A Conceptual Framework for Costing Perovskite Solar Cells Through Material Flow Cost Accounting †
Abstract
1. Introduction
Research Questions and Objective
- RQ1: Which chemicals are needed to fabricate a perovskite layer in a solar cell?
- RQ2: To what extent may material flow cost accounting (MFCA) be a suitable costing method for fabricating the perovskite solar cell layer throughout the supply chain?
- RO: Develop a conceptual framework for perovskite solar cell costing through MFCA.
2. Materials and Methods
2.1. The Use of Propositions
- Content propositions, denoted by Cpi, for i ∈; {1, 2, 3, …}. Content propositions identify elements (content) of entities, which are the building blocks in the framework.
- Association propositions, denoted by Apj, for j ∈ {1, 2, 3, …}. Association propositions define associations among the building blocks of the framework.
- Consequential propositions indicated by Cons_pk, for k ∈ {1, 2, 3, …}. Consequential propositions capture information of the following form: if p then q.
2.2. Delimitations
3. Literature Review
- Proposition Cp1: Worldwide dependence on fossil fuels should be reduced in favor of renewable energy sources, such as solar cell technology.
- ➢
- The consumption of coal simulates a Gartner–Hype CycleTM since its global demand appears to have increased over the decades, decreased for a while, and then increased again.
- Proposition Cp2a: Perovskite solar cells (PSCs) may be the technology of choice in embarking on solar cell technology.
- Proposition Cp2: In embarking on solar cell technology, perovskite solar cells (PSCs) may be the technology of choice based on the following advantages and disadvantages:
- ➢
- PSCs inhibit relatively simple manufacturing processes at lower temperatures.
- ➢
- Their manufacturing resides with Industry 4.0 technologies and exhibits aspects of the 6IR regarding renewable energy [37] and nanotechnology.
- ➢
- Their manufacturing supply chain involves mining raw materials, producing reagent species, equipment costs, and manufacturing devices.
- Proposition Cp3: Should a worldwide demand for PSCs emerge, suitable costing methods should be devised and employed to make their manufacturing economically feasible.
4. Findings
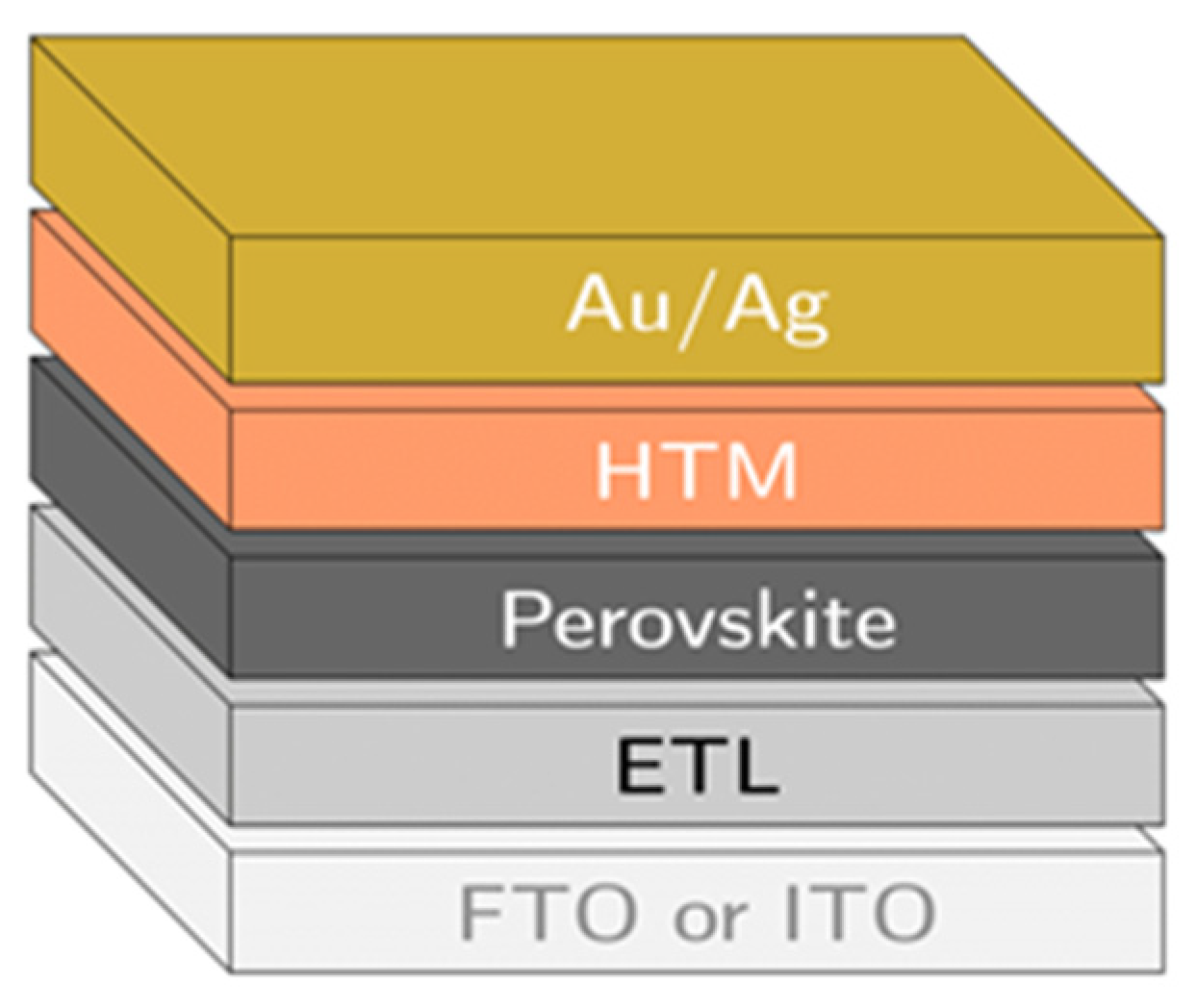
4.1. Fabrication of the Perovskite Layer in the PSC
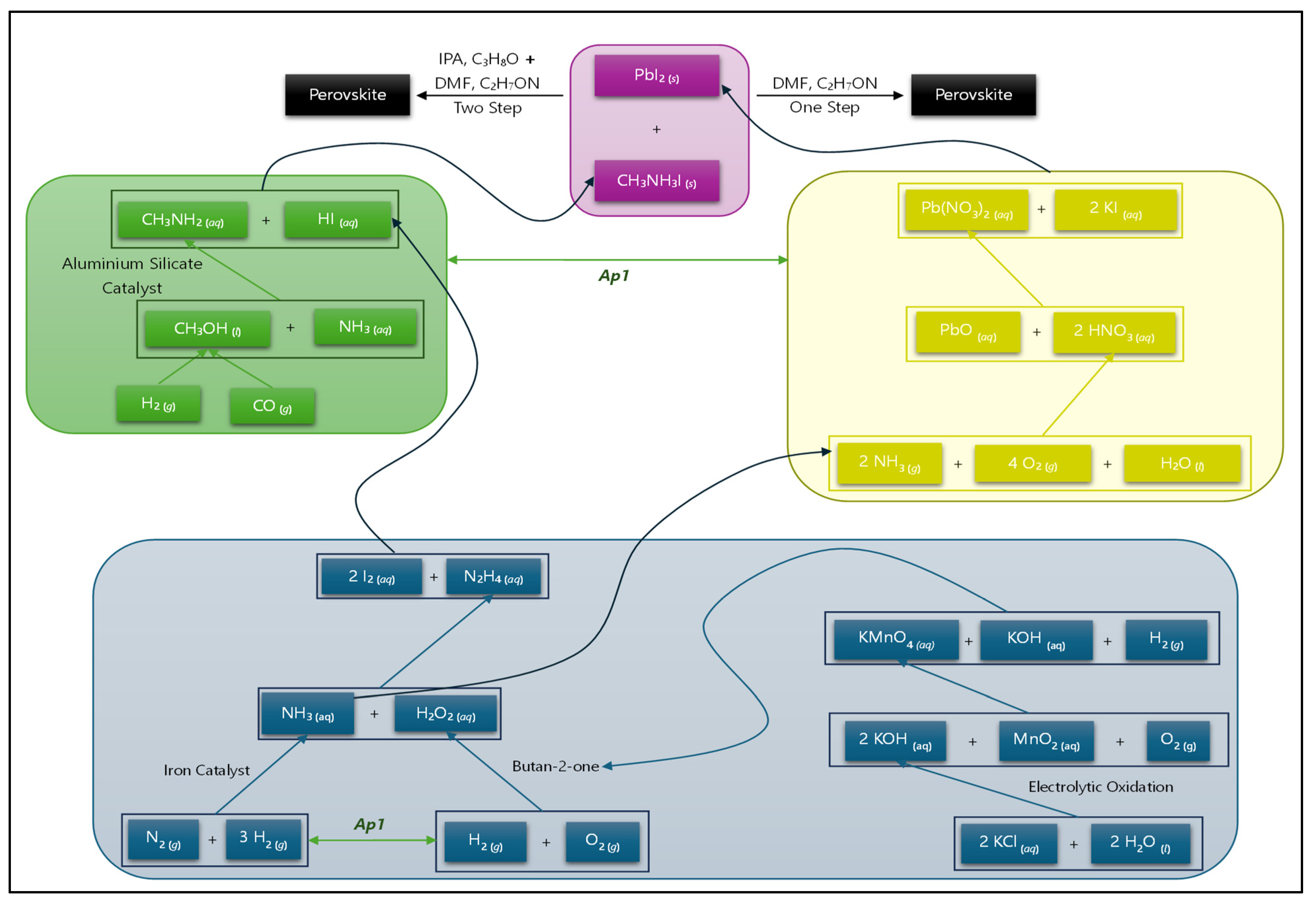
- Proposition Ap1: The fabrication of a PSC involves two or more groups of processes that may be executed in parallel.
- ➢
- Parallel execution may incur cost savings in placing a product on the market but may necessitate two or more sets of personnel to oversee each process.
- Proposition Cp4: The one-step method for fabricating PSCs may be preferred since the compound C3H8O is not needed, leading to reduced costs.
Production of the Chemicals
- Iodine (I2) is sourced from natural brines or caliche ore deposits [45,46]. Iodine from brines can be purified immediately; however, iodine from an ore deposit is first leached through water and further purified.We make the following observation:
- ❖
- Observation #1: Regarding reaction (2):
- ➢
- Several activities, stakeholders, and costs are involved. The costs are mining ore, e.g., iodine; transporting raw materials to the factory or laboratory; and fabricating the relevant materials. The wages and salaries of the mine personnel add to the cost of the reaction.
- ➢
- Hydrazine (N2H4): In the industrial production of hydrazine, the peroxide process uses a combination of ammonia (NH3) with hydrogen peroxide (H2O2) in the presence of a ketone catalyst (butane-2-one) to form hydrazine [49]. Equation (3) shows that water is produced as a byproduct and that ammonia, hydrogen peroxide, and butane-2-one should be produced for this synthesis to be viable.
- ❖
- Observation #2: Augmenting observation #1:
- ➢
- Several activities, stakeholders, and costs are involved. The costs are mining ore, e.g., iodine; transporting raw materials to the factory or laboratory; and fabricating the relevant materials.
- ➢
- The transport costs involved in moving the product from the factory to the laboratory, operational costs, and the remuneration of the personnel involved add to the cost of the product.
- ➢
- Ammonia: the Haber–Bosch process [50] is almost exclusively used in the industrial production of ammonia. Equation (4) gives the Haber–Bosch process.
- Nitrogen gas (N2): By purifying air, which contains 78% nitrogen [44], nitrogen is obtained on an industrial scale. We observe the following:
- ❖
- Observation #3: The cost of reaction (4), regarding forming nitrogen gas, stems from manufacturing or fabrication costs.
- Hydrogen gas (H2): This is produced from the steam reforming of natural gas, coal gasification, or the partial oxidation of other hydrocarbons [51]. A similar observation as in observation #3 may be made for nitrogen gas formation.
- An Iron catalyst is used in the form of magnetite (Fe3O4) from iron ores [52]. Observations #1, #2, and #3 also hold here, due to the underlying mining and manufacturing processes.
- Hydrogen peroxide: Produced by the anthraquinone process using an anthraquinone derivative and palladium (a catalyst) through a hydrogenation reaction in the presence of oxygen. This process is shown in Equation (5).
- Oxygen gas is distilled from the air in oxygen plants [53]. Consequently, this process is relatively inexpensive.
- Anthraquinone derivative: Anthraquinone is synthesized by acid catalysis of styrene [54]. Styrene can be obtained from the dehydrogenation of ethylbenzene over an iron oxide catalyst, which is obtained from the refining of crude oil [55]. This process may be costly; hence, observations #1, #2, and #3 hold.
- Palladium (Pd) is obtained from cooperite and polarite minerals. The usual mining and related processes apply here, leading to observations #1, #2, and #3.
- Butan-2-one (a catalyst): Synthesis starts with the oxidation of butan-2-ol using potassium permanganate (KMnO4). KMnO4 is produced through the process in Equation (6) [56].
- Potassium hydroxide: is produced from potassium chloride and electrolysis as per Equation (7) [57].
- Potassium chloride (KCl): This is obtained from underground mines. Underground deposits of sylvinite, carnallite, or potash are mined, and KCl is extracted [57]. Butan-2-ol is produced from the acid-catalyzed (sulfuric acid (H2SO4)) hydration (use of water) of but-1-ene or but-2-ene (both of which are obtained from the cracking of crude oil) [58]. In turn, H2SO4 is obtained from the contact process, which requires sulfur (S (s)) and oxygen (O2 (g)) [59]. Reaction (7) likewise involves several processes, including mining and manufacturing, leading to observations #1, #2, and #3.
- Potassium iodide: Produced by the reaction of KOH with HI, discussed earlier [57].
- Lead nitrate: Formed by the reaction of lead oxide (PbO) and nitric acid (HNO3) according to Equation (9) [44].
- Nitric acid: Produced through the Ostwald process in Equation (10), where all the reagent production has already been discussed.
- Methyl Amine (CH3NH2) is synthesized through the reaction of ammonia with methanol in the presence of an aluminium silicate catalyst, as shown in Equation (11) [62].
- Ammonia has been discussed before.
- Kaolinite (a clay mineral, Al2Si2O5(OH)4, the catalyst): Obtained from mining minerals such as feldspar [44]. Owing to manufacturing processes, observation #3 applies.
- Methanol: Produced from the combination of syngas (H2 and O2 gas combined) and CO or CO2 via a hydrogenation reaction, as per Equation (12) [63].
- CO and CO2: Obtained from burning natural gas or hydrocarbons [64].
- Dimethylformamide (C2H7ON): Requires the reaction of dimethylamine ((CH3)2NH) with carbon dioxide (CO2 gas) in the presence of a zinc chloride catalyst [65].
- Dimethylamine: Produced by the reaction between methanol and ammonia (Equation (14), all of which have been described earlier.
- Zinc chloride catalyst (ZnCl2): Reacting the zinc metal (Zn (s)) with hydrochloric acid (HCl (aq)), producing the catalyst and water as a byproduct.
- HCl: Reaction between hydrogen and chlorine gas [59].
- Chlorine gas is obtained from the electrolysis of brine (NaCl and KCl solution) to release Cl2 gas [66].
- Isopropyl alcohol (C3H8O): The hydration of propene, using sulfuric acid as a catalyst, is the main industrial method of synthesis for isopropyl alcohol [67].
- Propene: Obtained by steam cracking and fractional distillation of saturated hydrocarbons obtained from natural gas and crude oil [67].
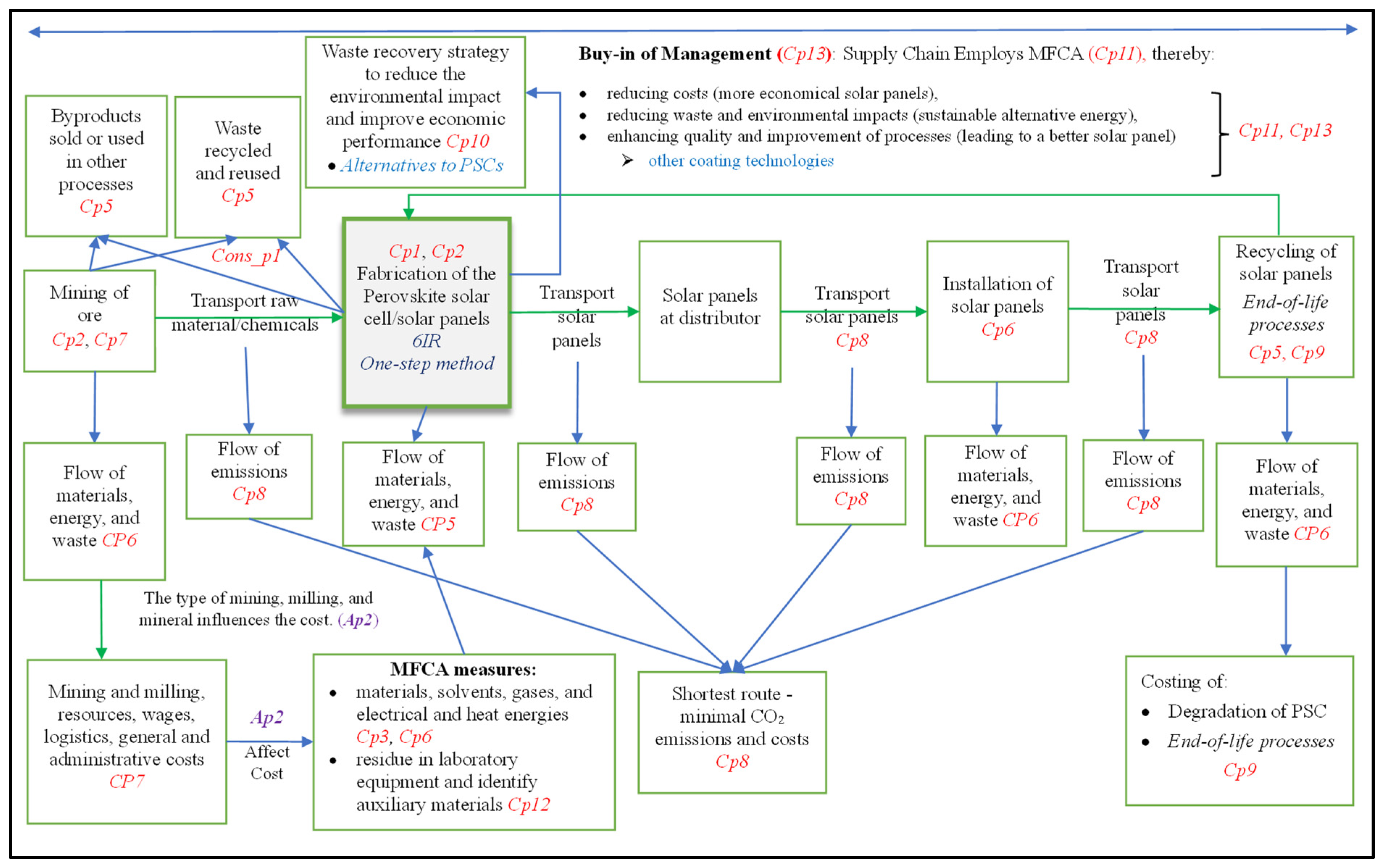
- Proposition Cp5: The chemical processes towards the fabrication of PSCs lead to the generation of additional compounds, as follows:
- ➢
- Byproducts, such as water or nitrogen, result, and these may be sold or used in other processes, reducing the cost of the process.
- ➢
- Waste, e.g., acid mine drainage (AMD), may be recycled and reused (cf. our observations #1 and #2).
- Proposition Cp6: The supply chain of chemical processes involves materials from mining, fabrication in a factory, and laboratory processes.
- ➢
- A costing method is needed for each process to follow the material flow of each product and chemical substance and to quantify it in physical and monetary units.
4.2. Mining of Ore
- Proposition Cp7: The cost of mining ore can, amongst others, be divided into mining and milling; resources, e.g., electricity and fuel; administrative, e.g., wages and salaries of personnel; logistics; general; and miscellaneous expenses.It also leads to the following associate proposition:
- Proposition Ap2: The types of mining, milling, and mineral recovery affect the cost of the rest of the supply chain.
4.3. Transporting the Raw Materials, Products, and Chemicals
- Proposition Cp8: The shortest route in transporting chemicals and products should be established to reduce CO2 emissions and costs.
4.4. Recycling of Perovskite Modules
- Proposition Cp9: Costing all end-of-life processes ought to be conducted since the business that sold the solar panel and the customer might not be the only responsible parties.
4.5. Perovskite Solar Cell Degradation
4.6. Material Flow Cost Accounting, a Match for Perovskite Solar Cell Fabrication
- Proposition Cp10a: MFCA traces and assesses the flow of materials and attempts to reduce waste at the source.
- Proposition Cp11: MFCA can be employed to cost the perovskite fabrication supply chain.
- Proposition Cons_p1: An MFCA waste recovery strategy to reduce an industry’s environmental impact and improve its economic performance facilitates waste reduction during fabrication.
- Proposition Cp10: MFCA traces and quantifies the flow of materials. It:
- ➢
- aims to reduce waste at the source;
- ➢
- assists in measuring materials, solvents, gases, and electrical and heat energies in fabricating the perovskite layer.
- Proposition Cp11: MFCA can achieve the following:
- ➢
- reduce costs (more economical solar panels—societal impact).
- ➢
- reduce waste and environmental impacts (sustainable alternative energy).
- ➢
- enhance quality and improvement of processes (leading to improved solar panels).
- Proposition Cp12: MFCA can measure material loss and residue in laboratory equipment during a process and can identify auxiliary materials.
- Proposition Cp13: It is vital for a PSC’s management and governance structures to buy into using MFCA to address costing aspects and environmental challenges incurred through the company’s operations.
4.7. Discussion of Findings
5. Discussion
6. Hypothetical Case
- End of Synthesis
- Validation of the MFCA framework for manufacturing PSCs
- End of validation
7. Conclusions
7.1. Advancing Theoretical Knowledge
7.2. Practical Implications
7.3. Novelty of the Work
7.4. Limitations
7.5. Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| COP | Conference of Parties |
| DMF | Dimethylformamide |
| ETL | Electron transporting layer |
| FA | Formamidinium |
| FTO | Fluorine-doped tin oxide |
| HTL | Hole transporting layer |
| IPA | Isopropyl alcohol |
| ITO | Indium doped tin oxide |
| MA | Methyl ammonium |
| MFCA | Material flow cost accounting |
| PSC | Perovskite solar cell |
Appendix A. Production of Chemicals
| Chemical | Equation | Byproducts | |
|---|---|---|---|
| CH3NH3I | CH3NH2 (aq) + HI (aq) → CH3NH3I (s) | (1) | |
| Hydrogen iodide (HI) | 2 I2 (aq) + N2H4 (aq) 4 HI (aq) + N2 (g) | (2) | Nitrogen gas (N2) |
| Iodine (Equation (2)) | 2 NH3 (g) + H2O2 (aq) N2H4 (aq) + 2 H2O (l) | (3) | Water |
| N2 (g) + 3 H2 (g) 2 NH3 (g) | (4) | ||
| Nitrogen gas, hydrogen gas and iron (Equation (4)) | H2 (g) + O2 (g) H2O2 (aq) | (5) | |
| 2 MnO2 (aq) + 4 KOH (aq) + O2 (g) 2 K2MnO4 (aq) + 2 H2O (l) 2 KMnO4 (aq) + 2 KOH (aq) + H2 (g) | (6) | ||
| 2 KCl (aq) + 2 H2O (l) 2 KOH (aq) + Cl2 (g) + H2 (g) | (7) | ||
| Lead iodide (PbI2) | Pb(NO3)2 (aq) + 2 KI (aq) PbI2 (s) + 2 KNO3 (aq) | (8) | |
| PbO (aq) + 2 HNO3 (aq) Pb(NO3)2 (aq) + H2O (l) | (9) | ||
| 2 NH3 (g) + 4 O2 (g) + H2O (l) 3 H2O (g) + 2 HNO3 (aq) | (10) | ||
| Methyl amine (CH3NH2) | NH3 (aq) + CH3OH (l) H2O (l) + CH3NH2 (aq) | (11) | |
| 3 H2 (g) + CO (g) CH3OH (l) | (12) | ||
| 3 H2 (g) + CO2 (g) CH3OH (l) + H2O (l) | (13) | ||
| Dimethylformamide (C2H7ON) | NH3 (aq) + CH3OH (l) → (CH3)2NH (aq) + 2H2O (l) | (14) | |
References
- United Nations Environment Programme; International Resource Panel. Global Resources Outlook 2024–Bend the Trend Pathways to a Liveable Planet as Resource Use Spikes; United Nations Environment Programme: Nairobi, Kenya, 2024. [Google Scholar]
- United Nations Climate Action. Renewable Energy–Powering a Safer Future. Available online: https://www.un.org/en/climatechange/raising-ambition/renewable-energy (accessed on 10 January 2025).
- United Nations. Conference of Parties (COP); United Nations: Geneva, Switzerland, 2022. [Google Scholar]
- United Nations Development Programme. Sustainable Development Goals Booklet; Sustainable Development Goals 2015; United Nations Development Programme: Geneva, Switzerland, 2015. [Google Scholar]
- Yi, R.; Chen, A. Blessing Or Curse Energy Sustainability: How does Climate Change Affect Renewable Energy Consumption in China? Environ. Dev. Sustain. 2024, 24. [Google Scholar] [CrossRef]
- Adwek, G.; Boxiong, S.; Ndolo, O.O.; Siagi, Z.O.; Chepsaigutt, C.; Kenmunto, C.M.; Arowo, M.; Shimmon, J.; Simiyu, P.; Yabo, A.C. The Solar Energy Access in Kenya: A Review Focusing on Pay-as-You-Go Solar Home System. Environ. Dev. Sustain. 2020, 22, 3897–3938. [Google Scholar]
- Igliński, B.; Skrzatek, M.; Kujawski, W.; Cichosz, M.; Buczkowski, R. SWOT Analysis of Renewable Energy Sector in Mazowieckie Voivodeship (Poland): Current Progress, Prospects and Policy Implications. Environ. Dev. Sustain. 2022, 24, 77–111. [Google Scholar]
- Ng, C.H.; Lim, H.N.; Hayase, S.; Zainal, Z.; Huang, N.M. Photovoltaic Performances of Mono- and Mixed-Halide Structures for Perovskite Solar Cell: A Review. Renew. Sustain. Energy Rev. 2018, 90, 248–274. [Google Scholar]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A Perspective on the Production of Dye-Sensitized Solar Modules. Energy Environ. Sci. 2014, 7, 3952. [Google Scholar] [CrossRef]
- Meillaud, F.; Boccard, M.; Bugnon, G.; Despeisse, M.; Hänni, S.; Haug, F.-J.; Persoz, J.; Schüttauf, J.-W.; Stuckelberger, M.; Ballif, C. Recent Advances and Remaining Challenges in Thin-Film Silicon Photovoltaic Technology. Mater. Today 2015, 18, 378–384. [Google Scholar]
- Weyand, S.; Kawajiri, K.; Mortan, C.; Zeller, V.; Schebek, L. Are Perovskite Solar Cells an Environmentally Sustainable Emerging Energy Technology? Upscaling from Lab to Fab in Life Cycle Assessment. ACS Sustain. Chem. Eng. 2023, 11, 14010–14019. [Google Scholar] [CrossRef]
- Fortune Business Insight. Perovskite Solar Cell Market Size, Share & Industry Analysis, by Type (Rigid and Flexible), by End-User (BIPV, Power Station, Transportation & Mobility, Consumer Electronics, and Others) and Regional Forecast, 2024–2032. 2025. Available online: https://www.fortunebusinessinsights.com/industry-reports/perovskite-solar-cell-market-101556 (accessed on 10 January 2025).
- Čulík, P.; Brooks, K.; Momblona, C.; Adams, M.; Kinge, S.; Maréchal, F.; Dyson, P.J.; Nazeeruddin, M.K. Design and Cost Analysis of 100 MW Perovskite Solar Panel Manufacturing Process in Different Locations. ACS Energy Lett. 2022, 7, 3039–3044. [Google Scholar] [CrossRef]
- Kajal, P.; Verma, B.; Vadaga, S.G.R.; Powar, S. Costing Analysis of Scalable Carbon-Based Perovskite Modules using Bottom Up Technique. Glob. Chall. 2021, 6, 2100070. [Google Scholar] [CrossRef]
- Bhardwaj, K.D. Manual on Material Flow Cost Accounting: ISO14051-2014; Asian Productivity Organization (APO): Tokyo, Japan, 2014; p. 37. [Google Scholar]
- Lee, K.; Gunarathne, N. An Exploration of the Implementation and Usefulness of Environmental Management Accounting: A Comparative Study between Australia and Sri Lanka. CIMA Res. Exec. Summ. 2019, 15, 1–26. [Google Scholar]
- van der Poll, H.J.; van der Poll, H.M. Manufacturing and Costing Perovskite Solar Cells for a Brighter Future. In Proceedings of the Academy for Global Business Advancement 2023 Conference, Le Méridien Dubai Hotel & Conference Centre (Dubai Airport), Dubai, United Arab Emirates, 20–22 May 2023; pp. 21–31. [Google Scholar]
- Saunders, M.N.K.; Lewis, P.; Thornhill, A. Research Methods for Business Students, 9th ed.; Pearson Education Limited: London, UK, 2024. [Google Scholar]
- Jaakkola, E. Designing Conceptual Articles: Four Approaches. AMS Rev. 2020, 10, 18–26. [Google Scholar]
- van der Poll, J.A.; van der Poll, H.M. Assisting Postgraduate Students to Synthesise Qualitative Propositions to Develop a Conceptual Framework. J. New Gener. Sci. 2023, 21, 146–158. [Google Scholar]
- Jabareen, Y. Building a Conceptual Framework: Philosophy, Definitions, and Procedure. Int. J. Qual. Methods 2009, 8, 49–62. [Google Scholar]
- Jacak, J.E.; Jacak, W.A. Routes for Metallization of Perovskite Solar Cells. Materials 2022, 15, 2254. [Google Scholar] [CrossRef]
- United Nations. COP26: Together for our Planet; United Nations: Geneva, Switzerland, 2021. [Google Scholar]
- Green, F.; van Asselt, H. Opinion: COP27 Flinched on Phasing Out ‘all Fossil Fuels’. What’s Next? United Nations: Geneva, Switzerland, 2022. [Google Scholar]
- United Nations Climate Change. COP 28: What Was Achieved and What Happens Next? United Nations: Geneva, Switzerland, 2023. [Google Scholar]
- International Energy Agency. World Energy Outlook 2017; International Energy Agency: Paris, France, 2017; pp. 1–782. [Google Scholar]
- International Energy Agency. Coal 2023: Analysis and Forecast to 2026; International Energy Agency: Paris, France, 2023; pp. 1–130. [Google Scholar]
- Hegedus, S.; Luque, A. Achievements and Challenges of Solar Electricity from Photovoltaics. In Handbook of Photovoltaic Science and Engineering; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 1–38. [Google Scholar]
- Ikeda, N.; Teshima, K.; Miyasaka, T. Conductive Polymer–carbon–imidazolium Composite: A Simple Means for Constructing Solid-State Dye-Sensitized Solar Cells. Chem. Commun. 2006, 16, 1733–1735. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic. Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar]
- Adams, G.R.; Okoli, O.I. A Review of Perovskite Solar Cells with a Focus on Wire-Shaped Devices. Renew. Energy Focus 2018, 25, 17–23. [Google Scholar]
- Onwubiko, I.; Khan, W.S.; Subeshan, B.; Asmatulu, R. Investigating the Effects of Carbon-Based Counter Electrode Layers on the Efficiency of Hole-Transporter-Free Perovskite Solar Cells. Energy Ecol. Environ. 2020, 5, 141–152. [Google Scholar]
- National Renewable Energy Laboratory. Best Research-Cell Efficiency Chart; National Renewable Energy Laboratory: Golden, CO, USA, 2024. [Google Scholar]
- Kim, B.J.; Kim, D.H.; Kwon, S.L.; Park, S.Y.; Li, Z.; Zhu, K.; Jung, H.S. Selective Dissolution of Halide Perovskites as a Step Towards Recycling Solar Cells. Nat. Commun. 2016, 7, 11735. [Google Scholar]
- Zuser, A.; Rechberger, H. Considerations of Resource Availability in Technology Development Strategies: The Case Study of Photovoltaics. Resour. Conserv. Recycl. 2011, 56, 56–65. [Google Scholar]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [PubMed]
- Duggal, A.S.; Malik, P.K.; Gehlot, A.; Singh, R.; Gaba, G.S.; Masud, M.; Al-Amri, J. A Sequential Roadmap to Industry 6.0: Exploring Future Manufacturing Trends. IET Commun. 2022, 16, 521–531. [Google Scholar]
- Mariotti, S.; Köhnen, E.; Scheler, F.; Sveinbjörnsson, K.; Zimmermann, L.; Piot, M.; Yang, F.; Li, B.; Warby, J.; Musiienko, A.; et al. Interface Engineering for High-Performance, Triple-Halide Perovskite–silicon Tandem Solar Cells. Science 2023, 381, 63–69. [Google Scholar] [PubMed]
- Laalioui, S.; Alaoui, K.B.; Dads, H.A.; Assali, K.E.; Ikken, B.; Outzourhit, A. Progress in Perovskite Based Solar Cells: Scientific and Engineering State of the Art. Rev. Adv. Mater. Sci. 2020, 59, 10–25. [Google Scholar]
- Patwardhan, S.; Cao, D.H.; Hatch, S.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G.; Schatz, G.C. Introducing Perovskite Solar Cells to Undergraduates. J. Phys. Chem. Lett. 2015, 6, 251–255. [Google Scholar]
- Cheng, J.; Liu, F.; Tang, Z.; Li, Y. Scalable Blade Coating: A Technique Accelerating the Commercialization of Perovskite-Based Photovoltaics. Energy Technol. 2021, 9, 2100204. [Google Scholar]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Taylor, F.S. The Evolution of the Still. Ann. Sci. 1945, 5, 185–202. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; pp. 1–1384. [Google Scholar]
- Krukowski, S.T.; Johnson, K.S. Iodine. In Industrial Minerals & Rocks: Commodities, Markets, and Uses; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2006; pp. 1–1548. [Google Scholar]
- Maekawa, T.; Igari, S.; Kaneko, N. Chemical and Isotopic Compositions of Brines from Dissolved-in-Water Type Natural Gas Fields in Chiba, Japan. Geochem. J. 2006, 40, 475–484. [Google Scholar]
- Mbedzi, M.D.; Van Der Poll, H.M.; Van Der Poll, J.A. Enhancing a Decision-Making Framework to Address Environmental Impacts of the South African Coalmining Industry. Energies 2020, 13, 4897. [Google Scholar] [CrossRef]
- Nyakuwanika, M.; Van Der Poll, H.M.; Van Der Poll, J.A. A Conceptual Framework for Greener Goldmining through Environmental Management Accounting Practices (EMAPs): The Case of Zimbabwe. Sustainability 2021, 13, 10466. [Google Scholar] [CrossRef]
- Schirmann, J.; Bourdauducq, P. Hydrazine. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2001; Volume 18, pp. 79–96. [Google Scholar]
- Appl, M. Ammonia. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2011; Volume 3, pp. 107–137. [Google Scholar]
- Subramani, V.; Sharma, P.; Zhang, L.; Liu, K. Catalytic steam reforming technology for the production of hydrogen and syngas. In Hydrogen and Syngas Production and Purification Technologies; Liu, K., Song, C., Subramani, V., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 14–126. [Google Scholar]
- Webmineral. Magnetite. Available online: http://webmineral.com/data/Magnetite.shtml (accessed on 20 December 2023).
- Drnevich, R.F.; Ecelbarger, E.J.; Portzer, J.W. Industrial Oxygen Plants: A Technology Overview for Users of Coal Gasification-Combined-Cycle Systems; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1981; pp. 1–96. [Google Scholar]
- Vogel, A. Anthraquinone. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; Volume 3, pp. 503–511. [Google Scholar]
- Lee, E.H. Iron Oxide Catalysts for Dehydrogenation of Ethylbenzene in the Presence of Steam. Catal. Rev. 1974, 8, 285–305. [Google Scholar]
- Reidies, A.H. Manganese Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; Volume 22, pp. 223–244. [Google Scholar]
- Schultz, H.; Bauer, G.; Schachl, E.; Hagedorn, F.; Schmittinger, P. Potassium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; Volume 29, pp. 639–704. [Google Scholar]
- Mazoyer, E.; Szeto, K.C.; Basset, J.; Nicholas, C.P.; Taoufik, M. High Selectivity Production of Propylene from 2-Butene: Non-Degenerate Pathways to Convert Symmetric Olefins Via Olefin Metathesis. Chem. Commun. 2012, 48, 3611–3613. [Google Scholar]
- Green, D.W.; Southard, M.Z. Perry’s Chemical Engineers’ Handbook, 9th ed.; McGraw Hill: New York, NY, USA, 2018. [Google Scholar]
- Saikumar, I.; Ahmad, S.; Baumberg, J.J.; Vijaya Prakash, G. Fabrication of Excitonic Luminescent Inorganic–organic Hybrid Nano- and Microcrystals. Scr. Mater. 2012, 67, 834–837. [Google Scholar]
- Ghazi, A.M.; Millette, J.R. Lead. In Environmental Forensics: Contaminant Specific Guide; Morrison, R.D., Murphy, B.L., Eds.; Academic Press: Cambridge, MA, USA, 1964; pp. 55–79. [Google Scholar]
- Corbin, D.R.; Schwarz, S.; Sonnichsen, G.C. Methylamines Synthesis: A Review. Catal. Today 1997, 37, 71–102. [Google Scholar]
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process Advantages of Direct CO2 to Methanol Synthesis. Front. Chem. 2018, 6, 446. [Google Scholar]
- Keeling, C.D. Industrial Production of Carbon Dioxide from Fossil Fuels and Limestone. Tellus 1973, 25, 174–198. [Google Scholar]
- Weissermel, K.; Arpe, H. Industrial Organic Chemistry, 4th ed.; Wiley-VCH: Hoboken, NJ, USA, 2003; pp. 1–491. [Google Scholar]
- Landolt, D.; Ibl, N. Anodic Chlorate Formation on Platinized Titanium. J. Appl. Electrocehm. 1972, 2, 201–210. [Google Scholar]
- Klabunde, J.; Bischoff, C.; Papa, A.J. Propanols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2018; pp. 1–14. [Google Scholar]
- Curry, J.A.; Ismay, M.J.L.; Jameson, G.J. Mine Operating Costs and the Potential Impacts of Energy and Grinding. Miner. Eng. 2014, 56, 70–80. [Google Scholar]
- Attari, M.Y.N.; Torkayesh, A.E. Developing Benders Decomposition Algorithm for a Green Supply Chain Network of Mine Industry: Case of Iranian Mine Industry. Oper. Res. Pers. 2018, 5, 371–382. [Google Scholar]
- Rodrigue, J.P. The Geography of Transport Systems, 6th ed.; Routledge: London, UK, 2024; p. 402. [Google Scholar]
- Goetz, K.P.; Taylor, A.D.; Hofstetter, Y.J.; Vaynzof, Y. Sustainability in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 13, 1–17. [Google Scholar] [PubMed]
- Wright, M.M.; Tan, E.C.D.; Tu, Q.; Martins, A.; Parvatker, A.G.; Yao, Y.; Sunol, A.; Smith, R.L. Life Cycle Inventory Availability: Status and Prospects for Leveraging New Technologies. ACS Sustain. Chem. Eng. 2024, 12, 12708–12718. [Google Scholar] [PubMed]
- Rodriguez-Garcia, G.; Aydin, E.; De Wolf, S.; Carlson, B.; Kellar, J.; Celik, I. Life Cycle Assessment of Coated-Glass Recovery from Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 15239–15248. [Google Scholar]
- Kadro, J.M.; Hagfeldt, A. The End-of-Life of Perovskite PV. Joule 2017, 1, 29–46. [Google Scholar]
- Ava, T.T.; Al Mamun, A.; Marsillac, S.; Namkoong, G. A Review: Thermal Stability of Methylammonium Lead Halide Based Perovskite Solar Cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, 1803792. [Google Scholar]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal Degradation of CH3NH3PbI3 Perovskite into NH3 and CH3I Gases Observed by Coupled Thermogravimetry–mass Spectrometry Analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar]
- Dragomir, V.D.; Gorgan, C.; Calu, D.; Dumitru, M. The Relevance and Comparability of Corporate Financial Reporting regarding Renewable Energy Production in Europe. Renew. Energy Focus 2022, 41, 206–215. [Google Scholar]
- Herzig, C.; Viere, T.; Schaltegger, S.; Burritt, R.L.; Lee, K. Environmental Management Accounting: Case Studies of South-East Asian Companies. Account. Forum 2012, 36, 310–312. [Google Scholar]
- Burritt, R.; Christ, K.; Schaltegger, S. Materials and energy accounting. In Routledge Handbook of Environmental Accounting; Bebbington, J., Larrinaga, C., O’Dwyer, B., Thomson., I., Eds.; Routledge: Abingdon, UK, 2021; p. 17. [Google Scholar]
- Chetanraj, D.B.; Senthil Kumar, J.P. Material Flow Cost Accounting for Aluminum Gravity Die Casting in Electrical Products Manufacturing. J. Bus. Ind. Mark. 2024, 39, 2556–2572. [Google Scholar]
- Higashida, A. Supply Chain MFCA Implementation: Emphasizing Evidence on Coordination. Sustain. Account. Manag. Policy J. 2020, 12, 695. [Google Scholar] [CrossRef]
- Usul, H.; Olgun, E.B. An Analysis of Material Flow Cost Accounting in Companies using Different Cost Accounting Systems. Heliyon 2025, 11, e42555. [Google Scholar] [CrossRef] [PubMed]
- ISO14051:2011; Environmental Management–Material Flow Cost Accounting–General Framework. International Organization for Standardization: Geneva, Switzerland, 2011.
- Wan, Y.K.; Ng, R.T.L.; Ng, D.K.S.; Tan, R.R. Material Flow Cost Accounting (MFCA)–Based Approach for Prioritisation of Waste Recovery. J. Clean. Prod. 2015, 107, 602–614. [Google Scholar] [CrossRef]
- Burritt, R.L.; Herzig, C.; Schaltegger, S.; Viere, T. Diffusion of Environmental Management Accounting for Cleaner Production: Evidence from some Case Studies. J. Clean. Prod. 2019, 224, 479–491. [Google Scholar] [CrossRef]
- Walz, M.; Guenther, E. What Effects does Material Flow Cost Accounting have for Companies?: Evidence from a Case Studies Analysis. J. Ind. Ecol. 2021, 25, 593–613. [Google Scholar]
- van der Poll, J.A. The Role of 4IR-5IR Leadership-Management in the Adoption of Formal Methods. Systems 2024, 12, 306. [Google Scholar] [CrossRef]
- van der Poll, J.A. Towards a Problematization Framework of 4IR Formalisms: The Case of QUALITY 4.0. In Proceedings of the International Conference on Intelligent Vision and Computing (ICIVC), Sur, Omen, 3–4 October 2021; Proceedings in Adaptation, Learning and Optimization. Springer: Cham, Switzerland, 2022; Volume 15, pp. 212–226. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Poll, H.J.; van der Poll, H.M.; van der Poll, J.A. A Conceptual Framework for Costing Perovskite Solar Cells Through Material Flow Cost Accounting. Sustainability 2025, 17, 2892. https://doi.org/10.3390/su17072892
van der Poll HJ, van der Poll HM, van der Poll JA. A Conceptual Framework for Costing Perovskite Solar Cells Through Material Flow Cost Accounting. Sustainability. 2025; 17(7):2892. https://doi.org/10.3390/su17072892
Chicago/Turabian Stylevan der Poll, Hendrik Johannes, Huibrecht Margaretha van der Poll, and John Andrew van der Poll. 2025. "A Conceptual Framework for Costing Perovskite Solar Cells Through Material Flow Cost Accounting" Sustainability 17, no. 7: 2892. https://doi.org/10.3390/su17072892
APA Stylevan der Poll, H. J., van der Poll, H. M., & van der Poll, J. A. (2025). A Conceptual Framework for Costing Perovskite Solar Cells Through Material Flow Cost Accounting. Sustainability, 17(7), 2892. https://doi.org/10.3390/su17072892




