Regulation of Thin-Layered g-C3N4 for Efficient Persulfate Photocatalysis of Ibuprofen Contaminated Groundwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipments
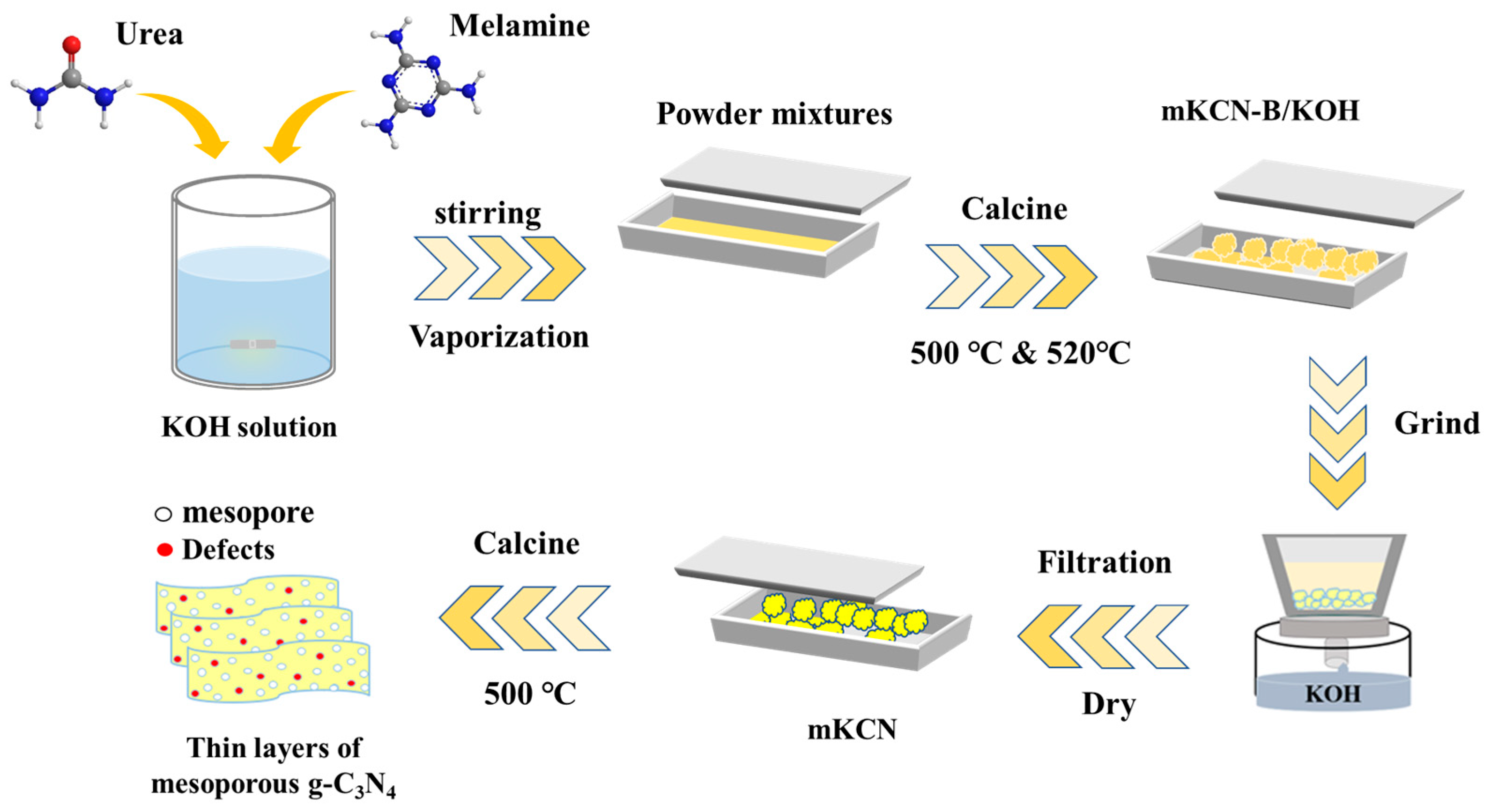
2.2. Preparation of Catalysts
2.3. Test Methods and Equipment
3. Results and Discussion
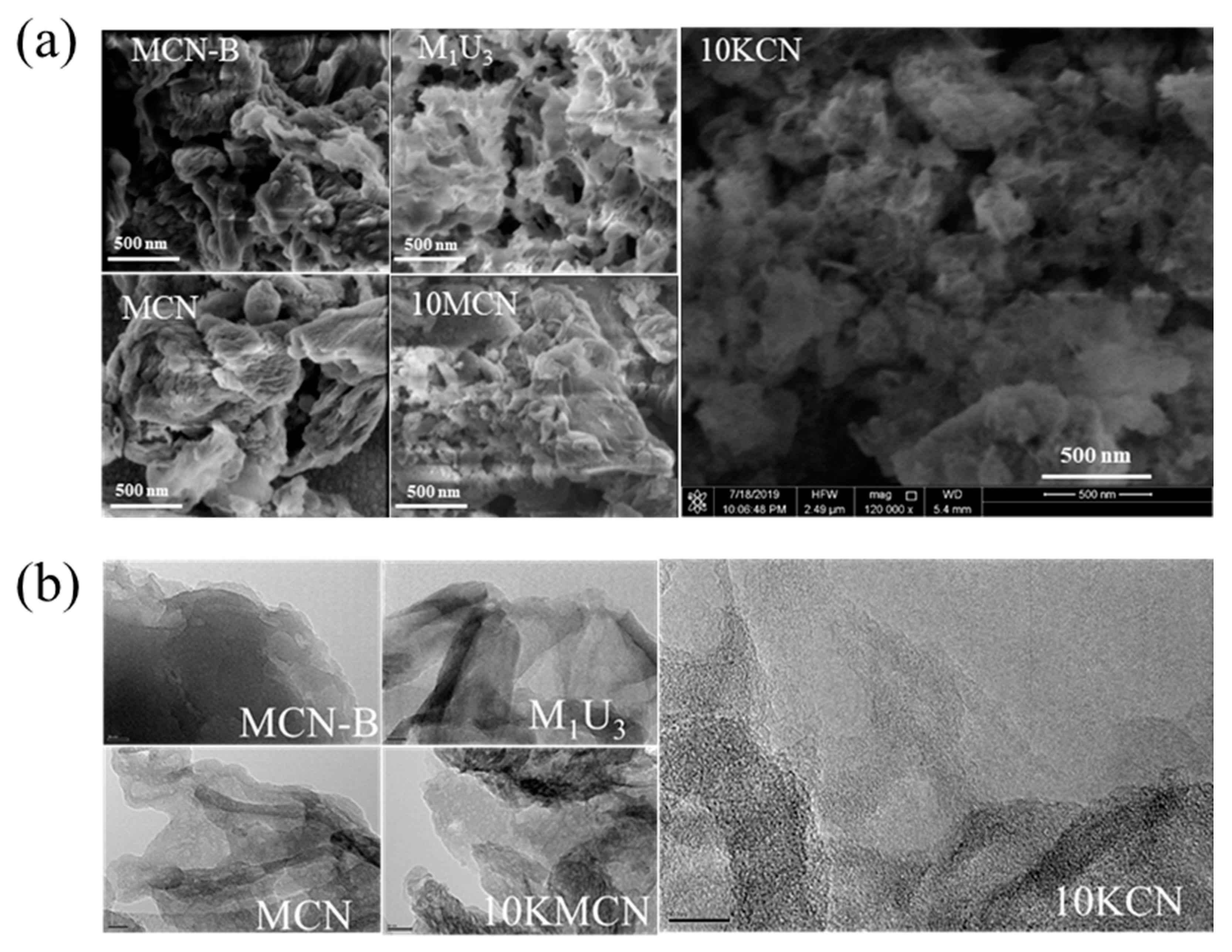
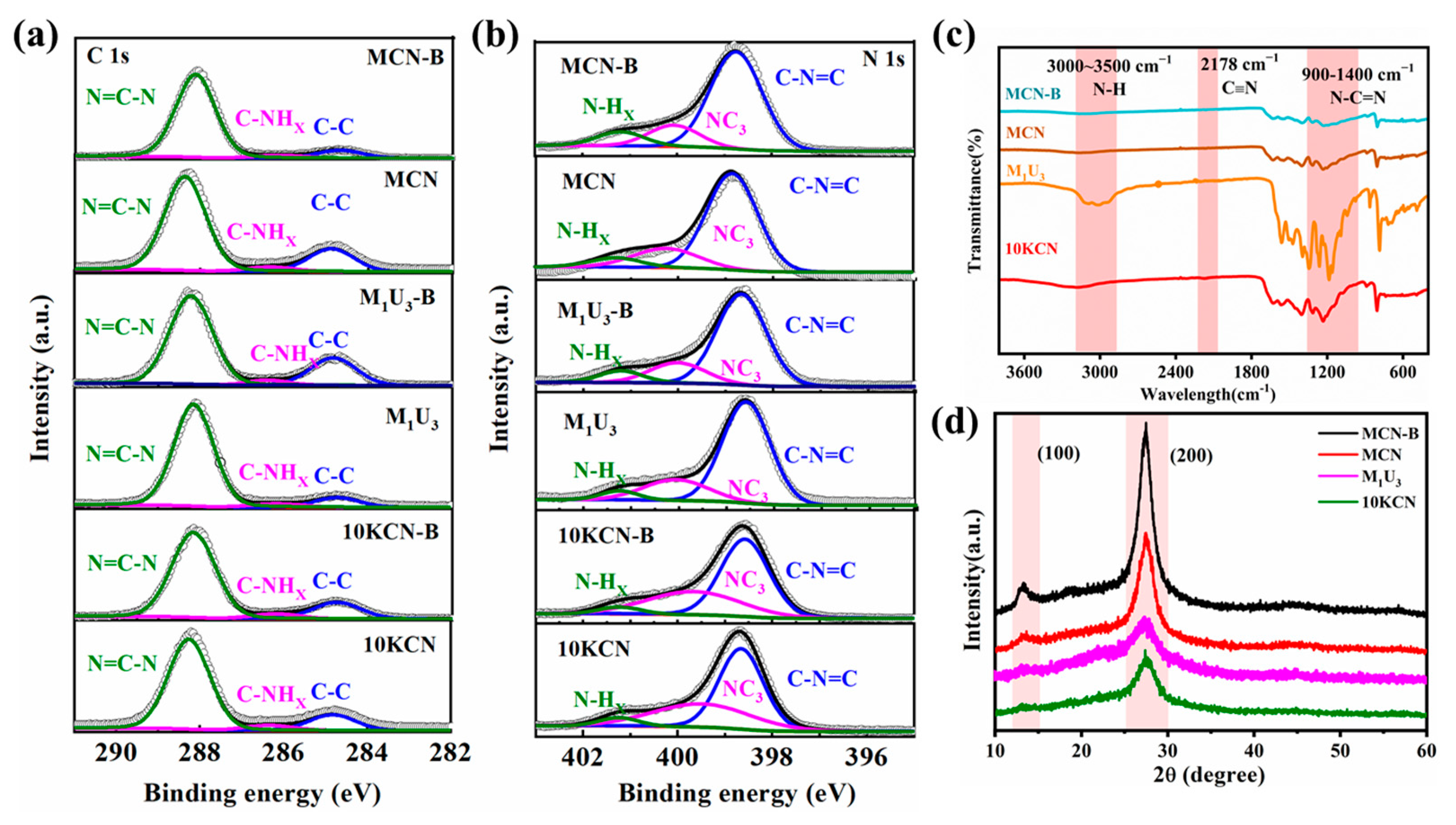
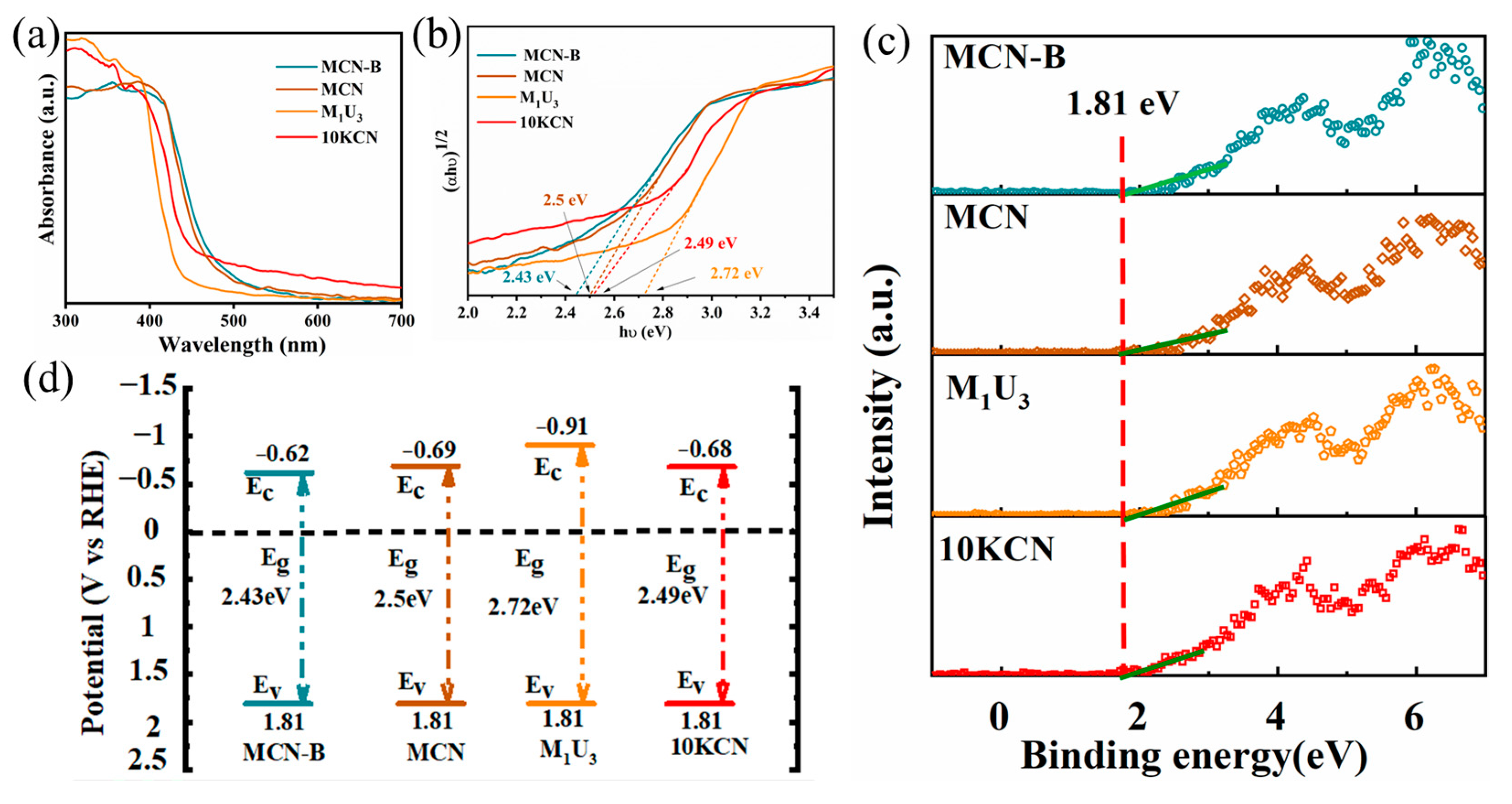
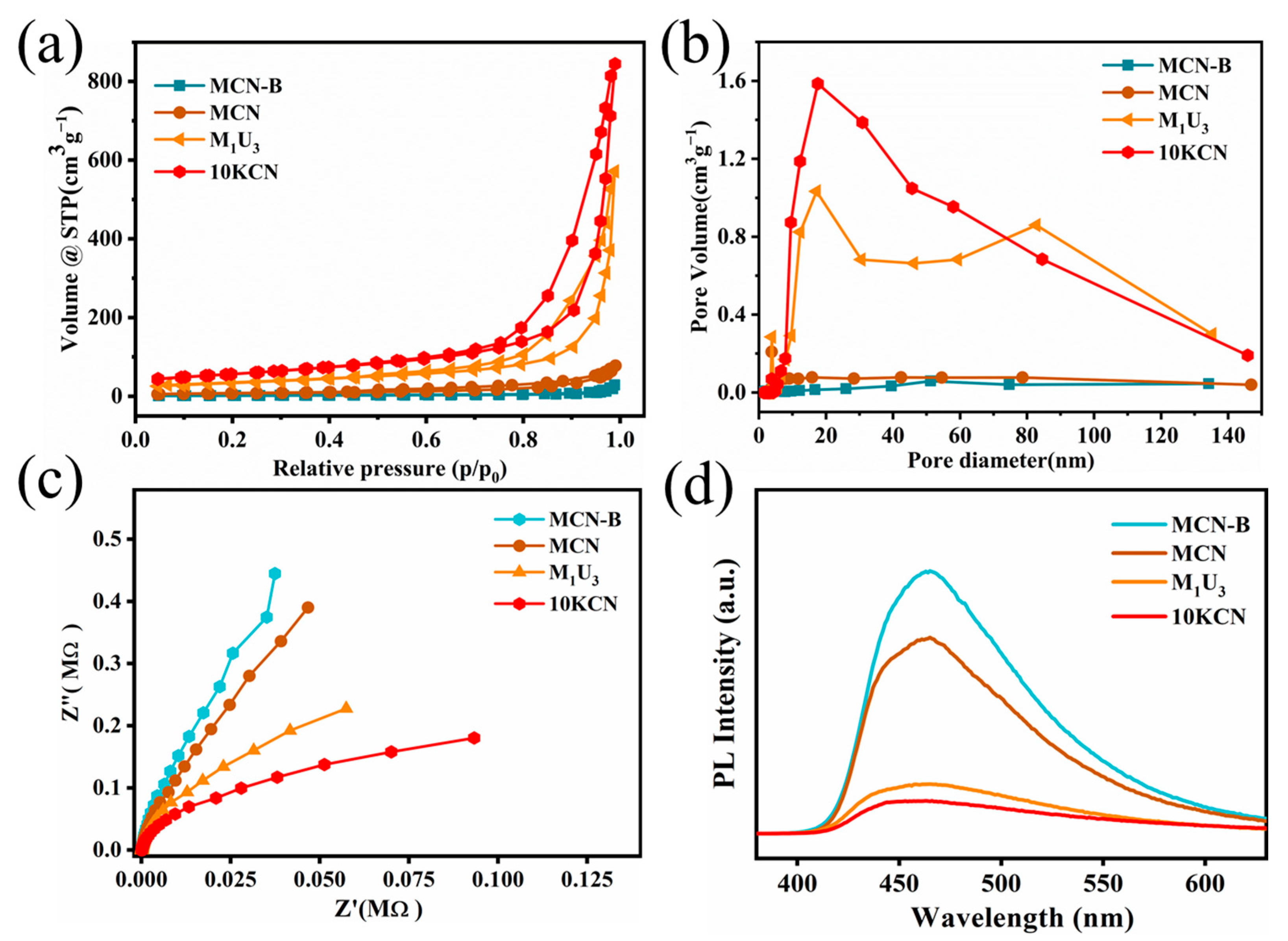
3.1. Material Characterization
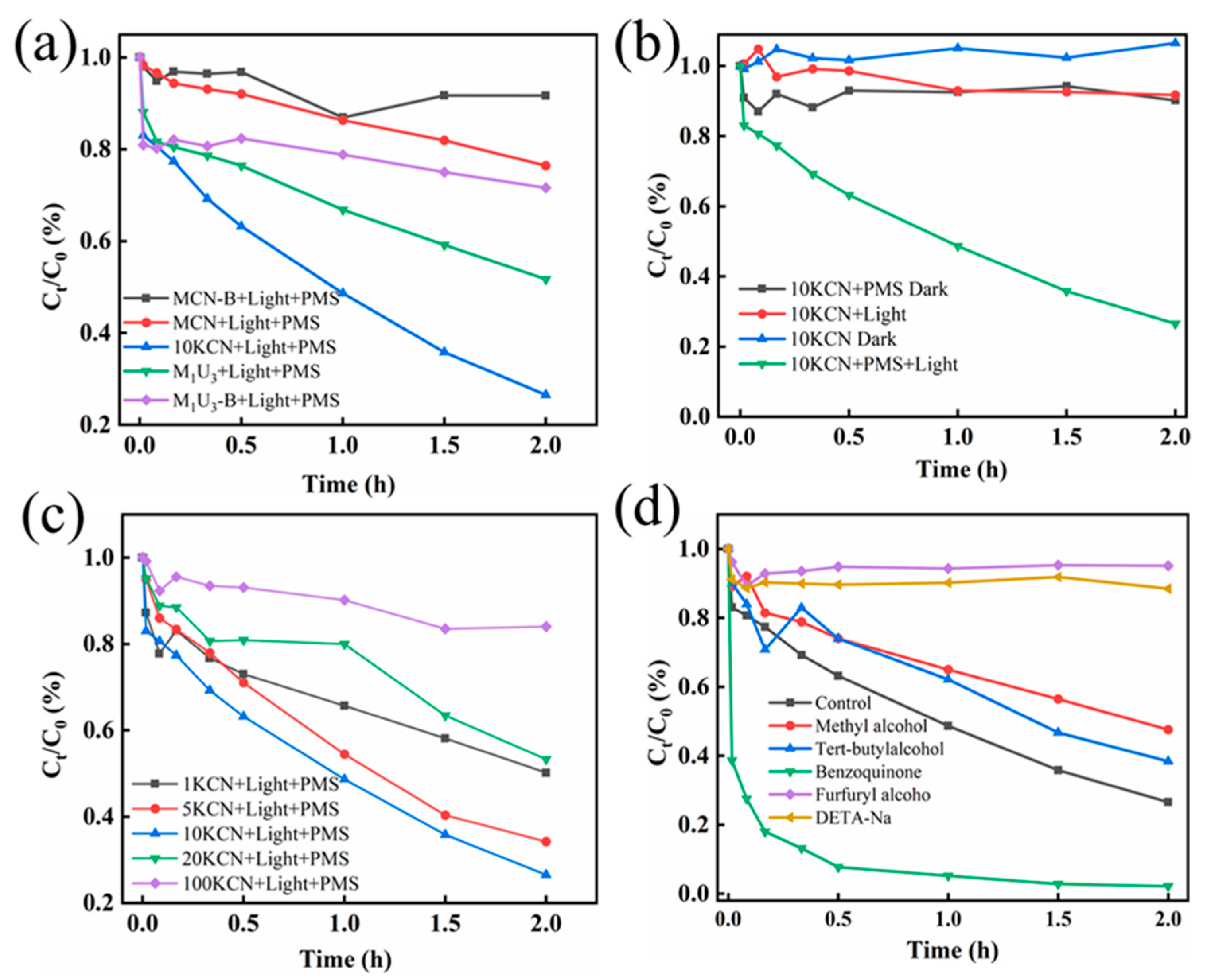
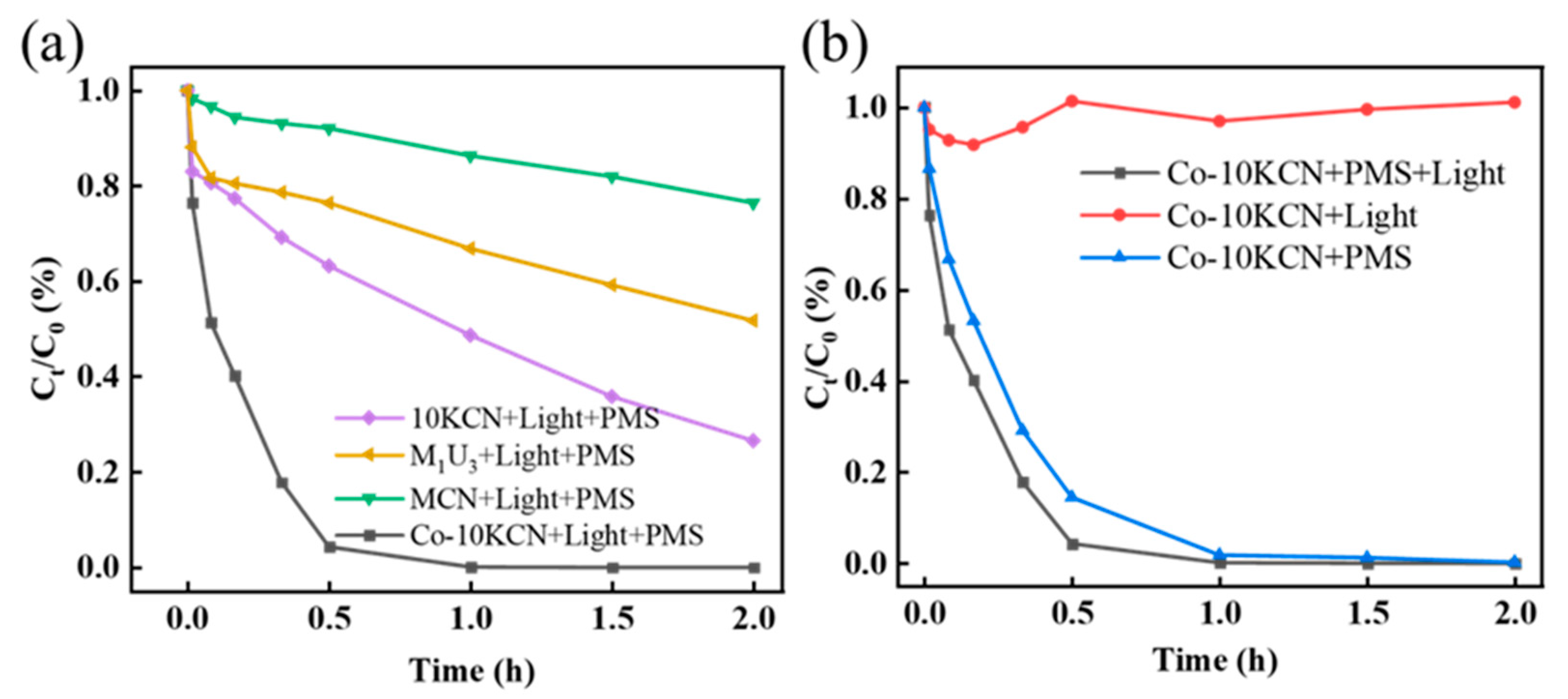
3.2. Activity of Photo-Catalytically Coupled PMS System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Zhang, X.; Feng, R.; Dong, X.; Lu, J.; Jiang, C. Critical roles of soil composition and pollutant properties on the degradation of PPCPs during ferrous/persulfate processes. Chem. Eng. J. 2024, 495, 153390. [Google Scholar] [CrossRef]
- Chen, X.; Lu, X.; Zhao, R.; Su, G.; Meng, J.; Li, Q.; Hua, Y.; Shi, B. Occurrence and risks of PPCPs of a typical mountainous region: Implications for sustainable urban water systems. Sci. Total Environ. 2024, 951, 175714. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, S.; Yang, B.; Deng, J.; Xiao, K.; Chen, H.; Zhuo, Q.; Sun, K. High-efficient degradation of ibuprofen as typical PPCP through a novel electrochemical approach of traditional peroxone method. Sep. Purif. Technol. 2024, 330, 125347. [Google Scholar] [CrossRef]
- Yao, W.; Qi, Y.; Han, Y.; Ge, J.; Dong, Y.; Wang, J.; Yi, Y.; Volmer, D.A.; Li, S.L.; Fu, P. Seasonal variation and dissolved organic matter influence on the distribution, transformation, and environmental risk of pharmaceuticals and personal care products in coastal zone: A case study of Tianjin, China. Water Res. 2024, 249, 120881. [Google Scholar]
- Hussain, S.; Aneggi, E.; Gelao, V.; Briguglio, S.; Mattiussi, M.; Baratta, W.; Zuccaccia, D.; Trovarelli, A.; Goi, D. Potential Residual Toxicity of the Ibuprofen Oxidative Degradation Products by HPLC–MS and Principal Component Analysis. ACS ES&T Water 2024, 4, 2057–2063. [Google Scholar]
- Merkus, V.I.; Sommer, C.; Smollich, E.; Sures, B.; Schmidt, T.C. Acute ecotoxicological effects on daphnids and green algae caused by the ozonation of ibuprofen. Sci. Total Environ. 2022, 847, 157611. [Google Scholar]
- Ellepola, N.; Ogas, T.; Turner, D.N.; Gurung, R.; Maldonado-Torres, S.; Tello-Aburto, R.; Patidar, P.L.; Rogelj, S.; Piyasena, M.E.; Rubasinghege, G. A toxicological study on photo-degradation products of environmental ibuprofen: Ecological and human health implications. Ecotoxicol. Environ. Saf. 2020, 188, 109892. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Dzionek, A.; Wojcieszyńska, D.; Borgulat, J.; Jałowiecki, Ł.; Guzik, U. Changes in Ibuprofen Toxicity and Degradation in Response to Immobilization of Bacillus thuringiensis B1(2015b). Molecules 2024, 29, 5680. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Qu, H.; Zhao, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Yu, G. The influence of nanoplastics on the toxic effects, bioaccumulation, biodegradation and enantioselectivity of ibuprofen in freshwater algae Chlorella pyrenoidosa. Environ. Pollut. 2020, 263 Pt B, 114593. [Google Scholar] [CrossRef]
- Menicagli, V.; Ruffini Castiglione, M.; Cioni, E.; Spano, C.; Balestri, E.; De Leo, M.; Bottega, S.; Sorce, C.; Lardicci, C. Stress responses of the seagrass Cymodocea nodosa to environmentally relevant concentrations of pharmaceutical ibuprofen: Ecological implications. J. Hazard. Mater. 2024, 476, 135188. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar]
- Zhou, H.; Wang, H.; Yue, C.; He, L.; Li, H.; Zhang, H.; Yang, S.; Ma, T. Photocatalytic degradation by TiO2-conjugated/coordination polymer heterojunction: Preparation, mechanisms, and prospects. Appl. Catal. B Environ. 2024, 344, 123605. [Google Scholar]
- Yang, H.; Wang, W.; Wu, X.; Siddique, M.S.; Su, Z.; Liu, M.; Yu, W. Reducing ROS generation and accelerating the photocatalytic degradation rate of PPCPs at neutral pH by doping Fe-N-C to g-C3N4. Appl. Catal. B Environ. 2022, 301, 120790. [Google Scholar]
- Chen, M.; Sun, M.; Cao, X.; Wang, H.; Xia, L.; Jiang, W.; Huang, M.; He, L.; Zhao, X.; Zhou, Y. Progress in preparation, identification and photocatalytic application of defective g-C3N4. Coord. Chem. Rev. 2024, 510, 215849. [Google Scholar]
- Khan, M.A.; Mutahir, S.; Shaheen, I.; Qunhui, Y.; Bououdina, M.; Humayun, M. Recent advances over the doped g-C3N4 in photocatalysis: A review. Coord. Chem. Rev. 2025, 522, 216227. [Google Scholar]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A review of the current status of graphitic carbon nitride. Crit. Rev. Solid. State Mater. Sci. 2020, 46, 189–217. [Google Scholar]
- Khan, J.; Sun, Y.; Han, L. A Comprehensive Review on Graphitic Carbon Nitride for Carbon Dioxide Photoreduction. Small Methods 2022, 6, e2201013. [Google Scholar]
- Xie, K.; Fang, J.; Li, L.; Deng, J.; Chen, F. Progress of graphite carbon nitride with different dimensions in the photocatalytic degradation of dyes: A review. J. Alloys Compd. 2022, 901, 163589. [Google Scholar]
- Dong, S.; Chen, X.; Su, L.; Wen, Y.; Wang, Y.; Yang, Q.; Yi, L.; Xu, W.; Yang, Q.; He, P.; et al. Integration of Atomically Dispersed Cu–N4 Sites with C3N4 for Enhanced Photo-Fenton Degradation over a Nonradical Mechanism. ACS EST Eng. 2022, 3, 150–164. [Google Scholar]
- Zhou, Y.; Ding, J.; Yan, W.; Meng, Y.; Yu, B.; Wen, T.; Yue, Y.; Zhong, Y.; Zhang, H. Well-dispersed copper and molybdenum co-doped g-C3N4 as an excellent persulfate activator for highly efficient removal of bisphenol a in wastewater. Sep. Purif. Technol. 2024, 335, 126195. [Google Scholar]
- Xu, X.; Xiao, Y.; Xu, X.; Carabineiro, S.A.C.; Zhu, J. Dual-site engineering of N vacancies and K single-atoms in C3N4: Enabling spatial charge transfer channels for photocatalysis. J. Mater. 2024, 11, 100969. [Google Scholar]
- Zhang, H.; Hu, X.; Tang, Y.; Zhang, H.; Li, K. Preparation of phosphorus-doped mesoporous g-C3N4 and its photocatalytic degradation of tetracycline hydrochloride. Microporous Mesoporous Mater. 2023, 360, 112733. [Google Scholar]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar]
- Zhao, W.; Shen, Q.; Nan, T.; Zhou, M.; Xia, Y.; Hu, G.; Zheng, Q.; Wu, Y.; Bian, T.; Wei, T.; et al. Cobalt-based catalysts for heterogeneous peroxymonosulfate (PMS) activation in degradation of organic contaminants: Recent advances and perspectives. J. Alloys Compd. 2023, 958, 170370. [Google Scholar]
- Li, N.; Wang, Y.; Cheng, X.; Dai, H.; Yan, B.; Chen, G.; Hou, L.; Wang, S. Influences and mechanisms of phosphate ions onto persulfate activation and organic degradation in water treatment: A review. Water Res. 2022, 222, 118896. [Google Scholar]
- Le, P.-N.-M.; Tran, H.-T.; Huynh, N.-D.-T.; Truong, C.-H.; Ngo, T.-H.; Dang, B.-T.; Luan, V.H.; Tseng, T.-H.; Johan, M.R.; Sagadevan, S.; et al. The synergy of zinc oxide supported magnetic cobalt ferrite: Efficient boosting the peroxymonosulfate activation towards the refractory organic pollutants elimination. J. Alloys Compd. 2023, 968, 172208. [Google Scholar]
- Gao, Y.-Q.; Rao, Y.-Y.; Ning, H.; Chen, J.-X.; Zeng, Q.; Tian, F.-X.; Gao, N.-Y. Comparative investigation of diclofenac degradation by Fe2+/chlorine and Fe2+/PMS processes. Sep. Purif. Technol. 2022, 297, 121555. [Google Scholar]
- Tian, Y.; Jia, N.; Zhou, L.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. Photo-Fenton-like degradation of antibiotics by inverse opal WO(3) co-catalytic Fe2+/PMS, Fe2+/H2O2 and Fe2+/PDS processes: A comparative study. Chemosphere 2022, 288 Pt 3, 132627. [Google Scholar]
- Liu, Y.; Wang, Y.; Wang, Q.; Pan, J.; Zhang, J. Simultaneous removal of NO and SO2 using vacuum ultraviolet light (VUV)/heat/peroxymonosulfate (PMS). Chemosphere 2018, 190, 431–441. [Google Scholar] [CrossRef]
- Hao, R.; Gu, X.; Liu, T.; Li, S. One pot synthesis of novel C3N4/C nanosheet composites under environmental benign molten salts for peroxymonosulfate activation with enhanced efficiency and reusability. J. Environ. Chem. Eng. 2024, 12, 113526. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Zhong, D.; Zhong, N. Electron utilization efficiency of ZVI core activating PMS enhanced by C-N/g-C3N4 shell. Appl. Catal. A General 2020, 608, 117828. [Google Scholar] [CrossRef]
- Hu, E.; Chen, Q.; Gao, Q.; Fan, X.; Luo, X.; Wei, Y.; Wu, G.; Deng, H.; Xu, S.; Wang, P.; et al. Cyano group-Functionalized Graphitic Carbon Nitride with Adsorption and Photoreduction Isosite Achieving Efficient Uranium Extraction from Seawater. Adv. Funct. Mater. 2024, 34, 2312215. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, J.; Wang, D.; Li, C.; Zhang, X.; Wen, C.; Song, Q.; Wang, J.; Zhu, G.; Zhu, Y. Bifunctional perylene diimide supramolecular photocatalyst for antibiotic removal and heavy metal chromium (Cr) recovery. Appl. Catal. B Environ. Energy 2025, 365, 124962. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, K.; Cui, M.; Liu, X.; Zhang, Q. Co-modification of carbon and cyano group defect in g-C3N4 for enhanced photocatalytic peroxymonosulfate activation: Combined experimental and theoretical analysis. Sep. Purif. Technol. 2023, 316, 123844. [Google Scholar] [CrossRef]
- Liu, X.; Cui, M.; Cui, K.; Ding, Y.; Chen, X.; Chen, C.; Nie, X. Construction of Li/K dopants and cyano group defects in graphitic carbon nitride for highly efficient peroxymonosulfate activation towards organic contaminants degradation. Chemosphere 2022, 294, 133700. [Google Scholar] [CrossRef]
- Dou, Y.; Zhu, C.; Zhu, M.; Fu, Y.; Wang, H.; Shi, C.; Huang, H.; Liu, Y.; Kang, Z. Highly mesoporous carbon nitride photocatalysts for efficient and stable overall water splitting. Appl. Surf. Sci. 2020, 509, 144706. [Google Scholar] [CrossRef]
- Chen, X.; Guo, T.; Yan, T.; Dai, Y.; Yin, L. Selective generation of hydroxyl and sulfate radicals under electric field regulation for micropollutants degradation: Mechanism and structure-activity relationship. J. Hazard. Mater. 2025, 481, 136513. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef]
- Liu, X.; Shao, P.; Gao, S.; Bai, Z.; Tian, J. Benzoquinone-assisted heterogeneous activation of PMS on Fe3S4 via formation of active complexes to mediate electron transfer towards enhanced bisphenol A degradation. Water Res. 2022, 226, 119218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, J.; Li, Y.; Huang, Y.; Huang, Z.; Yan, G.; Liang, S.; Zhang, Y.; Zeng, K.; Qi, J.; et al. Regulation of Thin-Layered g-C3N4 for Efficient Persulfate Photocatalysis of Ibuprofen Contaminated Groundwater. Sustainability 2025, 17, 2831. https://doi.org/10.3390/su17072831
Yang Y, Li J, Li Y, Huang Y, Huang Z, Yan G, Liang S, Zhang Y, Zeng K, Qi J, et al. Regulation of Thin-Layered g-C3N4 for Efficient Persulfate Photocatalysis of Ibuprofen Contaminated Groundwater. Sustainability. 2025; 17(7):2831. https://doi.org/10.3390/su17072831
Chicago/Turabian StyleYang, Yunchuan, Jie Li, Yong Li, Yanbin Huang, Zuyang Huang, Gangan Yan, Siran Liang, Yining Zhang, Ke Zeng, Junjie Qi, and et al. 2025. "Regulation of Thin-Layered g-C3N4 for Efficient Persulfate Photocatalysis of Ibuprofen Contaminated Groundwater" Sustainability 17, no. 7: 2831. https://doi.org/10.3390/su17072831
APA StyleYang, Y., Li, J., Li, Y., Huang, Y., Huang, Z., Yan, G., Liang, S., Zhang, Y., Zeng, K., Qi, J., Xiong, S., Liu, J., & Ng, H. Y. (2025). Regulation of Thin-Layered g-C3N4 for Efficient Persulfate Photocatalysis of Ibuprofen Contaminated Groundwater. Sustainability, 17(7), 2831. https://doi.org/10.3390/su17072831





