1. Introduction
The compound 1,3-butadiene is an essential building block in the production of synthetic rubbers and plastics such as styrene–butadiene rubber, polybutadiene rubber, and latex. It is primarily obtained as a by-product of steam cracking processes, yet its separation from C
4 hydrocarbon mixtures presents considerable technical challenges due to the similar boiling points of the components within these mixtures. Conventional distillation methods require an impractically high number of trays and elevated reflux ratios, making the separation process unfeasible through conventional distillation alone [
1]. Extractive distillation has emerged as the primary method for effectively separating 1,3-butadiene, capitalizing on the selective solubility and enhanced relative volatility provided by the addition of appropriate solvents to facilitate the separation process.
Historically, N,N-dimethylformamide (DMF) and N-methyl-2-pyrrolidone (NMP) have been the most widely used solvents in 1,3-butadiene extractive distillation. As aprotic dipolar solvents, they are particularly effective for this process due to their strong dipole moments and ability to induce favorable solvation interactions [
2]. While 1,3-butadiene is a non-polar molecule, its conjugated diene structure makes it more polarizable than simple alkanes. The key mechanism at play is dipole-induced dipole interactions, where the high dipole moment of these solvents induces a temporary dipole in 1,3-butadiene, leading to enhanced solubility.
Each of these solvents offers distinct advantages and limitations [
2,
3,
4,
5,
6]. However, despite their performance advantages, these solvents are associated with severe health and environmental risks. Prolonged exposure to DMF and NMP has been linked to adverse health effects, including skin irritation, liver toxicity, and increased cancer risks. Furthermore, these solvents may bioaccumulate in ecosystems, posing a long-term threat to biodiversity and environmental stability. Given these concerns, there is a pressing need to transition towards greener and safer alternatives to ensure sustainable industrial processes and responsible resource use [
7,
8].
The economic, environmental, and health concerns linked to the conventional solvents used in extractive distillation have fueled increasing interest in simulation-based approaches to evaluate and enhance process efficiency. Simulation software, such as Aspen Plus
®, provides a comprehensive platform for modeling extractive distillation operations, allowing for the integration of mass and energy balances, phase equilibrium relationships, and complex distillation configurations to systematically assess the feasibility of alternative solvents. Rigorous thermodynamic models, including the Non-Random Two-Liquid (NRTL) model in combination with the Redlich–Kwong equation of state, are frequently utilized to account for the non-ideal behavior of multi-component mixtures. These simulations not only enable the accurate forecasting of process performance but also assist in optimizing distillation column design, improving overall efficiency and reducing energy consumption. Several studies have employed computer-aided simulations to assess the effectiveness of conventional solvents like DMF and NMP in the extractive distillation of 1,3-butadiene from crude C
4 mixtures [
4,
9,
10].
Given the health, safety, and environmental concerns associated with solvent emissions and losses, the transition towards green solvents has gained significant attention within the field of chemical engineering. Since the 1990s, green chemistry principles have emphasized the substitution of toxic solvents with non-toxic, renewable, and energy-efficient alternatives. The pursuit of greener solvents is closely linked to the broader goal of reducing hazardous chemical exposure while ensuring high-performance industrial operations. The use of green solvents aligns with the UN’s Sustainable Development Goals (SDGs). By adopting green solvents, industries support sustainable industrial development (SDG 9) and pollution reduction (SDG 13), thus contributing to a healthier environment (SDG 3), reduced chemical pollution (SDG 6), and responsible resource use (SDG 12). As regulatory restrictions tighten and sustainability efforts intensify, the search for innovative solvent solutions remains a fundamental challenge for the chemical industry.
Our previous work has highlighted 1,2-propylene carbonate—commonly referred to as propylene carbonate (PC)—as a promising green alternative for 1,3-butadiene extraction, presenting a viable substitute for traditional hazardous solvents [
11]. Like DMF and NMP, PC is an aprotic dipolar solvent. Evaluations by Bello Forero et al. (2016) and the GSK Solvent Selection Guide have recognized propylene carbonate as a highly suitable and sustainable option with significant green chemistry benefits, positioning it as an ideal candidate for advancing environmentally friendly practices [
12,
13,
14]. PC has been widely used in various industries, including electronics, pharmaceuticals, cosmetics, and carbon capture technologies, but its use in extractive distillation remains unexplored.
Although PC demonstrates slightly lower selectivity towards 1,3-butadiene (1.30 compared to 1.32 for DMF), it offers an effective balance between selectivity, cost-efficiency, and industrial feasibility. Furthermore, its environmentally friendly characteristics and improved health and safety profile align well with existing regulatory policies, including REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals), in the European Union. Additionally, CEFIC (European Chemical Industry Council) advocates for the development and adoption of safer and more sustainable chemical alternatives, further reinforcing the significance of exploring PC as a viable solvent for industrial applications.
This study aims to bridge the gap between conceptual green solvent selection and practical industrial implementation by assessing the feasibility of integrating propylene carbonate (PC) into an existing 1,3-butadiene plant with minimal process modifications. PC has not been extensively explored in this context, unlike more commonly studied solvents such as DMF and NMP; thus, this work offers new insights into its performance and potential for use in industrial-scale applications. To achieve this, a comparative analysis of using PC and DMF is conducted using Aspen Plus® (v12.1) to assess both the operational and economic implications of transitioning to a more sustainable solvent. Additionally, this work examines potential enhancements in energy efficiency, process simplification, and overall sustainability benefits, aligning with the principles of green chemistry—specifically, principles 3, 5, and 6. The findings contribute to the broader initiative of developing sustainable chemical processes while ensuring industrial efficiency, without compromising performance.
1,3-Butadiene Process Overview
The 1,3-butadiene extraction process is a multi-stage procedure that consists of several interconnected distillation sections, including two extractive distillation columns, a conventional distillation unit, and a solvent purification section (see
Figure 1). These units work synergistically to achieve efficient 1,3-butadiene separation while facilitating solvent recovery and reuse. Process optimization techniques, such as heat integration strategies, further enhance the sustainability of the operation by minimizing total energy consumption. For instance, the hot solvent stream from the bottom of the stripping columns (T2 and T5) is redirected to a heat recovery circuit, where it is utilized to preheat subsequent distillation column streams, reducing external energy input requirements.
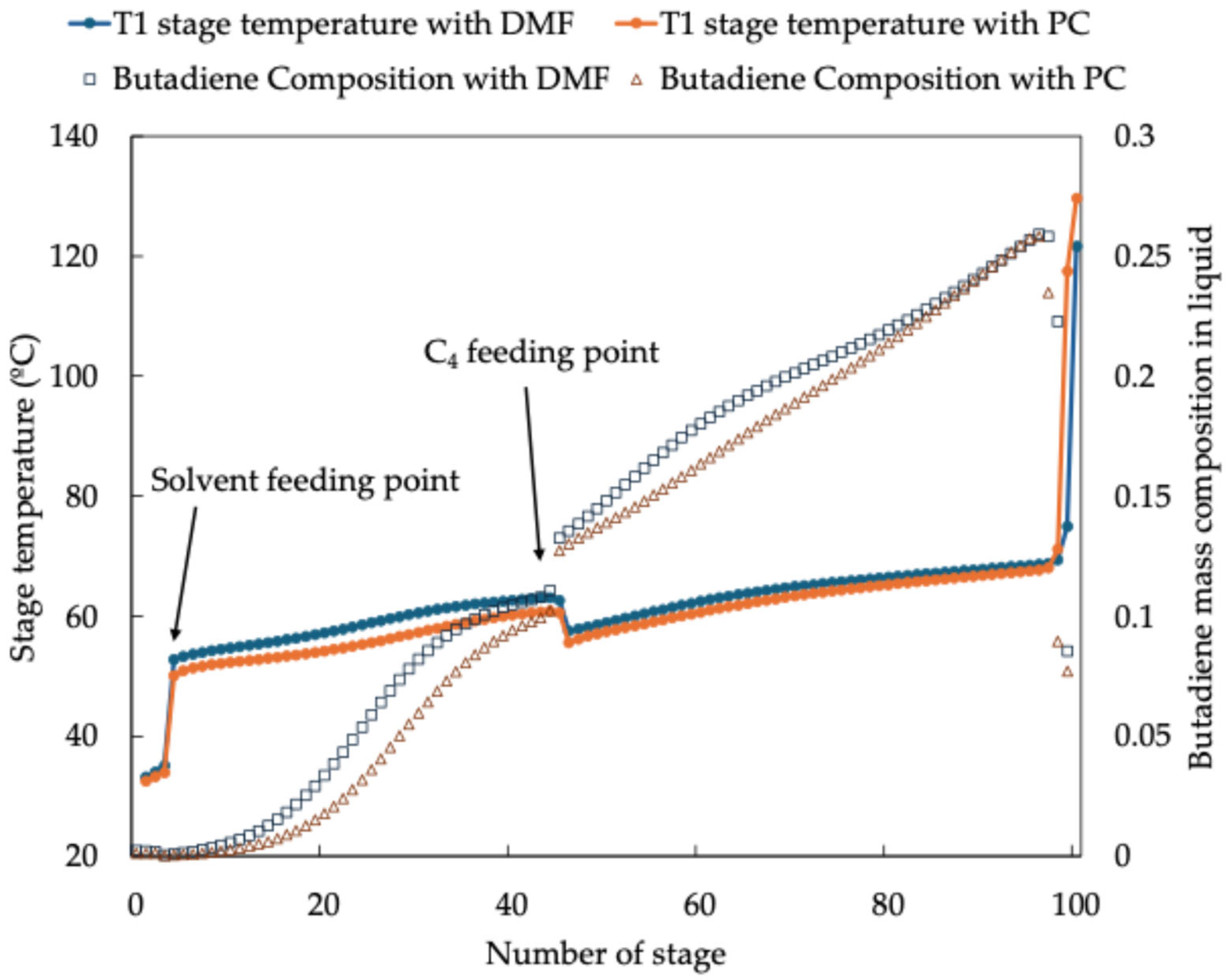
In the first extractive distillation unit (T1), the C4 feed stream is introduced into the primary distillation column, where lighter hydrocarbons with higher relative volatility, such as 1-butene, are removed as a raffinate stream. The bottom stream, enriched with 1,3-butadiene and other less volatile hydrocarbons, is directed towards a stripping unit (T2) for further purification. The stripping process effectively separates hydrocarbons and impurities from the solvent, producing an intermediate stream that undergoes additional refinement in a second extractive distillation column (T3). In this step, residual low-volatility impurities are eliminated, yielding high-purity 1,3-butadiene as the final overhead product.
The conventional distillation section serves to refine valuable by-products such as methylacetylene (propyne) and cis-2-butene. Additives, such as tert-butyl catechol (known as TBC) and silicone-based inhibitors, are commonly employed to suppress 1,3-butadiene polymerization during the distillation process. However, the complex polymerization mechanisms of butadienes (1,2-butadiene and 1,3-butadiene), including their potential for “popcorn” polymerization under specific reaction conditions, present operational challenges that must be managed carefully. Yet, this phenomenon did not seem to be essential for the execution of this work. Future research should consider incorporating polymerization kinetics into simulation studies to develop more comprehensive process models.
The final stage of the process involves solvent purification, where a portion of the second stripper’s (T5) bottom stream is directed to a dedicated distillation column (T8) for solvent purification. The implementation of an efficient solvent regeneration system is critical for minimizing solvent losses, reducing operational costs, and enhancing the overall sustainability of the process.
Ensuring the accuracy of a simulation that aims to closely replicate real plant operation requires not only precise input data but also a robust thermodynamic framework capable of reliably predicting phase behavior under industrial conditions. A key aspect of this modeling process is the accurate representation of the phase equilibrium, which dictates the distribution of components between phases and directly influences separation efficiency. To obtain reliable results, selecting an appropriate thermodynamic model and accurately determining binary interaction parameters are essential. In extractive distillation, where a solvent is introduced to enhance separation, the most critical interactions to characterize are those between the components of the mixture and the solvent. However, due to the scarcity of experimental data and literature-reported interaction parameters for certain key component pairs, new vapor-liquid equilibrium (VLE) data may have to be generated using predictive methods, such as the Conductor-like Screening Model for Real Solvents (COSMO-RS)—a quantum-chemistry-based approach—that was used in this study. This methodology improves the predictive accuracy of the simulation by generating equilibrium data through COSMO-RS, which may then be used to calculate the missing interaction parameters and better capture the non-ideal behavior of the system.
2. Materials and Methods
A steady-state model was developed in the Aspen Plus
® framework for both DMF and PC solvents. The initial step involved modeling the DMF-based process using real data from an operational plant. The feed specifications and operating conditions utilized in the simulations were sourced directly from the plant to ensure the highest possible accuracy and alignment between the simulated results and actual industrial performance. Typically, the 1,3-butadiene content in C
4 mixtures received from naphtha crackers, as was the case in this plant, is between 40–55 wt%. The solvent used for extraction was 97.0 wt% pure and contains 3.0 wt% of TAR (simulated as n-eicosane, C
20H
42) and dimer (4-vinylcyclohexene).
Table 1 shows a typical composition of the hydrocarbon feed stream.
When experimental data for specific molecular pair interactions are unavailable, or when dealing with emerging compounds such as PC, for which limited studies have been published, estimating missing interaction parameters becomes necessary. Unlike UNIFAC, one of the most widely used thermodynamic models for predicting phase equilibria, which requires predefined binary interaction parameters for every molecular substructure, COSMO-RS provides a more flexible and predictive approach. COSMO-RS can estimate missing interaction parameters and predict phase behavior across a broader range of compounds, including those containing novel functional groups or lacking sufficient experimental thermodynamic data [
15,
16]. Moreover, UNIFAC has the drawback of not discriminating between isomers, such as 1,3-butadiene and 1,2-butadiene—a crucial aspect in this analysis [
16].
In this study, both TURBOMOLE software (version 23.0.0 from BIOVIA TmoleX 2023, Vélizy-Villacoublay, France) and the COSMOtherm model (version 23.0.0 from BIOVIA COSMOtherm 2023) were employed within the COSMO-RS framework to model and predict molecular structures. The molecular structures were first geometrically optimized to generate the COSMO files, which were subsequently processed within the COSMOtherm package to obtain the equilibrium data and thermodynamic properties essential for an accurate simulation.
In addition to determining binary interaction parameters, understanding how components behave at different concentrations is fundamental for designing an efficient separation process. One of the most important thermodynamic properties influencing phase equilibrium calculations is the activity coefficient, which quantifies how much a liquid mixture deviates from ideal behavior. Particularly in extractive distillation, where a solvent is used to modify relative volatilities (
αij), accurate activity coefficient predictions are crucial for determining the feasibility and efficiency of the process, as can be seen in Equation (1):
where
i and
j are the key components (i.e., the components that define the desired separation),
y and
x are the vapor and liquid molar fractions, respectively, and
is the activity coefficient of the liquid phase.
The NRTL (Non-Random Two-Liquid) model is widely used to describe such non-ideal systems, as it is a local composition model that accounts for molecular interactions through binary interaction parameters. This model is particularly effective in representing the phase behavior of polar and strongly interacting compounds, making it well-suited for the extractive distillation of 1,3-butadiene. Given the system dependency of thermodynamic models, as highlighted in previous studies, selecting an appropriate model is critical for reliable simulations. In this study, the NRTL model integrated with the Redlich–Kwong (RK) equation of state was chosen to enhance the predictive accuracy of phase equilibrium calculations, by also accounting for non-idealities in the vapor phase.
With the newly predicted VLE data from COSMO-RS, it was then possible to use the NRTL equation to compute the missing binary interaction parameter,
bij, which is the key factor in describing the non-ideality of the liquid phase. In most cases, though not universally, the highest value of the activity coefficient is observed at infinite dilution, which represents the limiting behavior as the solute concentration approaches zero. As such, the infinite dilution activity coefficient (
) may serve as a useful parameter for assessing the effectiveness of a solvent in separation processes [
17]. Given its significance in describing the thermodynamic behavior of solutions,
is widely recognized as a critical factor in evaluating separation efficiency and solvent suitability [
18,
19]. To this end, the binary interaction parameters were calculated using the values of the activity coefficients at infinite dilution predicted by the COSMO-RS model. For a binary liquid mixture of components
i and
j, the NRTL equation at infinity dilution is given by:
where
The non-randomness parameter
α in the NRTL model was set to 0.3 for this study, which falls within the commonly used range of 0.2 to 0.47 for many binary mixtures, particularly for hydrocarbon systems. This value was selected based on established practices used in the modeling of similar hydrocarbon mixtures [
20]. The binary energetic terms
and
depend on the temperature (
T). In this study, the temperature dependence (Equations (4) and (5)) was correlated, setting
aij as equal to zero and obtaining the values of
bij. Indeed, by using activity coefficients at infinite dilution, we can determine only two parameters (
bij and
bji), while all other parameters (
aij,
aji, and
α) must be assigned fixed values.
The new binary interaction parameters (see
Table 2) were then implemented into the Aspen Plus
® properties framework. For certain component pairs, empirical values were already available from literature databases (see
Table 2), providing a well-established basis for thermodynamic modeling. These pre-existing parameters were incorporated into the simulation to maintain higher consistency with real data.
The next step was to assess the separation efficiency within the distillation columns. A crucial aspect of this evaluation involved determining the theoretical number of trays required to achieve the desired separation performance. The theoretical number of trays was determined by initially considering the actual number of trays in the distillation columns and systematically reducing them until significant differences in composition and temperature were observed between adjacent stages. This iterative approach ensured that only effective stages, which contribute meaningfully to the separation process, were included in the final design. If a stage exhibited negligible differences in temperature and composition when compared to adjacent stages, it was deemed ineffective contributing little to the separation process. Such stages were excluded from the final design as they added unnecessary complexity to the simulation. This approach is particularly critical in complex systems, such as the one studied here, which involves multiple recycles and interconnected equipment (see
Figure 1). Reducing the number of ineffective stages helps to streamline the simulation, improving computational efficiency and model convergence. Moreover, removing non-contributing stages minimizes the potential for numerical instability, which is often a concern in simulations with extensive recycle loops and highly coupled systems. By ensuring that only effective stages were included, the simulation more accurately represented the operational behavior of the distillation columns, leading to more reliable results.
4. Conclusions
This study has demonstrated the feasibility of replacing N,N-dimethylformamide with propylene carbonate, which is a greener solvent for 1,3-butadiene extraction, leveraging advanced thermodynamic modeling and process simulations. The transition to PC aligns with REACH regulations and CEFIC sustainability recommendations, offering an environmentally responsible alternative while maintaining industrial performance. Thus, this work is an effective contribution to the development of more sustainable industrial processes.
The Aspen Plus® simulations demonstrated that PC attains a separation efficiency comparable to that of DMF with minimal modifications to the existing process. However, due to PC’s lower selectivity towards 1,3-butadiene, a higher solvent-to-feed ratio was necessary, resulting in a moderate increase in operational costs. However, the elimination of column T6, due to the insolubility of methylacetylene in PC, led to capital cost reductions of approximately 4.7%, enhancing the overall economic attractiveness of this solvent transition. Furthermore, a cost analysis revealed that PC has a 21.4% lower solvent procurement cost and 12.2% lower make up cost compared to DMF, reinforcing its economic viability, particularly when considering long-term operational expenses and potential cost optimizations through solvent recovery and recycling strategies.
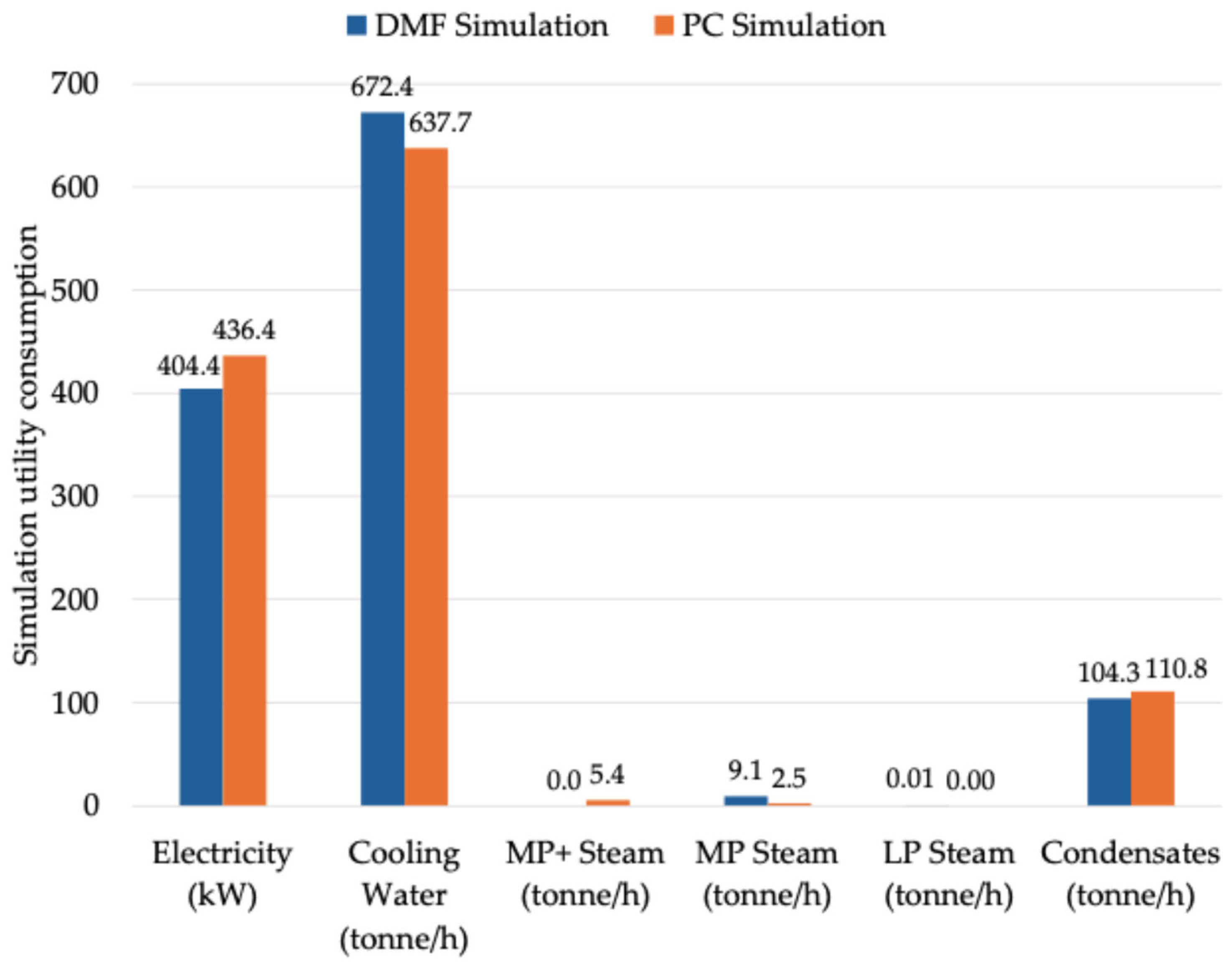
An analysis of energy consumption revealed that, while PC required 7.9% more electricity, overall heat consumption was reduced by 3.7%. Additionally, cost estimations from Aspen Plus® indicated that operating expenses were 2.7% higher for PC, suggesting that further optimization efforts could help bridge the cost gap. However, targeted process improvements, such as enhanced heat integration, optimized column configurations, and refined solvent recovery strategies, could reduce additional operational costs while maintaining the environmental benefits of PC.
Despite these differences, PC was found to be highly compatible with existing industrial configurations, indicating its strong potential as a sustainable alternative to conventional hazardous solvents. The elimination of hazardous solvent emissions, compliance with green chemistry principles, reductions in capital costs, and lower solvent acquisition costs reinforce the viability of PC in 1,3-butadiene extractive distillation, leading to a more sustainable process. However, the implementation of PC as a solvent in an existing plant requires adjustments due to its higher boiling point compared to DMF. Specifically, the use of MP vapor at higher pressures (18 bar g for PC compared to 12 bar g for DMF) will be necessary to achieve the desired operating conditions. Special attention should also be given to the increased temperatures, as some equipment in the existing plant may not be designed to withstand these higher conditions, requiring potential upgrades or modifications to ensure safe and efficient operation.
This study predominantly relies on simulation results, and while these provide valuable insights, further experimental work would offer a more robust validation of PC’s performance in 1,3-butadiene extractive distillation. Experimental data would allow for a more direct and accurate comparison between PC and DMF, improving the reliability of the performance evaluation and ensuring the practical feasibility of PC as a solvent in actual applications. We aim to pursue this experimental validation in future work to confirm our simulation findings and comprehensively assess the potential of PC in industrial settings.