Biotechnological Applications of the Ubiquitous Fungus Penicillium sp. 8L2: Biosorption of Zn(II) and Synthesis of ZnO Nanoparticles as Biocidal Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Preparation for Testing
2.2. Biosorption Tests
2.3. Experimental Design
2.4. Biosorption Mechanisms Study
2.5. Nanoparticle Synthesis: Recovery and Identification
2.6. Biocide Tests: Protocols
3. Results and Discussion
3.1. Optimal Operating Conditions for Zn(II) Biosorption
3.2. Kinetic and Equilibrium Tests
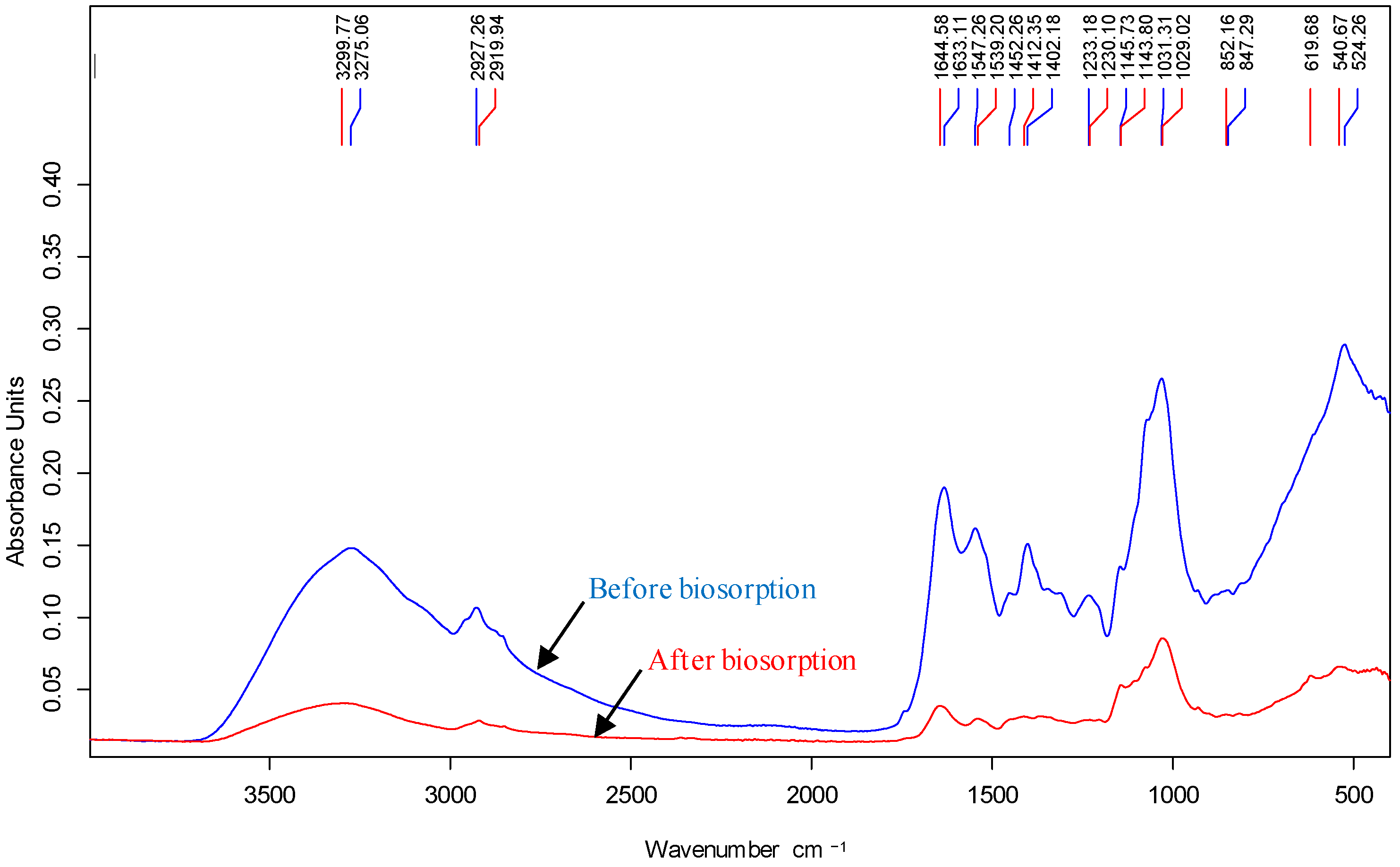
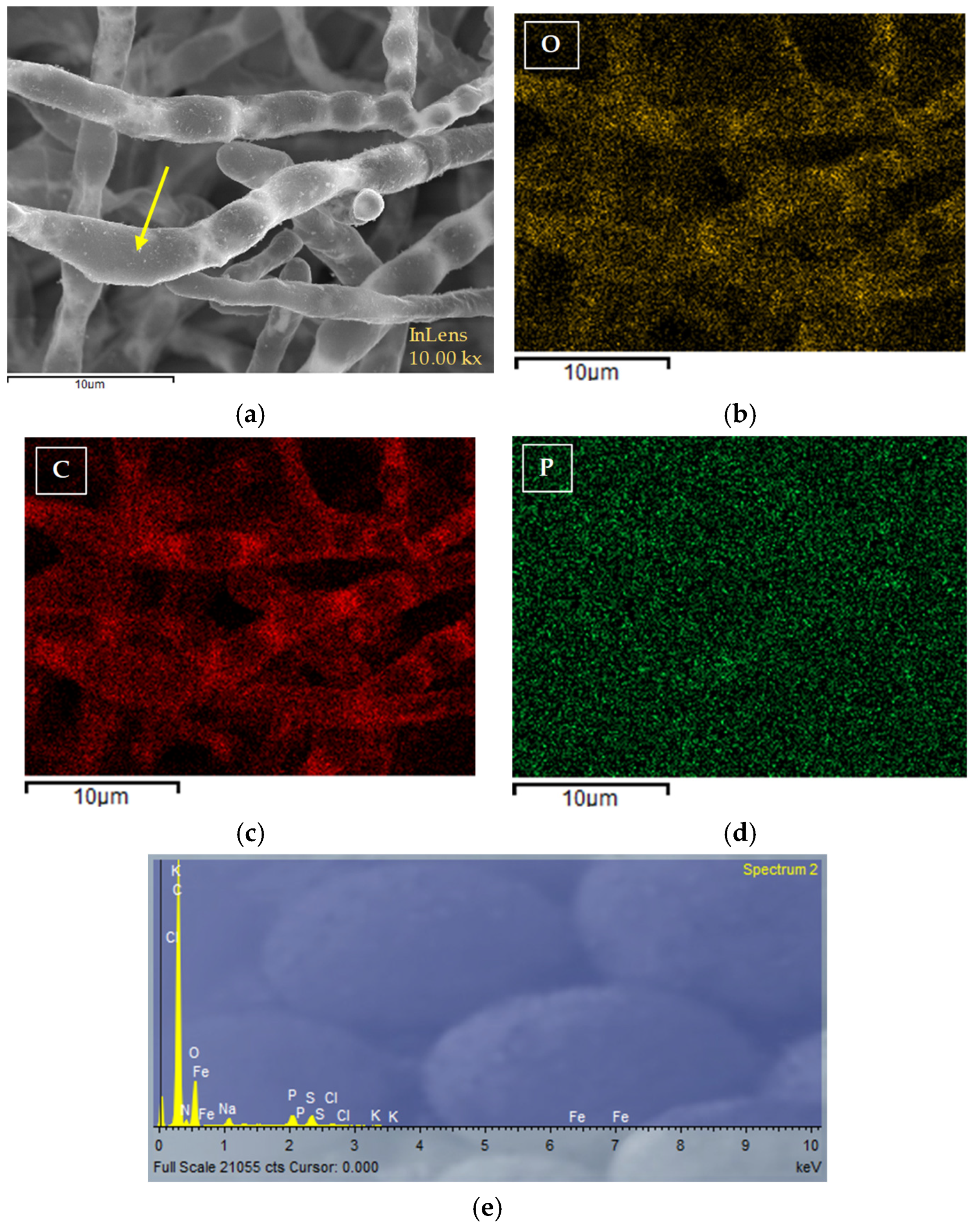
3.3. Biosorption Mechanisms
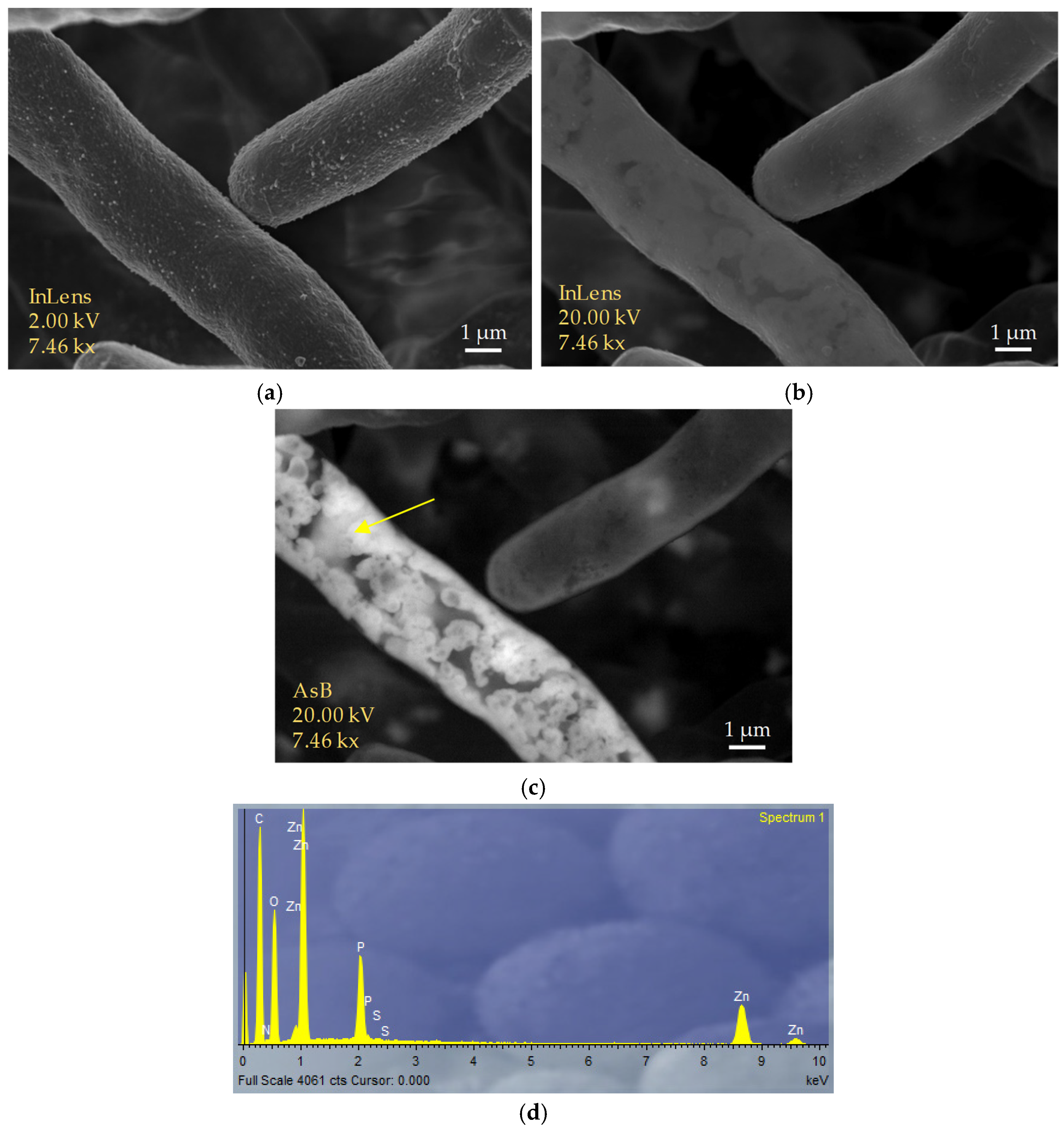
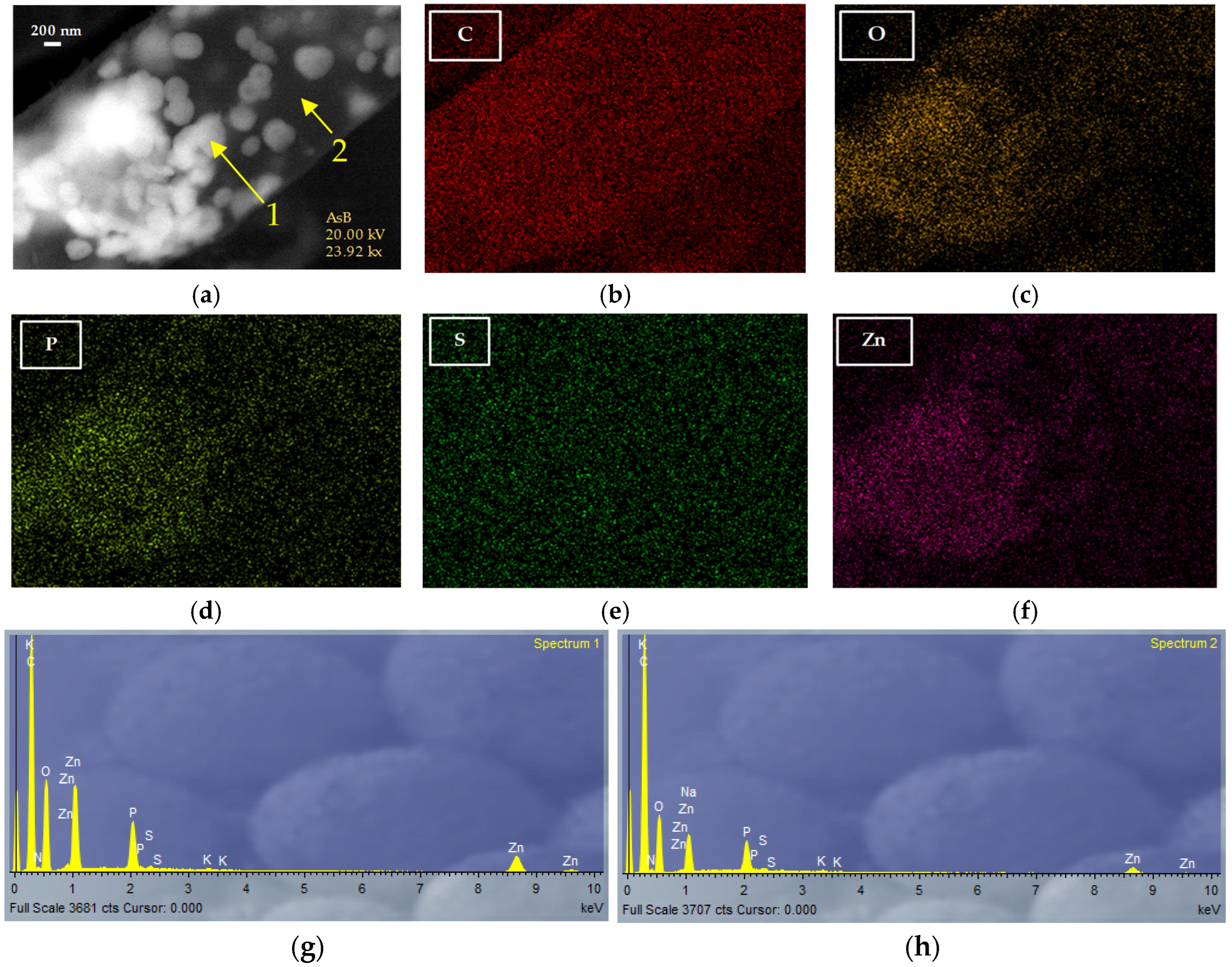
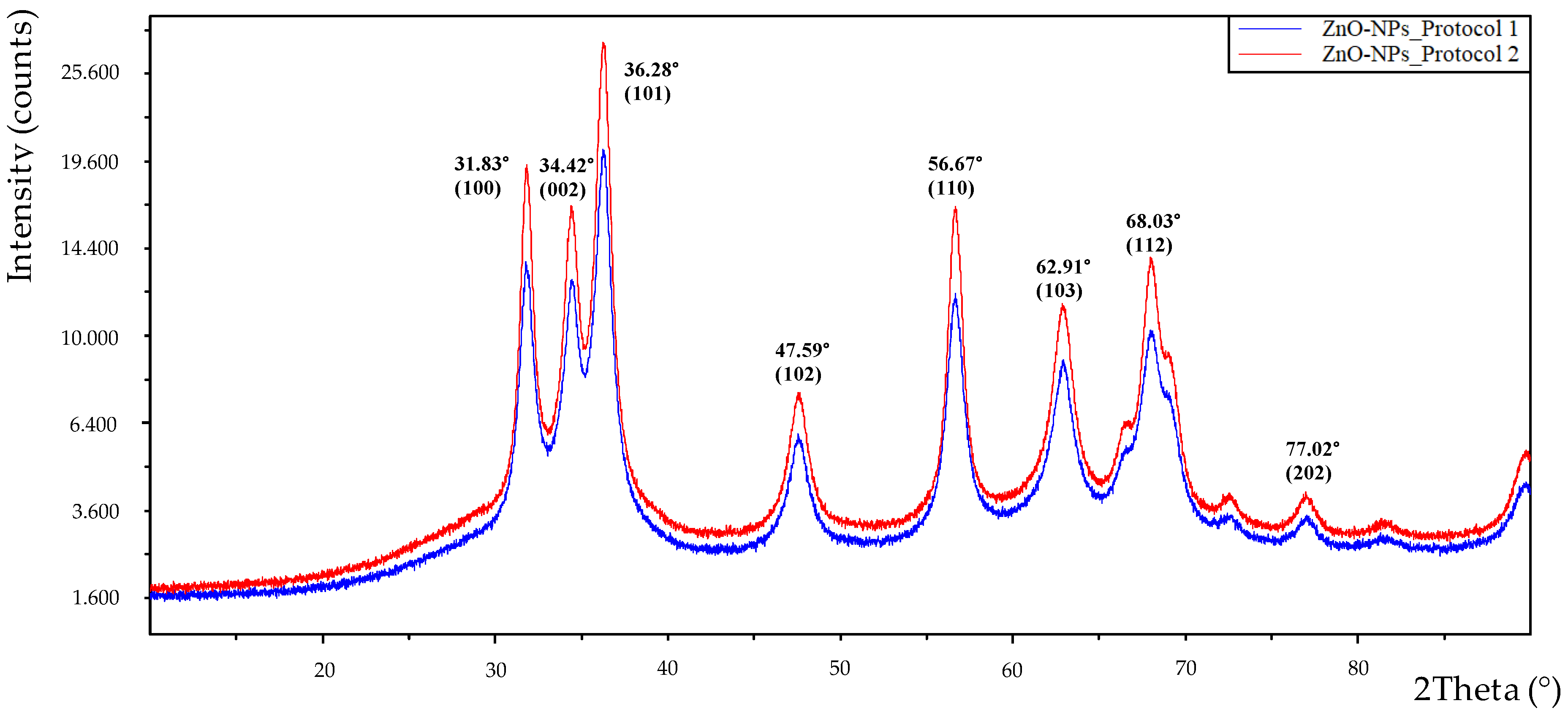
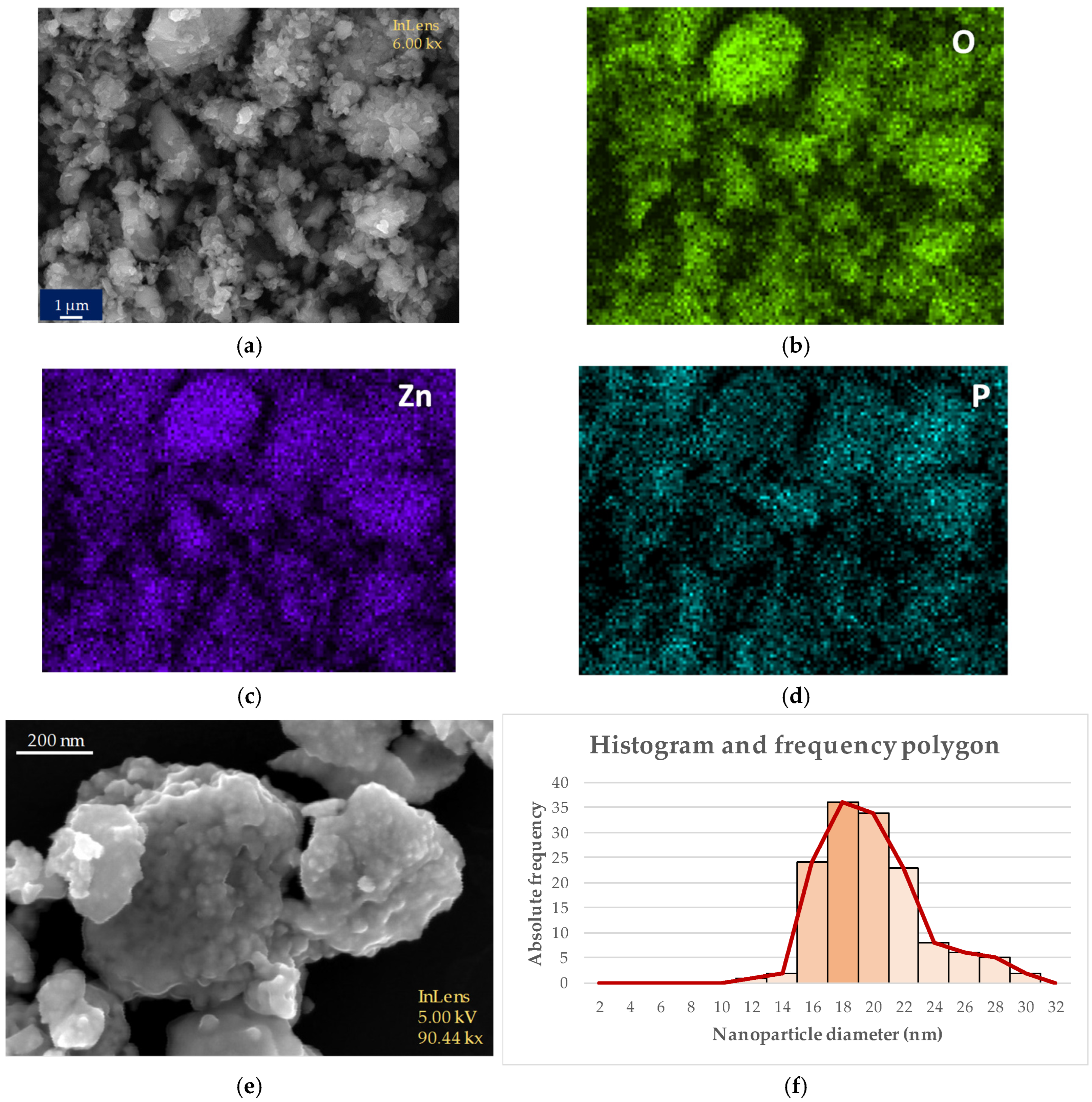
3.4. Characterization of Nanoparticles
3.5. Biocidal Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Effects of culture pH on cell surface properties and biosorption of Pb (II), Cd (II), Zn (II) of green alga Neochloris oleoabundans. J. Chem. Eng. 2023, 468, 143579. [Google Scholar] [CrossRef]
- Al Tanjil, H.; Akter, S.; Hossain, M.S.; Iqbal, A. Evaluation of physical and heavy metal contamination and their distribution in waters around Maddhapara Granite Mine, Bangladesh. Water Cycle 2024, 5, 286–296. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Registro Estatal de Emisiones y Fuentes Contaminantes (PRTR-España). Available online: https://prtr-es.es/ (accessed on 24 January 2025).
- Chen, M.; Wang, D.; Ding, S.; Fan, X.; Jin, Z.; Wu, Y.; Wang, Y.; Zhang, C. Zinc pollution in zones dominated by algae and submerged macrophytes in Lake Taihu. Sci. Total Environ. 2019, 670, 361–368. [Google Scholar] [CrossRef]
- Hu, J.; Yang, X.; Chi, H.; Liu, X.; Lu, N.; Liu, Y.; Yang, S.; Wen, X. Transport, pollution, and health risk of heavy metals in “soil-medicinal and edible plant-human” system: A case study of farmland around the Beiya mining area in Yunnan, China. Microchem. J. 2024, 207, 111958. [Google Scholar] [CrossRef]
- Chen, F.; Saqlain, L.; Ma, J.; Khan, Z.I.; Ahmad, K.; Ashfaq, A.; Sultana, R.; Muhammad, F.G.; Magsood, A.; Naeem, M.; et al. Evaluation of potential ecological risk and prediction of zinc accumulation and its transfer in soil plants and ruminants: Public health implications. Environ. Sci. Pollut. Res. 2022, 29, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Van, H.T.; Hoang, V.H.; Nga, L.T.Q.; Nguyen, V.Q. Effects of Zn pollution on soil: Pollution sources, impacts and solutions. Results Surf. Interfaces 2024, 17, 100360. [Google Scholar] [CrossRef]
- Kalousek, P.; Holátko, J.; Schreiber, P.; Pluháček, T.; Širůčková Lónová, K.; Radziemska, M.; Tarkowski, P.; Vyhnánek, T.; Hammerschmiedt, T.; Brtnický, M. The effect of chelating agents on the Zn-phytoextraction potential of hemp and soil microbial activity. Chem. Biol. Technol. Agric. 2024, 11, 23. [Google Scholar] [CrossRef]
- Guarino, F.; Improta, G.; Triassi, M.; Cicatelli, A.; Castiglione, S. Effects of zinc pollution and compost amendment on the root microbiome of a metal tolerant poplar clone. Front. Microbiol. 2020, 11, 1677. [Google Scholar] [CrossRef]
- Lobo, H.; Méndez-Fernández, L.; Martinez-Madrid, M.; Rodríguez, P.; Daam, M.A.; Espíndola, E.L.G. Bioaccumulation and chronic toxicity of arsenic and zinc in the aquatic oligochaetes Branchiura sowerbyi and Tubifex tubifex (Annelida, Clitellata). Aquat. Toxicol. 2021, 239, 105955. [Google Scholar] [CrossRef] [PubMed]
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Cent. J. 2011, 5, 64. [Google Scholar] [CrossRef]
- Anjum, A.; Garg, R.; Garg, R.; Gupta, D.; Eddy, N.O. Efficient sequestration of zinc and copper from aqueous media: Exploring strategies, mechanisms, and challenges. Int. J. Environ. Sci. Technol. 2024, 22, 5105–5126. [Google Scholar] [CrossRef]
- Dehkordi, M.M.; Nodeh, Z.P.; Dehkordi, K.S.; Salmanvandi, H.; Khorjestan, R.R.; Ghaffarzadeh, M. Soil, air, and water pollution from mining and industrial activities: Sources of pollution, environmental impacts, and prevention and control methods. Results Eng. 2024, 23, 102729. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Gholap, A.D.; Kumari, R.; Tanwar, R.; Kumar, V.; Khalid, M.; Faiyazuddin, M. A comprehensive review on the biomass-mediated synthesis of silver nanoparticles: Opportunities and challenges. J. Environ. Chem. Eng. 2025, 13, 115133. [Google Scholar] [CrossRef]
- Bordin, E.R.; Ramsdorf, W.A.; Domingos, L.M.L.; de Souza Miranda, L.P.; Mattoso Filho, N.P.; Cestari, M.M. Ecotoxicological effects of zinc oxide nanoparticles (ZnO-NPs) on aquatic organisms: Current research and emerging trends. J. Environ. Manag. 2024, 349, 119396. [Google Scholar] [CrossRef]
- Zango, Z.U.; Garba, A.; Shittu, F.B.; Iman, S.S.; Haruna, A.; Zango, M.U.; Wadi, I.A.; Bello, U.; Adamu, H.; Keshta, B.E.; et al. A state-of-the-art review on green synthesis and modifications of ZnO nanoparticles for organic pollutants decomposition and CO2 conversion. J. Hazard. Mater. Adv. 2025, 17, 100588. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Ruiz, E.; Abriouel, H.; Gálvez, A.; Ezzouhri, L.; Lairini, k.; Espínola, F. Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption. J. Chem. Eng. 2012, 210, 325–332. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Cuartero, M.; Castro, E. Biotechnological use of the ubiquitous fungus Penicillium sp. 8L2: Biosorption of Ag(I) and synthesis of silver nanoparticles. J. Environ. Manag. 2022, 316, 115281. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Moya, M.; Martín, C.; Ruiz, E. Biosorption, Recovery and Reuse of Cu(II) by Penicillium sp. 8L2: A Proposal Framed Within Environmental Regeneration and the Sustainability of Mineral Resources. Sustainability 2024, 16, 11001. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Moya, M.; Castro, E. Ag(I) Biosorption and Green Synthesis of Silver/Silver Chloride Nanoparticles by Rhodotorula mucilaginosa 1S1. Nanomaterials 2023, 13, 295. [Google Scholar] [CrossRef]
- Kumar, R.V.; Vinoth, S.; Baskar, V.; Arun, M.; Gurusaravanan, P. Synthesis of zinc oxide nanoparticles mediated by Dictyota dichotoma endophytic fungi and its photocatalytic degradation of fast green dye and antibacterial applications. S. Afr. J. Bot. 2022, 151, 337–344. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Moya, M.; Castro, E. Biocidal and synergistic effect of three types of biologically synthesised silver/silver chloride nanoparticles. World J. Microbiol. Biotechnol. 2024, 40, 18. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.J.; Espinola, F.; Moya, M.; Martín, C.; Ruiz, E. Cu(II) Biosorption and Synthesis of CuO Nanoparticles by Staphylococcus epidermidis CECT 4183: Evaluation of the Biocidal Effect. Appl. Sci. 2024, 14, 7623. [Google Scholar] [CrossRef]
- Fan, T.; Liu, Y.; Feng, B.; Zeng, G.; Yang, C.; Zhou, M.; Zhou, H.; Tan, Z.; Wang, X. Biosorption of cadmium(II), zinc(II) and lead(II) by Penicillium simplicissimum: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2008, 160, 655–661. [Google Scholar] [CrossRef]
- Saravanan, A.; Jeevanantham, S.; Kumar, P.S.; Varjani, S.; Yaashikaa, P.R.; Karishma, S. Enhanced Zn(II) ion adsorption on surface modified mixed biomass—Borassus flabellifer and Aspergillus tamarii: Equilibrium, kinetics and thermodynamics study. Ind. Crops. Prod. 2020, 153, 112613. [Google Scholar] [CrossRef]
- Tunali, S.; Akar, T. Zn(II) biosorption properties of Botrytis cinerea biomass. J. Hazard. Mater. 2006, 131, 137–145. [Google Scholar] [CrossRef]
- Areco, M.M.; Hanela, S.; Duran, J.; Afonso, M.S. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J. Hazard. Mater. 2012, 213–214, 123–132. [Google Scholar] [CrossRef]
- Kumar, Y.P.; King, P.; Prasad, V.S.R.K. Zinc biosorption on Tectona grandis L.f. leaves biomass: Equilibrium and kinetic studies. J. Chem. Eng. 2006, 124, 63–70. [Google Scholar] [CrossRef]
- Zhou, J.L. Zn biosorption by Rhizopus arrhizus and other fungi. Appl. Microbiol. Biotechnol. 1999, 51, 686–693. [Google Scholar] [CrossRef]
- Tao, Q.; Liu, Z.; Dai, Y.; Zhan, X. Biosorption properties of extracellular polymeric substances towards Zn(II) and Cu(II). Desalin. Water Treat. 2012, 45, 40–47. [Google Scholar] [CrossRef]
- Garip, S.; Bozoglu, F.; Servercan, F. Differentiation of Mesophilic and Thermophilic Bacteria with Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2007, 61, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Meng, Q.; Zhao, Q.; Ye, Z. Biosorption of Pb(II) and Zn(II) from aqueous solutions by living B350 biomass. Desalin. Water Treat. 2015, 55, 1832–1839. [Google Scholar] [CrossRef]
- Joshi, H.K.; Vishwakarma, M.C.; Kumar, R.; Sharma, H.; Bhandari, N.S.; Joshi, S.K. The biosorption of Zn2+ by various biomasses from wastewater: A review. J. Water Process Eng. 2023, 56, 104389. [Google Scholar] [CrossRef]
- Fathollahi, A.; Khasteganan, N.; Coupe, S.J.; Newman, A.P. A meta-analysis of metal biosorption by suspended bacteria from three phyla. Chemosphere 2021, 268, 129290. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.G.; Demeke, T.; Tefera, M.; Mulu, M.; Tesfaye, S. Biosynthesis and characterization of ZnO NPs using aqueous extract of Zehneria scabra L. leaf for comparing antibacterial activities and the efficacies of antioxidant activities. S. Afr. J. Chem. Eng. 2025, in press. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, G.; Zhang, Y.; Guo, J. Green synthesis of ZnO NPs with long-lasting and ultra-high antimicrobial activity. Surf. Interfaces 2024, 50, 104506. [Google Scholar] [CrossRef]
- Subba, B.; Rai, G.B.; Bhandary, R.; Parajuli, P.; Thapa, N.; Kandel, D.R.; Mulmi, S.; Shrestha, S.; Malla, S. Antifungal activity of zinc oxide nanoparticles (ZnO NPs) on Fusarium equiseti phytopathogen isolated from tomato plant in Nepal. Heliyon 2024, 10, e40198. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Kamel, A.G.; Eita, M.A.; Elbermawy, Y.; El-Shibiny, A. The cytotoxic potency of green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Origanum majorana. Mater. Lett. 2024, 367, 136654. [Google Scholar] [CrossRef]
- Dejene, B.K. Biosynthesized ZnO nanoparticle-functionalized fabrics for antibacterial and biocompatibility evaluations in medical applications: A critical review. Mater. Today Chem. 2024, 42, 102421. [Google Scholar] [CrossRef]
- He, W.; Yan, S.; Wang, Y.; Zhang, X.; Zhou, W.; Tian, X.; Sol, X.; Han, X. Biomimetic synthesis of mesoporous zinc phosphate nanoparticles. J. Alloys Compd. 2009, 477, 657–660. [Google Scholar] [CrossRef]
- Janani, M.; Viswanathan, D.; Pandiaraj, S.; Govindasamy, R.; Gomathi, T.; Vijayakumar, S. Review on phyto-extract methodologies for procuring ZnO NPs and its pharmacological functionalities. Process Biochem. 2024, 147, 186–212. [Google Scholar] [CrossRef]
- Kamaraj, C.; Naveenkumar, S.; Kumar, R.C.S.; Al-Ghanim, K.A.; Natesan, K.; Priyadharsan, A. Ice apple fruit peel assisted bio-synthesis of zinc oxide nanoparticles (ZnO NPs): An anticancer, antimicrobial, and larvicidal applications. J. Drug Deliv. Sci. Technol. 2025, 105, 106585. [Google Scholar] [CrossRef]
- Elankathirselvan, K.; Fathima H, A.; K, P.; Al-Ansari, M.M. Synthesis and characterization of Pyrus communis fruit extract synthesized ZnO NPs and assessed their anti-diabetic and anti-microbial potential. Environ. Res. 2024, 258, 119450. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.L.; Moreira, A.J.; Nóbrega, E.T.D.; Braga, S.; Argentin, M.N.; Da Cunha Camargo, I.L.B.; Azevedo, E.; Pereira, E.C.; Bernardi, M.I.B.; Mascaro, L.H. Selective inhibitory activity of multidrug-resistant bacteria by zinc oxide nanoparticles. J. Environ. Chem. Eng. 2024, 12, 111870. [Google Scholar] [CrossRef]
- Tran, G.T.; Nguyen, N.T.H.; Nguyen, N.T.T.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Formation, properties and applications of microalgae-based ZnO nanoparticles: A review. J. Environ. Chem. Eng. 2023, 11, 110939. [Google Scholar] [CrossRef]



| Run | F. 1 | F. 2 | R | F. 1 | F. 2 | R | |
|---|---|---|---|---|---|---|---|
| A: pH | B: (g/L) | qe (mg/g) | Run | A: pH | B: (g/L) | qe (mg/g) | |
| 1 | 6.21 | 0.20 | 44.77 | 14 | 6.21 | 0.20 | 52.49 |
| 2 | 5.19 | 0.50 | 43.56 | 15 | 5.19 | 0.50 | 41.02 |
| 3 | 5.19 | 0.08 | 55.55 | 16 | 5.19 | 0.08 | 53.32 |
| 4 | 6.62 | 0.50 | 36.50 | 17 | 6.62 | 0.50 | 41.89 |
| 5 | 5.19 | 0.50 | 40.03 | 18 | 5.19 | 0.50 | 43.15 |
| 6 | 6.21 | 0.80 | 34.21 | 19 | 6.21 | 0.80 | 37.40 |
| 7 | 5.19 | 0.50 | 40.87 | 20 | 5.19 | 0.50 | 42.74 |
| 8 | 5.19 | 0.50 | 40.72 | 21 | 5.19 | 0.50 | 36.39 |
| 9 | 4.24 | 0.80 | 33.30 | 22 | 4.24 | 0.80 | 32.04 |
| 10 | 4.24 | 0.20 | 38.43 | 23 | 4.24 | 0.20 | 35.35 |
| 11 | 5.19 | 0.90 | 36.54 | 24 | 5.19 | 0.90 | 35.95 |
| 12 | 3.83 | 0.50 | 33.72 | 25 | 3.83 | 0.50 | 34.33 |
| 13 | 5.19 | 0.50 | 38.98 | 26 | 5.19 | 0.50 | 43.16 |
| Type of Biomass | Biomass | qm (mg/g) * | Reference |
|---|---|---|---|
| Plant/fungi | Borassus flabellifer/Aspergillus tamarii | 49.79 54.74 | [28] |
| Fungi | Botrytis cinerea | 12.98 | [29] |
| Green alga | Ulva lactuva | 22.88 | [30] |
| Plant | Tectona grandis | 16.42 | [31] |
| Fungi | Rhizopus arrhizus Mucor racemosus Mycotypha africana Aspergillus nidulans Aspergillus niger Schizosaccharomyces pombe | 13.92 13.01 12.55 11.90 11.90 10.10 | [32] |
| Fungi | Penicillium simplicissimum | 52.50 65.60 76.90 | [27] |
| Fungi | Penicillium sp. 8L2 | 52.14 | This work |
| Bacteria | NPs | NPs + PVA * |
|---|---|---|
| B. cereus | 1000–2000 | 250–500 |
| S. epidermidis | 125–250 | 62.5–125 |
| E. coli | 250–500 | 62.5–125 |
| P. fluorescens | 1000–2000 | 500–1000 |
| R. mucilaginosa 1S1 | - | 62.5–125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobo, A.J.M.; Lozano, F.E.; Vilar, M.M.; Valenzuela, C.M.; Ramos, E.R. Biotechnological Applications of the Ubiquitous Fungus Penicillium sp. 8L2: Biosorption of Zn(II) and Synthesis of ZnO Nanoparticles as Biocidal Agents. Sustainability 2025, 17, 2379. https://doi.org/10.3390/su17062379
Cobo AJM, Lozano FE, Vilar MM, Valenzuela CM, Ramos ER. Biotechnological Applications of the Ubiquitous Fungus Penicillium sp. 8L2: Biosorption of Zn(II) and Synthesis of ZnO Nanoparticles as Biocidal Agents. Sustainability. 2025; 17(6):2379. https://doi.org/10.3390/su17062379
Chicago/Turabian StyleCobo, Antonio Jesús Muñoz, Francisco Espínola Lozano, Manuel Moya Vilar, Celia Martín Valenzuela, and Encarnación Ruiz Ramos. 2025. "Biotechnological Applications of the Ubiquitous Fungus Penicillium sp. 8L2: Biosorption of Zn(II) and Synthesis of ZnO Nanoparticles as Biocidal Agents" Sustainability 17, no. 6: 2379. https://doi.org/10.3390/su17062379
APA StyleCobo, A. J. M., Lozano, F. E., Vilar, M. M., Valenzuela, C. M., & Ramos, E. R. (2025). Biotechnological Applications of the Ubiquitous Fungus Penicillium sp. 8L2: Biosorption of Zn(II) and Synthesis of ZnO Nanoparticles as Biocidal Agents. Sustainability, 17(6), 2379. https://doi.org/10.3390/su17062379







