indigenous Microbial Degradation. Sustainability, 17(8), 3337. https://doi.org/10.3390/su17083337




