Effects of Long-Term Multi-Treatment Experiments on Organic Matter and Enzymatic Activity in Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Chemical Analysis of the Included Soil
- -
- pH in 1 M KCl and H2O by potentiometric method;
- -
- total organic carbon (TOC) and total nitrogen (TN) were assayed with the Vario Max CN analyser (Elementar, Langenselbold, Germany);
- -
- the amount of dissolved organic carbon (DOC) and dissolved total nitrogen (DTN) were measured in the solutions following the extraction with 0.004 M CaCl2. DOC and DTN were assayed with the Muli N/C 3100 Analityk Jena analyzer (Jena, Germany, assay sensitivity of 1 µg L−1) and expressed in mg kg−1 d.w. of the soil sample as well as the percentage share in the pool of TOC and TN, respectively;
- -
- humus fractions susceptible to oxidation [5,6]. The method is based on assaying the fractions of organic carbon susceptible to oxidation by acting on the soil sample with a 0.333 M solution (CL labile carbon) and (CNL non-labile carbon) as well as a 0.0333 M KMnO4 solution (CL1 labile carbon) in a neutral environment.
- -
- carbon pool size index CPI:
- -
- carbon management index CMI:where the lability indexand L is calculated as follows:where the L–lability of C (C in fraction oxidised by KMnO4; C remaining unoxidized by KMnO4).
2.2.2. The Activity of Enzymes in Soil
- -
- the activity of dehydrogenases (DEH) was assayed with the Thalmann [26] method after sample incubation with 2,3,5-triphenyltetrazolium chloride, and the measurement of absorbance of triphenylformazan (TPF) at 546 nm was expressed in mg TPF kg−1 24 h−1.
- -
- the activity of catalase (CAT) was assayed with the Johnson and Temple [27] method with a 0.3% solution of hydrogen peroxide as a substrate. The other H2O2 was determined with a titration of 0.02 M KMnO4 in acid conditions.
- -
- the activities of alkaline phosphatase (AlP) and acid phosphatase (AcP) were measured from the detection of p-nitrophenol (pNP) released after incubation (37 °C, 1 h) for a pH~6.5 for acid phosphatase and for a pH~11.0 for alkaline phosphatase [28].
- -
- the activity of β-glucosidase (BG) was measured with the Eivazi and Tabatabai [29] method, applying p-nitrophenyl-β-D-glucopyranoside as a substrate. The concentrations of p-nitrophenol were assayed with an immediate readout of the sample at 400 nm after alkalization with the buffer Tris/NaOH (pH 10.0) and CaCl2.
- -
- the activity of proteases (PRO) was assayed with the Ladd and Butlera [30] method, where the concentration of the amino acid tyrosine (Tyr) was assayed in the soil samples after incubation with sodium caseinate. Absorbance was measured with the spectrophotometer at a rem λ = 680.
- -
- enzymatic index of the soil pH from the activity of alkaline (AlP) and acid (AcP) phosphatase [31]:
- -
- the geometric mean GMea [32]:
- -
- to evaluate the total activity of soil enzymes (TEI) (total enzyme activity index), the following was calculated [33]:where Xi is the activity of soil enzyme i, and is the mean activity of enzyme i in all the samples.
- -
- the results of the metabolic activity index (MAI) [34] for the total soil activity are also presented:where , and Aij is the value of activity of each enzyme; Refj is the reference parameter—TOC; Acij is the value of the activity of each enzyme in the control soil; Refcj is the reference parameter in the control soil.
2.3. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Properties of the Soil
3.2. The Content of Available Macronutrients
3.3. The Activity of Enzymes
3.4. Enzymatic Indicators of Soil Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonet, S.S. Ochrona zasobów materii organicznej. [W:] Rola materii organicznej w środowisku. In Antropogeniczne Przekształcenia Pokrywy Glebowej Brodnickiego Parku Krajobrazowego; Gonet, S.S., Markiewicz, M., Eds.; PTSH: Wrocław, Poland, 2007; pp. 7–29. [Google Scholar]
- Jaskulska, I.; Lemanowicz, J.; Breza-Boruta, B.; Siwik-Ziomek, A.; Radziemska, M.; Dariusz, J.; Białek, M. Chemical and biological properties of sandy loam soil in response to long-term organic–mineral fertilisation in a warm-summer humid continental climate. Agronomy 2020, 10, 1610. [Google Scholar] [CrossRef]
- Nardi, S.; Morari, F.; Berti, A.; Tosoni, M.; Giardani, L. Soil organic matter properties after 40years of different use of organic and mineral fertilizers. Eur. J. Agron. 2004, 21, 357–367. [Google Scholar] [CrossRef]
- Mercik, S.; Stępień, M.; Stępień, W.; Sosulski, T. Dynamic of organic carbon content in soil depending on long-term fertilization and crop rotation. Soil Sci. Ann. 2005, 56, 53–60. [Google Scholar]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Łoginow, W.; Wiśniewski, W.; Gonet, S.S.; Cieścińska, B. Testowa metoda oceny podatności na utlenianie materii organicznej gleb. Zesz. Probl. Post. Nauk Rol. 1993, 411, 207–212. (In Polish) [Google Scholar]
- Gonet, S.S.; Debska, B. Dissolved organic carbon and dissolved nitrogen in soil under different fertilization treatments. Plant Soil Environ. 2006, 52, 55–63. [Google Scholar] [CrossRef]
- Khalid, M.; Soleman, N.; Jones, D.L. Grassland plants affect dissolved organic carbon and nitrogen dynamics in soil. Soil Biol. Biochem. 2007, 39, 378–381. [Google Scholar] [CrossRef]
- Gautam, A.; Sekaran, U.; Guzman, J.; Kovács, P.; Hernandez, J.L.G.; Kumar, S. Responses of soil microbial community structure and enzymatic activities to long-term application of mineral fertilizer and beef manure. Environ. Sustain. Indic. 2020, 8, 100073. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Lemanowicz, J.; Rydlewska, M.; Drabińska, O.; Ewert, K. Enzymatic activity of soil after applications distillery stillage. Agriculture 2022, 12, 652. [Google Scholar] [CrossRef]
- Yang, Z.; Ha, L. Analysis and comparison of nutrient contents in different animal manures from Beijing suburbs. Agric. Sci. 2013, 4, 50–55. [Google Scholar] [CrossRef]
- Li, C.-X.; Ma, S.-C.; Shao, Y.; Ma, S.-T.; Zhang, L.-L. Effects of long-term organic fertilization on soil microbiologic characteristics, yield and sustainable production of winter wheat. J. Integr. Agric. 2018, 17, 210–219. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Zielińska, A.; Jaskulska, I.; Rydlewska, M.; Klunek, K.; Polkowska, M. The Effect of enzyme activity on carbon sequestration and the cycle of available macro- (P, K, Mg) and microelements (Zn, Cu) in Phaeozems. Agriculture 2023, 13, 172. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Sharma, P.C.; Dixit, B.; Jat, M.L. Soil enzymes activity: Effect of climate smart agriculture on rhizosphere and bulk soil under cereal based systems of north-west India. Eur. J. Soil Biol. 2021, 103, 103292. [Google Scholar] [CrossRef]
- Jaskulska, I.; Lemanowicz, J.; Debska, B.; Jaskulski, D.; Breza-Boruta, B. Changes in soil organic matter and biological parameters as a result of long-term strip-till cultivation. Agriculture 2023, 13, 2188. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Li, X.G.; Jia, B.; Lv, J.; Ma, Q.; Kuzyakov, Y.; Li, F. Nitrogen fertilization decreases the decomposition of soil organic matter and plant residues in planted soils. Soil Biol. Biochem. 2017, 112, 47–55. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Haddad, S.A.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P. The role of an urban park’s tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil. Sci. Total Environ. 2020, 741, 140446. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Marks, M.; Jastrzębska, M.; Kostrzewska, M.K. (Eds.) Long-Term Experiments in Agricultural Studies in Poland; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego w Olsztynie: Olsztyn, Poland, 2018; p. 280. [Google Scholar]
- PN-R-04023; Chemical and Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- PN-R-04022; Chemical and Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- PN-R-04020; Chemical and Agricultural Analysis. Determination of the Content Available Magnesium. Polish Standards Committee: Warszawa, Poland, 1994.
- Schachtschabel, P. Das pflanzenverfügbare Magnesium des Boden und seine Bestimmung. J. Plant. Nutr. Soil Sci. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- Thalmann, A. Zur methodic derestimung der Dehydrogenaseaktivität und Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch 1968, 21, 249–258. [Google Scholar]
- Johnson, J.I.; Temple, K.L. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soc. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p–nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and peptide derivates as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Garcia-Ruiz, R.; Viñegla, B.; Carreira, J.A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 2004, 36, 1637–1644. [Google Scholar] [CrossRef]
- Tan, X.; Xie, B.; Wang, J.; He, W.; Wang, X.; Wei, G. County-scale spatial distribution of soil enzyme activities and enzyme activity indices in agricultural land: Implications for soil quality assessment. Sci. World J. 2014, 2014, 535768. [Google Scholar] [CrossRef]
- Picariello, E.; Baldantoni, D.; Muniategui-Lorenzo, S.; Concha-Granã, E.; De Nicola, F. A synthetic quality index to evaluate the functional stability of soil microbial communities after perturbations. Ecol. Indic. 2021, 128, 107844. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Guo, X.; Li, H.; Yu, H.; Li, W.; Ye, Y.; Biswas, A. Drivers of spatio-temporal changes in paddy soil pH in Jiangxi Province, China from 1980 to 2010. Sci. Rep. 2018, 8, 2702. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, J.; Zhu, Q.; Zhang, Z.; Lin, X. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci Rep. 2017, 7, 40093. [Google Scholar] [CrossRef]
- Minasny, B.; Hong, S.Y.; Hartemink, A.E.; Kim, Y.H.; Kang, S.S. Soil pH increase under paddy in South Korea between 2000 and 2012. Agric. Ecosyst. Environ. 2016, 221, 205–213. [Google Scholar] [CrossRef]
- Ciric, V.; Belic, M.; Nesic, L.; Seremesic, S.; Pejic, B.; Bezdan, A.; Manojlovic, M. The sensitivity of water extractable soil organic carbon fractions to land use in three soil types. Arch. Agron. Soil Sci. 2016, 62, 1654–1664. [Google Scholar] [CrossRef]
- Morelli, R.; Bertoldi, D.; Baldantoni, D.; Zanzotti, R. Labile, recalcitrant and stable soil organic carbon: Comparison of agronomic management in a vineyard of Trentino (Italy). BIO Web Conf. 2022, 44, 02007. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Poulton, P.R. Long-term management impacts on soil C, N and physical fertility Part I: Broadbalk experiment. Soil Tillage Res. 2006, 91, 30–38. [Google Scholar] [CrossRef]
- Huang, S.; Rui, W.; Peng, X.; Huang, Q.; Zhang, W. Organic carbon fractions affected by long-term fertilization in a subtropical paddy soil. Nutr. Cycl. Agroecosyst. 2010, 86, 153–160. [Google Scholar] [CrossRef]
- Mando, A.; Bonzi, M.; Woperejs, M.C.S.; Lompo, F.; Stroosnijder, L. Long-term effects of mineral and organic fertilization on soil organic matter fractions and sorghum yield under Sudano-Sahelian conditions. Soil Use Manag. 2006, 21, 396–401. [Google Scholar] [CrossRef]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzowa, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Filipek, T.; Skowron, P. New proposals for phosphorus management in Polish agriculture. W: Scientific basis to mitigate the nutrient dispersion into the environment. Pr. Zbior. Red. A Sapek. Falenty. Wydaw. IMUZ 2000, 151–159. [Google Scholar]
- Arbačauskas, J.; Vaišvila, Z.J.; Staugaitis, G.; Žičkienė, L.; Masevičienė, A.; Šumskis, D. The influence of mineral npk fertiliser rates on potassium dynamics in soil: Data from a long-term agricultural plant fertilisation experiment. Plants 2023, 12, 3700. [Google Scholar] [CrossRef]
- Balik, J.; Černy, I.; Kulhanek, M.; Sedlar, O.; Suran, P. Balance of potassium in two long-term field experiments with different fertilization treatments. Plant Soil. Environ. 2019, 65, 225–232. [Google Scholar] [CrossRef]
- Sienkiewicz, S.; Krzebietke, S.; Wojnowska, T.; Żarczyński, P.; Omilian, M. Effect of long-term differentiated fertilization with farmyard manure and mineral fertilizers on the content of available forms of P, K and Mg in soil. J. Elem. 2009, 14, 779–786. [Google Scholar] [CrossRef]
- Jabborova, D.; Sulaymanov, K.; Sayyed, R.Z.; Alotaibi, S.H.; Enakiev, Y.; Azimov, A.; Jabbarov, Z.; Ansari, M.J.; Fahad, S.; Danish, S.; et al. Mineral fertilizers improves the quality of turmeric and soil. Sustainability 2021, 13, 9437. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, X.; Wu, L.; Rensinga, C.; Xing, S. Short-term application of magnesium fertilizer affected soil microbial biomass, activity, and community structure. J. Soil Sci. Plant Nutr. 2021, 21, 675–689. [Google Scholar] [CrossRef]
- Cowan, J.A. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals 2002, 15, 225–235. [Google Scholar] [PubMed]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Li, W.X.; Guo, L.; Cai, Z.J.; Wang, B.R.; Chen, J.; Shen, R.F. Changes of acid and alkaline phosphatase activities in long-term chemical fertilization are driven by the similar soil properties and associated microbial community composition in acidic soil. Eur. J. Soil Sci. 2021, 104, 103312. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Tang, Z.; Wang, J.; Zhang, Y. Long-term organic fertilization reshapes the communities of bacteria and fungi and enhances the activities of C- and P-cycling enzymes in calcareous alluvial soil. Appl. Soil Ecol. 2024, 194, 105204. [Google Scholar] [CrossRef]
- Choudhary, M.; Meena, V.S.; Panday, S.C.; Mondal, T.; Yadav, R.P.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A. Long-term effects of organic manure and inorganic fertilization on biological soil quality indicators of soybean-wheat rotation in the Indian mid-Himalaya. Appl. Soil Ecol. 2021, 157, 103754. [Google Scholar] [CrossRef]
- Yang, O.; Norton, J.M. Short-term nitrogen fertilization affects microbial community composition and nitrogen mineralization functions in an agricultural soil. Appl. Environ. Microbiol. 2020, 86, e02278-19. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.; Gao, Y.; Li, Y.; Wang, H.; Ma, D.; Zhang, R.; Yang, S. Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in artemisia ordosica Community. Pedosphere 2010, 20, 229–235. [Google Scholar] [CrossRef]
- Datta, A.; Gujre, N.; Gupta, D.; Agnihotri, R.; Mitra, S. Application of enzymes as a diagnostic tool for soils as affected by municipal solid wastes. J. Environ. Manag. 2021, 286, 112169. [Google Scholar] [CrossRef]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2013, 27, 14–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Deng, S.; Zhang, J.; Hou, J.; Wang, C.; Fu, Z. Effect of different washing solutions on soil enzyme activity and microbial community in agricultural soil severely contaminated with cadmium. Environ. Sci. Pollut. Res. 2022, 29, 54641–54651. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The application of biochar from waste biomass to improve soil fertility and soil enzyme activity and increase carbon sequestration. Energies 2023, 16, 380. [Google Scholar] [CrossRef]
- Kamal, S.; Rehman, S.; Iqbal, H.M.N. Biotechnological valorization of proteases: From hyperproduction to industrial exploitation—A review. Environ. Prog. Sustain. Energy 2016, 36, 511–522. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. Forest Res. Papers 2017, 78, 39–44. [Google Scholar] [CrossRef]
- Cincotta, M.M.; Perdrial, J.N.; Shavitz, A.; Libenson, A.; Landsman-Gerjoi, M.; Perdrial, N.; Armfield, J.; Adler, T.; Shanley, J.B. Soil aggregates as a source of dissolved organic carbon to streams: An experimental study on the effect of solution chemistry on water extractable carbon. Front. Environ. Sci. 2019, 7, 172. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Series: Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and enzymatic soil properties influenced by cropping of primary wheat under organic and conventional farming systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]

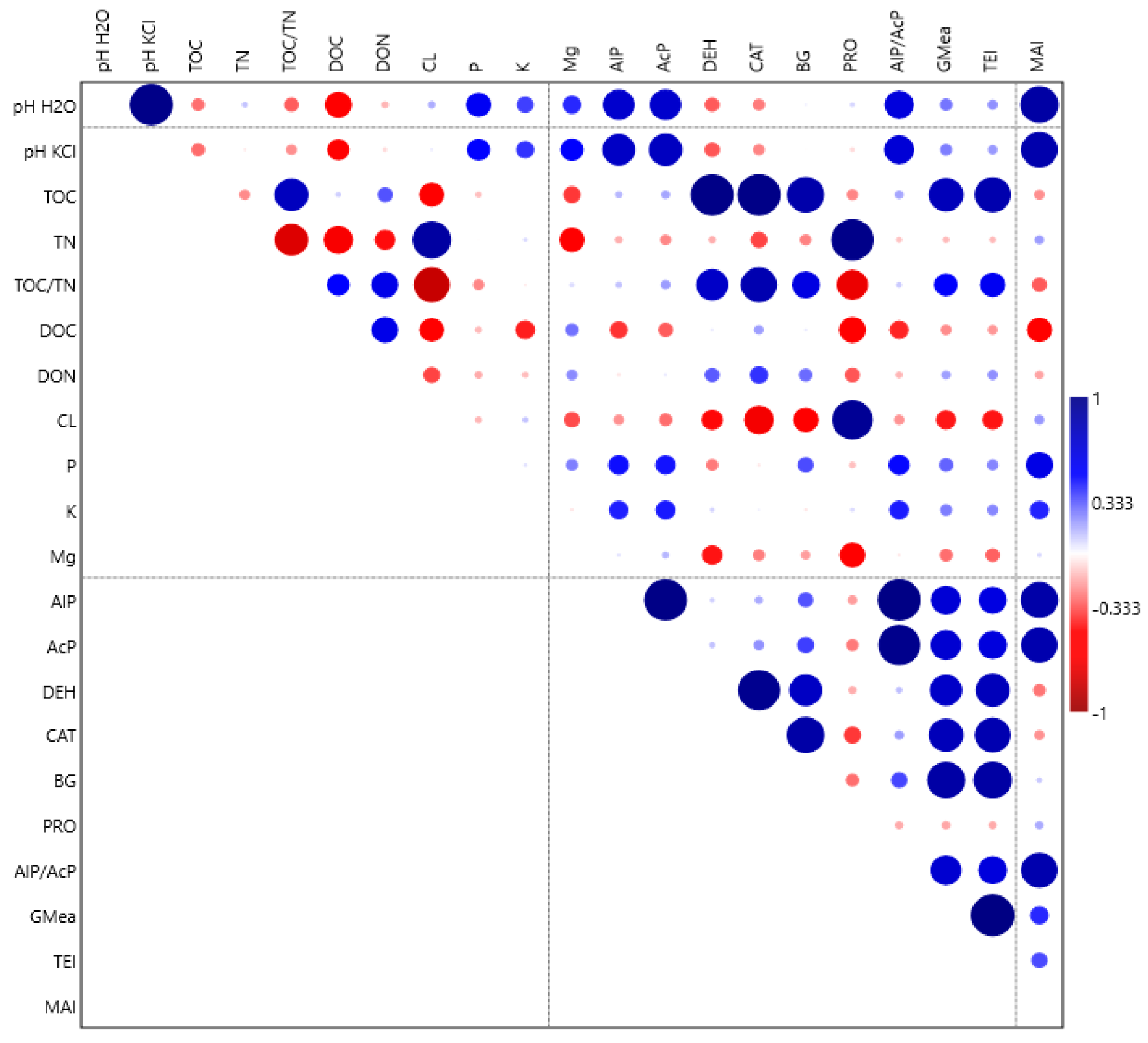
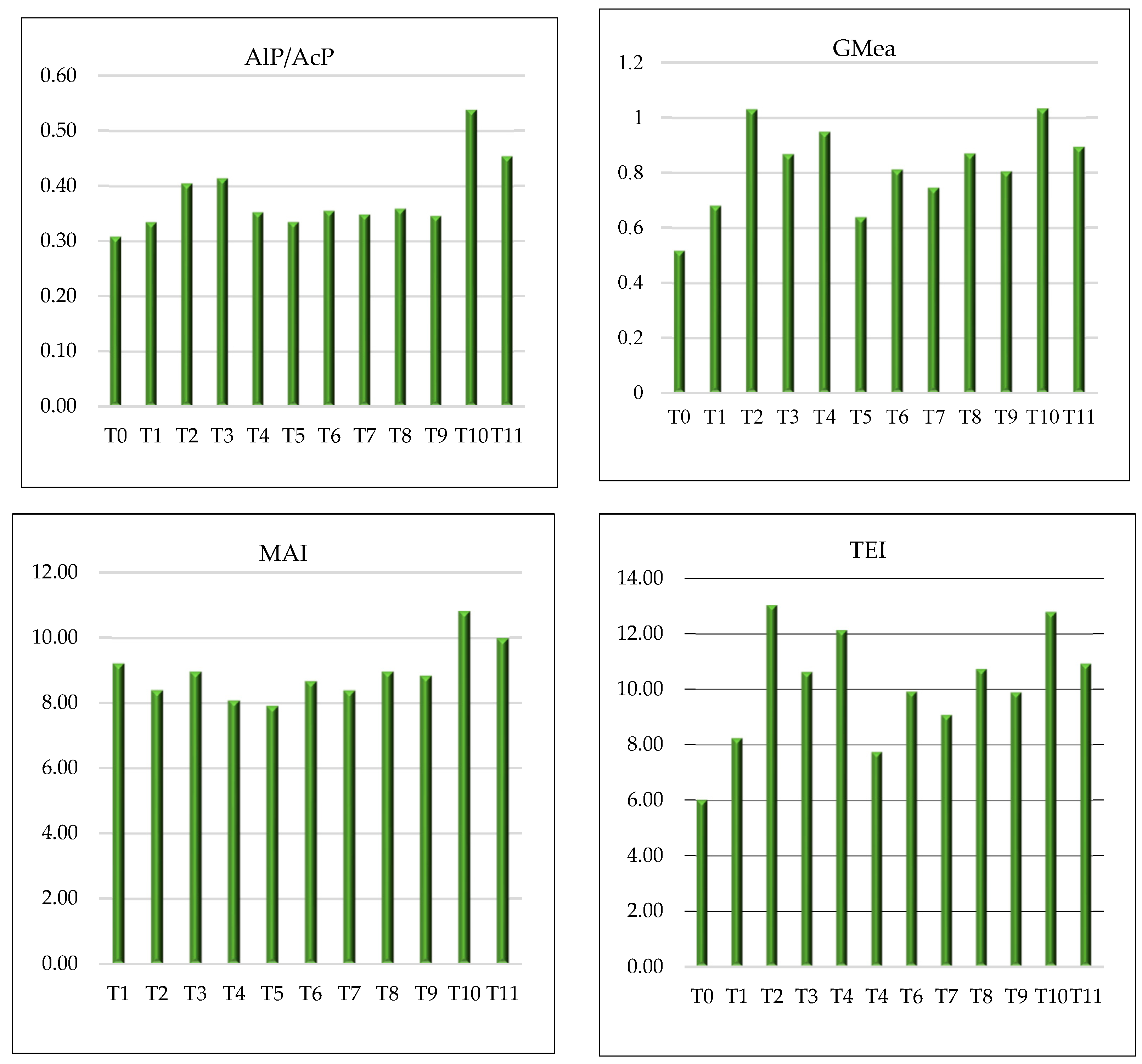

| Fertilisation | pH KCl | pH H2O | TOC g kg−1 | TN g kg−1 | TOC/ TN | DOC mg kg−1 | DTN mg kg−1 | DOC %TOC | DTN %TN |
|---|---|---|---|---|---|---|---|---|---|
| T0 | 5.3 | 6.3 | 5.67 | 0.53 | 10.7 | 75.65 | 7.28 | 1.33 | 1.37 |
| ±0.005 | ±0.005 | ±0.10 | ±0.005 | ||||||
| T1 | 4.5 | 6.0 | 5.07 | 0.94 | 5.4 | 64.55 | 6.30 | 1.27 | 0.70 |
| ±0.005 | ±0.005 | ±0.15 | ±0.005 | ||||||
| T2 | 4.4 | 5.8 | 8.85 | 0.68 | 13.0 | 67.95 | 6.41 | 0.80 | 0.94 |
| ±0.005 | ±0.005 | ±0.05 | ±0.01 | ||||||
| T3 | 4.2 | 5.6 | 6.75 | 0.55 | 12.3 | 78.20 | 6.60 | 1.16 | 1.20 |
| ±0.005 | ±0.005 | ±0.00 | ±0.005 | ||||||
| T4 | 4.2 | 5.6 | 8.56 | 0.50 | 17.1 | 80.05 | 11.41 | 0.92 | 2.28 |
| ±0.005 | ±0.005 | ±0.05 | ±0.01 | ||||||
| T5 | 4.4 | 5.6 | 5.57 | 0.46 | 12.1 | 79.50 | 8.02 | 1.43 | 1.74 |
| ±0.005 | ±0.005 | ±0.10 | ±0.005 | ||||||
| T6 | 6.52 | 0.57 | 11.4 | 78.40 | 7.58 | 1.20 | 1.33 | ||
| 4.4 | 5.7 | ±0.01 | ±0.005 | ±0.20 | ±0.005 | ||||
| T7 | 6.16 | 0.54 | 11.4 | 80.20 | 8.03 | 1.30 | 1.50 | ||
| 4.5 | 5.9 | ±0.005 | ±0.005 | ±0.10 | ±0.005 | ||||
| T8 | 6.82 | 0.58 | 11.7 | 82.10 | 10.87 | 1.20 | 1.88 | ||
| 4.6 | 5.9 | ±0.01 | ±0.005 | ±0.10 | ±0.005 | ||||
| T9 | 6.36 | 0.56 | 11.3 | 70.25 | 7.59 | 1.10 | 1.35 | ||
| 4.9 | 6.2 | ±0.01 | ±0.005 | ±0.05 | ±0.02 | ||||
| T10 | 6.72 | 0.58 | 11.6 | 67.10 | 8.08 | 1.00 | 1.39 | ||
| 5.4 | 6.5 | ±0.005 | ±0.000 | ±0.00 | ±0.005 | ||||
| T11 | 6.23 | 0.53 | 11.7 | 77.70 | 7.70 | 1.25 | 1.45 | ||
| 5.4 | 6.5 | ±0.005 | ±0.005 | ±0.10 | ±0.005 | ||||
| Mean | 6.607 | 0.585 | 11.642 | 75.137 | 7.993 | 1.163 | 1.428 | ||
| HSD | 0.039 | 0.028 | n.s. | 0.516 | 0.063 | 0.062 | n.s. |
| Fertilisation | CL1 g kg−1 | CL1 %TOC | CL g kg−1 | CNL g kg−1 |
|---|---|---|---|---|
| T0 * | 0.321 ± 0.001 | 5.7 | 1.07 ± 0.005 | 4.60 |
| T1 | 0.191 ± 0.000 | 3.8 | 0.64 ± 0.005 | 4.43 |
| T2 | 0.200 ± 0.000 | 2.3 | 0.61 ± 0.000 | 8.24 |
| T3 | 0.266 ± 0.000 | 3.9 | 0.64 ± 0.005 | 6.11 |
| T4 | 0.211 ± 0.000 | 2.5 | 0.56 ± 0.005 | 8.00 |
| T5 | 0.253 ± 0.003 | 4.5 | 0.65 ± 0.010 | 4.92 |
| T6 | 0.243 ± 0.003 | 3.7 | 2.07 ± 0.010 | 4.45 |
| T7 | 0.267 ± 0.003 | 4.3 | 0.64 ± 0.010 | 5.52 |
| T8 | 0.274 ± 0.004 | 4.0 | 0.80 ± 0.010 | 6.02 |
| T9 | 0.311 ± 0.001 | 4.9 | 2.05 ± 0.005 | 4.31 |
| T10 | 0.302 ± 0.002 | 4.5 | 1.05 ± 0.005 | 5.67 |
| T11 | 0.323 ± 0.003 | 5.2 | 1.07 ± 0.010 | 5.16 |
| Mean | 0.264 | 4.108 | 0.998 | 5.428 |
| HSD | n.s. | n.s. | n.s. | 3.791 |
| Fertilisation | P | K | Mg |
|---|---|---|---|
| mg kg−1 | |||
| T0 * | 73.52 ± 1.26 | 59.80 ± 3.58 | 10.32 ± 1.98 |
| T1 | 118.4 ± 3.59 | 168.2 ± 9.12 | 11.91 ± 2.59 |
| T2 | 128.7 ± 7.25 | 172.9 ± 7.56 | 12.11 ± 2.14 |
| T3 | 154.2 ± 9.37 | 195.4 ± 8.23 | 13.29 ± 1.89 |
| T4 | 89.31 ± 2.53 | 141.2 ± 6.12 | 15.04 ± 2.56 |
| T5 | 92.70 ± 3.57 | 175.9 ± 12.11 | 22.98 ± 3.45 |
| T6 | 142.7 ± 8.21 | 78.50 ± 5.73 | 19.63 ± 4.11 |
| T7 | 149.7 ± 7.11 | 67.90 ± 6.32 | 23.17 ± 4.96 |
| T8 | 163.8 ± 11.89 | 172.4 ± 9.63 | 21.75 ± 4.87 |
| T9 | 159.7 ± 10.46 | 179.2 ± 10.45 | 24.89 ± 3.28 |
| T10 | 168.9 ± 8.26 | 183.7 ± 9.58 | 17.56 ± 3.56 |
| T11 | 142.9 ± 7.63 | 211.5 ± 13.44 | 26.97 ± 2.31 |
| Mean | 132.0 | 150.6 | 18.30 |
| HSD0.05 | 4.170 | 11.62 | 3.151 |
| Fertilisation | DEH | CAT | AlP | AcP | BG | PRO |
|---|---|---|---|---|---|---|
| T0 * | 0.271 ± 0.08 | 0.063 ± 0.01 | 0.200 ± 0.09 | 0.649 ± 0.05 | 0.529 ± 0.08 | 16.23 ± 1.23 |
| T1 | 0.289 ± 0.09 | 0.089 ± 0.02 | 0.237 ± 0.07 | 0.709 ± 0.07 | 0.612 ± 0.07 | 37.57 ± 2.35 |
| T2 | 0.489 ± 0.08 | 0.265 ± 0.09 | 0.351 ± 0.07 | 0.868 ± 0.01 | 1.351 ± 0.11 | 22.43 ± 1.98 |
| T3 | 0.358 ± 0.04 | 0.183 ± 0.08 | 0.362 ± 0.08 | 0.875 ± 0.10 | 1.107 ± 0.09 | 18.56 ± 1.23 |
| T4 | 0.471 ± 0.04 | 0.251 ± 0.08 | 0.291 ± 0.06 | 0.826 ± 0.05 | 1.309 ± 0.12 | 19.65 ± 1.58 |
| T5 | 0.321 ± 0.05 | 0.138 ± 0.07 | 0.252 ± 0.07 | 0.753 ± 0.07 | 0.531 ± 0.11 | 15.22 ± 1.66 |
| T6 | 0.334 ± 0.04 | 0.169 ± 0.06 | 0.286 ± 0.09 | 0.806 ± 0.03 | 1.122 ± 0.10 | 19.56 ± 2.08 |
| T7 | 0.317 ± 0.03 | 0.156 ± 0.05 | 0.267 ± 0.11 | 0.767 ± 0.08 | 0.961 ± 0.09 | 17.72 ± 1.83 |
| T8 | 0.365 ± 0.05 | 0.198 ± 0.06 | 0.309 ± 0.11 | 0.861 ± 0.08 | 1.128 ± 0.11 | 20.08 ± 1.98 |
| T9 | 0.343 ± 0.06 | 0.172 ± 0.06 | 0.271 ± 0.09 | 0.784 ± 0.12 | 1.139 ± 0.12 | 19.05 ± 1.76 |
| T10 | 0.361 ± 0.04 | 0.181 ± 0.07 | 0.648 ± 0.12 | 1.203 ± 0.11 | 1.186 ± 0.11 | 20.19 ± 2.08 |
| T11 | 0.328 ± 0.03 | 0.161 ± 0.05 | 0.499 ± 0.11 | 1.099 ± 0.09 | 0.984 ± 0.09 | 17.96 ± 1.81 |
| Mean | 0.354 | 0.169 | 0.331 | 0.850 | 0.997 | 20.35 |
| HSD0.05 | 0.022 | 0.015 | 0.044 | 0.029 | 0.131 | 2.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratowicz-Maciejewska, K.; Lemanowicz, J.; Jaskulska, I. Effects of Long-Term Multi-Treatment Experiments on Organic Matter and Enzymatic Activity in Sandy Soil. Sustainability 2025, 17, 3252. https://doi.org/10.3390/su17073252
Kondratowicz-Maciejewska K, Lemanowicz J, Jaskulska I. Effects of Long-Term Multi-Treatment Experiments on Organic Matter and Enzymatic Activity in Sandy Soil. Sustainability. 2025; 17(7):3252. https://doi.org/10.3390/su17073252
Chicago/Turabian StyleKondratowicz-Maciejewska, Krystyna, Joanna Lemanowicz, and Iwona Jaskulska. 2025. "Effects of Long-Term Multi-Treatment Experiments on Organic Matter and Enzymatic Activity in Sandy Soil" Sustainability 17, no. 7: 3252. https://doi.org/10.3390/su17073252
APA StyleKondratowicz-Maciejewska, K., Lemanowicz, J., & Jaskulska, I. (2025). Effects of Long-Term Multi-Treatment Experiments on Organic Matter and Enzymatic Activity in Sandy Soil. Sustainability, 17(7), 3252. https://doi.org/10.3390/su17073252







