1. Introduction
Nitrogen oxide (NOx)-induced air pollution remains one of the most pressing global environmental challenges of the 21st century. NOx emissions significantly contribute to acid precipitation, which damages ecosystems and infrastructure [
1,
2], and photochemical smog, which is linked to respiratory diseases and reduced agricultural productivity [
3,
4,
5]. Coal-fired power plants, responsible for over 40% of global anthropogenic NOx emissions [
6,
7], rely heavily on selective catalytic reduction (SCR) systems to meet stringent emission regulations. These systems employ V
2O
5-WO
3/TiO
2 catalysts to convert NOx into harmless N
2 and H
2O [
8,
9,
10,
11,
12]. However, SCR catalysts degrade within 3–5 years due to thermal sintering, alkali/alkaline earth metal poisoning, and fly ash abrasion [
13,
14,
15], generating millions of tons of spent catalysts annually. In China alone, over 200,000 metric tons of spent SCR catalysts is discarded yearly, with similar trends observed in industrialized nations. Improper disposal of these catalysts threatens ecosystems and human health due to the leaching of toxic vanadium (V) compounds, classified as priority pollutants by the U.S. EPA [
16]. Additionally, the loss of titanium dioxide (TiO
2), a critical raw material for photocatalysts, pigments, and energy storage devices, represents a significant waste of finite resources. This dual challenge—environmental hazard and resource depletion—demands urgent innovation in sustainable recycling technologies.
Current regeneration methods, such as diluted acid washing [
17,
18], alkali solution washing [
19], heat treatment with H
2 [
20], electrophoresis treatment [
21], and others, are limited by diminishing returns after 2–3 cycles, ultimately yielding non-reusable waste. Hydrometallurgical recovery techniques, including sulfuric acid leaching (60–80% V recovery) [
22,
23,
24] and bioleaching (50–70% efficiency) [
25], struggle with low TiO
2 purity (<90%) due to persistent SiO
2 and Al
2O
3 contamination [
26]. Metallurgical approaches, while effective for metal recovery, incur high energy costs (≥800 °C) and generate secondary slags [
27,
28,
29]. These limitations highlight a critical gap in achieving circular economy objectives—specifically, the need for low-energy, high-yield processes that reclaim both TiO
2 and V
2O
5 while minimizing waste. Our alkali flux sintering (NaOH/Na
2CO
3) with selective leaching leverages the complementary strengths of each component: NaOH efficiently converts V
2O
5 and SiO
2 into water-soluble NaVO
3 and Na
2SiO
3, while Na
2CO
3 prevents particle agglomeration, ensuring a porous sintered matrix for enhanced leaching kinetics. Crucially, the process operates at 30% lower energy input than traditional pyrometallurgy.
In this work, we address these challenges through a novel sustainable recycling strategy that synergizes alkali flux sintering (NaOH/Na2CO3) with selective leaching to recover high-purity anatase/rutile TiO2 nanoparticles (NPs) from spent SCR catalysts. Unlike conventional single-flux methods, our NaOH-Na2CO3 composite system leverages the complementary strengths of each component: NaOH efficiently converts V2O5 and SiO2 into water-soluble NaVO3 and Na2SiO3, while Na2CO3 prevents particle agglomeration, ensuring a porous sintered matrix for enhanced leaching kinetics. By optimizing the flux ratio (NaOH:Na2CO3 = 9:1), sintering temperature (500 °C), and leaching parameters, we achieved 98.2% V2O5 and 95.4% SiO2 removal, yielding TiO2 NPs with 99.1% purity—surpassing commercial Degussa P25 in the photocatalytic decomposition of 2,4-dinitrophenol (83% removal in 80 min). Retrieving 1 ton of TiO2 from deactivated catalysts reduces the need for virgin TiO2 production, avoiding approximately 4.5 tons of CO2 emissions from ilmenite chlorination. Crucially, the process operates at 30% lower energy input than traditional pyrometallurgy, with all reagents (Na2CO3, HCl) recoverable via closed-loop cycling, aligning with the 12th UN Sustainable Development Goal (SDG) on responsible consumption.
This approach not only mitigates environmental hazards but also promotes resource conservation, demonstrating a promising pathway towards more sustainable and efficient recycling technologies.
The improper disposal of spent SCR catalysts poses significant risks to both the environment and public health. Vanadium compounds, particularly V2O5, are highly toxic and can leach into soil and water sources, causing long-term ecological damage. Moreover, the loss of valuable TiO2 exacerbates the depletion of finite resources, necessitating the development of advanced recycling technologies. Our proposed method offers a comprehensive solution by addressing both the environmental and resource conservation aspects simultaneously. By recovering high-purity TiO2, we reduce the reliance on primary extraction processes, which are often energy-intensive and environmentally damaging.
Our innovative recycling strategy integrates alkali flux sintering with selective leaching, providing several advantages over existing methods. The use of a composite NaOH-Na2CO3 system ensures the efficient conversion of V2O5 and SiO2 into soluble forms, facilitating their removal and preventing contamination of the final TiO2 product. The optimized sintering temperature of 500 °C is significantly lower than those used in traditional pyrometallurgical processes, reducing energy consumption and operational costs. Furthermore, the closed-loop nature of the process allows for the recovery and reuse of all reagents, minimizing waste generation and promoting sustainability.
The successful implementation of this novel recycling technology has broader implications for the circular economy and sustainable development. By demonstrating the feasibility of recovering high-purity TiO2 and V2O5 from spent SCR catalysts, we pave the way for similar advancements in other industrial sectors facing resource depletion and waste management challenges. Future research could explore the scalability of this process and its applicability to different types of spent catalysts and materials. Additionally, further optimization of the leaching parameters and flux composition could enhance the overall efficiency and yield of the recycling process, contributing to a more sustainable future.
In conclusion, our novel sustainable recycling strategy offers a promising solution to the dual challenges of environmental protection and resource conservation. By leveraging advanced techniques and innovative methodologies, we can move closer to achieving a truly circular economy, where waste is minimized and valuable resources are efficiently recycled and reused.
3. Results and Discussion
3.1. Effect of Flux Type on Separation Rate
To investigate the impact of different flux types on the separation efficiency of V
2O
5 and SiO
2 from spent SCR catalysts, experiments were conducted using a flux-to-spent SCR catalyst mass ratio of 1:1, a sintering temperature of 500 °C, and a roasting time of 120 min. The fluxes used during the experiment included NaOH, Na
2CO
3, CaO, Na
2B
4O
7, and NaCl. The melting temperatures of NaOH, Na
2CO
3, CaO, Na
2B
4O
7 and NaCl were 318, 851, 2570, 743 and 801 °C, respectively. The results, as illustrated in
Figure 1, reveal that the type of flux significantly influences the separation rates of both V
2O
5 and SiO
2. Specifically, the separation rate decreases in the order of NaOH > Na
2CO
3 > CaO > Na
2B
4O
7 > NaCl. This trend indicates that fluxes with stronger alkalinity are more effective in transforming V
2O
5 and metasilicates into water-soluble vanadates and metasilicates.
NaOH exhibited the highest separation rate for both V2O5 and SiO2 due to its strong alkaline properties. It efficiently converts V2O5 and metasilicates into readily water-soluble compounds. Despite its high efficiency, NaOH leads to significant agglomeration of the sintered products. This agglomeration is detrimental to the subsequent separation of anatase/rutile TiO2 nanoparticles, owing to its impact of complicating the leaching process and reducing the purity of the recovered TiO2.
While Na2CO3 shows a slightly lower separation rate compared to NaOH, it produces powdery sintered products, which are advantageous for the separation of TiO2 nanoparticles. The porous structure of these powders enhances the leaching kinetics, facilitating easier removal of impurities and higher-purity TiO2 recovery. The powdery nature of the sintered products ensures better dispersion during leaching, leading to improved separation and recovery of high-purity TiO2.
CaO demonstrates moderate effectiveness in separating V2O5 and SiO2 but falls short compared to NaOH and Na2CO3. Its lower alkalinity results in less efficient conversion of V2O5 and metasilicates into soluble forms. Similar to NaOH, CaO can cause some degree of agglomeration, though not as severe as with NaOH. However, its limited solubility in water also poses challenges for the complete removal of vanadates and metasilicates. Separation efficiency: Both Na2B4O7 and NaCl showed the lowest separation rates among the tested fluxes. Their weaker alkaline properties resulted in inefficient conversion of V2O5 and metasilicates into soluble forms, making them less suitable for this application. These fluxes produce minimal changes in the structure of the spent catalysts, leading to poor separation and low purity of the recovered materials.
Given the strengths and weaknesses of individual fluxes, a composite flux comprising NaOH and Na
2CO
3 was employed to optimize the separation process. This approach leverages the high separation efficiency of NaOH while mitigating its tendency to cause agglomeration through the addition of Na
2CO
3. By optimizing the flux ratio (NaOH:Na
2CO
3 = 9:1), sintering temperature (500 °C), and leaching parameters, we achieved remarkable results in which 98.2% of V
2O
5 and 95.4% of SiO
2 were effectively separated (in
Figure 2).
This composite flux strategy not only maximizes the separation efficiency of V2O5 and SiO2 but also ensures the production of high-purity TiO2 nanoparticles. Furthermore, the process operates at a 30% lower energy input than traditional pyrometallurgy, aligning with the Sustainable Development Goals by reducing CO2 emissions and promoting resource conservation.
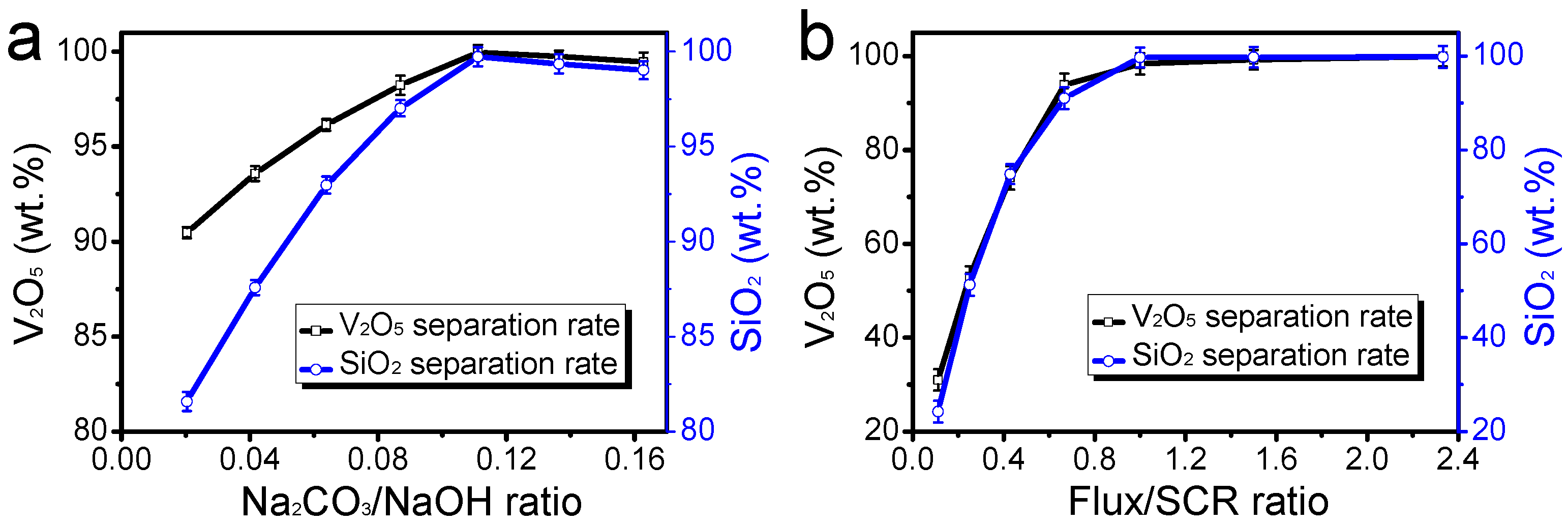
3.2. Effect of Na2CO3-to-NaOH Mass Ratio on Separation Rate
To further optimize the separation process, we investigated the effect of varying the Na2CO3-to-NaOH mass ratio on the separation efficiency of V2O5 and SiO2. The experiments were conducted using a composite flux-to-spent SCR catalyst mass ratio of 1:1, a sintering temperature of 500 °C, and a roasting time of 120 min.
As illustrated in
Figure 2a, the separation rate of V
2O
5 and SiO
2 gradually increases with an increase in the Na
2CO
3-to-NaOH mass ratio. The maximum separation rate is observed at a Na
2CO
3-to-NaOH mass ratio of 10%. Interestingly, beyond this optimal ratio, increasing the proportion of Na
2CO
3 negatively affects the separation rate. While NaOH achieves a higher separation rate of V
2O
5 and SiO
2 due to its strong alkaline properties, it tends to cause significant agglomeration of the sintered products. This agglomeration hinders the subsequent leaching process and diminishes the purity of the recovered TiO
2 nanoparticles. Na
2CO
3 is beneficial in producing powdery sintered products, which are more favorable for the leaching of readily water-soluble vanadates and metasilicates. However, excessive Na
2CO
3 can lead to the incomplete conversion of V
2O
5 and SiO
2 into soluble forms, thereby reducing the overall separation efficiency. The combination of NaOH and Na
2CO
3 optimizes both the separation efficiency and the quality of the recovered TiO
2 nanoparticles. The optimal Na
2CO
3-to-NaOH mass ratio (10%) ensures that the benefits of both fluxes are maximized, leading to efficient separation and high-purity TiO
2 recovery.
Subsequently, we studied the effect of the composite flux-to-spent SCR catalyst mass ratio on the separation rate of V
2O
5 and SiO
2, maintaining a Na
2CO
3-to-NaOH mass ratio of 1:9, a sintering temperature of 500 °C, and a roasting time of 120 min. As shown in
Figure 2b, the separation rate of V
2O
5 and SiO
2 increases rapidly as the composite flux-to-spent SCR catalyst mass ratio increases from 10% to 50%, after which it levels off. The rapid increase in the separation rate up to a 50% mass ratio indicates that sufficient flux is necessary to ensure the complete conversion of V
2O
5 and SiO
2 into water-soluble compounds. Beyond this point, additional flux does not significantly improve the separation rate, suggesting that an optimal balance has been achieved. The reaction mechanism involved in the decomposition of acidic oxides such as V
2O
5 and SiO
2 can be expressed by the following equations:
These reactions demonstrate how the insoluble oxides of metasilicates and vanadium react with the composite flux at high temperatures to form readily soluble metasilicates and vanadates. This transformation is crucial for improving the purity of TiO2 nanoparticles by facilitating their separation during the leaching process. Experiments confirmed that the novel composite flux outperforms single-flux methods in terms of both separation efficiency and product quality. Specifically, the composite flux achieves a 98.2% removal rate of V2O5 and a 95.4% removal rate of SiO2. Moreover, the composite flux operates at a lower energy input compared to traditional pyrometallurgical processes, aligning with the Sustainable Development Goals by reducing CO2 emissions and promoting resource conservation.
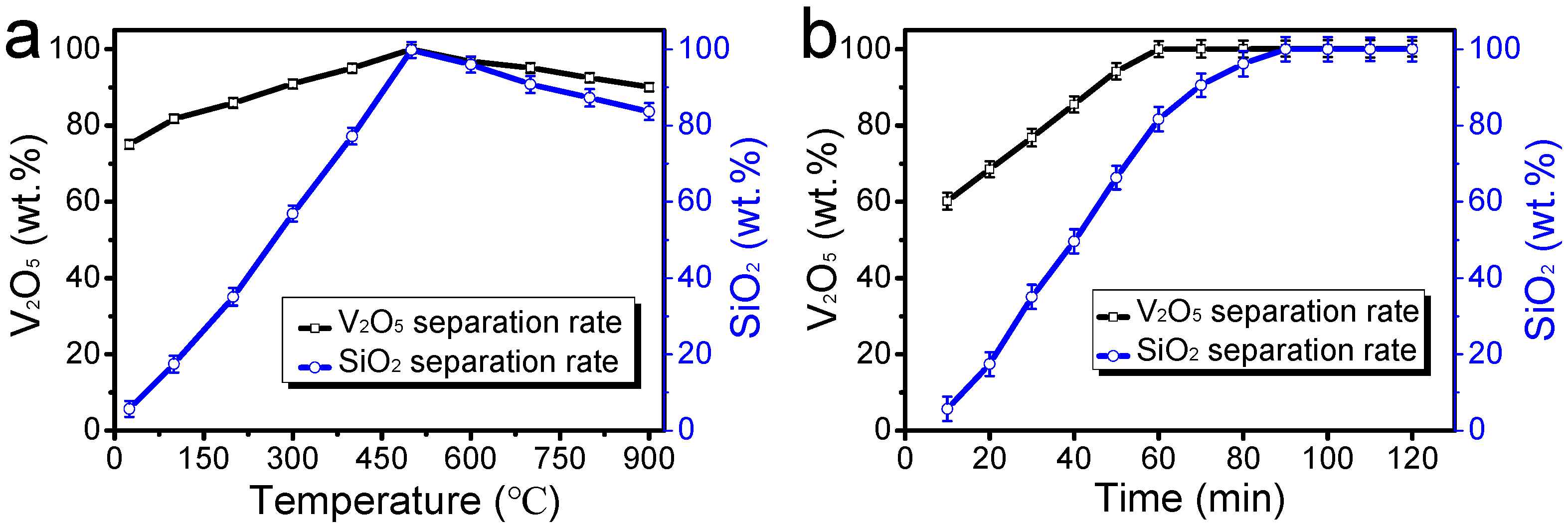
3.3. Effect of Sintering Temperature and Time on Separation Rate
Mixtures consisting of spent SCR catalysts and composite flux at a mixing ratio of 1:1 were sintered for 60 min under various temperatures. The other sintering and leaching process applies the same parameters as the previous. The effect of sintering temperature on separation rate is shown in
Figure 3.
As shown in
Figure 3, the separation rate of V
2O
5 and SiO
2 increased rapidly as the sintering temperature increased from room temperature to 500 °C and then decreased slightly. The maximum was obtained at the temperature of 500 °C. Although the sintering reactions (1–2) can be improved with an increase in temperature, high temperature also increases the particle size of the sintered SCR catalyst. The increase in the particle size deteriorated the monomeric liberation of the sodium metavanadate and sodium silicate particles in the sintered SCR catalyst feeding the leaching separation process. The monomeric liberation is essential for the sodium metavanadate and sodium silicate particles to be effectively separated by the leaching process [
32]. On the other hand, the conversion of anatase TiO
2 nanoparticles into the corresponding rutile was achieved when the spent SCR catalysts were annealed in a muffle furnace at 500 °C. The phase inversion of TiO
2 nanoparticles favored the transformation, transportation, and leaching of V
2O
5, which occupied the interstitial space in the TiO
2 lattice structure.
By keeping the spent SCR catalysts and the composite flux at a mixing ratio of 1:1, the mixtures were sintered at 500 °C for different times. The sintered SCR catalysts followed the same leaching process parameters specified above. The effect of sintering time on the separation rate is shown in
Figure 3b.
As shown in
Figure 3b, with increasing sintering time, the separation rate of V
2O
5 rapidly increased as the roasting time increased from 10 to 60 min, and then leveled off after the roasting time passed 60 min. Meanwhile, the separation rate of SiO
2 in the spent SCR catalysts increased as the roasting time increased from 10 min to 90 min, and then leveled off after the roasting time passed 90 min. V
2O
5 was favored over metasilicate to form a readily water-soluble substance in the sintering process. Hence, the optimal sintering time was determined to be 90 min.
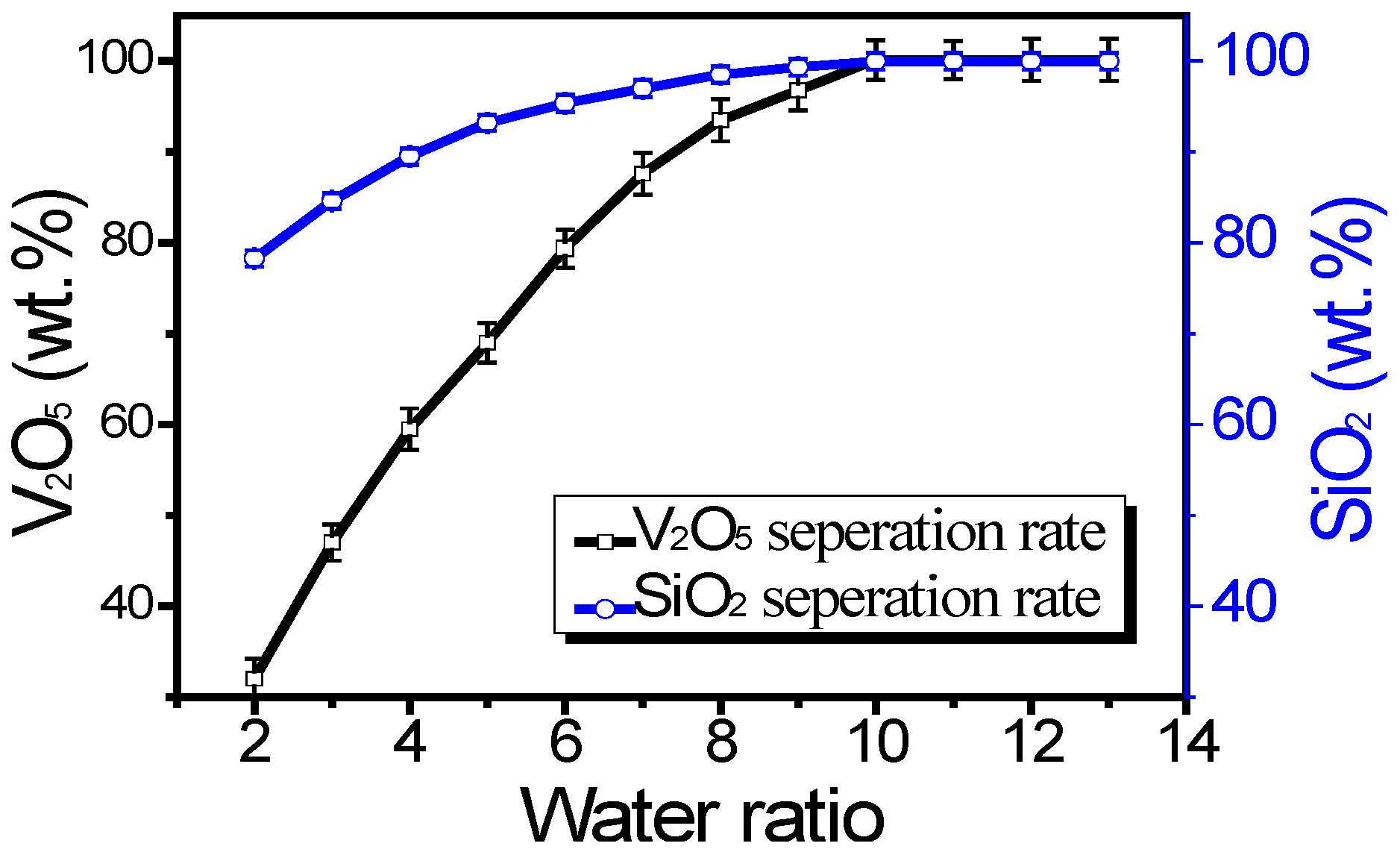
3.4. Effect of Water Ratio on Separation Rate
To further enhance the efficiency of separating V2O5 and SiO2 from spent SCR catalysts, we investigated the effect of varying the water-to-spent SCR catalyst ratios on iron recovery. The sintering process parameters remained consistent with those specified earlier: a composite flux-to-spent SCR catalyst mass ratio of 1:1, a sintering temperature of 500 °C, and a roasting time of 120 min. After sintering, various water ratios were tested at room temperature for 2 h.
As shown in
Figure 4, the separation rate of V
2O
5 and SiO
2 increases with an increase in the water-to-spent SCR catalyst ratio, up to a certain point. Specifically, the separation rate increases as the water ratio increases from 1 to 10 but levels off when the water ratio exceeds 10. As the water-to-spent SCR catalyst ratio increases from 1 to 10, the separation rate of V
2O
5 and SiO
2 improves. This is primarily due to the enhanced solubility and dispersion of vanadates and metasilicates in the aqueous phase. Vanadates and silicates can adsorb onto the surface of spent SCR catalysts. However, this adsorption decreases with decreasing concentrations of these compounds. Higher water ratios dilute the concentration of vanadates and silicates, reducing their tendency to re-adsorb onto the catalyst surface and promoting their dissolution into the aqueous phase.
Beyond a water ratio of 10, the separation rate levels off. This indicates that beyond this point, additional water does not significantly improve the separation efficiency. The system reaches a saturation point where further dilution no longer enhances the solubilization or dispersion of vanadates and silicates. Excessive water addition may lead to increased processing times, higher energy consumption for drying, and potential dilution of other valuable components, which could complicate downstream processes. The concentrations of vanadates and silicates decrease with increasing water ratio. This dilution reduces the driving force for re-adsorption onto the spent catalyst surface, facilitating their dissolution into the aqueous phase. The optimal water-to-spent SCR catalyst ratio (around 10) balances the need for sufficient water to dissolve and disperse vanadates and silicates while avoiding unnecessary excess water that does not contribute to further separation. Experiments confirmed that the separation rate of V
2O
5 and SiO
2 follows the trend observed in
Figure 4. The optimal water ratio of around 10 achieves the efficient separation of V
2O
5 and SiO
2, ensuring high-purity TiO
2 nanoparticles.
3.5. Separation of TiO2 Nanoparticles
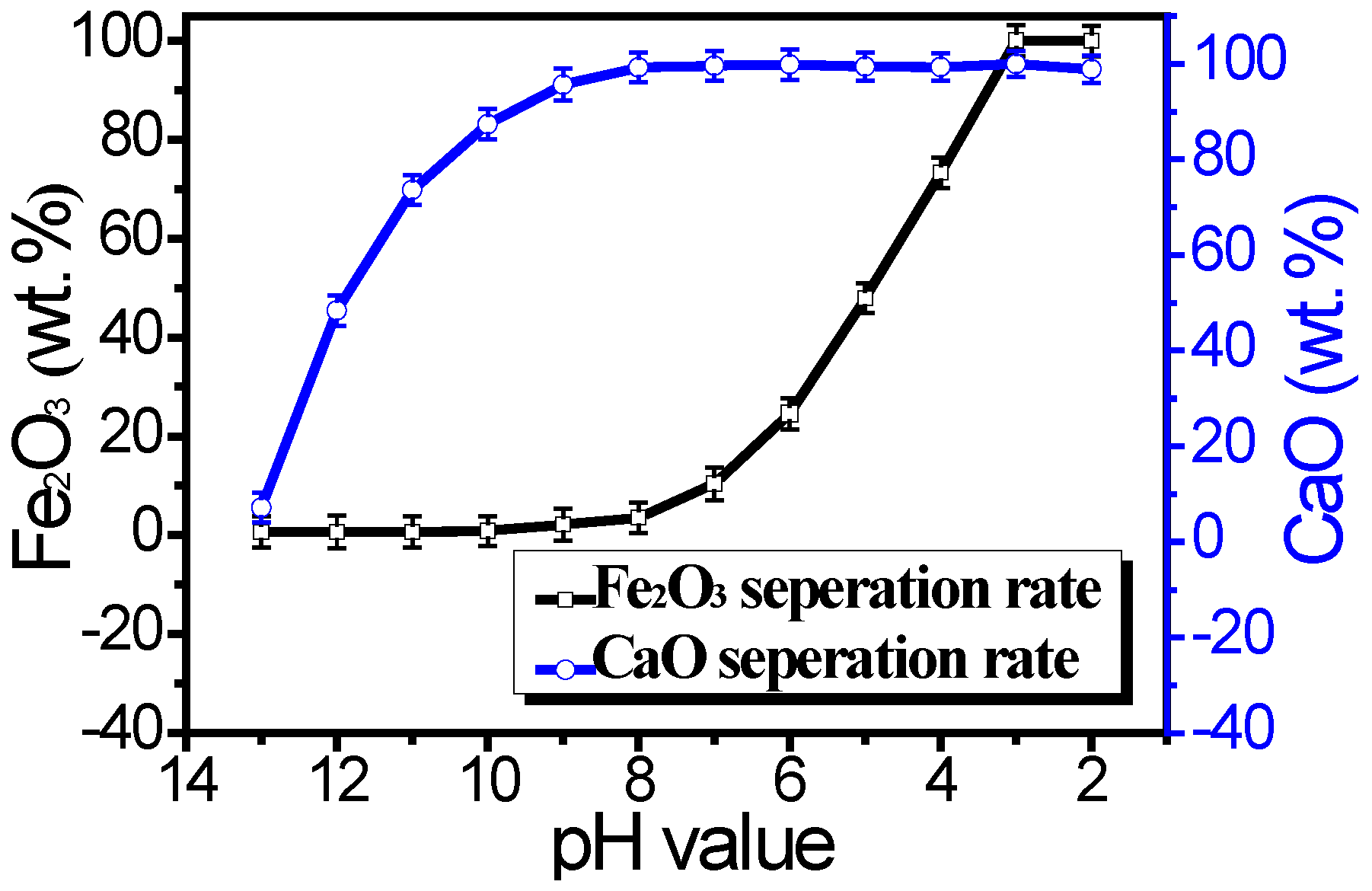
To further remove metal impurities such as iron, calcium, and magnesium from the filter residue, we immersed it in dilute hydrochloric acid with various pH values for 30 min.
Figure 5 illustrates the effect of the pH value of dilute hydrochloric acid on the separation rate of ferric oxide (Fe
2O
3) and calcium oxide (CaO). As the pH value decreases from 13 to 3, the separation rate of Fe
2O
3 and CaO significantly increases. Lower pH values indicate a stronger acidic environment, which facilitates the dissolution of metal oxides like Fe
2O
3 and CaO more effectively. In a highly acidic medium, the concentration of hydrogen ions (H
+) is higher, accelerating the dissolution reactions of metal oxides and thereby improving the separation efficiency. When the pH value drops below 3, the separation rate levels off, indicating that the system has reached a saturation point. Extremely low pH values may lead to unnecessary corrosion issues without providing additional benefits. Therefore, a pH of 3.0 is considered optimal.
After immersing the filter residue in dilute hydrochloric acid with a pH of 3.0 for a specified time, the undissolved substances were collected by centrifugation and repeatedly washed with deionized water. The samples were then dried and calcined. The recovered TiO2 nanoparticles were analyzed using XRD and FESEM to understand the experimental results discussed above.
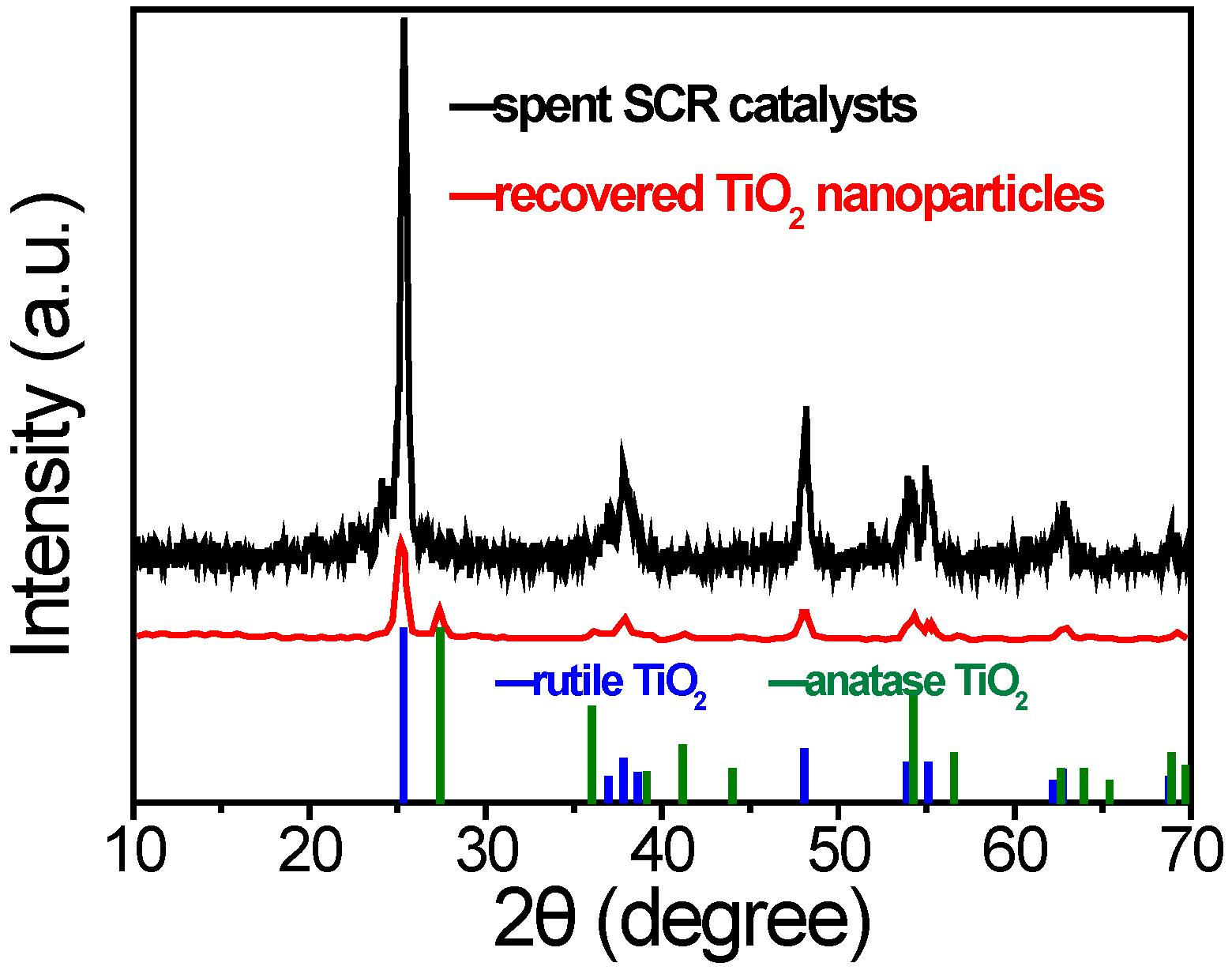
As shown in
Figure 6, the components of raw spent SCR catalyst are a mixture of anatase and rutile, along with several impurity peaks. In comparison to the raw spent SCR catalyst, the recycled product contained only anatase and rutile forms of TiO
2 without any additional impurity peaks, suggesting that TiO
2 nanoparticles were completely separated from other components, such as vanadates and silicates. Metals like iron, aluminum, calcium, and magnesium were not detected, resulting in high-purity TiO
2 nanoparticles containing both anatase and rutile phases [
33,
34].
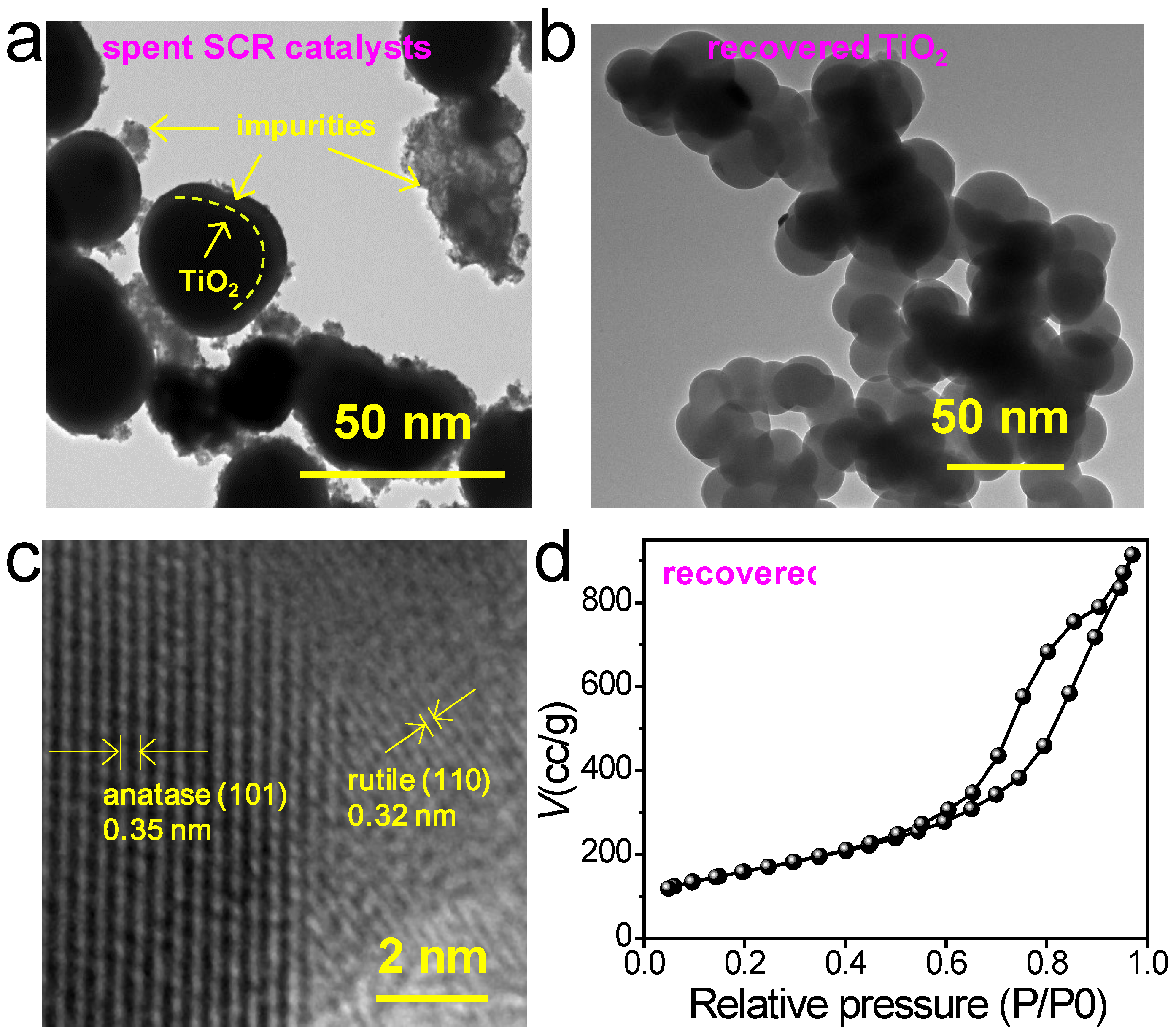
Figure 7a shows that the surface of the spent SCR catalyst is wrapped with other ingredients, making it uneven and impure. In contrast, the recovered TiO
2 products have a very clean and uniform surface without any impurities. Most of the nanoparticles maintain a nanosphere morphology diameter within the interval of 20 to 30 nm. The results suggest that our “NaOH + Na
2CO
3” sintering system can not only help to maintain the nanosphere morphology of TiO
2 in spent SCR catalysts but also recycle pure TiO
2 from spent SCR catalysts.
Figure 7c presents an HRTEM image of the as-prepared TiO
2 nanospheres. The 0.35 nm and 0.32 nm lattice fringes are affiliated with the d spacings of the (101) plane of anatase TiO
2 and the (110) crystal plane of rutile TiO
2 nanospheres.
Figure 7d plots the N
2 adsorption–desorption isotherm of the as-prepared TiO
2 nanospheres. The specific surface area of the prepared TiO
2 nanospheres was 55.5 m
2/g according to the results of the N
2 adsorption–desorption isotherm. Compared to the raw spent SCR catalyst, the as-prepared TiO
2 nanospheres possessed a higher specific surface area. Photocatalytic activity is influenced by several factors, including light absorption, carrier separation, and surface reactions. A larger specific surface area provides more active sites for these processes, thereby enhancing the overall efficiency of photocatalytic reactions.
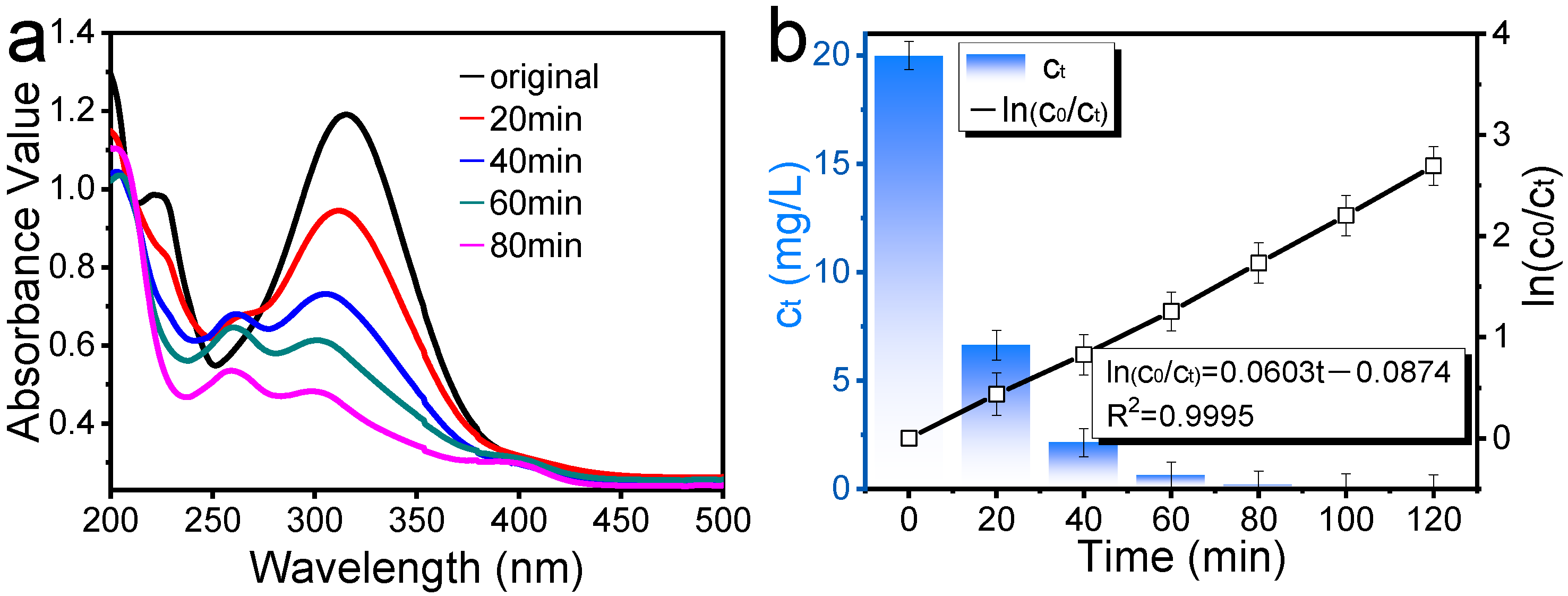
The photocatalytic performance of the recovered TiO
2 nanoparticles was assessed by monitoring the degradation of 2,4-dinitrophenol (DNP) aqueous solution under visible light irradiation. UV-vis spectra were observed during the degradation of the DNP solution under visible light irradiation. The photocatalytic degradation of DNP is shown in
Figure 8.
As shown in
Figure 8a, when the characteristic absorption peaks of DNP decrease as the reaction time goes on, a new absorption peak appears at 260 nm, indicating that nitrophenol has been oxidized, forming new substances. However, as the degradation progresses, the absorption peak at 260 nm also gradually disappears, suggesting that these new substances are being further photodegraded. It could be inferred that DNP was degraded in the process of photodegradation. Additionally, the degradation efficiencies of DNP could reach 83% in the degradation time of 80 min. To enhance the credibility of the experimental results, the photocatalytic performance of the original SCR catalyst was also assessed. Under the same degradation conditions, the degradation rate of DNP by the original SCR catalyst was only 17.2%.
Figure 8b shows the degradation kinetics of the prepared TiO
2 nanospheres for 2,4-dinitrophenol and their photocatalytic performance. As can be seen in
Figure 8b, the concentration of 2,4-dinitrophenol solution (
) decreased when the reaction time was prolonged, and 99.9 % of 2,4-dinitrophenol was oxidized in 120 min. The oxidation reaction of 2,4-dinitrophenol complies with first-order kinetics, and the first-order kinetics rate constant of photocatalysis is 0.0603 min
−1. The results disclosed that the recovered TiO
2 nanoparticles possessed powerful photooxidation ability and could be utilized as photocatalysts for practical applications [
35,
36], such as coating, plastic, papermaking, printing ink, cosmetics, mere catalysts, medicine, etc.
4. Conclusions
This work presents an eco-friendly strategy to address the dual challenges of hazardous waste management and sustainable resource recovery by reclaiming anatase/rutile TiO2 nanospheres from spent selective catalytic reduction (SCR) catalysts. Spent SCR catalysts, classified as hazardous due to toxic components like vanadium, pose significant environmental risks if landfilled. By employing a novel NaOH/Na2CO3 composite flux system, this method achieves the safe recovery of high-value TiO2 nanoparticles with a preserved nanospherical morphology and photocatalytic activity. The optimized sintering–leaching process minimizes environmental pollution by diverting the spent catalysts from landfills and preventing toxic leakage into ecosystems. The recovered TiO2 nanospheres exhibit exceptional photocatalytic performance, enabling their reuse in sustainable applications such as solar-driven water purification or air pollution control, thereby closing the material lifecycle loop. Meanwhile, recycling V2O5 reduces reliance on energy-intensive primary mining, aligning with circular economy principles.
This approach advances sustainability by transforming hazardous industrial waste into functional materials, reducing the carbon footprint associated with conventional TiO2 synthesis, and conserving finite natural resources. The method’s scalability and efficiency support the United Nations Sustainable Development Goals (SDGs), including responsible consumption and production (SDG 12), climate action (SDG 13), and industry, innovation, and infrastructure (SDG 9). By integrating waste valorization with resource efficiency, this work offers a blueprint for green chemistry solutions that reconcile industrial productivity with planetary boundaries.