Effect of Plant Part and Age on the Proximate, Chemical, and Elemental Characteristics of Elephant Grass Cultivar BRS Capiaçu for Combustion-Based Sustainable Bioenergy
Abstract
1. Introduction
2. Materials and Methods
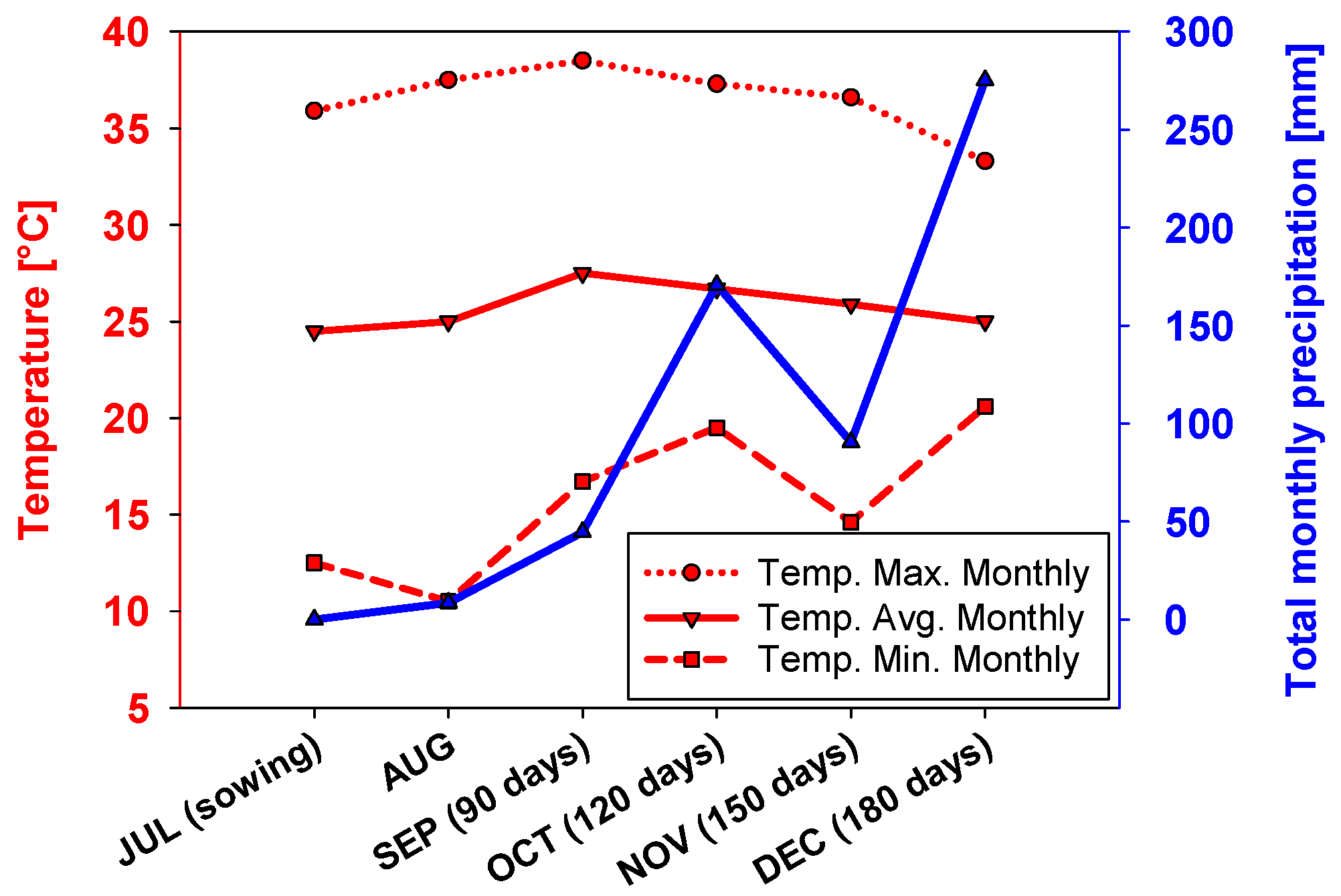
2.1. Biomass Production
2.2. Proximate and Density Analysis
2.3. Chemical Analysis
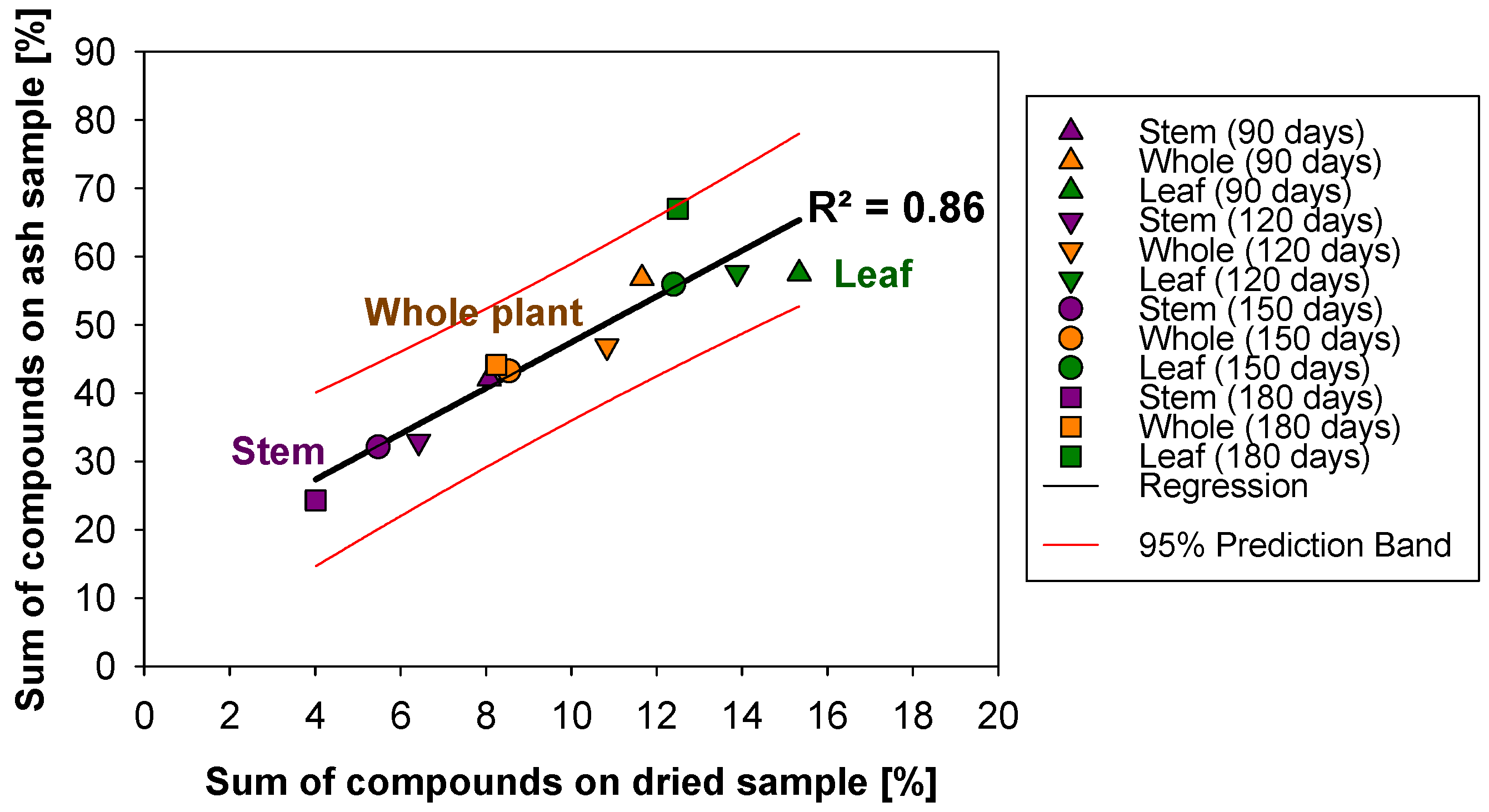
2.4. Elemental Analysis
3. Results and Discussion
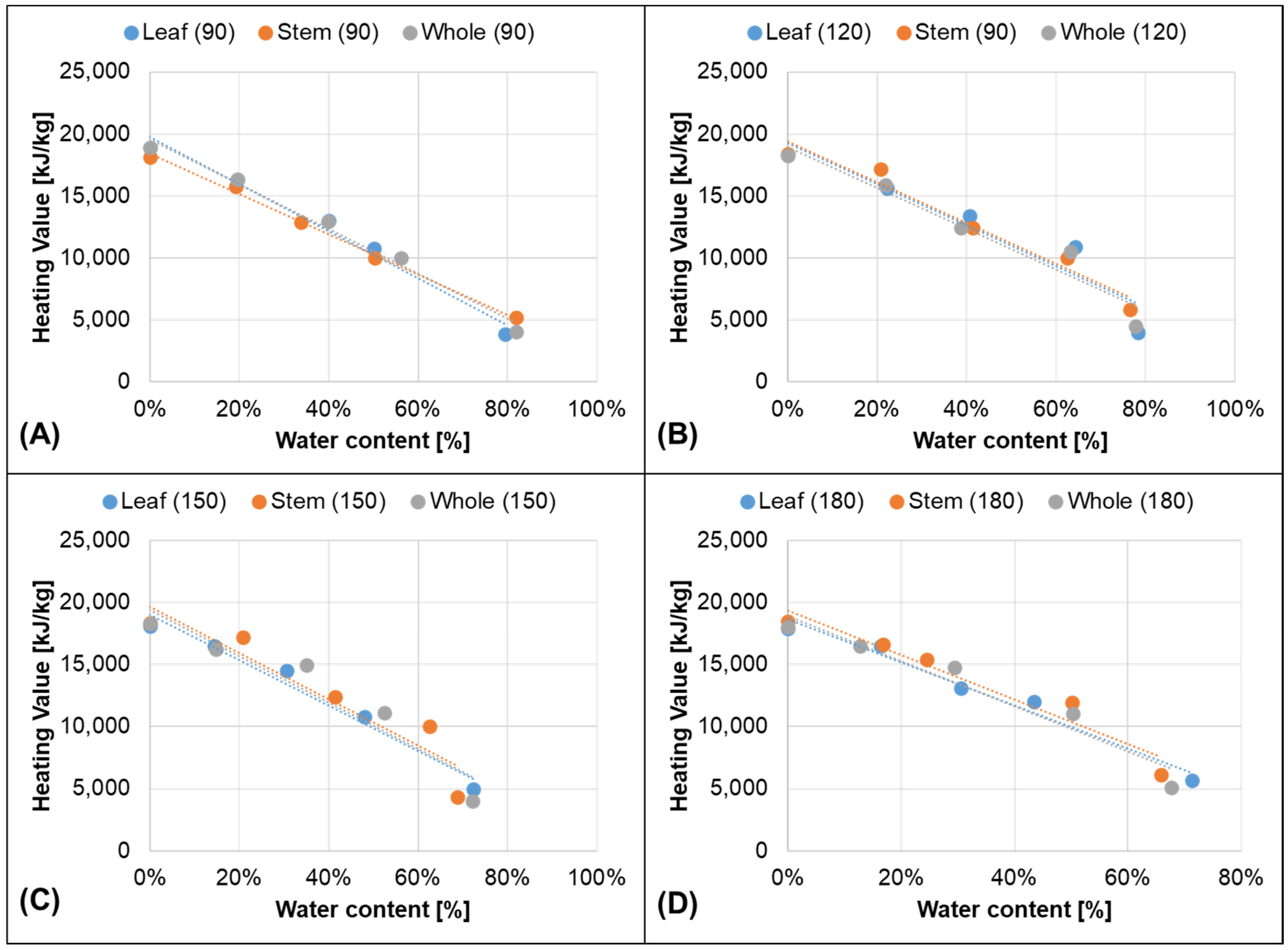
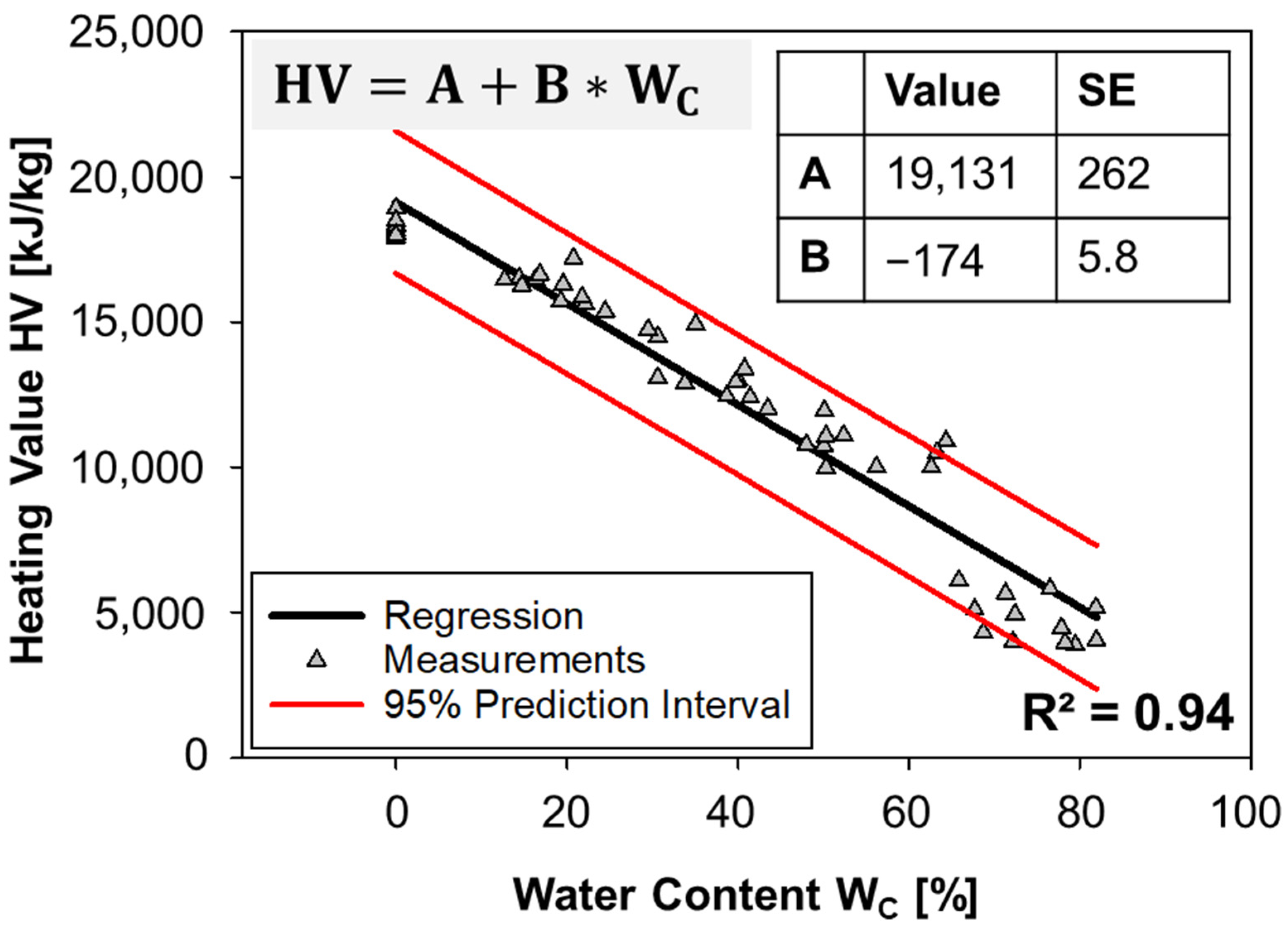
3.1. Proximate and Density Analysis: Effect of Water Content on Density and Heating Value
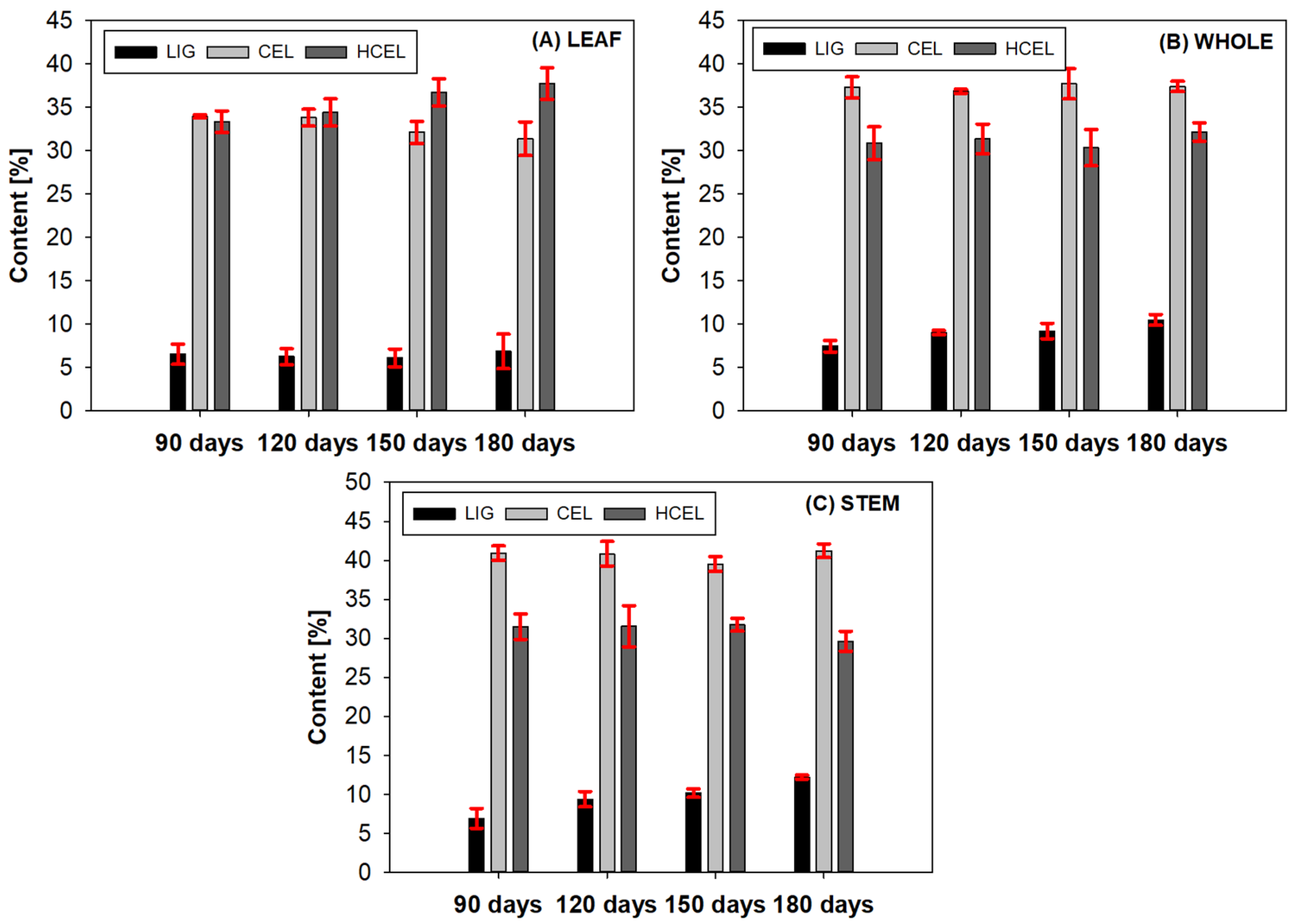
3.2. Chemical Analysis: Effect of Plant Age and Parts on Lignocellulosic Composition
3.3. Elemental Analysis: Ash Behaviour on Potential Challenges During Operation
3.4. Impact of Findings on the Selection of Plant Part and Age
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Age | Leaf | Whole | Stem | |||
|---|---|---|---|---|---|---|
| Water Content | Density | Water Content | Density | Water Content | Density | |
| 90 days | 79.46 ± 0.39 | 124.74 ± 7.74 | 81.91 ± 0.58 | 177.79 ± 4.38 | 81.85 ± 0.60 | 247.89 ± 6.53 |
| 90 days | 50.04 ± 1.53 | 70.53 ± 3.07 | 56.20 ± 1.60 | 100.00 ± 1.66 | 50.30 ± 1.13 | 123.68 ± 2.35 |
| 90 days | 40.03 ± 1.51 | 65.79 ± 1.66 | 39.76 ± 3.30 | 71.05 ± 1.66 | 33.79 ± 1.95 | 104.21 ± 2.68 |
| 90 days | 19.56 ± 0.64 | 60.32 ± 1.72 | 19.54 ± 1.23 | 64.74 ± 1.29 | 19.28 ± 0.44 | 88.42 ± 1.29 |
| 90 days | 0.00 ± 0.00 | 56.32 ± 2.68 | 0.00 ± 0.00 | 58.42 ± 1.97 | 0.00 ± 0.00 | 74.21 ± 1.97 |
| 120 days | 78.26 ± 0.55 | 128.95 ± 3.72 | 77.84 ± 0.95 | 184.53 ± 1.78 | 76.49 ± 1.14 | 263.16 ± 1.45 |
| 120 days | 64.32 ± 1.83 | 81.37 ± 1.72 | 63.29 ± 1.45 | 116.84 ± 2.86 | 62.60 ± 1.79 | 180.84 ± 2.57 |
| 120 days | 40.77 ± 0.57 | 56.84 ± 1.63 | 38.67 ± 1.58 | 82.32 ± 1.40 | 41.40 ± 1.73 | 106.84 ± 1.45 |
| 120 days | 22.13 ± 2.14 | 53.68 ± 1.00 | 21.76 ± 0.53 | 68.95 ± 1.79 | 20.76 ± 0.26 | 100.00 ± 1.94 |
| 120 days | 0.00 ± 0.00 | 49.47 ± 1.97 | 0.00 ± 0.00 | 58.95 ± 2.68 | 0.00 ± 0.00 | 87.37 ± 1.97 |
| 150 days | 72.42 ± 2.66 | 111.58 ± 4.28 | 72.14 ± 1.65 | 151.05 ± 6.36 | 68.70 ± 0.78 | 217.37 ± 7.55 |
| 150 days | 48.01 ± 1.11 | 82.11 ± 1.97 | 52.37 ± 0.34 | 113.47 ± 1.78 | 62.60 ± 1.79 | 163.89 ± 1.18 |
| 150 days | 30.61 ± 1.71 | 64.21 ± 2.11 | 35.05 ± 4.69 | 100.00 ± 1.66 | 41.40 ± 1.73 | 127.89 ± 2.11 |
| 150 days | 14.41 ± 0.29 | 57.89 ± 1.66 | 14.73 ± 1.76 | 83.68 ± 1.97 | 20.76 ± 0.26 | 112.95 ± 1.72 |
| 150 days | 0.00 ± 0.00 | 51.05 ± 2.68 | 0.00 ± 0.00 | 51.05 ± 2.68 | 0.00 ± 0.00 | 96.84 ± 3.07 |
| 180 days | 71.31 ± 1.13 | 120.00 ± 2.11 | 67.70 ± 0.96 | 151.89 ± 3.52 | 65.85 ± 1.02 | 216.84 ± 4.88 |
| 180 days | 43.49 ± 0.39 | 85.79 ± 2.11 | 50.30 ± 0.71 | 108.53 ± 2.18 | 50.11 ± 0.33 | 156.32 ± 2.68 |
| 180 days | 30.60 ± 0.26 | 62.11 ± 1.29 | 29.49 ± 0.31 | 88.95 ± 2.20 | 24.49 ± 0.30 | 128.42 ± 1.97 |
| 180 days | 16.32 ± 0.38 | 51.05 ± 1.29 | 12.71 ± 0.11 | 66.84 ± 3.00 | 16.83 ± 0.34 | 115.26 ± 1.97 |
| 180 days | 0.00 ± 0.00 | 45.79 ± 2.68 | 0.00 ± 0.00 | 72.11 ± 3.00 | 0.00 ± 0.00 | 100.53 ± 3.07 |
References
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass Energy and Biofuels: Perspective, Potentials, and Challenges in the Energy Transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Paletto, A.; Bernardi, S.; Pieratti, E.; Teston, F.; Romagnoli, M. Assessment of environmental impact of biomass power plants to increase the social acceptance of renewable energy technologies. Heliyon 2019, 5, e02070. [Google Scholar] [CrossRef]
- Janiszewska, D.; Ossowska, L. The Role of Agricultural Biomass as a Renewable Energy Source in European Union Countries. Energies 2022, 15, 6756. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Schmer, M.R.; Anderson, W.F.; Jin, V.; Balkcom, K.S.; Kiniry, J.; Coffin, A.; White, P. Dedicated Energy Crops and Crop Residues for Bioenergy Feedstocks in the Central and Eastern USA. Bioenerg. Res. 2016, 9, 384–398. [Google Scholar] [CrossRef]
- Lewandrowski, J.; Rosenfeld, J.; Pape, D.; Hendrickson, T.; Jaglo, K.; Moffroid, K. The greenhouse gas benefits of corn ethanol—Assessing recent evidence. Biofuels 2020, 11, 361–375. [Google Scholar] [CrossRef]
- Alves, F.G.d.S.; Silva, S.F.; Santos, F.N.d.S.; Carneiro, M.S.d.S. Elephant grass: A bioenergetic resource. Nucleus Animal. 2018, 10, 117–130. [Google Scholar] [CrossRef]
- Campos, B.B.; Diniz, R.H.S.; Silveira, F.A.d.; Ribeiro Júnior, J.I.; Fietto, L.G.; Machado, J.C.; Silveira, W.B.d. Elephant grass (Pennisetum purpureum Schumach) is a promising feedstock for ethanol production by the thermotolerant yeast Kluyveromyces marxianus CCT 7735. Braz. J. Chem. Eng. 2019, 36, 43–49. [Google Scholar] [CrossRef]
- Efetobor, U.J.; Ikpeseni, S.C.; Sada, S.O. Determination of proximate, ultimate and structural properties of elephant grass as biomass material for bio-oil production. J. Appl. Sci. Environ. Manag. 2022, 26, 1903–1907. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J.; Hayman, C. Thermal conversion of elephant grass (Pennisetum purpureum Schum) to bio-gas, bio-oil and charcoal. Bioresour. Technol. 2008, 99, 8394–8399. [Google Scholar] [CrossRef]
- de Conto, D.; Silvestre, W.P.; Baldasso, C.; Godinho, M. Performance of rotary kiln reactor for the elephant grass pyrolysis. Bioresour. Technol. 2016, 218, 153–160. [Google Scholar] [CrossRef]
- Morais, R.F.; Morais, C.S.B.; de Morais, L.F.; Almeida, J.C.C. Energy balance of elephant grass biomass for power generation by direct biomass combustion. Afr. J. Biotechnol. 2018, 17, 405–410. [Google Scholar] [CrossRef]
- Rocha, J.R.d.A.S.d.C.; Machado, J.C.; Carneiro, P.C.S.; Da Carneiro, J.C.; Resende, M.D.V.; Pereira, A.V.; Carneiro, J.E.d.S. Elephant grass ecotypes for bioenergy production via direct combustion of biomass. Ind. Crops Prod. 2017, 95, 27–32. [Google Scholar] [CrossRef]
- Ana, K.F.V.; Da Tatiane, C.B.; Rogerio, F.D.; Janeo, E.A.F.; Roberta, S.N.d.L.; Rafael, S.F.; Drieli, A.R.; Da Erik, S.O.; Da Bruna, R.S.M.; Geovana, C.E.; et al. Production potential and chemical composition of elephant grass (Pennisetum purpureum Schum.) at different ages for energy purposes. Afr. J. Biotechnol. 2017, 16, 1428–1433. [Google Scholar] [CrossRef]
- Johannes, L.P.; Minh, T.T.N.; Xuan, T.D. Elephant Grass (Pennisetum purpureum): A Bioenergy Resource Overview. Biomass 2024, 4, 625–646. [Google Scholar] [CrossRef]
- Alonso Pippo, W.; Alves, A. Corn Ethanol in Brazil: Analyzing the Prospect of Becoming a Widespread Practice in South America. Sugar Tech 2022, 24, 857–869. [Google Scholar] [CrossRef]
- Silva, A.L.d.; Castañeda-Ayarza, J.A. Macro-environment analysis of the corn ethanol fuel development in Brazil. Renew. Sustain. Energy Rev. 2021, 135, 110387. [Google Scholar] [CrossRef]
- Freitas, R.S.; Da Barbé, T.C.; Daher, R.F.; Vidal, A.K.F.; Stida, W.F.; Da Silva, V.B.; Da Menezes, B.R.S.; Pereira, A.V. Chemical Composition and Energy Yield of Elephant-Grass Biomass as Function of Five Different Production Ages. JAS 2017, 10, 343. [Google Scholar] [CrossRef]
- Pereira, A.V.; Lédo, F.J.d.S.; Machado, J.C. BRS Kurumi and BRS Capiaçu-New elephant grass cultivars for grazing and cut-and-carry system. Crop Breed. Appl. Biotechnol. 2017, 17, 59–62. [Google Scholar] [CrossRef]
- Beber, R.C.; Da Turini, C.S.; Beber, V.C.; Nogueira, R.M.; Pires, E.M. Elephant Grass Cultivar BRS Capiaçu as Sustainable Biomass for Energy Generation in the Amazon Biome of the Mato Grosso State. Energies 2024, 17, 5409. [Google Scholar] [CrossRef]
- Huang, S.; Liu, J.; Chen, S.; Wang, J.; Chen, Z.; Evrendilek, F.; Chen, T.; Huang, W.; Xie, W.; Sun, S. Converting and valorizing heavy metal-laden post-harvest hyperaccumulator (Pteris vittate L.) into biofuel via acid-pretreated pyrolysis and gasification. Chem. Eng. J. 2023, 468, 143490. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Z.; Liu, J.; Chen, Z.; Evrendilek, F.; Huang, W. Oxy-fuel and air combustion performances and gas-to-ash products of aboveground and belowground biomass of Sedum alfredii Hance. Chem. Eng. J. 2021, 422, 130312. [Google Scholar] [CrossRef]
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. A framework for the design and analysis of integrated multi-product biorefineries from agricultural and forestry wastes. Renew. Sustain. Energy Rev. 2021, 139, 110687. [Google Scholar] [CrossRef]
- Singh, Y.D.; Mahanta, P.; Bora, U. Comprehensive characterization of lignocellulosic biomass through proximate, ultimate and compositional analysis for bioenergy production. Renew. Energy 2017, 103, 490–500. [Google Scholar] [CrossRef]
- Rizvi, T.; Xing, P.; Pourkashanian, M.; Darvell, L.I.; Jones, J.M.; Nimmo, W. Prediction of biomass ash fusion behaviour by the use of detailed characterisation methods coupled with thermodynamic analysis. Fuel 2015, 141, 275–284. [Google Scholar] [CrossRef]
- Lacey, J.A.; Aston, J.E.; Thompson, V.S. Wear Properties of Ash Minerals in Biomass. Front. Energy Res. 2018, 6, 119. [Google Scholar] [CrossRef]
- Antunes, R.A.; de Oliveira, M.C.L. Corrosion in biomass combustion: A materials selection analysis and its interaction with corrosion mechanisms and mitigation strategies. Corros. Sci. 2013, 76, 6–26. [Google Scholar] [CrossRef]
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An overview of slagging and fouling indicators and their applicability to biomass fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Ardila, Y.C.; Figueroa, J.E.J.; Maciel, M.R.W. Mathematical models for predicting the higher heating value and ultimate analysis of biomass. Ind. Crops Prod. 2024, 208, 117777. [Google Scholar] [CrossRef]
- Kujawska, J.; Kulisz, M.; Oleszczuk, P.; Cel, W. Improved Prediction of the Higher Heating Value of Biomass Using an Artificial Neural Network Model Based on the Selection of Input Parameters. Energies 2023, 16, 4162. [Google Scholar] [CrossRef]
- Vargas-Moreno, J.M.; Callejón-Ferre, A.J.; Pérez-Alonso, J.; Velázquez-Martí, B. A review of the mathematical models for predicting the heating value of biomass materials. Renew. Sustain. Energy Rev. 2012, 16, 3065–3083. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.Y.; Kim, H.E.; Oh, K.C.; Kim, S.J.; La Cho, H.; Jeon, Y.K.; Kim, D. Calorific Value Prediction Model Using Structure Composition of Heat-Treated Lignocellulosic Biomass. Energies 2023, 16, 7896. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical review of predictive coefficients for biomass ash deposition tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Nhuchhen, D.R. Prediction of carbon, hydrogen, and oxygen compositions of raw and torrefied biomass using proximate analysis. Fuel 2016, 180, 348–356. [Google Scholar] [CrossRef]
- National Institute of Metereology. Metereological Historical Data for 2022-City: Sao José do Rio Claro, State of Mato Grosso. Available online: https://portal.inmet.gov.br/dadoshistoricos (accessed on 12 August 2024).
- ABNT. NBR 8633: Carvão Vegetal-Determinação do Poder Calorífico (Charcoal-Determination of Calorific Value); Associação Brasileira de Normas Técnicas: São Paulo, SP, Brazil, 1983. [Google Scholar]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Xing, P.; Mason, P.E.; Chilton, S.; Lloyd, S.; Jones, J.M.; Williams, A.; Nimmo, W.; Pourkashanian, M. A comparative assessment of biomass ash preparation methods using X-ray fluorescence and wet chemical analysis. Fuel 2016, 182, 161–165. [Google Scholar] [CrossRef]
- Kofman, P.; Kent, T. Harvesting and Processing Forest Biomass for Energy Production in Ireland: The ForestEnergy Programme; COFORT: Dublin, Ireland, 2007. [Google Scholar]
- Ungureanu, N.; Vladut, V.; Voicu, G.; Dinca, M.-N.; Zabava, B.-S. Influence of biomass moisture content on pellet properties-review. In Proceedings of the 17th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 23–25 May 2018; Latvia University of Agriculture: Jelgava, Latvia, 2018. [Google Scholar]
- Ismail, A.B.O.; Sulaiman, Y.R.; Ahmed, F.A.; Ali, H.A.M. Effect of Stages of Maturity on Nutritive Value of Some Range Herbage Species in Low-Rainfall Woodland Savanna Southern Darfur, Sudan. Open J. Anim. Sci. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Ndathi, A.J.N.; Wambua, S.M.; Bosma, L.; Kaindi, E.M.; Kioko, T.; Kadenyi, N.; Musyoki, G.K.; van Steenbergen, F.; Musimba, N.K.R. Forage value of vegetative leaf and stem biomass fractions of selected grasses indigenous to African rangelands. Anim. Prod. Sci. 2021, 61, 1476–1483. [Google Scholar] [CrossRef]
- Okumus, O.; Ciftci, B.; Uzun, S.; Kaplan, M. Determination of feed quality parameters of leaves and stems of different alfalfa genotypes. Curr. Trends Nat. Sci. 2021, 10, 118–121. [Google Scholar] [CrossRef]
- Egwu, U. Improvement of biomethane potential of Elephant grass (Napier grass, Pennisetum purpureum Schum) by addition of biomass ash and ash-extracts as supplements. Bioresour. Technol. Rep. 2021, 15, 100760. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Lee, W.-C.; Kuan, W.-C.; Sirisansaneeyakul, S.; Savarajara, A. Evaluation of different pretreatments of Napier grass for enzymatic saccharification and ethanol production. Energy Sci. Eng. 2018, 6, 683–692. [Google Scholar] [CrossRef]
- Marafon, A.C.; Amaral, A.F.C.; Machado, J.C.; Da Carneiro, J.C.; Bierhals, A.N.; Guimarães, V.d.S. Chemical composition and calorific value of elephant grass varieties and other feedstocks intended for direct combustion. Grassl. Sci. 2021, 67, 241–249. [Google Scholar] [CrossRef]
- Flyman, M.V.; Afolayan, A.J. Effect of plant maturity on the mineral content of the leaves of Momordica balsamina L. and Vigna unguiculata subsp. Sesquipedalis (L.). Verdc. J. Food Qual. 2008, 31, 661–671. [Google Scholar] [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123849052. [Google Scholar]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.-C.; Li, W.-Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Li, F.; Yu, B.; Zhao, W.; Wang, J.; Xu, M.; Fan, H.; Huang, J.; Fang, Y. Investigation on formation mechanisms of ash and deposit from cotton stalk vibrating grate boiler combustion based on their characteristics. Fuel 2022, 323, 124446. [Google Scholar] [CrossRef]
- Vlček, J.; Ovčačíková, H.; Velička, M.; Topinková, M.; Burda, J.; Matějková, P. The Corrosion Effect of Fly Ash from Biomass Combustion on Andalusite Refractory Materials. Minerals 2023, 13, 357. [Google Scholar] [CrossRef]
- Horák, J.; Kuboňová, L.; Dej, M.; Laciok, V.; Tomšejová, Š.; Hopan, F.; Krpec, K.; Koloničný, J. Effects of the type of biomass and ashing temperature on the properties of solid fuel ashes. Pol. J. Chem. Technol. 2019, 21, 43–51. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D. Predicting the behaviour of ash from agricultural wastes during combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Mayes, W.M.; Stewart, D.I. New insights into biomass combustion ash categorisation: A phylogenetic analysis. Fuel 2021, 287, 119469. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Martí-Rosselló, T.; Zhang, X. Experimental study on the ignition characteristics of cellulose, hemicellulose, lignin and their mixtures. J. Energy Inst. 2019, 92, 1303–1312. [Google Scholar] [CrossRef]
- Jung, S.-J.; Kim, S.-H.; Chung, I.-M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mishra, A.K.; Shah, S.N.; Bhat, M.A.; Jan, S.; Rahman, S.; Baek, K.-H.; Jan, A.T. Soil and Mineral Nutrients in Plant Health: A Prospective Study of Iron and Phosphorus in the Growth and Development of Plants. Curr. Issues Mol. Biol. 2024, 46, 5194–5222. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, C.F.; Brandão, L.E.; Gomes, L.L. Elephant grass biorefineries: Towards a cleaner Brazilian energy matrix? J. Clean. Prod. 2015, 96, 85–93. [Google Scholar] [CrossRef]


| Part | Ages [Days] | Density = f (WC) | R2 |
|---|---|---|---|
| Leaf | 90 | D = 148.75 ∗ Wc2 − 31.896 ∗ Wc + 54.514 | 0.9762 |
| Whole | 90 | D = 258.6 ∗ Wc2 − 71.948 ∗ Wc + 61.62 | 0.9915 |
| Stem | 90 | D = 296.21 ∗ Wc2 − 38.448 ∗ Wc + 78.28 | 0.9899 |
| Leaf | 120 | D = 215.7 ∗ Wc2 − 80.665 ∗ Wc + 53.869 | 0.9548 |
| Whole | 120 | D = 273.02 ∗ Wc2 − 68.567 ∗ Wc + 64.66 | 0.9691 |
| Stem | 120 | D = 499.78 ∗ Wc2 − 178.24 ∗ Wc + 102.19 | 0.9851 |
| Leaf | 150 | D = 95.24 ∗ Wc2 + 11.82 ∗ Wc + 53.316 | 0.9978 |
| Whole | 150 | D = 95.359 ∗ Wc2 + 35.858 ∗ Wc + 73.545 | 0.988 |
| Stem | 150 | D = 180.8 ∗ Wc2 + 48.847 ∗ Wc + 81.793 | 0.9232 |
| Leaf | 180 | D = 112.2 ∗ Wc2 + 23.003 ∗ Wc + 48.1 | 0.9778 |
| Whole | 180 | D = 130.79 ∗ Wc2 + 44.35 ∗ Wc + 59.414 | 0.9871 |
| Stem | 180 | D = 287.87 ∗ Wc2 − 39.587 ∗ Wc + 113.53 | 0.9771 |
| Part | Ages [Days] | Heating Value = f (WC) | R2 |
|---|---|---|---|
| Leaf | 90 | HV = −18,947 ∗ Wc + 19,735 | 0.9823 |
| Whole | 90 | HV = −18,085 ∗ Wc + 19,585 | 0.9872 |
| Stem | 90 | HV = −16,228 ∗ Wc + 18,397 | 0.9967 |
| Leaf | 120 | HV = −16,475 ∗ Wc + 19,215 | 0.8972 |
| Whole | 120 | HV = −16,440 ∗ Wc + 18,941 | 0.935 |
| Stem | 120 | HV = −16,387 ∗ Wc + 19,352 | 0.959 |
| Leaf | 150 | HV = −18,240 ∗ Wc + 18,998 | 0.9723 |
| Whole | 150 | HV = −18,673 ∗ Wc + 19,425 | 0.9088 |
| Stem | 150 | HV = −18,687 ∗ Wc + 19,684 | 0.8889 |
| Leaf | 180 | HV = −17,368 ∗ Wc + 18,651 | 0.9753 |
| Whole | 180 | HV = −18,204 ∗ Wc + 18,913 | 0.941 |
| Stem | 180 | HV = −17,959 ∗ Wc + 19,361 | 0.9413 |
| Part | Ages [Days] | NDF [%] | ADF [%] | LIG [%] | CEL [%] | HCEL [%] | HVV [kJ/kg] |
|---|---|---|---|---|---|---|---|
| Leaf | 90 | 73.73 ± 0.51 | 40.48 ± 1.14 | 6.52 ± 1.86 | 33.97 ± 1.15 | 33.35 ± 1.26 | 18,917 |
| Whole | 90 | 75.63 ± 0.93 | 44.76 ± 1.63 | 7.30 ± 0.66 | 36.92 ± 1.20 | 30.87 ± 2.17 | 18,151 |
| Stem | 90 | 79.30 ± 0.40 | 47.85 ± 1.56 | 6.55 ± 1.27 | 40.85 ± 0.92 | 31.49 ± 1.90 | 18,917 |
| Leaf | 120 | 74.48 ± 0.57 | 40.06 ± 1.46 | 6.24 ± 0.92 | 33.41 ± 0.92 | 34.42 ± 2.00 | 18,337 |
| Whole | 120 | 77.26 ± 1.68 | 45.89 ± 0.35 | 9.04 ± 0.23 | 36.86 ± 0.23 | 31.37 ± 1.94 | 18,352 |
| Stem | 120 | 81.78 ± 0.76 | 50.22 ± 2.53 | 9.70 ± 0.99 | 41.44 ± 1.57 | 31.56 ± 2.00 | 18,282 |
| Leaf | 150 | 74.97 ± 0.41 | 38.24 ± 1.52 | 6.16 ± 1.02 | 32.00 ± 1.02 | 36.74 ± 1.13 | 18,074 |
| Whole | 150 | 77.29 ± 0.45 | 46.30 ± 2.23 | 9.25 ± 0.89 | 37.48 ± 1.74 | 30.99 ± 2.14 | 18,352 |
| Stem | 150 | 81.45 ± 0.41 | 49.70 ± 0.71 | 9.96 ± 0.55 | 39.63 ± 0.96 | 31.75 ± 0.78 | 18,300 |
| Leaf | 180 | 75.98 ± 1.00 | 38.24 ± 1.52 | 7.42 ± 2.00 | 30.74 ± 2.00 | 37.75 ± 1.77 | 17,922 |
| Whole | 180 | 80.03 ± 0.79 | 47.87 ± 0.71 | 10.56 ± 0.62 | 37.53 ± 0.60 | 32.15 ± 0.23 | 18,500 |
| Stem | 180 | 83.05 ± 1.00 | 53.42 ± 0.81 | 12.23 ± 0.26 | 41.19 ± 0.87 | 29.63 ± 1.13 | 18,001 |
| Plant Age | |||||
|---|---|---|---|---|---|
| Test | Test-value | Num DF | Den DF | F-value | p-value |
| Wilks’ lambda | 0.5674 | 12 | 111.4131 | 2.2177 | 0.0153 |
| Pillai’s trace | 0.4536 | 12 | 132 | 1.9597 | 0.0328 |
| Plant part | |||||
| Test | Test-value | Num DF | Den DF | F-value | p-value |
| Wilks’ lambda | 0.1964 | 8 | 84 | 13.1948 | ~0 |
| Pillai’s trace | 0.8668 | 8 | 86 | 8.2233 | ~0 |
| Interaction Age*Plant part | |||||
| Test | Test-value | Num DF | Den DF | F-value | p-value |
| Wilks’ lambda | 0.3482 | 24 | 147.7305 | 2.1734 | ~0 |
| Pillai’s trace | 0.8361 | 24 | 180 | 1.9820 | ~0 |
| Leaf | 90 Days | 120 Days | 150 Days | 180 Days | |||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Unity | Dried | Ash | Dried | Ash | Dried | Ash | Dried | Ash |
| MgO | % | 0.59 | 3.08 | 0.71 | 6.27 | 0.82 | 7.08 | 1.04 | 14.22 |
| SiO2 | % | 2.33 | 11.89 | 2.18 | 10.64 | 2.08 | 13.37 | 3.21 | 17.99 |
| P2O5 | % | 1.17 | 5.96 | 0.98 | 6.19 | 0.82 | 4.18 | 0.91 | 5.90 |
| SO4 | % | 1.00 | 1.04 | 0.87 | 1.25 | 0.69 | 0.89 | 0.79 | 1.44 |
| Cl | % | 0.76 | 2.31 | 0.74 | 1.96 | 0.66 | 1.59 | 0.55 | 0.53 |
| K2O | % | 5.58 | 21.78 | 3.94 | 16.38 | 2.66 | 11.98 | 1.84 | 8.14 |
| CaO | % | 3.45 | 10.11 | 4.09 | 13.79 | 4.31 | 15.71 | 3.86 | 17.86 |
| MnO2 | % | 0.23 | 0.65 | 0.22 | 0.67 | 0.21 | 0.67 | 0.16 | 0.59 |
| FeO | % | 0.23 | 0.70 | 0.17 | 0.54 | 0.15 | 0.46 | 0.14 | 0.35 |
| TiO2 | ppm | 227.8 | 687.2 | 148.1 | 464.7 | 167.2 | 458.6 | 136.1 | 364.6 |
| CuO | ppm | 33.7 | 162.3 | 30.9 | 102.5 | 26.5 | 94.3 | 24.2 | 109.5 |
| ZnO | ppm | 112 | 349.5 | 110.4 | 306.9 | 87.2 | 311.1 | 81.4 | 290.6 |
| Br | ppm | 50.9 | 103.7 | 56 | 79.9 | 53.1 | 95.6 | 32.8 | 17.9 |
| Rb2O | ppm | 136 | 649.7 | 82.8 | 303.3 | 53.3 | 200.9 | 32.4 | 125.7 |
| SrO | ppm | 33 | 205.7 | 37.4 | 161.1 | 40.9 | 162.8 | 40.3 | 198.5 |
| Sum | % | 15.336 | 57.515 | 13.883 | 57.664 | 12.397 | 55.939 | 12.495 | 67.014 |
| Stem | 90 Days | 120 Days | 150 Days | 180 Days | |||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Unity | Dried | Ash | Dried | Ash | Dried | Ash | Dried | Ash |
| MgO | % | 0.65 | 7.28 | 0.56 | 6.46 | 0.49 | 6.77 | 0.37 | 4.97 |
| SiO2 | % | 1.32 | 8.89 | 1.13 | 5.64 | 1.02 | 6.73 | 0.70 | 5.08 |
| P2O5 | % | 0.77 | 4.60 | 0.64 | 3.58 | 0.57 | 3.28 | 0.49 | 2.88 |
| SO4 | % | 0.80 | 1.21 | 0.80 | 1.67 | 0.76 | 1.61 | 0.75 | 1.30 |
| Cl | % | 0.51 | 0.65 | 0.38 | 0.29 | 0.24 | 0.12 | 0.08 | 0.04 |
| K2O | % | 1.74 | 9.70 | 1.18 | 7.06 | 0.71 | 4.99 | 0.56 | 4.14 |
| CaO | % | 1.95 | 8.49 | 1.45 | 7.17 | 1.35 | 7.24 | 0.89 | 4.88 |
| MnO2 | % | 0.18 | 0.75 | 0.12 | 0.55 | 0.15 | 0.75 | 0.10 | 0.54 |
| FeO | % | 0.15 | 0.57 | 0.17 | 0.50 | 0.18 | 0.63 | 0.08 | 0.47 |
| TiO2 | ppm | 155.5 | 611.3 | 183.6 | 484.9 | 147 | 693.3 | 75.5 | 395.3 |
| CuO | ppm | 15.5 | 54.1 | 15.4 | 53.7 | 12.7 | 49.8 | 12.2 | 63.7 |
| ZnO | ppm | 95.4 | 357.3 | 68.2 | 159.5 | 25.8 | 60.8 | 17.7 | 76 |
| Br | ppm | 39.2 | 36.4 | 23.9 | 10.4 | 11.4 | 91.7 | 2.9 | 10 |
| Rb2O | ppm | 39.9 | 155.6 | 24.4 | 81.8 | 15.3 | 63.3 | 12.3 | 54.6 |
| SrO | ppm | 17.5 | 68 | 13.7 | 42.1 | 12.9 | 44.6 | 8.8 | 35.2 |
| Sum | % | 8.074 | 42.138 | 6.421 | 32.906 | 5.478 | 32.121 | 4.01376 | 24.2883 |
| Whole | 90 Days | 120 Days | 150 Days | 180 Days | |||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Unity | Dried | Ash | Dried | Ash | Dried | Ash | Dried | Ash |
| MgO | % | 0.69 | 6.66 | 0.69 | 6.05 | 0.61 | 7.36 | 0.64 | 8.03 |
| SiO2 | % | 2.22 | 12.49 | 2.01 | 10.22 | 1.89 | 11.23 | 1.66 | 10.29 |
| P2O5 | % | 0.96 | 6.38 | 0.82 | 4.42 | 0.67 | 3.59 | 0.68 | 4.18 |
| SO4 | % | 0.88 | 1.23 | 0.81 | 1.37 | 0.69 | 1.42 | 0.75 | 1.45 |
| Cl | % | 0.58 | 1.42 | 0.56 | 1.03 | 0.40 | 0.45 | 0.32 | 0.28 |
| K2O | % | 3.22 | 16.81 | 2.50 | 12.17 | 1.45 | 7.70 | 1.33 | 7.50 |
| CaO | % | 2.69 | 10.37 | 2.95 | 10.25 | 2.45 | 10.22 | 2.49 | 11.23 |
| MnO2 | % | 0.21 | 0.74 | 0.18 | 0.61 | 0.17 | 0.62 | 0.15 | 0.58 |
| FeO | % | 0.21 | 0.74 | 0.30 | 0.78 | 0.20 | 0.69 | 0.22 | 0.64 |
| TiO2 | ppm | 214.9 | 740.7 | 205 | 557.4 | 180.6 | 620.5 | 171.6 | 561.9 |
| CuO | ppm | 23.2 | 80.3 | 22 | 74.1 | 18.3 | 63.9 | 15.3 | 68 |
| ZnO | ppm | 67.7 | 287 | 91 | 256.9 | 39.7 | 149.8 | 47.6 | 142.8 |
| Br | ppm | 43.2 | 78.6 | 36.2 | 43.4 | 28.8 | 26.1 | 17 | 8.2 |
| Rb2O | ppm | 85.1 | 372.6 | 48.8 | 186.8 | 29.1 | 114.7 | 21.7 | 83.4 |
| SrO | ppm | 26.5 | 121.9 | 25.1 | 93.8 | 23.1 | 79.4 | 24.3 | 83.3 |
| Sum | % | 11.653 | 56.838 | 10.833 | 46.89 | 8.538 | 43.282 | 8.239 | 44.171 |
| Leaf 90 Days | Leaf 180 Days | Whole 90 Days | Whole 180 Days | Stem 90 Days | Stem 180 Days | |
|---|---|---|---|---|---|---|
| Basic-to-acidic ratio (B/A) | 2.98 | 2.25 | 2.75 | 2.65 | 2.91 | 2.83 |
| Slagging/fouling inclination | Severe | Severe | Severe | Severe | Severe | Severe |
| Bed agglomeration index (BAI) | 0.03 | 0.04 | 0.04 | 0.09 | 0.06 | 0.11 |
| Agglomeration inclination | Yes | Yes | Yes | Yes | Yes | Yes |
| Fouling index (FU) | 64.95 | 18.31 | 46.27 | 19.84 | 28.24 | 11.70 |
| Fouling inclination | Severe | High | Severe | High | High | High |
| Slag viscosity index (SR) | 46.12 | 35.69 | 41.29 | 34.09 | 35.22 | 32.98 |
| Molten ash inclination | Severe | High | Severe | High | High | High |
| Chlorine index | 2.307 | 0.532 | 1.421 | 0.276 | 0.649 | 0.0383 |
| Slagging/fouling inclination | Severe | Severe | Severe | High | Severe | Low |
| Stem 90 Days | Whole 90 Days | Leaf 90 Days | Stem 180 Days | Whole 180 Days | Leaf 90 Days | |
|---|---|---|---|---|---|---|
| Water content | 81.85 ± 0.30 | 81.91 ± 0.29 | 79.45 ± 0.20 | 65.84 ± 0.46 | 67.70 ± 0.48 | 71.31 ± 0.57 |
| Volatile content | 78.60 ± 0.91 | 76.54 ± 0.59 | 76.11 ± 0.92 | 83.60 ± 0.94 | 80.56 ± 0.85 | 77.80 ± 0.52 |
| Fixed Carbon Content | 19.89 ± 0.81 | 20.01 ± 0.59 | 19.62 ± 0.98 | 15.06 ± 0.98 | 16.30 ± 0.82 | 17.70 ± 0.52 |
| Ash content | 1.51 ± 0.13 | 3.45 ± 0.35 | 4.27 ± 0.11 | 1.34 ± 0.19 | 3.13 ± 0.19 | 4.50 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beber, R.C.; Turini, C.d.S.; Beber, V.C.; Nogueira, R.M.; Pires, E.M. Effect of Plant Part and Age on the Proximate, Chemical, and Elemental Characteristics of Elephant Grass Cultivar BRS Capiaçu for Combustion-Based Sustainable Bioenergy. Sustainability 2025, 17, 2741. https://doi.org/10.3390/su17062741
Beber RC, Turini CdS, Beber VC, Nogueira RM, Pires EM. Effect of Plant Part and Age on the Proximate, Chemical, and Elemental Characteristics of Elephant Grass Cultivar BRS Capiaçu for Combustion-Based Sustainable Bioenergy. Sustainability. 2025; 17(6):2741. https://doi.org/10.3390/su17062741
Chicago/Turabian StyleBeber, Roberto C., Camila d. S. Turini, Vinicius C. Beber, Roberta M. Nogueira, and Evaldo M. Pires. 2025. "Effect of Plant Part and Age on the Proximate, Chemical, and Elemental Characteristics of Elephant Grass Cultivar BRS Capiaçu for Combustion-Based Sustainable Bioenergy" Sustainability 17, no. 6: 2741. https://doi.org/10.3390/su17062741
APA StyleBeber, R. C., Turini, C. d. S., Beber, V. C., Nogueira, R. M., & Pires, E. M. (2025). Effect of Plant Part and Age on the Proximate, Chemical, and Elemental Characteristics of Elephant Grass Cultivar BRS Capiaçu for Combustion-Based Sustainable Bioenergy. Sustainability, 17(6), 2741. https://doi.org/10.3390/su17062741







