Propagation of Hinoki Cypress (Chamaecyparis obtusa) Through Tissue Culture Technique as a Sustainable Method for Mass Cloning of Selected Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Surface Sterilization of Explants
2.2. Adventitious Bud Induction
2.3. Shoot Elongation from Induced Adventitious Buds
2.4. Rooting of Elongated Shoots
2.5. In Vitro Growth and Ex Vitro Acclimatization of Regenerated Plants
2.6. Statistical Analyses
3. Results
3.1. Surface Sterilization of Explants for In Vitro Culture Initiation
3.2. Effect of PGRs on Adventitious Bud Induction and Response from Different Clones
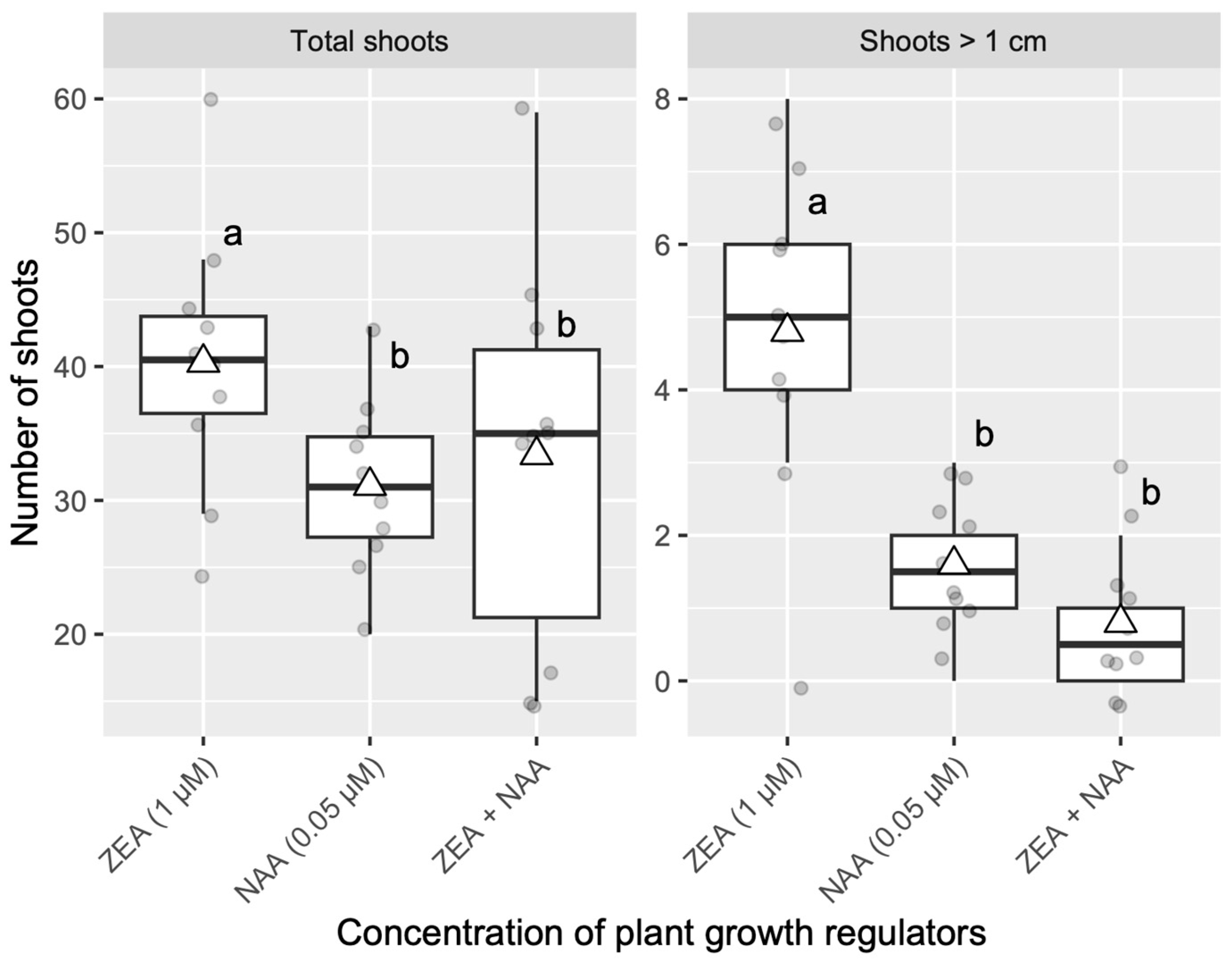
3.3. Effect of PGRs on Shoot Elongation from Adventitious Buds
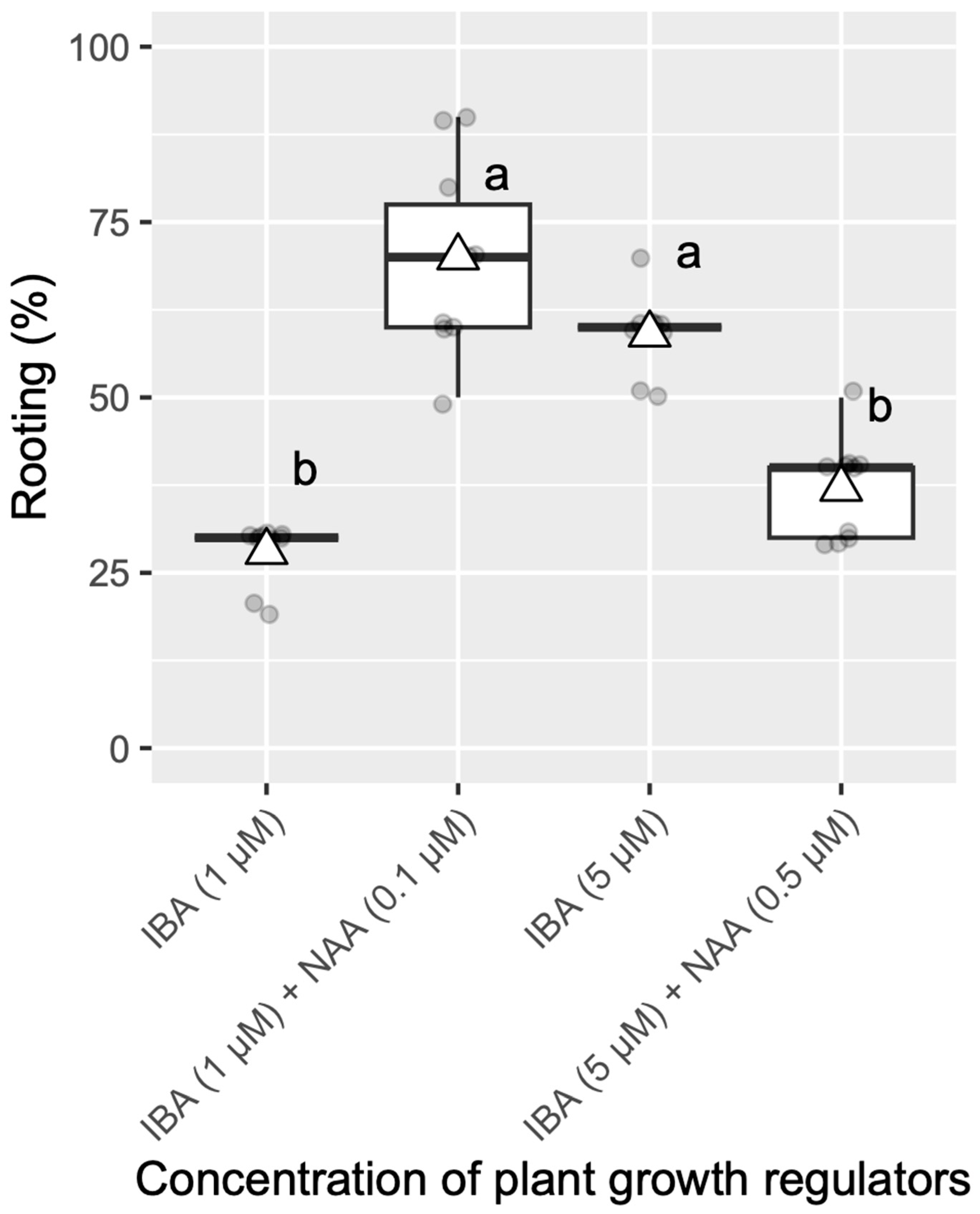
3.4. Effect of PGRs on Rooting and Response from Different Clones
3.5. In Vitro Growth and Ex Vitro Acclimatization of Regenerated Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Ruhsam, M.; Milne, R.; Graham, S.W.; Li, J.; Tao, T.; Zhang, Y.; Mao, K. Incomplete lineage sorting and local extinction shaped the complex evolutionary history of the Paleogene relict conifer genus, Chamaecyparis (Cupressaceae). Molec. Phylogen. Evolut. 2022, 172, 107485. [Google Scholar]
- Forestry Agency (Ed.) Statistical Directory of Forest and Forestry of Japan 2024 (Shinrin Ringyo Tokei Yoran); Nihon Shinrin Ringyo Shinkokai: Tokyo, Japan, 2024; pp. 1–261. (In Japanese) [Google Scholar]
- Ishi, K. Hinoki cypress (Chamaecyparis obtusa). In Biotechnology in Agriculture and Forestry, Trees III; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 16, pp. 471–478. [Google Scholar]
- Asakawa, S.; Katsuta, M.; Yokoyama, T. Seeds of Woody Plants in Japanese Gymnospermae; Japan Forest Tree Breeding Association: Tokyo, Japan, 1981; 150p. (In Japanese) [Google Scholar]
- Kobayashi, K. Cone and seed insects of Japanese conifers. In Proceedings of the 17th IUFRO World Congress, Kyoto, Japan, 6–17 September 1981; Div. 2. pp. 455–461. [Google Scholar]
- Maeta, T. Effects of gamma-rays irradiation on interspecific hybridization between Chamaecyparis obtusa S. et Z. and C. pisifera S. et Z. Hoshasen Ikussyujo Kenkkyu Hokoku 1982, 5, 1–87. (In Japanese) [Google Scholar]
- Ishii, K.; Maruyama, E.; Hosoi, Y. Plant regeneration by somatic embryogenesis from in vitro-cultured shoots of hinoki cypress (Chamaecyparis obtusa Sieb. et Zucc.). Propag. Ornam. Plants. 2003, 3, 19–22. [Google Scholar]
- Osada, T.; Okano, M. Japanese cedar and cypress pollinosis updated: New allergens, cross-reactivity, and treatment. Allergol. Int. 2021, 70, 281–290. [Google Scholar] [PubMed]
- Yamamoto, C.; Fukuhara, N. Cone and seed yields after open-, self-, intraspecific-, and interspecific-pollinations in Chamaecyparis obtusa (Sieb. et Zucc.) Endl. and C. pisifera (Sieb. et Zucc.). Endl. Bull. For. Forest Prod. Res. Inst. 1980, 311, 65–92. [Google Scholar]
- Maeta, T.; Yamamoto, C. Interspecific hybridization among Chamaecyparis species. In Proceedings of the 17th IUFRO World Congress, Kyoto, Japan, 6–17 September 1981; Div. 2. pp. 169–176. [Google Scholar]
- Ohguro, T.; Okamura, M. Morphological and cytological studies on hybridization among Chamaecyparis obtusa Sieb. et Zucc., C pisifera Sieb. et Zucc., and C. lawsoniana Parl. Bull. For. Tree. Breed. Inst. 1997, 5, 59–87. (In Japanese) [Google Scholar]
- Fukuhara, N. Fertility in interspecific-crossing between hinoki (Chamaecyparis obtusa Endl.) and sawara (C. pisifera Endl.) and the identification of the hybrids. Bull. For. Prod. Res. Inst. 1989, 354, 1–38. [Google Scholar]
- Fukuhara, N. Meiotic observation in the pollen mother cell of interspecific hybrid between Chamaecyparis obtusa and C. pisifera. J. Jpn. For. Soc. 1978, 60, 437–441. [Google Scholar]
- Taniguchi, T.; Kurita, M.; Itahana, N.; Kondo, T. Somatic embryogenesis and plant regeneration from immature zygotic embryos of hinoki cypress (Chamaecyparis obtusa Sieb. et Zucc.). Plant Cell Rep. 2004, 23, 26–31. [Google Scholar] [CrossRef]
- Maruyama, E.; Ishii, K.; Hosoi, Y. Efficient plant regeneration of hinoki cypress (Chamaecyparis obtusa Sieb. et Zucc.) via somatic embryogenesis. J. For. Res. 2005, 10, 73–77. [Google Scholar] [CrossRef]
- Ishii, K. In vitro plantlet formation from adventitious buds on juvenile seedlings of hinoki cypress (Chamaecyparis obtusa). Plant Cell Tiss. Org. Cult. 1986, 7, 247–255. [Google Scholar] [CrossRef]
- Okamura, M.; Senda, M.; Kondo, T. Tissue culture of hinoki (Chamaecyparis obtusa). Bull. National For. Tree. Breed. Center 1995, 13, 119–129. [Google Scholar]
- Ishii, K. Liquid culture and transformation of hinoki cypress (Chamaecyparis obtusa Sieb. et Zucc.). J. For. Res. 2002, 7, 99–104. [Google Scholar] [CrossRef]
- Min, J.Y.; Park, D.J.; Jeong, M.J.; Song, H.J.; Kim, Y.D.; Kang, Y.M.; Karigar, C.S.; Choi, M.S. In vitro propagation of Chamaecyparis obtusa Sieb. et Zucc. Prop. Ornam. Plants 2010, 10, 117–121. [Google Scholar]
- Kim, J.A.; Lee, N.-N.; Kim, Y.W. In vitro plantlets regeneration by multi-shoots induction and rooting in Chamaecyparis obtusa. J. Plant Biotechnol. 2019, 46, 303–309. [Google Scholar] [CrossRef]
- Ide, Y.; Yamamoto, S. Adventitious root formation on in vitro microcuttings of hinoki. J. Jpn. For. Soc. 1986, 68, 296–298. [Google Scholar]
- Okamura, M.; Senda, M.; Kondo, T. Rooting from the encapsulated adventitious buds of hinoki, Chamaecyparis obtusa. J. Jpn. For. Soc. 1994, 76, 601–603. [Google Scholar]
- Ishii, K.; Yoshioka, H.; Takijiri, F. Regeneration of hinoki cypress by tissue culture from adult tree. Trans. 100th Mtg. Jpn. For. Soc. 1989, 523–524. (In Japanese) [Google Scholar]
- Hosoi, Y.; Maruyama, E.T. Multiple buds formation and plant regeneration from leaflets of Chamaecyparis obtusa and multiple buds formation from leaflets of Chamaecyparis pisifera. Kanto Shinrin Kenkyu 2018, 69, 7–10. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryogenesis in sawara cypress (Chamaecyparis pisifera Sieb. et Zucc.) for stable and efficient plant regeneration, propagation and protoplast culture. J. For. Res. 2002, 7, 23–34. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Maruyama, E.T.; Tsuruta, M.; Katsuki, T. Tissue culture response and in vitro plant regeneration of ‘Haruka’ (Cerasus Sato-zakura Group ‘Haruka’), a new cultivar of Japanese flowering cherry. In Vitro Cell. Dev. Biol.-Plant 2024, 60, 183–193. [Google Scholar]
- Bonga, J.M.; von Aderkas, P. (Eds.) In Vitro Culture of Trees; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 55–71. [Google Scholar]
- Szendrák, E.; Read, P.E.; Yang, G. Prevention and elimination of contamination for in vitro culture of several woody species. In Pathogen and Microbial Contamination Management in Micropropagation; Cassells, A.C., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 233–236. [Google Scholar]
- Kurz, M.L.; Webb, D.T.; Vidaver, W.E. Micropropagation of yellow cedar (Chamaecyparis nootkatensis). Plant Cell Tiss. Org. Cult. 1989, 18, 297–312. [Google Scholar]
- Yokoyama, T.; Arai, M.; Takeuchi, M. Induction and subculture of bud forming tissues from twig sections of Chamaecyparis pisifera var. filifera. Plant Tiss. Cult. Letters 1988, 6, 63–67. [Google Scholar]
- Spanos, K.A.; Pirrie, A.; Woodward, S. Micropropagation of Cupressus sempervirens L. and Chamaecyparis lawsoniana (A. Murr.) Par. Silvae Genet. 1997, 46, 291–295. [Google Scholar]
- Khamushi, M.; Dehestani, M.; Zarei, A.; Kamali, K. An efficient protocol for micropropagation of old cypress of Abarkuh (Cupressus sempervirens var. horizontalis [Mill.]) under in vitro conditions. Plant Cell Tiss. Org. Cult. 2019, 138, 597–601. [Google Scholar]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryo culture for propagation, artificial seed production, and conservation of sawara cypress (Chamaecyparis pisifera Sieb. et Zucc.). J. For. Res. 2003, 8, 1–8. [Google Scholar]
- Misson, J.P.; de Cannière, C.; Andre, P. Western red cedar (Thuja plicata D. Don ex Lambert). In Biotechnology in Agriculture and Forestry, Trees III; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 16, pp. 479–490. [Google Scholar]
- Nour, K.A.; Thorpe, T. In vitro shoot multiplication of easter white cedar (Thuja occidentalis). In Vitro Cell. Dev. Biol.-Plant 1993, 29, 65–71. [Google Scholar]
- Gaspar, T.; Revers, C.; Debergh, P.; Maene, L.; Paques, M.; Boxus, P. Vitrification: Morphological and ecological aspects. In Cell and Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1987; Volume 1, pp. 152–166. [Google Scholar]
- Ziv, M. Vitrification: Morphological and physiological disorders of in vitro plants. In Micropropagation, Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 45–69. [Google Scholar]
- Capuana, M.; Giannini, R. Micropropagation of young and adult plants of cypress (Cupressus sempervirens L.). J. Hortic. Sci. 1997, 72, 453–460. [Google Scholar] [CrossRef]
- Giovanelli, A.; de Carlo, A. Micropropagation of Mediterranean cypress (Cupressus sempervirens L.). In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 93–105. [Google Scholar]
- George, E.F.; Hall, M.A.; de Klerk, G.-J. (Eds.) Plant Propagation by Tissue Culture 3rd Edition; Springer: Dordrecht, The Netherlands, 2008; pp. 355–401. [Google Scholar]
- Maruyama, T.E.; Hosoi, Y. Progress in somatic embryogenesis of Japanese pines. Front. Plant Sci. 2019, 10, 31. [Google Scholar]
- FFPRI (Forestry and Forest Products Research Institute); Department of Forest Molecular Genetics and Biotechnology (Eds.) Manual for the Propagation of Pollen-Free Sugi (Cryptomeria japonica) via Somatic Embryogenesis; Asahi Publishers: Ibaraki, Japan, 2024; Version 1.2; pp. 11–15. (In Japanese) [Google Scholar]
- Barberini, S.; Danti, R.; Lambardi, M. Somatic plant regeneration from selected common cypress (Cupressus sempervirens L.) clones resistant to the bark canker disease. Plant Cell Tiss. Organ Cult. 2016, 124, 393–403. [Google Scholar]




| CLONE CODE | Clone Name | Number of Tested Explants | Number of Aseptic Explants | Percentage of Aseptic Explants | Number of Non-Aseptic Explants | Percentage of Non-Aseptic Explants |
|---|---|---|---|---|---|---|
| Na14-14 | ‘Nangouhi 14-14’ | 68 | 68 | 100.00 | 0 | 0.00 |
| Na18 | ‘Nangouhi 18’ | 124 | 116 | 93.55 | 8 | 6.45 |
| NaS | ‘Nangouhi S’ | 112 | 112 | 100.00 | 0 | 0.00 |
| Isa | ‘Kenisahaya 1’ | 80 | 58 | 72.50 | 22 | 27.50 |
| Na14 x Isa | ‘Nangouhi 14’ x ‘Kenisahaya 1’ | 112 | 104 | 92.86 | 8 | 7.14 |
| Isa x Na14 | ‘Kenisahaya 1’ x ‘Nangouhi 14’ | 116 | 112 | 96.55 | 4 | 3.45 |
| SKZ3 | ‘‘ShizuokaKenZairai 3’ | 124 | 113 | 91.13 | 11 | 8.87 |
| SKZ5 | ‘ShizuokaKenZairai 5’ | 100 | 89 | 89.00 | 11 | 11.00 |
| SKZ6 | ‘ShizuokaKenZairai 6’ | 80 | 79 | 98.75 | 1 | 1.25 |
| SKZ8 | ‘ShizuokaKenZairai 8’ | 73 | 67 | 91.78 | 6 | 8.22 |
| No7-5 | ‘Nojiri 7-5’ | 85 | 60 | 70.59 | 25 | 29.41 |
| Total | 1074 | 978 | 91.10 | 96 | 8.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, T.E.; Tsuruta, M.; Matsumoto, A.; Kusano, R.; Hakamata, T. Propagation of Hinoki Cypress (Chamaecyparis obtusa) Through Tissue Culture Technique as a Sustainable Method for Mass Cloning of Selected Trees. Sustainability 2025, 17, 3039. https://doi.org/10.3390/su17073039
Maruyama TE, Tsuruta M, Matsumoto A, Kusano R, Hakamata T. Propagation of Hinoki Cypress (Chamaecyparis obtusa) Through Tissue Culture Technique as a Sustainable Method for Mass Cloning of Selected Trees. Sustainability. 2025; 17(7):3039. https://doi.org/10.3390/su17073039
Chicago/Turabian StyleMaruyama, Tsuyoshi E., Momi Tsuruta, Asako Matsumoto, Ryouichi Kusano, and Tetsuji Hakamata. 2025. "Propagation of Hinoki Cypress (Chamaecyparis obtusa) Through Tissue Culture Technique as a Sustainable Method for Mass Cloning of Selected Trees" Sustainability 17, no. 7: 3039. https://doi.org/10.3390/su17073039
APA StyleMaruyama, T. E., Tsuruta, M., Matsumoto, A., Kusano, R., & Hakamata, T. (2025). Propagation of Hinoki Cypress (Chamaecyparis obtusa) Through Tissue Culture Technique as a Sustainable Method for Mass Cloning of Selected Trees. Sustainability, 17(7), 3039. https://doi.org/10.3390/su17073039







