Production of Soft Magnetic Materials Fe-Si and Fe-Si-Al from Blends of Red Muds and Several Additives: Resources for Advanced Electrical Devices
Abstract
1. Introduction
1.1. RM Waste Management Strategies
1.2. Soft Magnetic Materials
1.3. Aims of the Investigation
2. Materials and Methods
2.1. Materials and Blend Compositions
2.2. Experimental
3. Results
3.1. Set I: Blends of RMA with Fe2O3 and Red MS
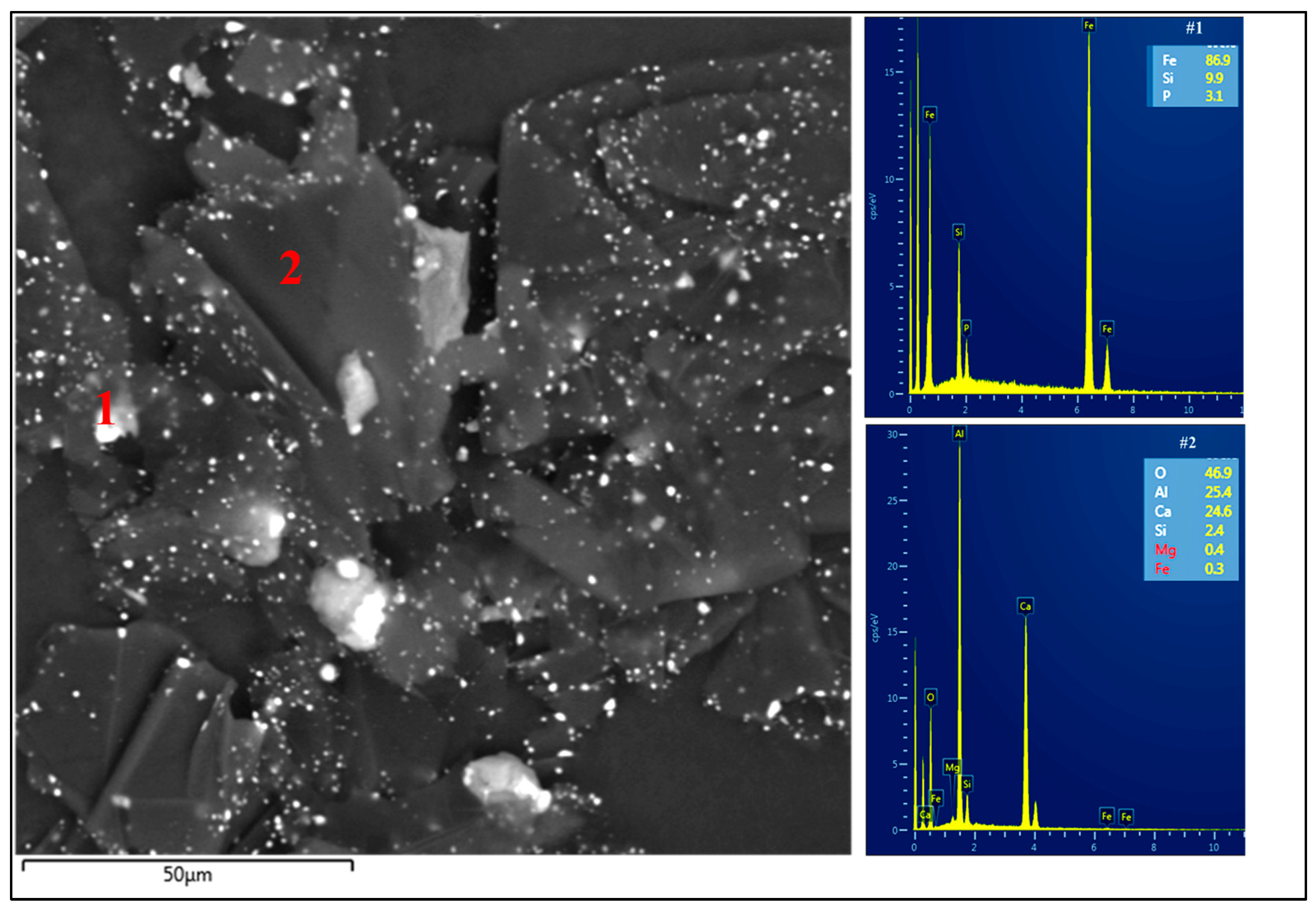
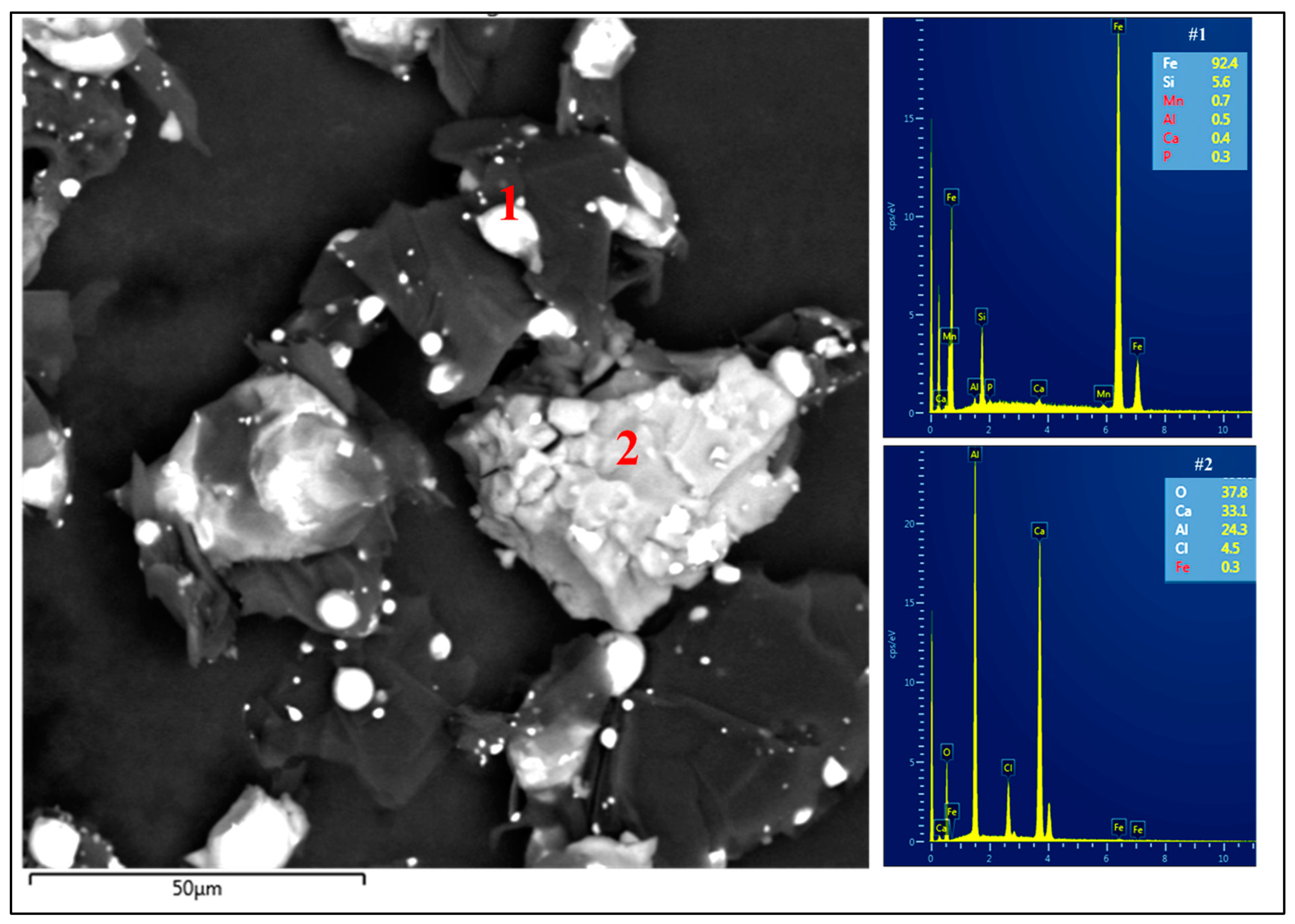
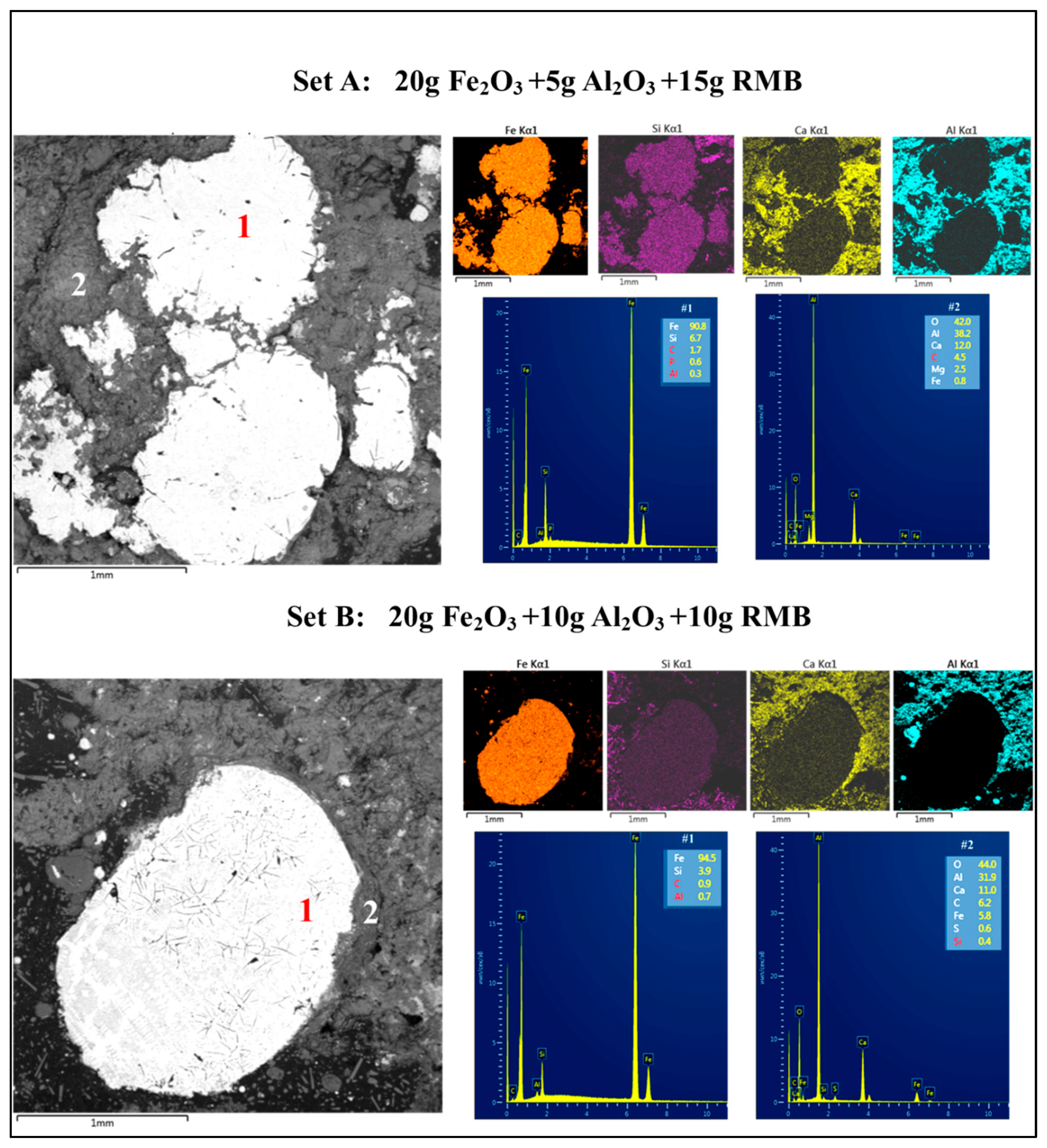
3.2. Set II: Blends of RMB with Al2O3, Fe2O3, and Red MS
3.2.1. Blends of RMB with Al2O3 and Fe2O3
3.2.2. Blends of RMB with Al2O3 and Red MS
3.3. Set III: Blends of RMB with MS (Black) and Al2O3
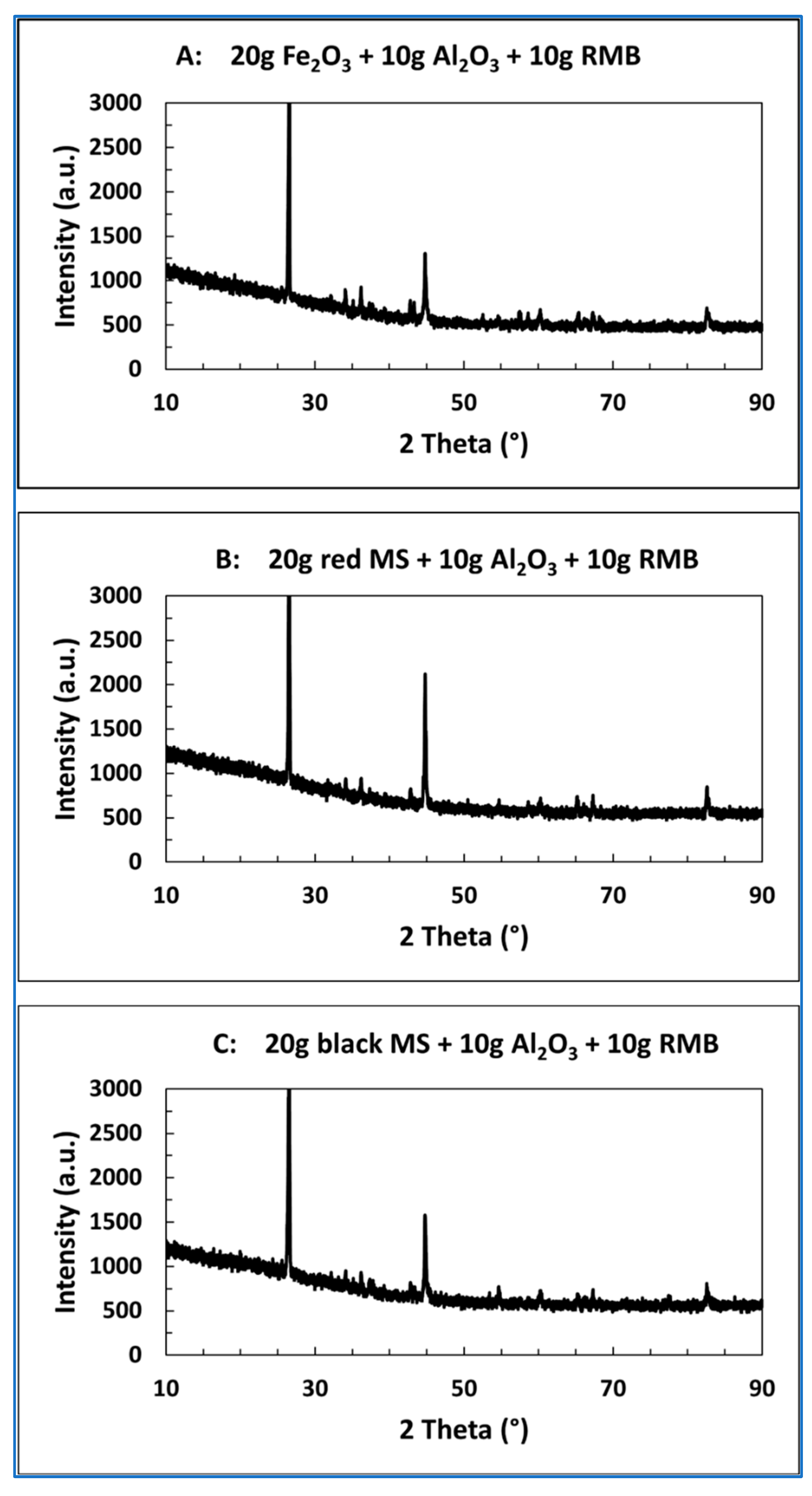
3.4. X-Ray Diffraction Investigations
4. Discussion
5. Conclusions
- The carbothermic reduction behavior of two RMs and their blends with three iron-oxides (Fe2O3, black and red MS) and Al2O3 additives was investigated at 1600–1650 °C, 30 min as Sets I, II and III. These were followed by detailed characterization of the reduction products, especially the metallic phases.
- Si levels in the iron-rich metallic droplets showed a wide variation: Set I (5.6 to 9.0 wt.%), Set II (3.9 to 6.7 wt.%) and Set III (6.5 to 6.6 wt.%), thereby indicating the key role of initial blend compositions on Si pick-up by metallic droplets.
- A broad variation was observed in the particulate sizes of the metallic droplets/regions generated: Set I (0.5 to 15 μm), Set II (60 to 500 μm), and Set III (30 to 150 μm). Non-wetting and repulsive interaction between Al2O3 and Fe played a key role in the assimilation of small droplets and subsequent growth of metallic regions. The Al2O3 content in the slag can also affect the interfacial tension between the slag and the steel.
- The formation of Fe-Si was observed in 8 out of 10 blends investigated. With Si levels ranging between 3.9 to 6.7 wt.%, these metallics will be a highly suitable raw material for producing SMMs (optimal range: 3.2 to 6.5 wt.% Si). The formation of Fe-Si-Al alloys, another type of SMM, was observed in 4 out of 10 blends investigated.
- This study presents a new approach for recycling RMs and their transformation into valuable SMMs for the energy sector. It will have a positive influence on the sustainable developments in the field impacting resource recovery, conservation, and economic/environmental sustainability.
- Industrial waste such as RMs have little value, high disposal rates and extensive transport costs. The novel approach to RM recycling developed in this study will help conserve the natural environment and resources and reduce the burden on waste storage facilities while closing the loop of a sustainable economy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahinroosta, M.; Karimi, Z.; Allahverdi, A. Recycling of red mud for value-added applications: A comprehensive review. Encycl. Renew. Sustain. Mater. 2020, 2, 561–582. [Google Scholar]
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef]
- Li, Z.; Gu, H.; Hong, B.; Wang, N.; Chen, M. An innovative process for dealkalization of red mud using leachate from Mn containing waste. J. Environ. Chem. Eng. 2022, 10, 107222. [Google Scholar] [CrossRef]
- Xue, S.G.; Wu, Y.J.; Li, Y.W.; Kong, X.F.; Zhu, F.; William, H.; Li, X.F.; Ye, Y.Z. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review. J. Cent. South Univ. 2019, 26, 268–288. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.; Liu, X.; Zhang, N. Utilization of red mud in road base and subgrade materials: A review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Uthayakumar, M.; Arumugaprabu, V. Development and sustainability of industrial waste-based red mud hybrid composites. J. Clean. Prod. 2019, 230, 862–868. [Google Scholar] [CrossRef]
- Hua, Y.; Heal, K.V.; Friesl-Hanl, W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review. J. Hazard. Mater. 2017, 325, 17–30. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, C.; Wu, Y. Characterization of red mud derived from a combined Bayer process and bauxite calcination method. J. Hazard. Mater. 2007, 146, 255–261. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recyc. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Mayes, W.M.; Jarvis, A.P.; Burke, I.T.; Walton, M.; Feigl, V.R.; Klebercz, O.; Gruiz, K. Dispersal and attenuation of trace contaminants downstream of the Ajka bauxite residue (red mud) depository failure. Hungary. Environ. Sci. Technol. 2011, 45, 5147–5155. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.-H.; Kumar, P.; Kim, K.H.; Lee, S.S.; Bhattacharya, S.S. Solid waste management: Scope and the challenge of sustainability. J. Clean. Prod. 2019, 228, 658–678. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Snars, K.; Gilkes, R.J. Evaluation of bauxite residues (red muds) of different origins for environmental applications. Appl. Clay Sci. 2009, 46, 13–20. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.; Zinoveev, D.; Jayasankar, K.; Burmistrov, I.; Kravchenko, M.; Mukherjee, P.S. Red Mud as a Secondary Resource of Low-Grade Iron: A Global Perspective. Sustainability 2022, 14, 1258. [Google Scholar] [CrossRef]
- Rai, S.; Bahadur, S.; Chaddha, M.J.; Agnihotri, A. Disposal practices and utilization of red mud (Bauxite Residue): A review in Indian context and abroad. J. Sustain. Metall. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Samal, S.; Ray, A.K.; Bandopadhyay, S.A. Proposal for resources, utilization and processes of red mud in India—A review. Int. J. Miner. Process. 2013, 118, 43–55. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, P.; Liang, M.; Jiang, H.; Yao, Z.; Zhang, X.; Yu, S. Utilization of red mud as an alternative mineral filler in asphalt mastics to replace natural limestone powder. Constr. Build. Mater. 2020, 237, 117821. [Google Scholar] [CrossRef]
- Li, H.; Shi, B.; Fu, X.; Zhang, H.; Yang, H. Preparation and application of red mud-based zero-valent iron heterogeneous Fenton catalyst: A new idea for red mud recycling. J. Environ. Chem. Eng. 2023, 11, 109998. [Google Scholar] [CrossRef]
- Shen, H.; Lou, B.; Liu, B.; Zhang, J.; Zhang, X.; Liu, J.; Zhang, R.; Chen, M.; Zhang, S. In-situ preparation of alumina-based cermet after reduction of iron oxide in red mud with aluminum dross. Ceram. Int. 2024, 50, 21630–21637. [Google Scholar] [CrossRef]
- Wang, K.; Dou, Z.; Liu, Y.; Li, X.; Lv, G.; Zhang, T.A. Summary of research progress on separation and extraction of valuable metals from Bayer red mud. Environ. Sci. Pollut. Res. 2022, 29, 89834–89852. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Investigation of mechanical and thermal activation on metal extraction from red mud. Sustain. Mater. Technol. 2021, 27, e00246. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Wang, N.; Hannian, G. Selective extraction of rare earth elements from red mud using oxalic and sulfuric acids. J. Environ. Chem. Eng. 2022, 10, 108650. [Google Scholar] [CrossRef]
- Zhang, D.R.; Chen, H.R.; Zhao, X.J.; Xia, J.L.; Nie, Z.; Zhang, R.Y.; Wen-Sheng Shu, W.S.; Pakostova, E. Fe(II) bio-oxidation mediates red mud transformations to form Fe(III)/Al (hydr)oxide adsorbent for efficient As(V) removal under acidic conditions. Chem. Eng. J. 2022, 439, 135753. [Google Scholar] [CrossRef]
- Tuazon, D.S.; Corder, D. Life cycle assessment of seawater neutralised red mud for treatment of acid mine drainage. Resour. Conserv. Recycl. 2008, 52, 1307–1314. [Google Scholar] [CrossRef]
- Li, C.; Yu, J.; Li, W.; He, Y.; Qiu, Y.; Li, P.; Wang, C.; Huang, F.; Wang, D.; Shiqiu Gao, S. Immobilization, enrichment and recycling of Cr (VI) from wastewater using a red mud/carbon material to produce the valuable chromite (FeCr2O4). Chem. Eng. J. 2018, 350, 1103–1113. [Google Scholar] [CrossRef]
- Lu, H.J.; Liang, P.; Gan, D.Q. Research on flow and sedimentation law of filling slurry and mechanical characteristics of backfill body. Rock Soil Mech. 2017, 38 (Suppl. S1), 263–270. [Google Scholar]
- Chen, X.Z.; Yang, X.C.; Guo, L.J.; Peng, X. Effect of curing age on the determination of cement content in cemented tailings backfill by EDTA titration method. Nonferrous Met. Eng. 2018, 9, 93–99. [Google Scholar]
- Feng, X.-P.; Liu, X.-M.; Sun, H.-H.; Bai, X.; Niu, X.-L. Study on the High Use Ratio of Red Mud in Cementitious Material. Multipurp. Utiliz. Miner. Resour. 2007, 4, 35–37. [Google Scholar]
- Dodoo-Arhin, D.; Konadu, D.S.; Annan, E.; Buabeng, F.P.; Yaya, A.; Agyei-Tuffour, B. Fabrication and Characterisation of Ghanaian Bauxite Red Mud-Clay Composite Bricks for Construction Applications. Am. J. Mater. Sci. 2013, 3, 110–119. [Google Scholar]
- Krivenko, P.; Kovalchuk, O.; Pasko, A.; Croymans, T.; Hult, M.; Lutter, G.; Vandevenne, N.; Schreurs, S.; Schroeyers, W. Development of alkali activated cements and concrete mixture design with high volumes of red mud. Construct. Build. Mater. 2017, 151, 819–826. [Google Scholar] [CrossRef]
- Tang, W.C.; Wang, Z.; Donne, S.W.; Forghani, M.; Liu, Y. Influence of red mud on mechanical and durability performance of self-compacting concrete. J. Hazard. Mater. 2019, 379, 120802. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, D.; Hou, J.; He, B.; Xiao, B. Preparation of glass-ceramics from red mud in the aluminium industries. Ceram. Int. 2008, 34, 125–130. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Pichór, W.; Kłosek-Wawrzyn, E. Influence of Firing Temperature on Phase Composition and Color Properties of Ceramic Tile Bodies. Materials 2021, 14, 6380. [Google Scholar] [CrossRef]
- Chiara, B.; Chiara, C.; Tam, P.; WAI, T.; Dimitrios, P. Review of Technologies in the Recovery of Iron, Aluminium, Titanium and Rare Earth Elements from Bauxite Residue (Red Mud). In Proceedings of the 3rd International Symposium on Enhanced Landfill Mining, Lisbon, Portugal, 8–10 February 2016; pp. 259–276. [Google Scholar]
- Pepper, R.A.; Couperthwaite, S.J.; Millar, G.J. Comprehensive examination of acid leaching behaviour of mineral phases from red mud: Recovery of Fe, Al, Ti, and Si. Miner. Eng. 2016, 99, 8–18. [Google Scholar] [CrossRef]
- Fanghai, L.; Xiangdong, S.; Fang, H.; Jiawei, W.; Haifeng, W. Co-Treatment of spent pot-lining and red mud for carbon reutilization and recovery of iron, aluminium and sodium by reductive roasting process. Metall. Mater. Trans. B 2020, 51, 1564–1575. [Google Scholar]
- Li, Y.; Wang, J.; Wang, X.; Wang, B.; Luan, Z. Feasibility study of iron mineral separation from red mud by high gradient superconducting magnetic separation. Phys. C Supercond. 2011, 471, 91–96. [Google Scholar] [CrossRef]
- Valeev, D.; Zinoveev, D.; Kondratiev, A.; Lubyanoi, D.; Pankratov, D. Reductive smelting of neutralized red mud for iron recovery and produced pig iron for heat resistant castings. Metals 2020, 10, 32. [Google Scholar] [CrossRef]
- Debadatta, D.; Pramanik, K. A study on chemical leaching of iron from red mud using sulphuric acid. Res. J. Chem. Environ. 2013, 17, 50–56. [Google Scholar]
- Liu, X.; Gao, P.; Yuan, S.; Lv, Y.; Han, Y. Clean utilization of high iron red mud by suspension magnetization roasting. Miner. Eng. 2020, 157, 106553. [Google Scholar] [CrossRef]
- Vakilchap, F.; Mousavi, S.M.; Shojaosadati, S.A. Role of Aspergillus niger in recovery enhancement of valuable metals from produced red mud in Bayer process. Biores. Technol. 2016, 218, 991–998. [Google Scholar] [CrossRef]
- Vachon, P.; Tyagi, R.D.; Auclair, J.C.; Wilkinson, K.J. Chemical and biological leaching of aluminium from red mud. Environ. Sci. Technol. 1994, 28, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kasliwal, P.; Sai, P.S.T. Enrichment of titanium dioxide in red mud: A kinetic study. Hydrometallurgy 1999, 53, 73–87. [Google Scholar] [CrossRef]
- Deep, A.; Malik, P.; Gupta, B. Extraction and separation of Ti(IV) using thiophosphinic acids and its recovery from ilmenite and red mud. Sep. Sci. Technol. 2001, 36, 671–685. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Sheng, L.; He, C.; Sun, W.; He, Q. Enhancing Cd(II) sorption by red mud with heat treatment: Performance and mechanisms of sorption. J. Environ. Manag. 2020, 255, 109866. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, X.; Luo, L.; Zhou, Y.; Wei, J.; Chen, A.; Tang, L.; Wu, H.; Deng, Y.; Zhang, F. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. Int. Biodeterior. Biodegrad. 2017, 118, 73–81. [Google Scholar] [CrossRef]
- Vilarinho, I.S.; Dias, A.C.; Carneiro, J.; Pinto, C.; Labrincha, J.A.; Seabra, M.P. Red mud valorization in stoneware pastes: Technical and environmental assessment. Sustain. Mater. Technol. 2023, 38, e00762. [Google Scholar] [CrossRef]
- Hadfield, R.A. Metallurgy and Its Influence on Modern Progress; Van Nostrand: New York, NY, USA, 1926. [Google Scholar]
- Fiorillo, F.; Bertotti, G.; Appino, C.; Pasquale, M. Soft magnetic materials. In Wiley Encyclopaedia of Electrical and Electronics Engineering; Webster, J.G., Ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; Wiley-IEEE Press: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lamichhane, T.N.; Sethuraman, L.A.; Dalagan, L.A.; Wang, H.; Keller, J.; Paranthaman, M.P. Additive manufacturing of soft magnets for electrical machines—A review. Mater. Today Phys. 2020, 15, 100255. [Google Scholar] [CrossRef]
- Lucarini, S.; Hossain, M.; Garcia-Gonzalez, D. Recent advances in hard-magnetic soft composites: Synthesis, characterisation, computational modelling, and applications. Compos. Struct. 2022, 279, 114800. [Google Scholar] [CrossRef]
- Fortunati, S.; Cucale, S.; Schneider, J.; Franke, A.; Rudolf, K. Developments in the field of electrical steels over the last years. In Proceedings of the International Conference on Magnetism and Metallurgy WMM16, Rome, Italy, 13–15 June 2016. [Google Scholar]
- Silveyra, J.M.; Ferrara, E.; Huber, D.L.; Monson, T.C. Soft magnetic materials for a sustainable and electrified world. Science 2018, 362, 6413. [Google Scholar] [CrossRef] [PubMed]
- Birčáková, Z.; Kollár, P.; Weidenfeller, B.; Füzer, J.; Fáberová, M.; Bureš, R. Reversible and irreversible DC magnetization processes in the frame of magnetic, thermal and electrical properties of Fe-based composite materials. J. Alloys Compd. 2015, 645, 283–289. [Google Scholar] [CrossRef]
- Baricco, M.; Mastrandrea, E.; Antonione, C.; Viala, B.; Degauque, J.; Ferrara, E.; Fiorillo, F. Grain growth and texture in rapidly solidified Fe (Si) 6.5 wt.% ribbons. Mater. Sci. Eng. A 1997, 226–228, 1025–1029. [Google Scholar] [CrossRef]
- Azuma, D.; Ito, N.; Ohta, M. Recent progress in Fe-based amorphous and nanocrystalline soft magnetic materials. J. Magnet. Magn. Mater. 2020, 501, 166373. [Google Scholar] [CrossRef]
- Electric Vehicles—Worldwide. Available online: https://www.statista.com/outlook/mmo/electric%20vehicles/worldwide (accessed on 6 January 2025).
- Singh Raman, R.K. Characterisation of ‘rolled-in’, ‘fragmented’ and ‘red’ scale formation during secondary processing of steels. Eng. Fail. Anal. 2006, 13, 1044–1050. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.; Li, K.; Jayasankar, K.; Maslennikov, N.; Zinoveev, D.; Kargin, J.; Leybo, D.; Kravechenko, M.; Mukherjee, P.S. Innovative Transformation and Valorization of Red Mill Scale Waste into Ferroalloys: Carbothermic Reduction in the Presence of Alumina. Sustainability 2023, 15, 16810. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-Ul Haq, M.; Wang, Y.; Seetharaman, S.; Sahajwalla, V. Chemical interactions of alumina–carbon refractories with molten steel at 1823 K (1550 C): Implications for refractory degradation and steel quality. Metall. Mater. Trans. B 2011, 42, 677–684. [Google Scholar] [CrossRef]
- Khanna, R.; Sahajwalla, V. An atomistic model for the graphite–alumina/liquid iron system: Monte-Carlo simulations on carbon dissolution. Acta Mater. 2005, 53, 1205–1214. [Google Scholar] [CrossRef]
- Rai, S.; Wasewar, K.; Agnihotri, A. Treatment of alumina refinery waste (red mud) through neutralization techniques: A review. Waste Manag. Res. 2017, 35, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Song, M.; Luo, X.; Qu, M.; Wang, X. A modeling study of different kinds of sessile droplets on the horizontal surface with surface wettability and gravity effects considered. Energy Storage Sav. 2022, 1, 22–32. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.; Ikram-ul-haq, M.; Burmistrov, I.; Cayumil, R.; Belov, V.A.; Rogachev, S.O.; Leybo, D.V.; Mukherjee, P.S. An innovative route for valorising iron and aluminium oxide rich industrial wastes: Recovery of multiple metals. J. Environ. Manag. 2021, 295, 113035. [Google Scholar] [CrossRef]
- Khanna, R.; Kongkarat, S.; Seetharaman, S.; Sahajwalla, V. Carbothermic reduction of alumina at 1823 K in the presence of molten steel: A Sessile Drop Investigation. ISIJ Int. 2012, 52, 993–999. [Google Scholar] [CrossRef][Green Version]
- Schiemann, M.; Wirtz, S.; Scherer, V.; Bärhold, F. Spray roasting of iron chloride FeCl2: Laboratory scale experiments and a model for numerical simulation. Powder Technol. 2012, 228, 301–308. [Google Scholar] [CrossRef]
- Kumar, K.S.; Sah, R.; Sekhar, V.R.; Vishwanath, S.C. Development and use of mill scale briquettes in BOF. Ironmak. Steelmak. 2017, 44, 134–139. [Google Scholar] [CrossRef]
- Environment and Climate Change, Worldsteel Association. 2021. Available online: https://worldsteel.org/steel-topics/environment-and-climate-change/ (accessed on 6 January 2025).
- Paswan, D.; Malathi, M.; Minj, R.K.; Bandopadhyay, D. Mill scale: A potential raw material for iron and steel making. Steel World 2015, 21, 54–56. [Google Scholar]
- Grudinsky, P.; Zinoveev, D.; Yurtaeva, A.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Iron recovery from red mud using carbothermic roasting with addition of alkaline salts. J. Sustain. Metall. 2021, 7, 858–873. [Google Scholar] [CrossRef]
- Rai, S.; Nimje, M.T.; Chaddha, M.J.; Modak, D.; Rai, K.R.; Agnihotri, A. Recovery of iron from bauxite residue using advanced separation techniques. Miner. Engg. 2019, 2134, 222–231. [Google Scholar] [CrossRef]





| Blends | Fe2O3 | Al2O3 | SiO2 | CaO | MgO | Na2O | SO32− | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| RMA | 36.9 | 11.8 | 8.7 | 23.8 | 1.0 | 0.3 | 0.1 | 0.4 | 3.5 |
| 20 g RMA + 20 g Fe2O3 | 68.5 | 5.9 | 4.4 | 11.9 | 0.5 | 0.1 | 0.1 | 0.2 | 1.8 |
| 20 g RMA + 20 g red MS | 68.5 | 5.9 | 4.4 | 11.9 | 0.5 | 0.1 | 0.1 | 0.2 | 1.8 |
| Blends | Fe2O3 | Al2O3 | SiO2 | CaO | MgO | Na2O | SO32− | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| RMB | 50.0 | 11.2 | 8.7 | 10.7 | 0.6 | 3.8 | 0.1 | 0.3 | 4.1 |
| 20 g Fe2O3 + 5 g Al2O3 + 15 g RMB | 68.8 | 16.7 | 3.3 | 4.0 | 0.2 | 1.4 | 0.01 | 0.1 | 1.5 |
| 20 g Fe2O3 + 10 g Al2O3 + 10 g RMB | 62.5 | 27.8 | 2.2 | 2.7 | 0.2 | 1.0 | 0.0 | 0.1 | 1.0 |
| 20 g red MS + 5 g Al2O3 + 15 g RMB | 68.8 | 16.7 | 3.3 | 4.0 | 0.2 | 1.4 | 0.1 | 0.1 | 1.5 |
| 20 g red MS + 10 g Al2O3 + 10 g RMB | 62.5 | 27.8 | 2.2 | 2.7 | 0.2 | 1.0 | 0.0 | 0.1 | 1.0 |
| Blends | Fe2O3 | Al2O3 | SiO2 | CaO | MgO | Na2O | SO32− | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| 20 g MS + 20 g RMB | 75.0 | 5.6 | 4.4 | 5.3 | 0.3 | 1.9 | 0.1 | 0.2 | 2.0 |
| 20 g MS + 5 g Al2O3 + 15 g RMB | 68.8 | 16.7 | 3.3 | 4.0 | 0.2 | 1.4 | 0.1 | 0.1 | 1.5 |
| 20 g MS + 10 g Al2O3 + 10 g RMB | 62.5 | 27.8 | 2.2 | 2.7 | 0.2 | 1.0 | 0.0 | 0.1 | 1.0 |
| S.N. | Blends | Fe | Si | Al | Size |
| 1. | RMA | 86.9 | 9.0 | - | 1–3 μm |
| 2. | 20 g RMA + 20 g Fe2O3 | 92.3 | 5.6 | - | 0.5–4 μm |
| 3. | 20 g RMA + 20 g red MS | 92.4 | 5.6 | 0.5 | 5–15 μm |
| 4. | 20 g Fe2O3 + 5 g Al2O3 + 15 g RMB | 90.3 | 6.7 | 0.03 | 60–300 μm |
| 5. | 20 g Fe2O3 + 10 g Al2O3 + 10 g RMB | 94.5 | 3.9 | 0.7 | 60–300 μm |
| 6. | 20 g red MS + 5 g Al2O3 + 15 g RMB | 88.7 | 6.6 | 1.8 | 100–500 μm |
| 7. | 20 g red MS + 10 g Al2O3 + 10 g RMB | 90.5 | 6.3 | 1.4 | 100–500 μm |
| 8. | 20 g MS + 20 g RMB | 96.7 | - | - | 30–150 μm |
| 9. | 20 g MS + 5 g Al2O3 + 15 g RMB | 88.7 | 6.6 | 1.8 | 30–150 μm |
| 10. | 20 g MS + 10 g Al2O3 + 10 g RMB | 90.5 | 6.5 | 1.4 | 30–150 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanna, R.; Konyukhov, Y.; Zinoveev, D.; Li, K.; Maslennikov, N.; Burmistrov, I.; Kargin, J.; Kravchenko, M.; Mukherjee, P.S. Production of Soft Magnetic Materials Fe-Si and Fe-Si-Al from Blends of Red Muds and Several Additives: Resources for Advanced Electrical Devices. Sustainability 2025, 17, 1795. https://doi.org/10.3390/su17051795
Khanna R, Konyukhov Y, Zinoveev D, Li K, Maslennikov N, Burmistrov I, Kargin J, Kravchenko M, Mukherjee PS. Production of Soft Magnetic Materials Fe-Si and Fe-Si-Al from Blends of Red Muds and Several Additives: Resources for Advanced Electrical Devices. Sustainability. 2025; 17(5):1795. https://doi.org/10.3390/su17051795
Chicago/Turabian StyleKhanna, Rita, Yuri Konyukhov, Dmitri Zinoveev, Kejiang Li, Nikita Maslennikov, Igor Burmistrov, Jumat Kargin, Maksim Kravchenko, and Partha Sarathy Mukherjee. 2025. "Production of Soft Magnetic Materials Fe-Si and Fe-Si-Al from Blends of Red Muds and Several Additives: Resources for Advanced Electrical Devices" Sustainability 17, no. 5: 1795. https://doi.org/10.3390/su17051795
APA StyleKhanna, R., Konyukhov, Y., Zinoveev, D., Li, K., Maslennikov, N., Burmistrov, I., Kargin, J., Kravchenko, M., & Mukherjee, P. S. (2025). Production of Soft Magnetic Materials Fe-Si and Fe-Si-Al from Blends of Red Muds and Several Additives: Resources for Advanced Electrical Devices. Sustainability, 17(5), 1795. https://doi.org/10.3390/su17051795









