Abstract
The extraction of metallic nanoparticles (MNPs) from waste electrical and electronic equipment (WEEE) has gained extensive attention from researchers for eco-friendly, reliable, and sustainable alternative protocol over the traditional linear economic approach (make-use-dispose) for boosting the circular economy. A plethora of MNPs including metals/metal oxide nanoparticles having a size dimension ranging from 1–100 nanometers (nm) have been extracted from these WEEE by using different chemical, physical, and biological methods. Recovery of certain precious MNPs can be achieved by dismantling and recycling electronic waste items in the form of gold (Au), platinum (Pt), zinc oxide (ZnO), silver (Ag), and copper oxide (CuO). These MNPs provide a huge range of applications such as antibacterial, therapeutic, target drug delivery, and biotechnological applications. This comprehensive review provides in-depth knowledge of the synthesis of MNPs using different techniques from WEEE and delves into their potential applications in biomedical fields with in-depth mechanisms. This article also discussed global challenges and opportunities in this area for adopting the concept of circular economy to conserve natural resources for future generations and hence create a greener environment and protect our planet.
1. Introduction
The emergence of various electronic devices, escalating urbanization, and industrial development have improved peoples’ quality of life. Through the use of electronic and electrical equipment, information technology has undergone a significant development that has made significant contributions to modern society. However, the increased usage of electronic and electrical equipment by present-day society has increased the challenge of the discharge of waste disposal systems [1,2]. Computers, smartphone screens, as well as other household and commercial gadgets represent a significant portion of the solid garbage [3,4,5]. If managed improperly, the presence of numerous poisonous metals and hazardous substances in the waste electrical and electronic equipment (WEEE) delivers significant fatal hazards to the human and natural environment. The reality that electronic devices are discarded even before they reach their end-of-life (EoL) and that a tiny proportion of EoL electronics end up in a formal recycling facility is a fascinating fact. Most of these electronic devices are either burned in a waste-to-energy operation or end up in landfills [6,7,8].
It has been estimated that 61.3 million metric tons (Mt) of electronic waste will be generated in 2023 as compared to 59.4 million Mt in 2022 and with a significant manifold rise up to 74.7 million Mt by the end of 2030, as shown in Figure 1 e-waste continues to be under threat, and the disposal of it has emerged as one of the world’s most significant problems [9]. In addition to being a possible worldwide hazard, it is seen as a secondary source of key metals. A complex structure and design are created by the combined amalgamation of materials such as valuable, rare, and hazardous. In the later 1980s, as the dangers posed by e-waste development and management tactics increased, strict environmental restrictions were developed.
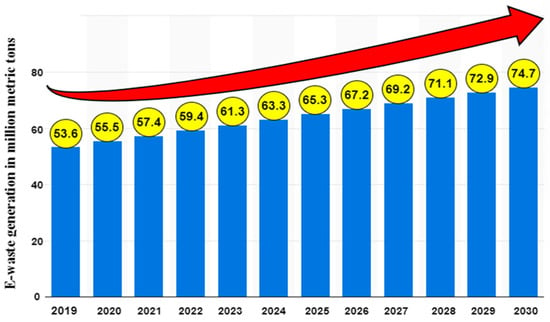
Figure 1.
Global estimation of e-waste generation by 2030 [10].
Later, as a result of a sharp increase in the cost of treating hazardous waste, the activity of illegal trafficking started at reduced costs in underdeveloped nations to eliminate the expenditures associated with treating hazardous waste. Since there are fewer and longer-lasting electronic items in some developing nations due to budgetary limitations on both a local community and across the nation, the development of e-waste in those nations is not as concerning. The main source of e-waste problems in developing countries is the importing of e-waste and electronic goods from developed western countries. In the majority of developing countries, e-waste is disposed of unofficially by the stakeholders and ends up in the backyards of low-income groups [11,12,13].
These processes are costly and seen as unprofitable techniques to extract metallic nanoparticles (MNPs) from WEEE. Numerous nanoparticles have been synthesized over the past few decades using a variety of techniques and used to enhance technology for environmentally friendly uses, which include water treatment, the detection of residual contaminants, soil/water treatment, followed by many more [14,15,16].
The study of sustainable inputs for nanoparticle extraction as well as the application of green synthesis approaches is driven by the discipline of materials science along with the engineering field’s growing interest in enhancing the ecological viability of the techniques involved in their production [17,18]. Highly hazardous waste products that are created in large quantities can be divided into wastes emanating from industries and wastes created by consumers, determined by the source of emission. Wastes from both industries and consumers can be used as raw materials for the creation and application of green synthesis methods that result in goods with value-added synthesis of MNPs [19,20,21].
Even though recycling WEEE is economically advantageous due to the substantial amounts of precious and semiprecious metals, there is still a need for a more effective and environmentally friendly approach for WEEE management that implements a larger portion of the waste materials (rather than only the metal pieces) and produces value-added items [22,23,24]. The highest concentrations of precious metals and a sizeable fraction of base metals are found in printed circuit boards (PCB) and cell phones, as illustrated in Figure 2. PCB is a crucial part of every electrical and electronic device and the main source of WEEE [25,26]. Metallic nanoparticles have demonstrated several qualities in the domain of nanotechnology, and this has opened up numerous new avenues for research. Metallic nanoparticles are unique and have the proper functional groups [27,28]. It can be created and altered so that it can bind to ligands, antibodies, and therapeutics. Metal nanoparticles are nanosized metals that range in size from 10 to 100 nm. Metallic nanoparticles are special due to their optical as well as surface plasmon resonance abilities [29]. While a solution of 20 nm gold nanospheres has a red ruby hue, a solution of 200 nm nanospheres exhibits a bluish colour, hence the colour of gold is indeed golden yellow. In the areas of catalysis, photography, and medical applications such as anticancer and anti-microbial drugs, researchers have focused a lot of attention on noble metals, particularly silver and gold [30,31]. The integration of nontoxic noble metal nanoparticles into biological systems has exerted a profound influence on medical and biological studies. The noble metallic nanoparticles attracted the most attention because of their distinctively low toxicity, simplicity of synthesis, and preferential association with biological molecules. The various kinds of metallic nanoparticles and their derivatives, such as those made of silver (Ag), gold (Au), copper (Cu), nickel (Ni), platinum (Pt), titanium (Ti), and zinc (Zn), have recently drawn a lot of attention due to their potent antibacterial capabilities. In the same way, metal oxides, including silver oxide (Ag2O), copper oxide (Cu2O), magnesium oxide (MgO), titanium dioxide (TiO2), and zinc oxide (ZnO), have a variety of special qualities, spectral activity, and outstanding antibacterial ability. The employment of noble metal nanoparticles in very sensitive investigative assays, radiotherapy advancement, gene delivery, thermal ablation, and drug delivery is regarded to make them more specialized and multifunctional agents with a variety of biological applications.
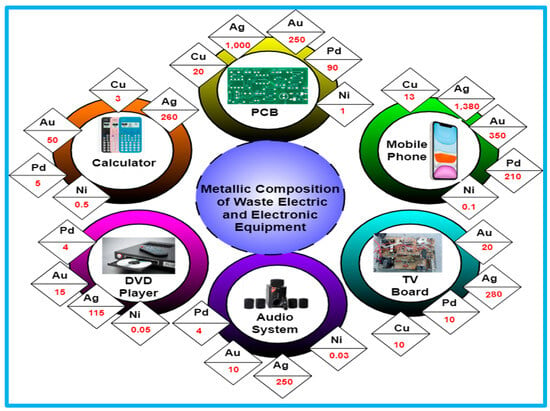
Figure 2.
Metallic composition of different WEEE by weight in percentage.
Two fundamental strategies, which include numerous sub-preparation techniques, can be used to synthesize MNPs in natural or artificial ways as shown in Figure 3. The “top to bottom” tactic is the first approach, which involves breaking down large-scale solid substances into smaller components by using external energy using physical, chemical, and thermal methods [32]. The second technique, referred to as “from the bottom up,” consists of molecules or atoms of either liquid or gas that are brought together and combined [33]. Pathogens that are resistant to antibiotics are becoming a severe hazard to human health. The pathogenic bacteria that cause these illnesses and infections include Geobacter, Staphylococcus, Enterococcus, and Streptococcus. When they reach the critical stage, these bacterial disorders may cause the infected person to die away. To prepare the advanced-level drugs or antibiotics required to control these bacterial disorders, new approaches are required. In recent years, nanotechnology has offered tremendous possibilities in many technical, environmental, medicinal, and other disciplines. For instance, silver nanoparticles exhibit outstanding antibacterial qualities against harmful viruses, bacteria, and other microorganisms [34,35]. As a result, it is a common antibacterial agent in the textile industry.
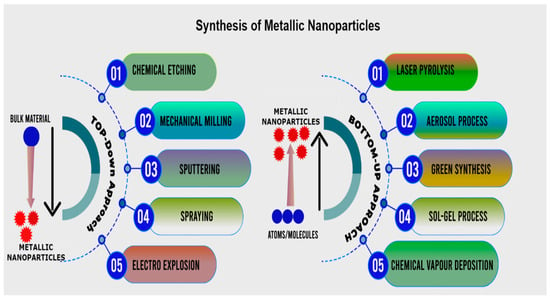
Figure 3.
Various approaches for the synthesis of MNPs.
This can be done by using eco-friendly technologies, developing green policies, and fostering innovative corporate cultures. Figure 4 depicts the circular economy concepts used in managing e-waste. By applying the principles of the circular economy, waste management may be done in any country or location sustainably [36,37]. The practices listed above can be used to improve e-waste management by carrying out the circular economy idea and sustainable development objectives. However, responsible consumption of resources may be influenced by cognition, conduct, way of life, morality, community, environmental ethics, and psychology [38,39]. The characteristics, benefits as well and drawbacks of metallic nanoparticles are enumerated in this review.
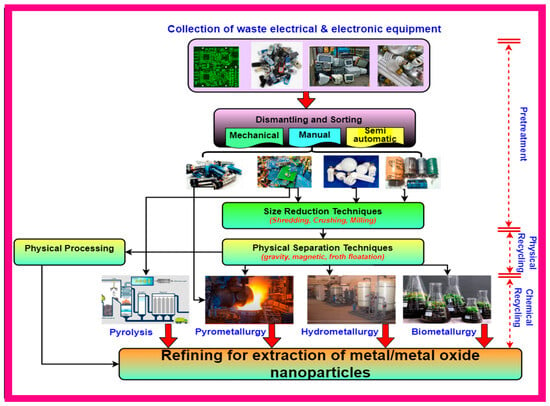
Figure 4.
Effective WEEE management by extracting metallic nanoparticles.
This review also discusses the synthesis of metallic nanoparticles derived from electronic and electrical waste equipment. It gives the readers in-depth details on synthesis using different methods, and characterization, with a focus on different applications as well as possible side effects alongside their future prospective. Recent progress has paved the door for these MNPs to deliver drugs and target specific sites.
1.1. Objective and Contributions
The purpose of this in-depth review is to investigate the extraction of metallic nanoparticles and metal oxides from waste electrical and electronic devices and to address the issues raised below.
- To discuss and analyze different techniques for the extraction of MNPs from WEEE and study their adverse impact on the ecological system.
- To identify how the synthesis of MNPs will enhance the concept of circular economy and discuss different steps that must be followed for implementing circular economy.
- To provide an in-depth insight to the readers of the recent applications of these metallic nanoparticles in the field of medicine, target drug delivery, and biotechnology.
1.2. Organization of the Paper
The rest of this comprehensive review is arranged as follows: Section 2 describes the research methodology to carry out this study. Section 3 provides the different extraction techniques from WEEE for enhancing the circular economy. Section 4 discusses and provides the comparison of MNPs extracted from waste devices with certain potential biomedical applications of MNPs discussed in Section 5. Discussion of this review article is presented in Section 6 along with future perspectives for the academician and researcher and a conclusion is provided in Section 7.
2. Methodology
From several electronic databases, which are extremely authentic, the pertinent research articles, book chapters, proceedings, websites of regulatory authorities, and government reports were gathered. As shown in Figure 5, the research publications (from journals and conferences) were typically accessed via Science Direct, MDPI, Elsevier, PubMed, Springer, Taylor & Francis, and Google Scholar. Additionally, books and pertinent book chapters were looked up for the same. The paper’s title and structure were chosen based on a variety of keywords, including “waste electrical and electronics equipment” OR “extraction of metallic nanoparticles” AND “application in biotechnology” OR “drug delivery” AND “circular economy” and several search criteria are implemented: (1) timespan: 2019–2025, (2) Language: English; (3) Document type: Article, review; (4) Source type: Journals and conferences. The idea of the circular economy by implementing e-waste management, predicted challenges, potential future perspective applications, and developments. The extraction of MNPs from WEEE is the primary concern when writing the paper. Additionally, it made care to consider all the important aspects, including new publications, novel and affordable extraction techniques from e-waste, and potential green processes. To ensure the highest level of quality, it was the idea of every co-author who also took part in brainstorming while this article was being written.
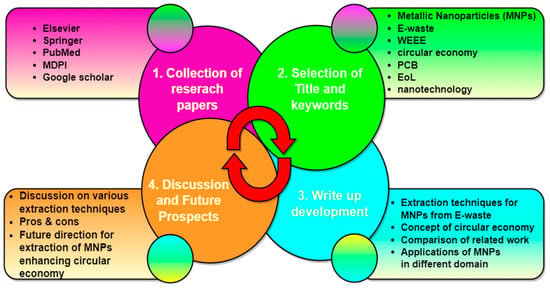
Figure 5.
Adopted structural outline for writing the present review article.
3. Extraction of Metallic and Metal Oxides Nanoparticles
Metallic nanoparticles can be synthesized using either a top-down or bottom-up strategy. The attenuation of substances with additional self-assembly steps that result in the development of nanostructures is one of these methods. In the course of self-assembly, units are combined into substantial, stable structures through nanoscale physical forces. Examples of processes of this type include the creation of nanoparticles using colloidal dispersion with quantum dots. Whereas top-down techniques nanostructures involve ball milling and applying substantial plastic deformation onto macroscopic structures that may be externally controlled [40,41,42]. The top-down process starts with a pattern created on a big scale, which is subsequently reduced to a nanoscale. This method is quick to build but slow and unsuitable for large-scale production. To enhance the circular economy urban mining, a concept related to resource efficiency, is the process of recovering resources from the human-made environment, which is a major origin of resources with percentages of elements frequently equal to or greater than occurring naturally [43,44]. Concerning essential raw materials, WEEE is regarded as the main component in urban mining. To safeguard the environment, save resources, and reap financial rewards, urban mining activities require systematic handling of manmade resource stocks and waste.
Urban mining is a good idea for the environment, but from the standpoint of resource recovery, it continues to be in the early stages because so many valuable materials end up in landfills. In many nations, where it necessitates a significant amount of consumer and collector awareness, effective e-waste management is absent [45]. The collecting typically takes place in both stationary and mobile settings. The main priority in the collecting of E-waste has been cost reduction. Source separation is essential since it will cut labor requirements and processing time in numerous manners. Service shops, community centers, and locations where municipal waste is gathered can all easily establish permanent collection sites. Ensuring consumer participation could result in elevated E-waste collection rates. To boost WEEE collections together with recycling, awareness is crucial. Economic incentives like free collection services and transportation of WEEE to recycling facilities could increase WEEE collection The best synthesis technique must be chosen from the wide range of available options, each with advantages and drawbacks to produce MNPs with the correct size and morphology and to successfully scale up for use in industrial applications. Based on several publications, the advancement of nanoparticle synthesis techniques beyond the laboratory level should take into consideration the clarification of issues relating to the production quantification of MNPs, determining a production time for a particular amount of nanoparticles, standardization of the conditions needed that produce specific monodisperse particles, along with their stability. The most common extracted metals are Au, Zn, Cu, Pd, Ti, Fe, and Pt, while the potential precursors are represented by the respective metallic salts, such as silver nitrate (AgNO3), copper(II) sulfate (CuSO4), palladium(II) chloride (PdCl2) and cadmium sulfate (CdSO4). The metallic and their oxide nanoparticles can be extracted from WEEE by adopting several different techniques:
- (a)
- Physical Methods: Physical techniques that rely on exposing particles to physical exposure to get the nanoparticles (such as dispersion, evaporation/condensation, and laser ablation) [46].
- (b)
- Chemical Methods: These techniques involve the synthesis of MNPs through chemical action [47].
- (c)
- Electrochemical Methods: To enable the deposition of metallic parts via their soluble ionic forms within e-waste to an electrode surface, these approaches make use of electrical energy. To recover particular metals in their metallic form, the procedure can involve electroplating or electrowinning [48,49].
- (d)
- Biological Methods: Biological techniques are which involve bioreduction utilizing microorganisms and plant extracts [50,51].
3.1. Pre-Processing of WEEE
The first and most important step in every form of e-waste recycling is pre-processing, which can be accomplished physically by utilizing mechanical processes that require disintegrating. Pre-treatment is known as dismantling, and it is typically done with a hammer, tools like screwdrivers, and conveyor beds to separate the components into distinct categories for recycling. The second stage of the pre-treatment process for e-waste involves mechanically crushing the materials [52]. After being placed in crushers and grinding machines, the e-waste can either be treated further using an electrical magnetic separator for the separation of non-metallic components. Sometimes, froth flotation is also used to concentrate metals in e-waste because it is capable of distinguishing between metallic and non-metallic particles of crushed waste PCB with sizes ranging from 75 nm to 1 mm. The pre-treated waste electronic and electrical components are then subjected to different processes for further recovery of precious metals employing metallurgical techniques described below:
3.2. Metallurgical Process
3.2.1. Pyrometallurgical Process
The pyrometallurgical treatment of e-waste consumes a lot of energy although it is ineffective at recovering precious metals. Within a blast furnace, pyrometallurgical procedures include melting, burning, and incineration, along with pyrolysis [53]. Pyrolysis is used to treat e-waste, which produces 70% metal-rich remnants, and 23% oil, alongside 5% gases when heated to a temperature of approximately 900 °C (or greater in some cases). These procedures cost a lot of money, use a lot of energy, and produce hazardous substances including dioxins as well as furans. This is due to the presence of halogenated flame retardants inside e-wastes, particularly in PCB [54,55].
3.2.2. Hydrometallurgical Process
Powerful acids are utilized for leaching base metals out of e-waste, while chemical reagents including cyanide, thiosulfate, and halide have been employed to leach out valuable metals. Chemical reagents constitute the main component of the hydrometallurgical procedure. These procedures use huge amounts of chemical reagents, create a wide range of by-products, and generate a lot of effluent waste. It is important to highlight that cyanide leaching is favored for recovering precious metals both from ores and e-waste because of its potential for high recovery and affordable price. However, this approach has significant drawbacks, including an increase in the amount of effort required to cleanse the effluent before disposal and the fact that cyanide is one of the restricted substances because of its toxicity [56].
3.2.3. Biohydrometallurgy and Bioleaching
Bioleaching is the most common term used to describe bio-metallurgical processes. Due to its benefits including low operational cost, minimal use of energy, lower chemical reagent usage, and simple manageability of secondary waste pollutants, this process has drawn a lot of interest as a promising sustainable approach to recovering metals from e-waste. For ores that have significant metal concentration and e-waste, nevertheless, this process has not yet been fully industrialized because it takes a while to leach metals. This bio-metallurgy is fully sustainable and poses no environmental risks, in contrast to pyrometallurgy together with hydrometallurgy. This approach recovers base metals by the use of microbiological processes [57,58]. Numerous organizations have expressed a great deal of intrigue in adopting this technique because of its environmentally friendly nature. The two processes used in this bio-metallurgical procedure are “bioleaching” and “biosorption”. Researchers are focusing primarily on the bioleaching procedure. The bioleaching technique has been widely used in mining over the last 40 years to extract Ag, Co, Au, and Zn, along with precious metals from substances of low quality for commercial uses [59,60].
Metal solubility during bioleaching heavily depends on the interactions of metals and microbes. Many patents created demonstrations employing various leachates. Investigations using sulfur, iron-oxidizing microorganisms, and heterotrophic fungi just as leachates revealed extraction rates of 50–90% of various metals according to various experimental setups. Researchers even experimented with using thiosulphate for extracting sulfide ores through the Fe3+ ion method [61,62]. These techniques have been successful at extracting metals from ores, but little is known about how well they work with microorganisms or how to recover metals from e-waste. These different acidophiles such as Acidithiobacillusthiooxidans, AspergillusNiger, Desulfovibriodesulphuricans, Thermoplasmaacidiphilum, and Penicilliumsimplicissimum, etc. are being utilized in the extraction of metals from e-waste and provide efficient results [63,64]. Biometallurgy has many advantages over other processes already in use, including a high rate of MNPs extraction from e-waste, the use of fewer chemicals and operational costs, and a lower environmental impact. Éllen F. Rodrigues et al. [65] reported Aspergillus niger used in a bioleaching process to recover copper and gold from computer PCB trash. Three bioleaching techniques with one or two phases or with wasted medium were examined in an incubator shaker with varying PCB waste contents (2.5 to 10 g/L) at 30 °C and 160 rpm. The source of carbon was glucose. A stirred tank reactor was used to test the optimal condition. The existence of gluconic, citric, and oxalic acids was verified by the Fourier-transform infrared (FTIR) spectrum. After 14 days of the procedure, Aspergillus niger demonstrated a bioleaching efficiency of up to 100% for copper and 42.5% for gold utilizing the two-step approach with 2.5 g/L PCB waste. Furthermore, María E. Díaz et al. [66] reported leaching with citric acid, three strains of Aspergillus niger, Candida orthopsilosis, Sphingomonas species., and their respective consortia biologically lixivize Cu, Ag, and Au through PCB of mobile phones. 0.5 g of PCB was added to mineral media containing the microorganisms, and the same quantity of PCB was added to the treatments with 1 M citric acid. At room temperature, each treatment was incubated for 35 days. The findings demonstrated that the addition of PCB can enhance the dry biomass of Sphingomonas MXB8 and the consortium of C. orthopsilosis MXL20 and Aspergillus niger MXPE6 by 147% and 126%, respectively. Sphingomonas species. MXB8/C. orthopsilosis MXL20 and Sphingomonas species. MXB8 leached 54%, 44.2%, and 35.8% of Ag during the bioleaching of metals when Aspergillus niger MXPE6 was inoculated.
3.3. Physical Methods
3.3.1. Plasma Method
The plasma process is another technique used to create nanoparticles. In the current instance, a radio frequency heating coil generates plasma. Microwaves along with plasma-spray fabrication are the two most popular plasma processes. An atomic plasma can also supply the energy required to cause the evaporation of the micrometer-sized nanoparticles [67]. The solid dust rapidly evaporates due to its thermal plasma’s temperatures, which are around 10,000 K. During the cooling phase, when the plasma state is present, nanoparticles grow as individuals. Using induction plasma torches, the electromagnetic radiation produced by the induction coil utilizes the energy inherent within the plasma. Although the costs of producing nanomaterials are often very low, the process also has other drawbacks, such as the difficulty in generating more complicated structures with accurate morphologies and the expensive equipment expenses [68].
3.3.2. Laser Ablation
A technique for creating many kinds of MNPs is laser ablation. Nanoparticles can be produced utilizing laser ablation, using a powerful laser beam, an optical focusing mechanism, and a target feeding system. The characteristics of the nanoparticles created by laser ablation are influenced by several variables, including the laser’s wavelength, which affects the metal charge, the period of the laser pulses, the laser flux, the ablation period, and the efficacy of the liquid medium, whether surfactants are present or not [69]. While it was discovered that the effectiveness of femtosecond ablation under water was poorer than that in air, the effectiveness of ablation for nanosecond pulsations turned out to be comparable in air as well as water. The absence of any chemical reagents required for the MNPs’ synthesis is a significant benefit of the laser ablation process over alternative techniques [70,71]. As a result, employing this method can produce pure, untainted colloidal metals, however, the method’s major drawbacks include the high costs of the required equipment, the technique’s high energy consumption, and its overall low yield rate. This approach allows for the generation of various MNPs types and the tuning of their attributes for various purposes [72].
3.3.3. Aerosol Technique
The coating applications often use aerosol-based techniques. After undergoing pyrolysis, chemical precursors applied on a surface within a heated liquid transform into nanoparticles [73]. The process produces MNPs with a small size distribution and enables the synthesis of complicated structures, however, it requires expensive equipment just like the plasma approach [74,75].
3.3.4. Gas Phase Synthesis
Gas-phase synthesis may be employed to develop metallic nanoparticles. The most common approach for creating metallic or metal oxide nanoparticles is gas-phase condensation. The material that needs to be vaporized, the vacuum chamber in combination with the heating element, alongside a cooling chamber coupled to the dust collecting mechanism, and the pumping system make up the primary components of the condensing system. A flow of matter is created by heating and putting pressure on the material inside the vacuum chamber [76]. This flow of matter then collides with the gas inside the cooling chamber to create spherical nanoparticles. The coolant’s reactiveness or inertness will depend on its use. Low melting point MNPs are frequently created via inert gas condensation. Super-cooled metallic vapors condense forming nanometer-sized particles that can be deposited on a substrate or investigated in situ while floating in a stream of inert gas. The technique produces highly pure nanoparticles, but its principal limitations include the inability to produce materials with higher melting temperatures or more complexity [77,78].
3.3.5. Spark Discharge
The method is based on an electric charge between two electrodes made of the metal that will cause it to be vaporized. The idea is to vaporize just enough metal to create nanoparticles by taking advantage of the electric arc effect [79]. Although the two procedures differ slightly in that the discharge in the arc process is continuous and the discharge in spark ionization is brief, spark ionization is frequently identified as an arc discharge. When the initial expenditures of the equipment are not taken into account, the approach produces high-quality MNPs and is moderately cost-effective [80].
3.3.6. Mechanical Dispersion
It has been demonstrated that colloidal mills, whose concept is based on producing sufficiently strong centrifugal forces within a small space across a rotating rotor alongside a stationary stator, can be used to achieve the greatest degree of dispersion. This method results in the mechanical tearing of larger fragments found in suspensions or emulsions [81,82]. Using a ball mill or mortar is the most basic way to disperse material. Because an aggregation process that is the opposite of dispersion, which is connected with the expansion of particles occurs constantly along with the dispersion procedure, these techniques, in theory, do not enable the creation of genuinely colloidal systems. The ease of installation and technology, the versatility in material grinding, and the availability of raw materials in large quantities are all advantages of mechanical grinding techniques. The method has drawbacks such as the possibility for contamination of powders created with abrasive materials, the challenge of getting materials with regulated morphology, the challenge of regulating the product’s composition during the grinding process, as well as the crystal defects caused by the method [83].
3.4. Chemical Methods
The most popular technique for creating nanoparticles is chemical synthesis. Employing organic or inorganic substances, the metal precursor is chemically reduced in this process. Metallic ions are reduced in various solutions using a variety of reducing agents, namely ascorbate, sodium citrate, as well as polyethylene glycol (PEG) [84]. Electrocatalysts are frequently made from a variety of wastes using wet-chemical techniques, including hydrothermal/solvothermal synthesis and the sol-gel process. These procedures essentially involve chemical reactions taking place in solutions under various pressures, temperatures, and chemical compositions [85,86]. It is crucial to safeguard nanoparticles that might be absorbed by or entrapped on surfaces throughout the synthesis in addition to employing stabilizers for dispersing nanoparticles to prevent agglomeration.
3.4.1. Hot Injection Method
Hot injection refers to a batch technique that involves chemical precursors being quickly injected directly into a heated reactor that also contains an amalgam of solvents with ligands to produce metal nanoparticles. The approach results in the achievement of MNPs with regulated size and shape, a limited size distribution, and excellent crystallinity [87,88]. The method’s primary flaws are its high related costs, use of hazardous chemicals, and challenging scale-up. By using this technique MNPs of different sizes having distinct morphology can be extracted from e-waste.
3.4.2. Thermal Decomposition
Another simple and affordable method to create stable, monodisperse MNPs with small dimensions is thermal decomposition which does not require any stabilizer. The technique produces a disproportionately large amount of monodispersed MNPs. The method’s disadvantages include the frequent usage of surfactants along with organic precursors as well as the high temperature that must be maintained for a considerable period [89,90].
3.4.3. Sol-Gel Method
Many nanomaterials can be synthesized using the sol-gel approach, including metal oxide nanoparticles. It is quick, easy, and affordable to obtain metallic nanoparticles using this process. The high purity of the finished product, uniformity of the nanoparticles, along low processing temperature are just a few of the many benefits of the sol-gel technique [84,91]. On the contrary, there are some drawbacks, especially the relatively high price of the organo-metallic precursors, and the utilization of some hazardous chemicals, This method can be used to produce metal oxide nanoparticles such as titanium dioxide (TiO2), tin oxide (SnO), aluminum (III) oxide (Al2O3), and zinc oxide (ZnO) [92].
3.4.4. Chemical Vapor Deposition
A chemical reaction coupled with several volatile precursors is required for chemical vapor deposition. In this process, high temperatures are required, and even the byproducts are hazardous gases. The process enables the synthesis of highly pure and homogenous MNPs but is highly substrate dependant. MNPs like SnO, TiO2, ZnO, nickel oxide (NiO), Al2O3, etc. can be produced using this technique [93,94,95].
3.4.5. Turkevich Method
The Turkevich method, which incorporates reducing chloroauric acid (HAuCl4) using sodium citrate within an aqueous solution at boiling point, is the most widely used technique for creating spherical Au nanoparticles. This process merely requires changing the ratio of the two reactants to produce nanoparticles with diameters ranging from 10 to 150 nm. The method’s main benefit is the ability to regulate the citrate/chloroauric acid molar ratio to adjust the size of the MNPs [96,97].
3.4.6. The Tollens Method
The Tollens method, which is utilized to create size-controlled Ag nanoparticles, represents a straightforward procedure for the synthesis of Ag nanoparticles. The Tollens reagent is reduced using an aldehyde. The enhanced approach produces Ag nanoparticle films (50–200 nm), colloidal silver (20–50 nm), and nanoparticles of different shapes with an acid molar ratio by reducing Ag ions by saccharides in the presence of ammonia [98,99].
3.4.7. The Polyol Method
The polyol approach is a crucial method of synthesis since it offers a great deal of control over the metallic nanoparticles’ size and geometry. Manganese (II, III) oxide (Mn3O4), ZnO, tin (IV) oxide (SnO2), lead (II) oxide (PbO), or TiO2 are just a few of the oxides of metallic nanoparticles whose fabrication has been reported in numerous works. Despite the reaction taking a while to complete, the approach enables the synthesis of regulated size and shape [100].
4. Comparative Analysis of MNPs Extracted from WEEE
An intriguing topic in the field of nanoscience over the past ten years has been the development of new synthesis techniques for nanomaterials including metal and their oxides nanoparticles, carbon nanotubes, and quantum dots, etc. Two distinct fundamental concepts of synthesis, specifically top-down as well as bottom-up techniques, have been studied in the literature to produce MNPs with the appropriate sizes, shapes, and characteristics verified by absorbance spectroscopy, transmission electron microscope (TEM), and scanning electron microscopy (SEM) [101,102]. This section particularly delves into different synthesis approaches for MNPs extracted from WEEE to enhance the concept of the circular economy.
John Watt et al. [103] reported the application of ultrasonic to a metal surface while a two-part surfactant solution is presented on the surface enabling the direct synthesis of nanoparticles using bulk metal. Material is ejected as a result of the implosive bursting of cavitation bubbles close adjacent to the bulk metal surface, which creates strong microjets. A surfactant bilayer structure in the form of nanoparticles traps and stabilizes this released substance. Gold is used to extract by this approach in detail, but it is also shown to work with various metals and their alloys, where it is broadly applicable. It is demonstrated that nanoparticles can be created irrespective of their overall metal form factor, and the technique is expanded to address the recovery of gold through electronic waste as depicted in Figure 6.
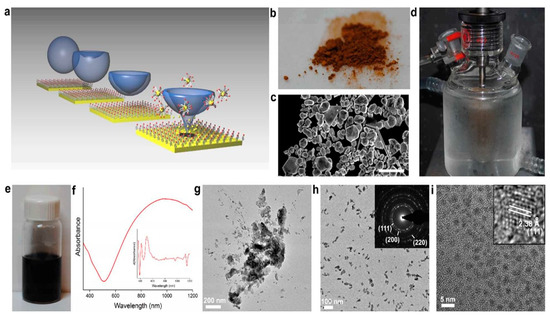
Figure 6.
Schematic for the recovery of gold nanoparticles (a) collapse of the cavitation bubble that causes a pit to arise and material to be ejected. When DDAB is present in water and bulk gold is ultrasonically agitated, (b) powder form bulk gold, (c) SEM analysis showing hexagonal and plate type particles, (d) Ultrasonication setup arrangement having glass cooling jacket operating at 18 watts for 6 h, (e) resultant blue solution of finely divided gold, (f) ultra violet spectrum of the resultant solution, (g) Transmission electron microscopy (TEM) images with different morphologies, (h) TEM images of rod-like gold nanoparticles, (i) TEM image of high resolution with nanoparticles population having a size of 2.0 ± 0.3 nm [103].
Rania Seif El-Nasr et al. [104] investigated that discarded computer PCB were selectively dissolved at room temperature into ammonium salt solutions. The impact of the variables governing copper recovery has been duly taken into account. Reducing leachate solutions utilizing L-ascorbic acid reductant along with cetyltrimethylammonium bromide (CTAB) as a chemical modifier around room temperature shows that copper nanoparticles were synthesized. For verification of the structure of the created copper nanoparticles, characterization tests were carried out. Copper nanoparticles’ perfect crystallinity phase was seen through X-ray diffraction (XRD) analysis, and their spherical form as well as 5–32 nm particle size were visible in TEM images.
Víctor Oestreicher et al. [105] provided a mechanism for recovering gold nanoparticles from electronic waste through a sequence of physically and chemically distinct procedures that have been carefully planned because of the complexity of the chosen WEEE and the desired result. In the first phase, microprocessor pins were mechanically separated, concentrating gold into a metallic fraction. After that, a two-step hydrometallurgical procedure was carried out to produce an Au (III) enriched solution. For this reason, a specific synthetic process was created and tailored to the high acidity along with the ionic strength of the solution, which was employed as a secondary raw material to make Au nanoparticles. With the help of two readily accessible reducing agents viz. sodium citrate along with ascorbic acid and a polymeric stabilizer, high-purity Au nanoparticles with a mix of distinct spherical and triangular forms could be produced. Also, other researchers like Ali Shokri et al. [106] also investigate the extraction of copper-tin (Cu-Sn) nanoparticles from the PCB of obsolete computers. By performing a thermogravimetric analysis (TGA) upon 22.7 mg of crushed powder PCB, the sample was heated using a heat source from room temperature to 1350 °C at a rate of 10 °C/s. Using a horizontal tube furnace comprising an argon atmosphere, an average flow rate of 1 L/min, 10 gms of the sample were heated to various temperatures. SEM and TEM analysis demonstrating the thermal transformation process revealed that the tin-lead (Sn-Pb) alloy contained in the trash started to melt at 900 °C, covering the Cu particles existing because of the alloy’s high moisture content on Cu. Because of the substantial solubility of Sn within Cu, the diffusion of Sn into the material was then activated, whereas the poor solubility of Pb within Cu caused the separation of Pb.
Whereas extraction of gold nanoparticles is reported by Javier Su-Gallegos et al. [107] by the Turkevich method. Au nanoparticles are synthesized from a gold coating that is extracted from processor pins with the least amount of waste possible. The procedure included four key steps: (a) the physical removal of pins; (b) acid digestion extraction of gold coatings; (c) moderate conditions for the synthesis of HAuCl4; and (d) the Turkevich method for synthesizing Au nanoparticles. Due to the tiny size of Au coatings, HAuCl4 could be synthesized using less hydrochloric acid (HCl) and nitric acid (HNO3) than when using aqua regia. Low nitrogen dioxide (NO2) emission, simple post-treatment along with purification, minimal synthesis cost, and excellent yields are a few of the many benefits of this method. TEM and even ultraviolet-visible (UV-Vis) spectroscopy were used to analyze gold nanoparticles made from HAuCl4. Extraction of gold coatings, which are recovered from pins of old processors by the reaction fo concentrated HNO3 acid with this waste material is performed and the XRD analysis shows that patterns of Au, SnO2, and CuO are produced. Acid digestion of gold coating with concentrated HNO3 is performed in a setup, and the extracted gold nanoparticles are analyzed with TEM spectroscopy.
Another work proposed by Mudila Dhanunjaya Rao et al. [108] describes a method through which e-waste can be extracted for gold and copper by a two-stage leaching and solvent extraction technique. In this, a new hydrometallurgical method is available for the commercially viable recovery of gold and copper using discarded PCB of mobile phones.
This procedure involves releasing the metallic fractional amounts from trimmed PCB, a 2-stage acid leaching procedure to bulk separate the copper along with gold out of the rest of the metals that exist, and then solvent extraction by employing extremely selective phenolic oxime along with amide extractants to purify the copper along with gold-containing solutions. In this process, 3 M nitric acid was used for fully dissolving the base metals at 30 °C, while copper solvent extractants (ACORGA M5640) mixed with kerosene were used in the solvent extraction process to achieve the selective separation of copper through this leaching liquid. Another work reported by Sabah M. Abdelbasir et al. [109] that extracts Cu and copper (II) oxide CuO nanoparticles from a waste PCB is described here. Cu and CuO nanoparticles are extracted from e-waste using sustainable processes. It is recommended to use ammoniacal ammonium salt leaching for selective and efficient recovery of copper from waste PCB. By using XRD, FTIR, as well as UV-visible investigations, the nanoparticles are verified. Cu and CuO nanoparticles had typical particle sizes of 460 nm and 50 nm, respectively, according to TEM images. The robust antibacterial activity of the produced nanoparticles has been evaluated against the five distinct bacterial along with fungal diseases. In comparison with Cu and CuO separately, a 1:1 mixture of copper and copper oxide nanoparticles showed good bactericidal action. Additionally, during visible light illumination, the nanoparticle mixture was utilized like a photocatalyst for the decomposition of rhodamine B (Rhod-B) dye.
Satya Sai Srikant et al. [110] investigated the recovery of Au, Ag, and Cu nanoparticles from WEEE such as capacitors, small transformers, and PCB of mobile phones, etc. The process involved microwave heating as the preliminary steps after being disassembled, removed, and recovered by a cutter, the PCB specimens with integrated circuits (ICs) and pogo pins served as the basis for the current investigations on the recovery of nano-gold. The integrated chips (ICs) and pogo pins taken from scrapped circuit boards were first broken into pieces measuring roughly 10 mm by 10 mm, and then these pieces were subsequently grounded to a fine particle form measuring roughly 250–300 mm. To extract precious metals like gold, copper, and silver nanoparticles from precipitated WEEE, a careful process involving microwave heating together with acid leaching (at first with HNO3, then with aqua leaching) of mashed e-waste specimens was required. After thoroughly shaking out the leaching solutions in a mixture of potassium chloride, hydrogen peroxide (H2O2), and β-cyclodextrin, the precipitated metals which included gold, silver, and copper colloids were dissolved to create colloidal solutions
Another investigation reported by Narayanasamy Marappa et al. [111] demonstrates the chemical makeup of precious metals including Au−, Ag−, Zn−, and Cu− can be extracted from e-waste. As a result, the current research highlights PCB as a key source of valuable metal in e-waste that has both an adverse effect on the environment as well as on humans. Utilizing two-step leaching techniques, native isolates Frankia species. along with Frankia casuarinae had been subjected to bioleaching of gold and valuable elements carrying e-waste. A significant degree of metal leaching inside the culture solution of Frankia metabolites along with organic acids generated by the leaching of metals was discovered to make the two-step bioleaching technique effective. The FTIR spectra and the low potential of hydrogen (pH) of the growing media were used to assess the existence of organic acid groups with functional properties.
The initial metal content and the substantial rise in metal concentration induced by leaching were both assessed by the atomic absorption spectrometry (AAS) analysis. Au−, Zn−, Cu−, and Ag− were the precious metal elements with the highest initial concentrations in e-waste (0.04, 0.1, 0.35, and 0.05 mg/g, respectively).
Frankia casuarinae extracted the highly precious metal gold more than Frankia species. Better copper recovery, at 94%, had been achieved by Frankia bio leaching of the bio-oxidized e-waste with 0.2% low pulp densities, while higher gold extraction, at 75%, for Frankia. Casuarinae the XRD and SEM results verified the findings. Other work proposed by Gaurav Sapra et al. [112] demonstrates that silica nanoparticles can be extracted from waste photo voltaics (PV) modules by utilizing thermal and chemical treatment. Voltage-current (V-I) characteristics are used to identify the kind of doping that occurs on the silicon wafer’s back and front surfaces. The configuration of recovered silicon is determined using XRD analysis. Additional nanoparticles of silica are created from a silicon wafer, and the outcome is consistently determined with UV-vis spectrophotometry. The recycling process is conducted using the following methodology: The PV module sample is first dissolved in an organic solvent, such as toluene, tetrahydrofuran, or trichloro-ethylene. Toluene is one of the chemicals that exhibits swelling around ethylene vinyl acetate (EVA) resin in an hour at ambient temperature. A PV module specimen measuring 7.8 cm by 2.4 cm is used for the experiment. Further, the glass was manually scraped off the sample surface when the EVA resin swelled out. At last, when the glass has been removed, place the piece of the solar cell devoid of the glass into a furnace and heat it there for four hours at 500 °C to break down the EVA layer. The sample is heated, and silicon is extracted along with certain metal impurities.
Another work investigated by Prashant Ram Jadhao et al. [113] shows the current work, which exhibits technologies for the extraction of metallic components and the creation of useful gases from e-waste. The procedure entails fixed bed pyrolysis lasting 10–60 min at temperatures between 200–600 °C. At a pyrolysis temperature of about 400 °C in 20 min, 35 weight % of combustible gases and 60 weight % of solid products were produced under optimal working circumstances. The solid result is an amalgam of metals along with other solid waste material, whereas the gaseous product was made up of methane (CH4), carbon dioxide (CO2), and carbon monoxide (CO). Around 90% of this metallic component was recovered when the solid product was treated with ultrasonication, producing a solid residue behind it. Additionally, nearly 100% of the valuable metals (like Au, Pt, Pd, and Ag) were transferred to the metallic fraction. To be able to effectively handle e-waste, recover the metallic component, and produce useful gases, this process integrates mild temperature pyrolysis with ultrasonication.
A different process investigated by Marta O. N. Amuanyena et al. [114] provides a mechanism to extract magnetic iron oxide nanoparticles by using moringa seed proteins from e-waste materials. The purpose of the investigation is to evaluate the effectiveness of nanoparticles of magnetic iron oxide treated with seed proteins coming from Moringa oleifera as a biological adsorbent for the extraction of Au(III), Pd(II), as well as Pt(IV) through aqueous solutions. With the help of FTIR, SEM, and TGA, many functional groups critical for the adsorption, shape, and thermal stability, along with the surface charges of these nanoparticles were identified. The valuable metal ions percentage that was recovered was assessed using the batch adsorption technique and plasma optical emission spectroscopy (ICP-OES). At 25 °C room temperature, impacts of pH, starting adsorbate concentration, adsorption agitation duration, and adsorbent dosage were investigated.
Pt (IV), Au (III), along with Pd (II) likewise produced maximum recoveries of 99.8%, and 87.7%, followed by 72.7%, respectively, at pH 2.5, ten mg/L initial adsorbate concentrations, 120 min of agitation, and also 0.065 g of adsorbent dose.
An industrial technique was proposed by Maksym Tatariants et al. [115] for the extraction of SnO2 from through-hole solder joint (THSJ) obtained from waste PCB. This investigation uses a sustainable leaching approach to recover Sn and Pb from the extracted THSJ in the form of SnO2 nanoparticles along with lead (IV) oxide (PbO2) microstructures as high-value products. A leaching solution was prepared using nitric acid, and SnO2 with PbO2 precursor was precipitated from the solution using ultrasound treatment. As other metal particles (Al, Ni, and Pd,) began to precipitate over time, they served as catalysts (or doping agents), accelerating the process of precipitation of SnO2 as well as PbO2. SnO2 and PbO2 precursors changed into nanoparticles and also micro cross/cubic nanostructures under the influence of sound waves, temperature, along catalysts; the generation and degree of refinement of these structures were regulated by varying the treatment period. The PbO2 microstructures were removed from the SnO2 nanoparticles using a micro-filtration method. By neutralizing the residual acidic solution with sodium hydroxide (NaOH) around pH 8, copper was further extracted as Copper (II) hydroxide (Cu(OH)2) from it. The created nano/micro products, retrieved metals, and extracted THSJ were all examined using SEM, TEM, and XRD. The findings revealed that the average diameters of the SnO2 and PbO2 were somewhat 7 nm and 1 μm, respectively, while the manufacturing costs were 6 times less than those of their commercial counterparts. For the large-scale synthesis of SnO2 with PbO2 a sustainable layout is proposed by the authors that also fulfilled the concept of circular economy.
A technique proposed by Burçak Ebin et al. [116] for the extraction of nickel (Ni) and nickel oxide (NiO) nanoparticles produced by reduction and calcination method by utilizing the waste Nickel metal hydride (NiMH) battery. By combining precipitation, reduction, along calcination processes with a raffinate solution obtained from a small-scale spent NiMH recovery operation, nanoparticles were created. By utilizing baking soda in a straightforward precipitation process, the recovery of Ni from the solution was 99.8%. The precipitate was characterized using X-ray diffraction, FTIR, and thermal gravimetric measurement techniques. By hydrogen reduction along with calcination of the precipitate around 400 °C, across 30–90 min residence periods, metallic and oxide nanoparticles were produced. Particle characterization techniques made it possible the detect the crystal structure, size, morphological characteristics, particle size, and surface area. Based on the findings, spherical Ni nanoparticles possess a crystallite size of close to 37 nm along with a particle size of approximatelminy 100 nm. By extending the residence time, the nanoparticle aggregation is reduced. NiO nanoparticles are smaller and have finer crystallites than the Ni nanoparticles created using identical temperatures and residence periods for metallic samples. However, a green synthesis technique is proposed by Pietrogiovanni Cerchier et al. [117] for the extraction of Cu nanoparticles. The synthesis of metallic copper (Cu) nanoparticles with the usage of ultrasound and environmentally friendly materials is described. For this investigation’s raw material, copper was retrieved from the pretreatment of PCB. L-ascorbic acid along with sodium borohydride was utilized as a reducing agent, and the effects of ultrasound, reducing agents, along with capping agents were also examined. The synthesis was carried out in aqueous solutions at ambient conditions including in the presence of air to make the procedure environmentally friendly. XRD, TEM, SEM, and UV spectroscopy, were used to characterize the nanoparticles. Results indicate that implementing ultrasound to create copper nanoparticles using L-ascorbic acid as a reducing agent produces a reaction time of 10 min as opposed to many hours when doing the synthesis without ultrasound.
This technique creates nanoparticles that are around 5 nm in size and are stable in liquids for days. Furthermore, obtaining nanoparticles of exceptional purity can be made possible by employing Cu nitrate from the leaching solution derived from electronic trash. All the recent extraction techniques from WEEE for the synthesis of metal and metal oxide nanoparticles to enhance the concept of circular economy are presented in Table 1.

Table 1.
Summary of synthesizing metallic and metal oxide nanoparticles from WEEE by different techniques.
5. Potential Applications of MNPs
Considering their special size-dependent features, nanoparticles are frequently utilized in many different industrial sectors. These nanomaterials are extremely fascinating for medicine and pharmacology because of their potential to modify the characteristics of nanoparticles. The use of nanoparticles in medicine is connected to the development of particular nanostructures that serve as cutting-edge therapeutic and diagnostic tools [118,119]. Because of their special size-dependent characteristics, MNPs are widely used in many different industries. Because of their exceptional qualities, they are especially desirable for use in pharmacology and medicine, where their qualities can be precisely adjusted to improve therapeutic and diagnostic potential. Nanoparticles are designed into certain nanostructures in the medical industry, which makes them cutting-edge instruments for diagnosis and treatment. Nanoparticles have a wide range of uses, including drug delivery systems, agriculture, environmental, proton therapies, bioimaging, and tissue engineering [120].
5.1. Drug Delivery
Targeted delivery of chemotherapy drugs to tumor cells is difficult because the majority of chemotherapeutic medications spread throughout the body cause toxicity and lead to poor patient behavior. Metallic nanoparticles are used to image cancer cells by active and passive targeting. Because of their small size, metallic nanoparticles may interact along with bio molecules on both the surface of cells as well as within them, improving therapeutic targeting [102].
Gold, nickel, platinum, and iron metallic nanoparticles in a range of sizes and morphologies between 10 and 100 nm have been investigated as diagnostic and medication-delivery agents. Gold nanoparticles have demonstrated a strong potential for usage as prospective drug delivery carriers among various kinds of noble nanoparticles made of metal. It is not difficult to find Au MNPs of different sizes [121,122]. Additionally, its surface is readily functionalized by H-bonding, covalent bonding, as well as electrostatic interactions to conjugate targeted agents with active biomolecules.
Additionally, it is simple to load multiple medicines to increase therapeutic efficacy. MNPs are derived from their capacity to enhance the blood circulation time of medicines, enhance the water solubility of hydrophobic drug compounds, and suppress or eliminate rapid renal drug excretion. The potential of multifunctional nanoparticles to provide concurrent cancer therapeutics along with diagnostics is greater than that of conventional nanoparticles.
Several instances include the co-delivery of several bioactive(s) using imaging agents and target-specific delivery via surface ligand decoration. Three key objectives in drug delivery are to direct the therapeutic agent to the point of action, reduce the drug’s negative effects on tissues that are healthy, and regulate drug release to prevent the overdosing/underdosing cycle. MNPs provide a strategy for achieving these objectives. As a result, the coating on MNP’s surface has been adjusted to regulate drug loading, distribution, and release in the intended location.
5.2. Gene Delivery
Providing exogenous ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) to prevent or cure diseases is known as gene therapy. Gene therapy effectiveness is decreased by popular viral vectors that frequently cause host immune systems to respond. Utilizing non-viral methods, such as metallic nanoparticles, can alleviate the aforementioned issues. According to recent research, Au MNPs of various forms, such as nanospheres or nanorods, shield nucleic acids against degradation by nucleases. The special characteristics of Au MNPs coupled to oligonucleotides make them suitable as gene-regulating agents [123,124,125]. One of the most popular nanoparticles for gene delivery is PEG gold. The effectiveness of a delivery system derived from PEGylated gold nanoparticles for plasmid DNA-mediated electroporation was assessed.
Following intravenous injection, gene expression was 100 times more improved by DNA-PEGylated gold nanoparticles than with naked DNA. The DNA was liberated and passed across the cellular membranes, and the transgenes were stable in circulation. Additionally, effective gene delivery vectors made of gold nanoparticles customized with amino acids have been used without endangering cells. Strong and controllable surface Plasmon absorption has been seen in the near-infrared (NIR) spectroscopy range using gold nanorods [126]. As a result, the use of gold nanorods in a controlled gene release system opens up a wide range of potential applications in gene therapy.
5.3. Protein Delivery
An enhancing amount of research is encouraging the use of nanoparticles as protein carriers. The structure as well as the morphology of proteins bound to Au MNPs might be investigated using the molecular probe organothiol. It has been discovered that insulin-functionalized Au MNPs are helpful for transmucosal medication delivery for the cure of diabetes in rat models. By coating Au MNPs using a non-toxic biopolymer, like chitosan, which strongly adsorbed insulin on their surface, it is possible to increase the effectiveness of insulin delivery [127,128]. Due to their non-antigenic nature, protein nanoparticles can also be employed in a wide range of targeted therapies, including lung delivery, cancer therapy, tumor therapy, and vaccinations. For regulated and sustained release, protein nanoparticles can be integrated into biodegradable polymers in a microsphere. Numerous uses for physiologically active chemical carriers based on noble metals offer hope for more successful methods for treating cancer and other modern diseases. However, the biggest issue with employing these tiny particles in vivo is difficulties with their breakdown and removal from the body. Therefore, the primary objective of scientists looking into this subject should be the enhancement of the pharmacokinetics of these nanoparticles.
5.4. Radiation Therapy for Curing Cancer
Delivering an excessive amount of radiation into cancer cells while simultaneously protecting the healthy tissue around them is the major objective of radiation treatment or proton therapy. Two strategies can be used to accomplish this goal: increasing the cancer cells’ radiation sensitivity or adjusting the dose to the tumor volume. A drawback of radiation therapy (RT) is that because gamma and X-rays have exponential dose accumulation with tissue depth, some of the radiation dosage is administered in front or behind the tumor. The formation of reactive oxygen species (ROS) can be increased by high atomic number nanoparticles, such as Au NPs, to enhance the effectiveness of RT. By using this method, nanoparticles can lower the overall radiation exposure while increasing the dose delivered locally to the tumor. Additionally, the negative effects can be minimized [129,130]. By taking advantage of the tumor’s leaky vasculature or focusing on the overexpressed receptors on the tumor cells, metal nanoparticles can reach the target locations. MNPs therapies can increase the accumulation of therapeutic agents in two different ways: passively and actively. The tumor vasculatures frequently have aberrant branching and leaky spots with pore diameters ranging from 100 nm to several hundred nanometers. The reason is that there were fewer pericytes because endothelial cells developed quickly. The body accumulates inert MNPs in the tumor as part of the passive targeting mechanism as a result of this leaky vasculature. The enhanced permeability and retention (EPR) effect is used to describe this. The medicinal delivery system is improved by active targeting, which targets certain tissues by functionally altering the MNP’s surface. Targeted intra-tumor drug release may come from the surface modification of MNPs with tumor-targeting ligands like antibodies, folic acid, and peptides, or the insertion of tumor-targeting ligands inside MNPs via disulfide bonding. Additionally, B. Khodashenas et al. [131] demonstrated the production and efficacy of the biopolymer of gold/gelatin nanoparticles as a medication delivery system for methotrexate (MTX). The chemical reduction approach was used to create gold nanorods in three different sizes (20, 50, and 100 nm length) and two distinct shapes of gold particles, including spherical Au nanoparticles (50 & 100 nm). The impact of Au nanoparticle’s size and shape on cellular uptake, drug release rate, along entrapment efficiency was examined. A gelatin biopolymer was applied to the surfaces of gold nanoparticles and Au nanorods, and the stability and characteristics of the resulting compounds were examined. The Au nanoparticles and rod complexes coated in gelatin were loaded with MTX, a chemotherapeutic drug. FTIR, SEM, TEM, and dynamic light scattering (DLS), spectroscopy were used to investigate the physicochemical characteristics of the gelatin-coated Au nanoparticles/Au nanorods complexes. In vitro studies were conducted to examine MTX release behavior from the complexes at pH values of 7.4 and 5.4 and temperatures of 37 and 40 °C.
5.5. Bioimaging Applications
Due to the employment of X-ray technology for medical imaging, several noninvasive techniques have been created and successfully applied in numerous medical research fields, including drug development, drug delivery, and diagnostic analysis, along with cellular biology research. Clinical imaging investigations are conducted in a variety of scientific fields and have a substantial impact on the advancement of medicine. There are numerous modified molecular bioimaging techniques, including positron emission tomography, magnetic resonance imaging (MRI), optical imaging, ultrasound imaging, and many others. These have been modified for use in biological treatments both in vitro along in vivo [132,133]. Because of the near-infrared absorption of light by biological tissue, noble metal nanoparticles like silver, gold, and platinum can be useful in immediate actuation and tracking. Owing they have strong near-infrared light absorption, frequently observed noble metallic nanoparticles are being utilized for in vivo imaging therapy. As a result, these nanoparticles can be thought of as highly effective contrast agents [134].
The standard of bio-imaging by offering higher contrast, higher resolution images to detect disorders at the molecular level has been improved. Additionally, using various nanoparticles functionalized with imaging agents and therapeutic compounds, theranostic treatment is another option provided by nanotechnology for health diagnosis and treatment. Several imaging approaches currently use nanomaterials exclusively as the imaging signal source rather than a contrast goal. The application of various nanomaterials in imaging modalities, such as MRI, Raman-based, luminescence upconversion imaging, CT (computed tomography), and fluorescence imaging are a few advancements in bioimaging [135].
5.6. Therapeutic Applications
The metallic nanoparticles possess plenty of noteworthy and beneficial properties for therapeutic applications. According to the investigation, metal nanoparticles have been approved as a human immunodeficiency virus (HIV) preventive-therapeutic agent silver nanoparticles can directly combat viral infection by interacting with glycoprotein. This kind of connectivity stops several forms of viral binding and successfully lowers the HIV infection rate. Therefore, it is widely known that MNPs may have been employed as effective antiviral medications to combat influenza, herpesvirus, and pulmonary syncytial viruses. Prospective noble metal nanoparticle production must be expanded upward to commercial medical levels. MNPs were recently created and are currently being tested extensively in multiple directions. At the moment, it is primarily employed to treat malignant cells. Metal nanoparticles demonstrate their potency as cutting-edge agents for potential tumor treatment approaches [136,137].
5.7. Catalysis Applications
Metallic nanoparticle-based catalysts are extremely active, and selective, and have a long lifespan for a variety of processes. Heterogeneous catalysts and homogeneous catalysts are the two forms of catalysts that are immobilized on inorganic support [138]. The applications include hydrogenation, hydrogen peroxide synthesis, water gas shift, and oxidation reaction. Metallic nanoparticles wrapped by stabilizers make up homogenous catalysts and the applications include the hydrogenation of olefins and nitrile [139].
5.8. Paint Industry
To take full advantage of its photocatalytic activity along with ultraviolet (UV) protection, titanium dioxide nanoparticles are utilized in the paint industry. Nano silicon dioxide (SiO2) can increase the abrasion, scratching, and micro as well as macro hardness of paints [140]. The nanopowder that is made up of fillers that are 40 to 60 nm in size and are therefore effective fillers will be used in the nano paints. These fillers include zirconium dioxide (ZrO2) and SiO2. The homogenous dispersion of the nanoparticles will increase dependability and offer a scratch-resistant structure. The nanoparticles will contain alumina compounds that will shield them from ultraviolet rays. The composite coating that will offer the unimolecular structure is part of the alumina’s performance. It will offer a superior finish and greater chemical resistance. The UV curable coating along with the ninefold module statement will be provided by this obvious nanoparticle.
5.9. Cosmetics Applications
Nanocosmetics has emerged as a field where nanotechnology has potential uses. Nanoscaled chemicals are used in cosmetic formulations to provide effects that last longer, penetrate the skin more deeply, offer greater UV protection, and have more color. The improved antibacterial capabilities of nanosilver are being combined by cosmetic makers in a variety of applications, such as utilizing it to act as a preservative in cosmetic items [141,142]. Due to the inclusion of Ag nanoparticles, some manufacturers claim that their deodorants can provide up to 24 h of antibacterial protection. Nanosized Au particles have been used in several toothpaste formulae since it is also believed that they have antimicrobial properties against many oral bacterial types [143,144].
5.10. Wound Dressing Applications
Generally involving the outer layer of skin, a wound is an either internal or external break-in or harm to human tissue. Inflammation, proliferation, and remodeling are some of the complex processes of healing. Maintaining the stages of healing depends in large part on the type of dressing used. Different types of polymers, both synthetic and natural in origin, are used in dressings [145,146]. Many different materials have been employed over the years, including cotton swabs, sponges, fins, etc. However, popular synthetic dressings have drawbacks such as poor mechanical strength and hazardous breakdown of byproducts. Thus for the protection as a result of the product’s inclusion Ag and Au nanoparticles have been used.
The inclusion of MNPs into chitosan scaffolds, including chitosan/Ag, chitosan/zinc oxide (ZnO), chitosan/Au, and chitosan/titanium oxide (TiO), has been the subject of much research. The aforementioned dressing materials are being added to certain toothpaste formulas because research has shown that they have enhanced antimicrobial properties, modified cytotoxicity, and enhanced healing due to the incorporation of metallic nanoparticles [147]. These dressing materials also claim to possess disinfecting effects regarding a variety of oral bacteria strains.
5.11. Food Industry Applications
Targeting pathogens, such as Staphylococcus aureus, Escherichia coli, Salmonella enterica, Enterococcus faecalis, Bacillus cereus, and Fusarium oxysporum, can be detected using the metallic nanoparticles to be sensing platforms, resulting in a combination response pattern that is selective for each pathogen [148]. A variety of sensing technologies, including colorimetric chemosensors, will be used to measure the reaction. Because of the dual roles that nanostructures play as receptors and indicators that result in a color change, they have the potential to be used as straightforward and effective sensing components in a colorimetric sensor system [149]. The materials used to package MNPs-food are often constructed of polyethylene terephthalate (PET), polypropylene (PP), as well as non-degradable polyethylene (PE), which prevents the infiltration of water and oxygen molecules. To further ensure food safety and maintain food quality, coatings with flavors, antioxidants, antibacterial agents, emitting sachets, and preservatives are also used. Additionally, it includes bio-based packaging, antimicrobial active packaging, improved food stability, and color, the ability to detect food spoilage organisms, the potential to prevent oxidation, and active ultraviolet (UV) protection. For instance, silica nanoparticles in plastic inhibit gaseous exchange, extending the shelf life of the product, while nano-sized iron particles have improved reactivity and bioavailability. Silver nanoparticles have higher antibacterial capabilities [150]. With effective spoiling management, the addition of MNPs has greatly extended the shelf life of canned food that is preserved. The ingestion of MNPs in food packaging can inhibit the growth of several bacteria, including Lactobacillus plantarum, Staphylococcus aureus, Escherichia coli, and yeasts. Mass/heat transfer, nanobiotechnology, and molecular synthesis are some of the MNPs-based nano-food packaging formulations. Those polymeric nanocomposites can be utilized to protect nano-foods from packaging barriers such as moisture, CO2, oxygen, and ethanol while also enhancing flavor and emission emissions. Intelligent packaging, biopolymers that degrade, and active packaging are a few more benefits of MNPs nano packaging.
6. Discussion
Heavy metals found in e-waste cause water pollution as well as soil damage. For example, heavy metals within the soil have the potential to contaminate groundwater by incorrect recycling and rainfall runoff, which can affect surrounding water bodies. We can lessen the environmental effects of recycling e-waste by using sustainable and eco-friendly ways, and we can also make money by recovering metals using various techniques. The main methods for treating WEEE are pyrometallurgical along hydrometallurgical procedures, although they are less environmentally friendly than the bio-metallurgical process, which involves bioleaching metals from electronic waste [151,152]. Although bioleaching has enormous potential for growth in the future, more study is still required before it can be used to eliminate huge amounts of e-waste. We can make money by recovering valuable metals from e-waste by using the most environmentally friendly method of treatment. We may mitigate the negative effects of radioactive isotopes, mercury, lead, and other toxic substances on human health. The establishment of a circular economic system that is sustainable and conscientious of the environment depends on the accurate, safe, and efficient (i.e., economically feasible) recycling of WEEE. We should incorporate eco-design into our initiatives as investigators and designers of new methodologies and technologies by making electronic and electrical devices easier to gather, reuse, and discard for sustainable development supporting the 6 R’s (reduce, reuse, recycle, rethink, repair, and refuse) of circular economy as depicted in Figure 7. It is well known that the current and dominant linear economy structure, referred to as “extract-produce-use-dump”, has been challenged for years as being unsustainable and aggressive towards the environment since it will deplete natural resources for both materials as well as energy. This sort of economy depends heavily on raw materials, hence there is a risk related to their supply and pricing. Additionally, it results in a significant loss of earth’s resources and subsequent economic losses.
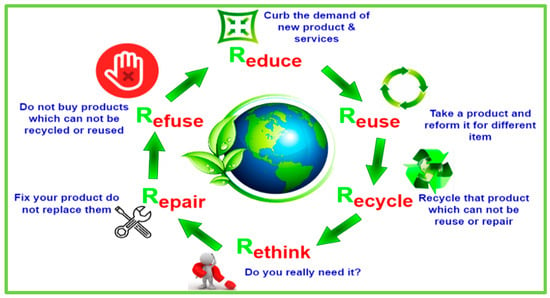
Figure 7.
The 6 R’s of the circular economy for sustainability.
The majority of the components that make up e-waste can be recycled using current technologies; however, further research on this subject is required to discover more effective, economically viable, and environmentally friendly recycling techniques that can then be used widely [153,154]. All advancements in recycling methods, however, are pointless without a widespread awareness among society including the presence of adequate infrastructure for collecting e-waste. Only a few developed nations now possess the necessary technologies to launch secure and effective recycling facilities. It is essential to invest in emerging nations to ensure that they can run a professional and effective recycling business. Different options for employing waste products as inputs for the creation of MNPs have been illustrated and addressed throughout this review in the framework of research. Although recycling waste into cutting-edge technologies for ecological functions may seem like an appealing full-circle strategy at first glance, it contains substantial gaps in understanding about engineered nanomaterials which need to be addressed before transferring these ideas through the lab over to the real world; specifically, regarding energy usage, generation of secondary wastes, the future and transport behavior, exposure routes throughout various environments, and toxic substances [155,156]. However, just because MNPs can be created using environmentally friendly methods doesn’t mean that they are inherently safe or that their usage or release into the environment won’t have a negative impact. Studies conducted in the past ten years have revealed that created nanoparticles (engineered MNPs) utilized in a variety of settings, such as food production and packaging, have the potential to significantly disrupt epigenetic pathways and raise many concerns about their potential to cause disease [157]. Reducing dependence on virgin resources, prolonging material lifecycles, and eliminating waste are all given top priority in the circular economy paradigm. By reprocessing products like PET or aluminum with little energy and maintaining material integrity, mechanical recycling, for example, is highly compatible with these objectives; but, contamination hazards may restrict its efficacy. By separating materials into monomers before reuse, chemical recycling, on the other hand, tackles complicated or mixed waste streams (such as multilayered plastics) and makes closed-loop systems possible. However, to prevent sustainability from being compromised by its high energy demand and potentially harmful consequences, solvent recovery and the incorporation of renewable energy are necessary. The slow processing speed of bioleaching limits scalability, but it offers near-zero energy consumption and purifying environments by using microorganisms to remove metals from low-grade ores or e-waste. High purity and efficiency are attained by hydrometallurgy, which uses aqueous solutions enabling selective metal recovery. However, to reduce the hazards of acid waste, it relies on water recycling and green chemistry. Although traditional pyrometallurgy is effective for bulk metals like steel, its high emissions and reliance on fossil fuels make it incompatible with circular principles unless it is modified to include carbon capture along with waste heat recovery.
Throughout this review article synthesis techniques of MNPs derived from WEEE are discussed along with their applications in protein delivery, drug delivery, bioimaging, gene delivery, therapeutic, paint, catalysis, and food industry. At first ultrasonic method is used to synthesize gold nanoparticles from SIM card waste having sizes 5 to 100 nm with spheroid morphology. Then Cu MNPs are synthesized by implementing a hydrometallurgical approach which proves to be economical and environmentally friendly. Cu-Sn nanoparticles are also synthesized from the PCB of the computer cryo-mill technique which is proved to be again eco-friendly approach as it does not emit Pb and Sb during the process. Cu and CuO nanoparticles are recovered from the waste PCB of old computers by the hydrometallurgical process. Different techniques are provided for the synthesis of MNPs some use acid leaching, pyrolysis, chemical, and thermal treatment, along bioleaching. Researchers could gain knowledge about these processes along with their pros and cons for extracting MNPs from WEEE for enhancing the concept of circular economy as discussed in subsequent sections. At last all the general applications of extracted MNPs are summarized for the readers describing the potential areas such as drug delivery, bioimaging, food industry, and environmental monitoring are discussed holistically.
7. Conclusions and Future Prospects
Considering the illustration given in this review paper, it is clear that nanomaterials of all kinds and metallic/metal oxide nanoparticles more specifically can be useful tools for identifying and even characterizing different applications as discussed earlier. This area should be one of ongoing research in the quest for a safer ecological system, given the possible threats to consumers’ health and the deletion of natural resources. Nanoparticles can be used in a variety of applications, but choosing the right synthesis method involves carefully evaluating all of its unique benefits and drawbacks. Because of their outstanding chemical, electrical, optical, and magnetic properties, metallic nanoparticle production is crucial. It has been demonstrated that producing nanoparticles with a constrained variation in size using radiolytic and photolytic technologies has many benefits. In addition, green synthesis and hydrometallurgical techniques can be used in conjunction to create nanoparticles from different WEEE sources enhancing the prime objective of a circular economy. The peculiarity of this kind of approach is that growth kinetics may be investigated in addition to the creation of metal nanoparticles. Metal nanoparticles of Cd and Ag were created in aqueous solutions along with viscous media. Metal nanoparticles were stabilized using a viscous medium. By blasting the particles with laser irradiation with ethylene glycol along with glycerol media, uniform sizes of particles can be created. Metallic nanoparticles are used in a wide variety of products, including imaging agents, delivery systems, artificial inhibitors, and sensors. Enhancing the therapeutic efficiency of nanoparticles while lowering their level of toxicity remains one of the primary issues facing the field of nanobiotechnology.
Featuring the swift advancement of nanomaterials, new therapeutic approaches are being investigated with the possibility that they use noble MNPs to solve current issues. The effect on human health must be properly studied before widespread use. Individualized treatment can benefit greatly from the use of nanotechnology. However, further research is required for that enhancement. It is undeniable that market demand for employing nanomaterials in industry, cosmetics, as well as healthcare is growing. Therefore, safety precautions must be taken to safeguard both human health and the environment. This amply demonstrates the requirement for more thorough research on the safety implications of metallic nanoparticles before their usage in healthcare. Each conceivable usage of metal nanoparticles concerning humans will require further study. On the whole, gold, palladium, platinum, silver, and nanoparticles of selenium are in a prime position to move from the bench of a laboratory toward the clinical setting in the very distant future. Metallic nanoparticle creation is complex, and today, even cancer treatment uses them extensively. A recent development in site-specific targeting is believed to have been made possible by these nanoparticles. Metallic nanoparticles highlight their strength as a brand-new agent for potential cancer treatment techniques. Whether noble MNPs are harmful or biocompatible? Since it is important to prevent damage from healthy tissue, it is crucial to experimentally examine nanoparticle interactions and adjust the nanoparticles for optimum biocompatibility with the cell. MNPs have proven to be effective weapons against cancer. Although it requires characterization and optimization to realize their full potential. That is now crucial to begin converting these structures towards a clinical field to combat cancer. By diminishing the requirement for dangerous reagents and improving nanoparticle biocompatibility, green synthesis techniques like plant-mediated and microbial-assisted nanoparticle recovery provide an environmentally acceptable substitute for conventional chemical and electrochemical processes. To assess the possible cytotoxic, genotoxic, and immunotoxic consequences of recycled nanoparticles, however, particularly when employed in biomedical applications such as drug delivery and biosensing, comprehensive toxicity studies are necessary. Furthermore, to comprehend the chance, stability, and possible bioaccumulation associated with these nanoparticles in ecosystems, long-term environmental impact evaluations are crucial. Standardized procedures for recovering, purifying, and reusing nanoparticles would improve the incorporation of sustainable nanotechnology into the waste management and medical fields. By tackling these important issues, future developments will promote a more responsible, economical, and ecologically friendly method of using metallic nanoparticles produced from e-waste to achieve sustainable development goals.
The circular economy’s fundamental principles can be advanced by the extraction of MNPs from WEEE. The environmental issues caused by inappropriate e-waste disposal are becoming more urgent as its number keeps increasing. By adopting circular economy principles, we can turn this trash into useful resources. We can save precious metals, ease the burden on conventional mining operations, and lower the overall negative environmental effect by extracting MNPs from old electrical gadgets. Additionally, this strategy increases the number of opportunities for nanoparticle recovery by extending the useful lives of electronic products through refurbishment along with repair. Reusing precious materials, not only adds value economically, but it also encourages breakthroughs in recycling along with materials recovery processes. To create effective recycling systems, cooperation between a variety of stakeholders, which includes governments, businesses, and consumers becomes very crucial. We may move forward with a more environmentally friendly and accountable approach that balances economic growth along with environmental sustainability through implementing a circular economy perspective in the extraction of MNPs from e-waste.
Author Contributions
S.K.: Writing—original draft, Methodology, Data curation, Validation, Software; H.S. (Harbinder Singh): Conceptualization, Supervision, Writing—review & editing, Formal analysis, Investigation, Resources; H.S. (Harjeevan Singh): Writing—review & editing, Data curation; H.S. (Himanshi Soni): Writing—review & editing, Visualization; M.B.: Writing—review & editing, Formal analysis, Funding acquisition; J.S.: Conceptualization, Supervision, Writing—review & editing, Formal analysis, Investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors gratefully acknowledge Chandigarh University, and Rayat Bahra University India for providing all the necessary facilities to carry out this work.
Conflicts of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
- Ongondo, F.O.; Williams, I.D.; Cherrett, T.J. How are WEEE doing? A global review of the management of electrical and electronic wastes. Waste Manag. 2011, 31, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Cheshmeh, Z.A.; Bigverdi, Z.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Gheibi, M. A comprehensive review of used electrical and electronic equipment management with a focus on the circular economy-based policy-making. J. Clean. Prod. 2023, 389, 136132. [Google Scholar] [CrossRef]
- Ismail, H.; Hanafiah, M.M. Life cycle assessment of e-waste management: Current practices and future research agenda towards sustainability. In Waste Management and Resource Recycling in the Developing World; Elsevier: Amsterdam, The Netherlands, 2023; pp. 237–252. [Google Scholar]
- Baxter, J.; Lyng, K.A.; Askham, C.; Hanssen, O.J. High-quality collection and disposal of WEEE: Environmental impacts and resultant issues. Waste Manag. 2016, 57, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, I.; Wright, N.; Kellner, R.; Bains, N.; Geraghty, K.; Goosey, M.; Lightfoot, L. An integrated approach to electronic waste (WEEE) recycling. Circuit. World 2007, 33, 52–58. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J.; Zeng, X. Rare Earth Elements Recovery from Waste Fluorescent Lamps: A Review. In Critical Reviews in Environmental Science and Technology; Taylor and Francis Inc.: Abingdon, UK, 2015; Volume 45, pp. 749–776. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Innocenzi, V.; De Michelis, I.; Medici, F.; Vegliò, F. Rare earth elements recovery from fluorescent lamps: A new thermal pretreatment to improve the efficiency of the hydrometallurgical process. J. Clean. Prod. 2017, 153, 287–298. [Google Scholar] [CrossRef]
- Ankit; Saha, L.; Kumar, V.; Tiwari, J.; Sweta; Rawat, S.; Singh, J.; Bauddh, K. Electronic waste and their leachates impact on human health and environment: Global ecological threat and management. Environ. Technol. Innov. 2021, 24, 102049. [Google Scholar] [CrossRef]
- Nithya, R.; Sivasankari, C.; Thirunavukkarasu, A. Electronic waste generation, regulation and metal recovery: A review. Environ. Chem. Lett. 2021, 19, 1347–1368. [Google Scholar] [CrossRef]
- Ghimire, H.; Ariya, P.A. E-Wastes: Bridging the Knowledge Gaps in Global Production Budgets, Composition, Recycling and Sustainability Implications. Sustain. Chem. 2020, 1, 154–182. [Google Scholar] [CrossRef]
- Manikandan, S.; Inbakandan, D.; Nachiyar, C.V.; Karthick Raja Namasivayam, S. Towards sustainable metal recovery from e-waste: A mini review. Sustain. Chem. Environ. 2023, 2, 100001. [Google Scholar] [CrossRef]
- Jain, M.; Kumar, D.; Chaudhary, J.; Kumar, S.; Sharma, S.; Singh Verma, A. Review on E-waste management and its impact on the environment and society. Waste Manag. Bull. 2023, 1, 34–44. [Google Scholar] [CrossRef]
- Barragan, J.A.; De León, C.P.; Castro, J.R.A.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; Larios-Durán, E.R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega 2020, 5, 12355–12363. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.; Yadav, S.; Khan, J. Bionanofactories for the Environmental Friendly Fabrication of Silver Nanoparticles: Application to the Analysis of Antimicrobial Agents. Curr. Pharm. Anal. 2024, 20, 98–114. [Google Scholar] [CrossRef]
- Gautam, P.; De, A.K.; Sinha, I.; Behera, C.K.; Singh, K.K. Genesis of copper oxide nanoparticles from waste printed circuit boards and evaluation of their photocatalytic activity. Environ. Res. 2023, 229, 115951. [Google Scholar] [CrossRef]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Deep, A.; Szulejko, J.E.; Vellingiri, K.; Kumar, S.; Kwon, E.E.; Yun, S.-T. Recovery of nanomaterials from battery and electronic wastes: A new paradigm of environmental waste management. Renew. Sustain. Energy Rev. 2018, 82, 3694–3704. [Google Scholar] [CrossRef]
- Balaji, R.; Prabhakaran, D.; Thirumarimurugan, M. Recent approaches towards e-waste printed circuit boards for Nanoparticle synthesis: A review. Int. J. Environ. Anal. Chem. 2021, 104, 291–305. [Google Scholar] [CrossRef]
- Birloaga, I.; De Michelis, I.; Ferella, F.; Buzatu, M.; Vegliò, F. Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manag. 2013, 33, 935–941. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, B.; Wei, Y.; Zhou, S.; Wang, H. Recycling potential of waste printed circuit boards using pyrolysis: Status quo and perspectives. Process Saf. Environ. Prot. 2023, 173, 437–451. [Google Scholar] [CrossRef]
- Holgersson, S.; Steenari, B.M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size Waste Electric and Electronic Equipment (WEEE) printed circuit boards—Part 1: Internet routers, mobile phones and smartphones. Resour. Conserv. Recycl. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Dutta, D.; Panda, R.; Kumari, A.; Goel, S.; Jha, M.K. Sustainable recycling process for metals recovery from used printed circuit boards (PCBs). Sustain. Mater. Technol. 2018, 17, e00066. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S.; Mousavi, S.M. Content evaluation of different waste PCBs to enhance basic metals recycling. Resour. Conserv. Recycl. 2018, 139, 298–306. [Google Scholar] [CrossRef]
- Adie, G.U.; Sun, L.; Zeng, X.; Zheng, L.; Osibanjo, O.; Li, J. Examining the evolution of metals utilized in printed circuit boards. Environ. Technol. 2017, 38, 1696–1701. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, B.; Pan, D.; Wu, Y.; Zuo, T. Recovery of waste printed circuit boards through pyrometallurgical processing: A review. Resour. Conserv. Recycl. 2017, 126, 209–218. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Venkatesh, N. Metallic Nanoparticle: A Review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.-Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Chong, K.F.; Ali, G.A.M. The recent progress on silver nanoparticles: Synthesis and electronic applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kumar, P.; Singh, A.K.; Agrawal, S.; Albukhaty, S.; Bhattacharya, I.; Tiwari, K.N.; Mishra, S.K.; Tripathi, A.K.; AlMalki, F.A.; et al. Green synthesis of silver nanoparticles using Trema Orientalis (L.) extract and evaluation of their antibacterial activity. Green Chem. Lett. Rev. 2025, 18, 2444679. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, R.; Devi, S.; Sharma, P.; Chaudhary, N.R.; Negi, S.; Tandel, N.; Marepally, S.; Pied, S.; Tyagi, R.K. Biogenically synthesized green silver nanoparticles exhibit antimalarial activity. Discov. Nano 2024, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Rabalao, T.M.; Ndaba, B.; Roopnarain, A.; Vatsha, B. Towards a circular economy: The influence of extraction methods on phytosynthesis of metallic nanoparticles and their impact on crop growth and protection. JSFA Rep. 2022, 2, 208–221. [Google Scholar] [CrossRef]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.-M. Optimized Synthesis Approaches of Metal Nanoparticles with Antimicrobial Applications. J. Nanomater. 2020, 2020, 6651207. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green Chemistry Based Benign Routes for Nanoparticle Synthesis. J. Nanoparticles 2014, 2014, 302429. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Matinde, E.; Simate, G.S.; Ndlovu, S. Mining and metallurgical wastes: A review of recycling and re-use practices. J. S. Afr. Inst. Min. Metall. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Zhang, Z.; Malik, M.Z.; Khan, A.; Ali, N.; Malik, S.; Bilal, M. Environmental impacts of hazardous waste, and management strategies to reconcile circular economy and eco-sustainability. Sci. Total Environ. 2022, 807, 150856. [Google Scholar] [CrossRef] [PubMed]
- Makhathini, T.P.; Bwapwa, J.K.; Mtsweni, S. Various Options for Mining and Metallurgical Waste in the Circular Economy: A Review. Sustainability 2023, 15, 2518. [Google Scholar] [CrossRef]
- Zeng, X.; Mathews, J.A.; Li, J. Urban Mining of E-Waste is Becoming More Cost-Effective Than Virgin Mining. Environ. Sci. Technol. 2018, 52, 4835–4841. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.J.; Williams, P.T. Separation and recovery of materials from scrap printed circuit boards. Resour. Conserv. Recycl. 2007, 51, 691–709. [Google Scholar] [CrossRef]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, P.; Das, A.K. Unconventional Physical Methods for Synthesis of Metal and Non-metal Nanoparticles: A Review. Proc. Natl. Acad. Sci. India Sect. A—Phys. Sci. 2019, 89, 199–221. [Google Scholar] [CrossRef]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of Chemical Synthesis in the Synthesis of Nanomaterial and Nanoparticles by the Chemical Deposition Method: A Review. BioNanoSci. 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. Electrochemical method for the synthesis of silver nanoparticles. J. Nanoparticle Res. 2009, 11, 1193–1200. [Google Scholar] [CrossRef]
- Singaravelan, R.; Bangaru Sudarsan Alwar, S. Electrochemical synthesis, characterisation and phytogenic properties of silver nanoparticles. Appl. Nanosci. 2015, 5, 983–991. [Google Scholar] [CrossRef]
- Husseiny, M.I.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M.A. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Ji, X.; Yang, M.; Wan, A.; Yu, S.; Yao, Z. Bioleaching of Typical Electronic Waste—Printed Circuit Boards (WPCBs): A Short Review. Int. J. Environ. Res. Public Health 2022, 19, 7508. [Google Scholar] [CrossRef]
- Yin, H.; Xing, P. Pyrometallurgical routes for the recycling of spent lithium-ion batteries. In Recycling of Spent Lithium-Ion Batteries: Processing Methods and Environmental Impacts; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 57–83. [Google Scholar]
- Holzer, A.; Windisch-Kern, S.; Ponak, C.; Raupenstrauch, H. A Novel pyrometallurgical recycling process for lithium-ion batteries and its application to the recycling of LCO and LFP. Metals 2021, 11, 149. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Holzer, A.; Ponak, C.; Raupenstrauch, H. Pyrometallurgical lithium-ion-battery recycling: Approach to limiting lithium slagging with the InduRed reactor concept. Processes 2021, 9, 84. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2013, 4, 3974–3983. [Google Scholar] [CrossRef]
- Awad, M.A.; Eisa, N.E.; Virk, P.; Hendi, A.A.; Ortashi, K.M.; Mahgoub, A.S.; Elobeid, M.A.; Eissa, F.Z. Green synthesis of gold nanoparticles: Preparation, characterization, cytotoxicity, and anti-bacterial activities. Mater. Lett. 2019, 256, 126608. [Google Scholar] [CrossRef]
- Patel, A.; Enman, J.; Gulkova, A.; Guntoro, P.I.; Dutkiewicz, A.; Ghorbani, Y.; Rova, U.; Christakopoulos, P.; Matsakas, L. Integrating biometallurgical recovery of metals with biogenic synthesis of nanoparticles. Chemosphere 2021, 263, 128306. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Bhainsa, K.C.; D’Souza, S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Gupta, R.K. Nanotechnology and potential of microorganisms. Crit. Rev. Biotechnol. 2005, 25, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, É.F.; Rovaris, B.C.; Valerio, A.; de Oliveira, D.; Hotza, D. Bioleaching of Printed Circuit Board Waste to Obtain Metallic Nanoparticles. Sustainability 2024, 16, 9837. [Google Scholar] [CrossRef]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Mendoza-López, M.R. Microbial Bioleaching of Ag, Au and Cu from Printed Circuit Boards of Mobile Phones. Curr. Microbiol. 2019, 76, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N. Generation of Metal Nanoparticles Using Reactive Plasma Arc Evaporation. In Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 787–790. [Google Scholar]
- Wei, X.; Yokoi, A.; Muto, H. Nano/Microcomposite Particles: Preparation Processes and Applications. In Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 781–785. [Google Scholar]
- Grigoropoulos, C.P. Laser synthesis and functionalization of nanostructures. Int. J. Extrem. Manuf. 2019, 1, 012002. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Ren, X.-L.; Zheng, M.-L.; Jin, F.; Liu, J.; Dong, X.-Z.; Zhao, Z.-S.; Duan, X.-M. Plasmon-enhanced nanosoldering of silver nanoparticles for high-conductive nanowires electrodes. Opto-Electron. Adv. 2021, 4, 200101. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Sugioka, K. Laser ablation in liquids for nanomaterial synthesis: Diversities of targets and liquids. J. Phys. Photon 2021, 3, 042002. [Google Scholar] [CrossRef]
- Naser, H.; Alghoul, M.A.; Hossain, M.K.; Asim, N.; Abdullah, M.F.; Ali, M.S.; Alzubi, F.G.; Amin, N. The role of laser ablation technique parameters in synthesis of nanoparticles from different target types. J. Nanoparticle Res. 2019, 21, 249. [Google Scholar] [CrossRef]
- An, W.-J.; Thimsen, E.; Biswas, P. Aerosol-chemical vapor deposition method for synthesis of nanostructured metal oxide thin films with controlled morphology. J. Phys. Chem. Lett. 2010, 1, 249–253. [Google Scholar] [CrossRef]
- Gautam, M.; Kim, J.O.; Yong, C.S. Fabrication of aerosol-based nanoparticles and their applications in biomedical fields. J. Pharm. Investig. 2021, 51, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, E.K.; Grass, R.N.; Stark, W.J. Chemical Aerosol Engineering as a Novel Tool for Material Science: From Oxides to Salt and Metal Nanoparticles. Aerosol Sci. Technol. 2010, 44, 161–172. [Google Scholar] [CrossRef]
- Grammatikopoulos, P.; Steinhauer, S.; Vernieres, J.; Singh, V.; Sowwan, M. Nanoparticle design by gas-phase synthesis. Adv. Phys. X 2016, 1, 81–100. [Google Scholar] [CrossRef]
- Huttel, Y.; Martínez, L.; Mayoral, A.; Fernández, I. Gas-phase synthesis of nanoparticles: Present status and perspectives. MRS Commun. 2018, 8, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Popok, V.N.; Kylián, O. Gas-Phase Synthesis of Functional Nanomaterials. Appl. Nano 2020, 1, 4. [Google Scholar] [CrossRef]
- Efimov, A.A.; Arsenov, P.V.; Borisov, V.I.; Buchnev, A.I.; Lizunova, A.A.; Kornyushin, D.V.; Tikhonov, S.S.; Musaev, A.G.; Urazov, M.N.; Shcherbakov, M.I.; et al. Synthesis of nanoparticles by spark discharge as a facile and versatile technique of preparing highly conductive Pt nano-ink for printed electronics. Nanomaterials 2021, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Tao, W.; Liu, J.; Gao, Y.; Zhang, L.; Li, Y. Tailoring the dispersion of nanoparticles and the mechanical behavior of polymer nanocomposites by designing the chain architecture. Phys. Chem. Chem. Phys. 2017, 19, 32024–32037. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Zou, W.; Li, J.; Zheng, R.; Wei, W.; Ni, B.-J.; Chen, H. Integrating electrodeposition with electrolysis for closed-loop resource utilization of battery industrial wastewater. Green Chem. 2022, 24, 3208–3217. [Google Scholar] [CrossRef]
- Lasfargues, M.; Geng, Q.; Cao, H.; Ding, Y. Mechanical dispersion of nanoparticles and its effect on the specific heat capacity of impure binary nitrate salt mixtures. Nanomaterials 2015, 5, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Chen, Z.; Yun, S.; Wu, L.; Zhang, J.; Shi, X.; Wei, W.; Liu, Y.; Zheng, R.; Han, N.; Ni, B.-J. Waste-Derived Catalysts for Water Electrolysis: Circular Economy-Driven Sustainable Green Hydrogen Energy. Nano-Micro Lett. 2023, 15, 4. [Google Scholar] [CrossRef]
- Wang, R.; Luo, S.; Zheng, R.; Shangguan, Y.; Feng, X.; Zeng, Q.; Liang, J.; Chen, Z.; Li, J.; Yang, D.; et al. Interfacial Coordination Bonding-Assisted Redox Mechanism-Driven Highly Selective Precious Metal Recovery on Covalent-Functionalized Ultrathin 1T-MoS2. ACS Appl. Mater. Interfaces 2023, 15, 9331–9340. [Google Scholar] [CrossRef] [PubMed]
- Zacharaki, E.; Kalyva, M.; Fjellvåg, H.; Sjåstad, A.O. Burst nucleation by hot injection for size controlled synthesis of ε-cobalt nanoparticles. BMC Chem. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Boadi, N.O.; Saah, S.A.; Helliwell, M.; Awudza, J.A.M. Hot-Injection Synthesis of PbE (E= S, Se) Nanoparticles from Dichalcogenoimidophosphinato Lead (II) Complexes. ChemistrySelect 2019, 4, 13908–13911. [Google Scholar] [CrossRef]
- Shahjuee, T.; Masoudpanah, S.M. Thermal Decomposition Synthesis of MgFe2O4 Nanoparticles for Magnetic Hyperthermia. J. Supercond. Nov. Magn. 2019, 32, 1347–1352. [Google Scholar] [CrossRef]
- Ilhan, S.; Izotova, S.G.; Komlev, A.A. Synthesis and characterization of MgFe2O4 nanoparticles prepared by hydrothermal decomposition of co-precipitated magnesium and iron hydroxides. Ceram. Int. 2015, 41, 577–585. [Google Scholar] [CrossRef]
- Dubey, R. Temperature-dependent phase transformation of TiO2 nanoparticles synthesized by sol-gel method. Mater. Lett. 2018, 215, 312–317. [Google Scholar] [CrossRef]
- Yue, S.; Yan, Z.; Shi, Y.; Ran, G. Synthesis of zinc oxide nanotubes within ultrathin anodic aluminum oxide membrane by sol–gel method. Mater. Lett. 2013, 98, 246–249. [Google Scholar] [CrossRef]
- Singh, C.; Shaffer, M.; Kinloch, I.; Windle, A. Production of aligned carbon nanotubes by the CVD injection method. Phys. B Condens. Matter 2002, 323, 339–340. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Inoue, S.; Matsumura, Y. Thermal decomposition products of various carbon sources in chemical vapor deposition synthesis of carbon nanotube. Diam. Relat. Mater. 2017, 75, 1–5. [Google Scholar] [CrossRef]
- Zou, W.; Li, J.; Wang, R.; Ma, J.; Chen, Z.; Duan, L.; Mi, H.; Chen, H. Hydroxylamine mediated Fenton-like interfacial reaction dynamics on sea urchin-like catalyst derived from spent LiFePO4 battery. J. Hazard. Mater. 2022, 431, 128590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, R.; Zou, W.; Wei, W.; Li, J.; Wei, W.; Ni, B.-J.; Chen, H. Integrating high-efficiency oxygen evolution catalysts featuring accelerated surface reconstruction from waste printed circuit boards via a boriding recycling strategy. Appl. Catal. B Environ. 2021, 298, 120583. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Chen, H.; Ni, B.-J. Eco-designed electrocatalysts for water splitting: A path toward carbon neutrality. Int. J. Hydrogen Energy 2023, 48, 6288–6307. [Google Scholar] [CrossRef]
- Chen, Z.; Zou, W.; Zheng, R.; Wei, W.; Wei, W.; Ni, B.-J.; Chen, H. Synergistic recycling and conversion of spent Li-ion battery leachate into highly efficient oxygen evolution catalysts. Green Chem. 2021, 23, 6538–6547. [Google Scholar] [CrossRef]
- Ammar, S.; Fiévet, F. Polyol synthesis: A versatile wet-chemistry route for the design and production of functional inorganic nanoparticles. Nanomaterials 2020, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Thanh, N.T.K. Recent development for synthesis of magnetic nanoparticles for biomedical applications. Int. J. Nanosci. 2011, 10, 883–890. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.; Austin, M.J.; Simocko, C.K.; Pete, D.V.; Chavez, J.; Ammerman, L.M.; Huber, D.L. Formation of Metal Nanoparticles Directly from Bulk Sources Using Ultrasound and Application to E-Waste Upcycling. Small 2018, 14, e1703615. [Google Scholar] [CrossRef]
- El-Nasr, R.S.; Abdelbasir, S.; Kamel, A.H.; Hassan, S.S. Environmentally friendly synthesis of copper nanoparticles from waste printed circuit boards. Sep. Purif. Technol. 2020, 230, 115860. [Google Scholar] [CrossRef]
- Oestreicher, V.; García, C.S.; Pontiggia, R.; Rossi, M.B.; Angelomé, P.C.; Soler-Illia, G.J. E-waste upcycling for the synthesis of plasmonic responsive gold nanoparticles. Waste Manag. 2020, 117, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Pahlevani, F.; Levick, K.; Cole, I.; Sahajwalla, V. Synthesis of copper-tin nanoparticles from old computer printed circuit boards. J. Clean. Prod. 2017, 142, 2586–2592. [Google Scholar] [CrossRef]
- Su-Gallegos, J.; Magallón-Cacho, L.; Ramírez-Aparicio, J.; Borja-Arco, E. Synthesis of Gold Nanoparticles from Gold Coatings Recovered from E-Waste Processors. Materials 2022, 15, 7307. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Recycling copper and gold from e-waste by a two-stage leaching and solvent extraction process. Sep. Purif. Technol. 2021, 263, 118400. [Google Scholar] [CrossRef]
- Abdelbasir, S.M. Synthesis of Cu and CuO Nanoparticles from E-waste and Evaluation of Their Antibacterial and Photo-catalytic Properties. Res. Sq. 2023, 30, 89690–89704. [Google Scholar]
- Srikant, S.S.; Mahapatra, R.P.; Rao, R.B. Extraction of nano-metals with judicious combination of microwave heating and acid leaching process from E-waste. J. Microw. Power Electromagn. Energy 2021, 55, 236–247. [Google Scholar] [CrossRef]
- Marappa, N.; Ramachandran, L.; Dharumadurai, D.; Nooruddin, T. Recovery of Gold and Other Precious Metal Resources from Environmental Polluted E-waste Printed Circuit Board by Bioleaching Frankia. Int. J. Environ. Res. 2020, 14, 165–176. [Google Scholar] [CrossRef]
- Sapra, G.; Chaudhary, V.; Kumar, P.; Sharma, P.; Saini, A. Recovery of silica nanoparticles from waste PV modules. Mater. Today Proc. 2019, 45, 3863–3868. [Google Scholar] [CrossRef]
- Jadhao, P.R.; Ahmad, E.; Pant, K.; Nigam, K. Environmentally friendly approach for the recovery of metallic fraction from waste printed circuit boards using pyrolysis and ultrasonication. Waste Manag. 2020, 118, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Amuanyena, M.O.N.; Kandawa-Schulz, M.; Kwaambwa, H.M. Magnetic Iron Oxide Nanoparticles Modified with Moringa Seed Proteins for Recovery of Precious Metal Ions. J. Biomater. Nanobiotechnol. 2019, 10, 142–158. [Google Scholar] [CrossRef]
- Tatariants, M.; Yousef, S.; Skapas, M.; Juskenas, R.; Makarevicius, V.; Lukošiūtė, S.-I.; Denafas, G. Industrial technology for mass production of SnO2 nanoparticles and PbO2 microcube/microcross structures from electronic waste. J. Clean. Prod. 2018, 203, 498–510. [Google Scholar] [CrossRef]
- Ebin, B. Simple Preparation of Ni and NiO Nanoparticles Using Raffinate Solution Originated from Spent NiMH Battery Recycling. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2554–2563. [Google Scholar] [CrossRef]
- Cerchier, P.; Dabalà, M.; Brunelli, K. Green synthesis of copper nanoparticles with ultrasound assistance. Green Process. Synth. 2017, 6, 311–316. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, P.K.; Malviya, R. Biomedical Applications and Patents on Metallic Nanoparticles. Nanosci. Nanotechnol.-Asia 2020, 11, 153–162. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, H.; Feder-Kubis, J.; Nguyen, D.D. Recent advances in nanobiosensors for sustainable healthcare applications: A systematic literature review. Environ. Res. 2023, 238, 117177. [Google Scholar] [CrossRef] [PubMed]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 137, pp. 115–170. [Google Scholar]
- Singla, S.; Rajput, K.; Kanjariya, P.; Ravi, K.; Anand, G.; Kumar, N.; Bansal, N.; Sharma, G. Structural, thermal, and optical properties of gold nanoparticle-doped bismuth borate glasses: Effect of concentration. J. Mater. Sci. Mater. Electron. 2024, 35, 1–10. [Google Scholar] [CrossRef]
- Mastrobattista, E.; van der Aa, M.A.E.M.; Hennink, W.E.; Crommelin, D.J.A. Artificial viruses: A nanotechnological approach to gene delivery. Nat. Rev. Drug Discov. 2006, 5, 115–121. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Jafari, S.; Khosroushahi, A.Y. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res. Lett. 2014, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Huang, X.; Min, J.; Le, S.; Wang, Q.; Wang, X.; Dogan, A.A.; Liu, X.; Zhang, P.; Draz, M.S.; et al. Nanoparticle Delivery Systems for DNA/RNA and their Potential Applications in Nanomedicine. Curr. Top. Med. Chem. 2019, 19, 2507–2523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, M.; Xi, J.; Li, J.; Ma, R.; Ren, L.; Bai, Z.; Qi, K.; Li, X. Gold Nanorods-Based Photothermal Therapy: Interactions Between Biostructure, Nanomaterial, and Near-Infrared Irradiation. Nanoscale Res. Lett. 2022, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Han, Y.; Zhang, X.; Ma, H.; Li, L.; Yu, R.; Liu, H. Application of New Radiosensitizer Based on Nano-Biotechnology in the Treatment of Glioma. Front. Oncol. 2021, 11, 633827. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, Z.; Li, L.; Zhou, L.; Yuan, S. Application of nanomedicine in radiotherapy sensitization. Front. Oncol. 2023, 13, 1088878. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ardjmand, M.; Rad, A.; Esfahani, M. Gelatin-coated gold nanoparticles as an effective pH-sensitive methotrexate drug delivery system for breast cancer treatment. Mater. Today Chem. 2021, 20, 100474. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Geppert, M.; Himly, M. Iron Oxide Nanoparticles in Bioimaging—An Immune Perspective. Front. Immunol. 2021, 12, 688927. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Gupta, D.; Roy, I.; Gandhi, S. Metallic nanoparticles for CT-guided imaging of tumors and their therapeutic applications. OpenNano 2023, 12, 100146. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 558493. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Navalón, S.; García, H. Nanoparticles for Catalysis. Nanomaterials 2016, 6, 123. [Google Scholar] [CrossRef]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef]
- Kaiser, J.-P.; Diener, L.; Wick, P. Nanoparticles in paints: A new strategy to protect façades and surfaces? J. Phys. Conf. Ser. 2013, 429, 012036. [Google Scholar] [CrossRef]
- Umar, S.; Ibrahim, M.N.M.; Umar, K.; Ahmad, N. Editorial: Design and Synthesis of Metallic Nanoparticles for Targeted Therapy and Diagnostics. Front. Chem. 2020, 8, 597800. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Setapar, S.H.M.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 495283. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Varshney, S.; Nigam, A.; Pawar, S.J.; Mishra, N. An overview on biomedical applications of versatile silica nanoparticles, synthesized via several chemical and biological routes: A review. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 72–88. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Vega Baudrit, J.R. Silver nanoparticles: Technological advances, societal impacts, and metrological challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, P.; Sidhu, H.K.; Aggarwal, N.; Nagireddi, S.; Anand, G.; Sharma, N.; Sharma, A.; Malhotra, S.; Panwar, R.S. Heavy metal detection in industrial waste water using Ficus Benjamina leaf extract–mediated Ag nanoparticles. Biomass Convers. Biorefinery 2022, 1, 1–5. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Ghulam, S.T.; Abushammala, H. Challenges and Opportunities in the Management of Electronic Waste and Its Impact on Human Health and Environment. Sustainability 2023, 15, 1837. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z. A Review of current progress of supercritical fluid technologies for e-waste treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.T.; Zgârian, R.G.; Fierascu, I.; Baroi, A.M.; Răileanu, S.; Fierăscu, R.C. Metallic and Metal Oxides Nanoparticles for Sensing Food Pathogens—An Overview of Recent Findings and Future Prospects. Materials 2022, 15, 5374. [Google Scholar] [CrossRef] [PubMed]
- Magoda, K.; Mekuto, L. Biohydrometallurgical Recovery of Metals from Waste Electronic Equipment: Current Status and Proposed Process. Recycling 2022, 7, 67. [Google Scholar] [CrossRef]
- Lakshmi, V.S.; Satya, C.H.R.; Aravind, D.N. Electronic waste: Overview, recycling and metal extraction methods. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1136, 012069. [Google Scholar] [CrossRef]
- Murthy, V.; Ramakrishna, S. A Review on Global E-Waste Management: Urban Mining towards a Sustainable Future and Circular Economy. Sustainability 2022, 14, 647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).