Effect of High Temperature on CO2 Gasification Kinetics of Sub-Bituminous Coal Fly Ash
Abstract
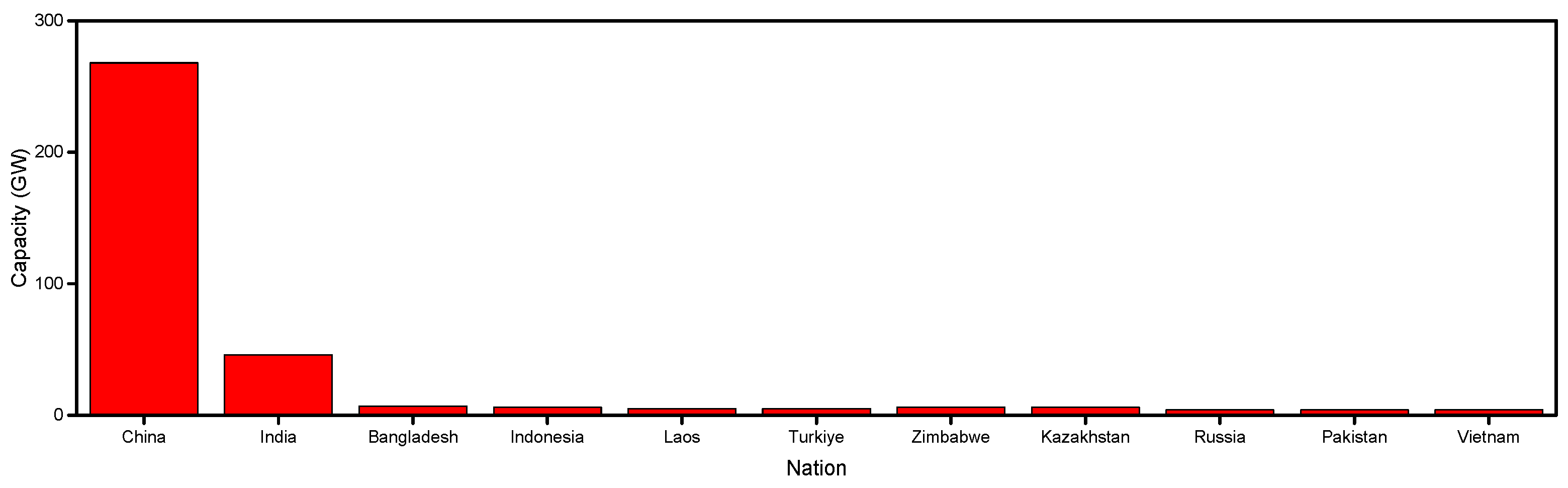
1. Introduction
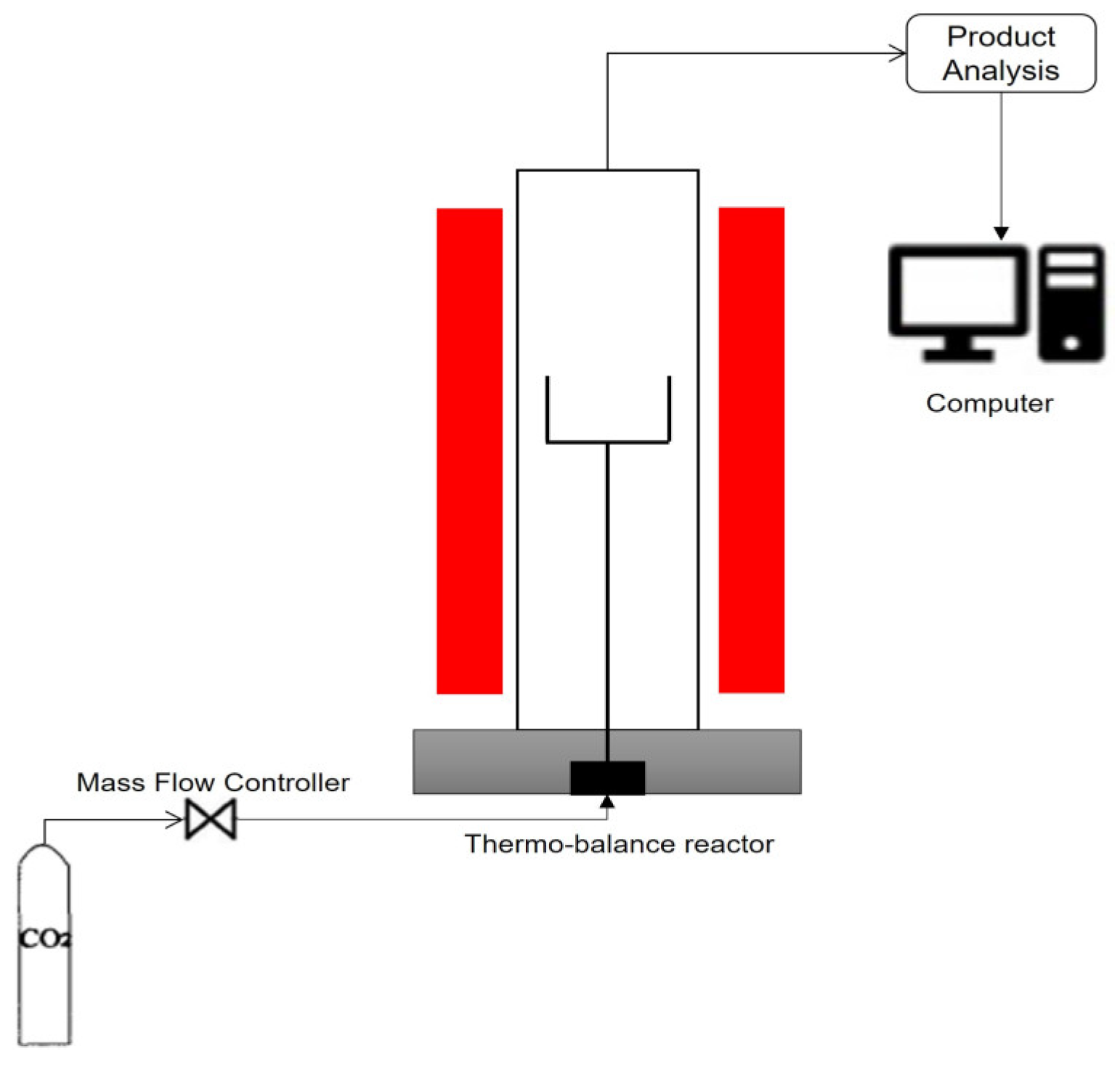
2. Experimental Method and Apparatus
2.1. Fly Ash Preparation
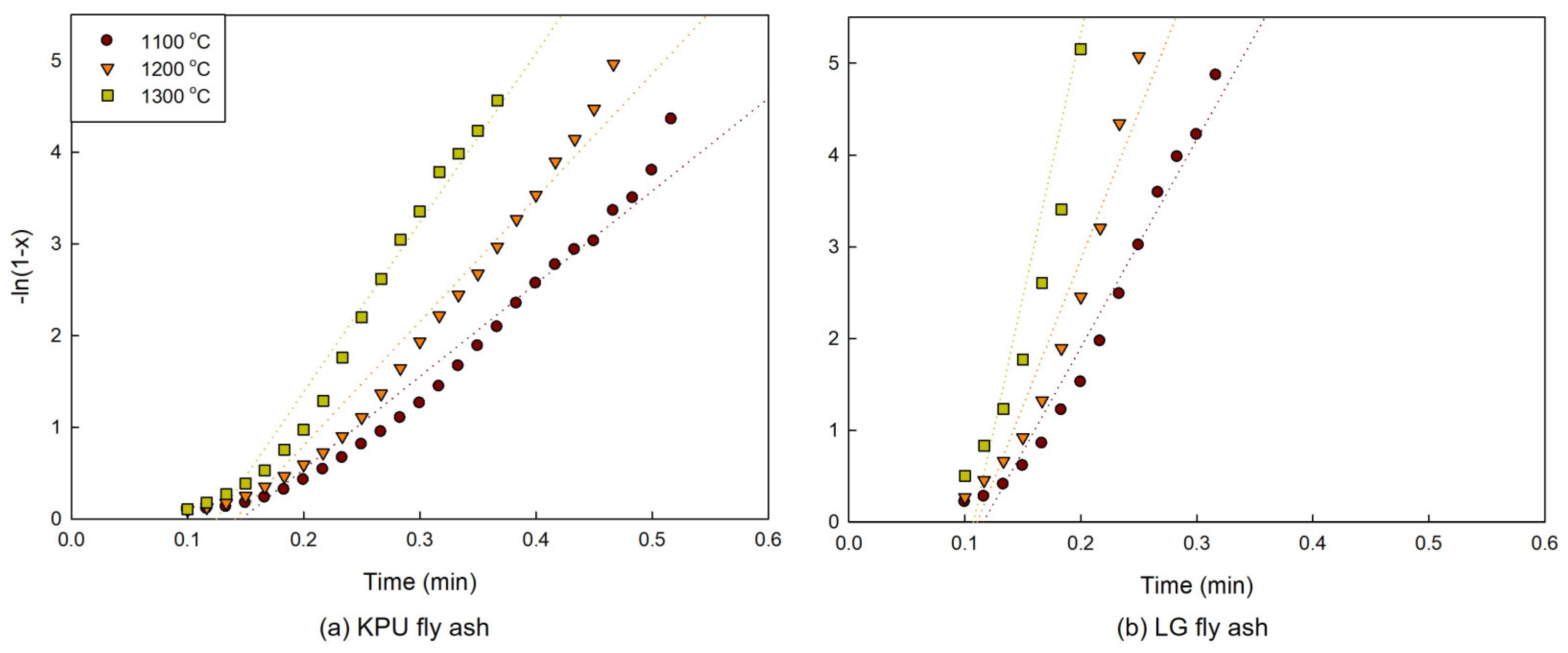
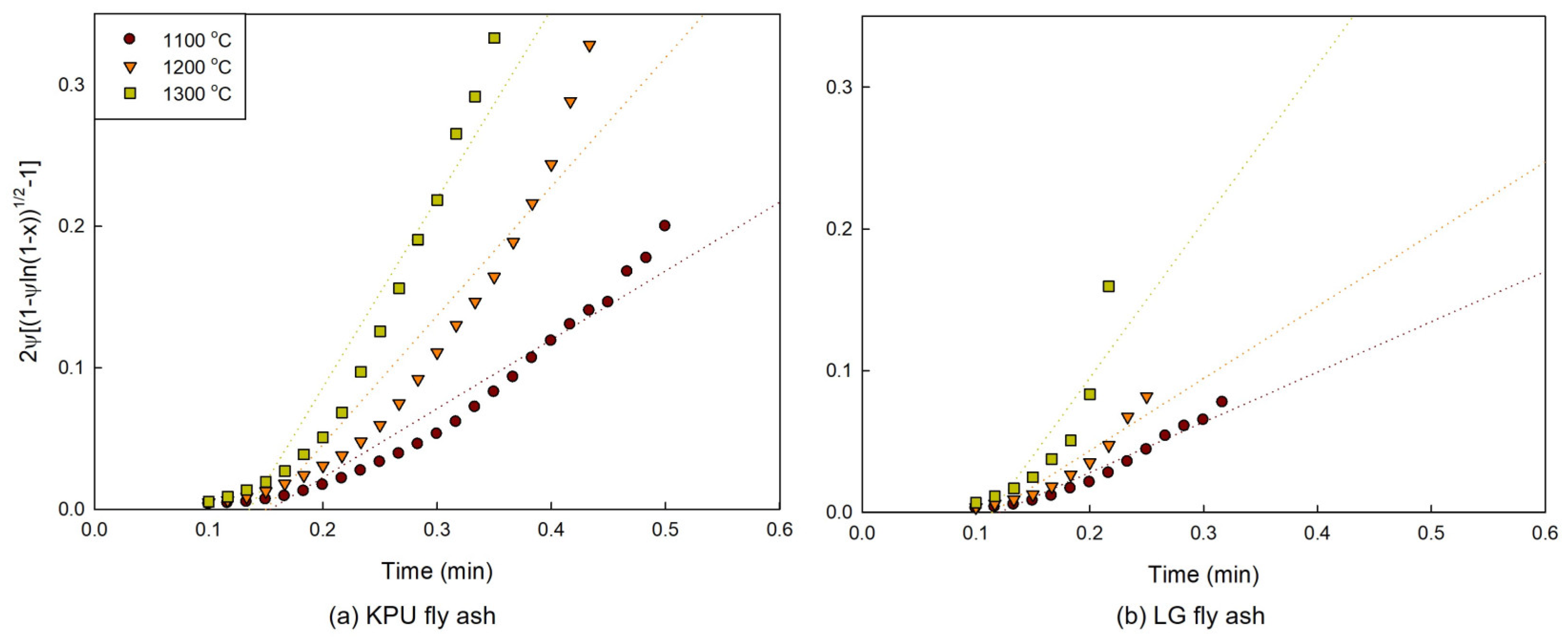
2.2. Kinetic Modeling
3. Results
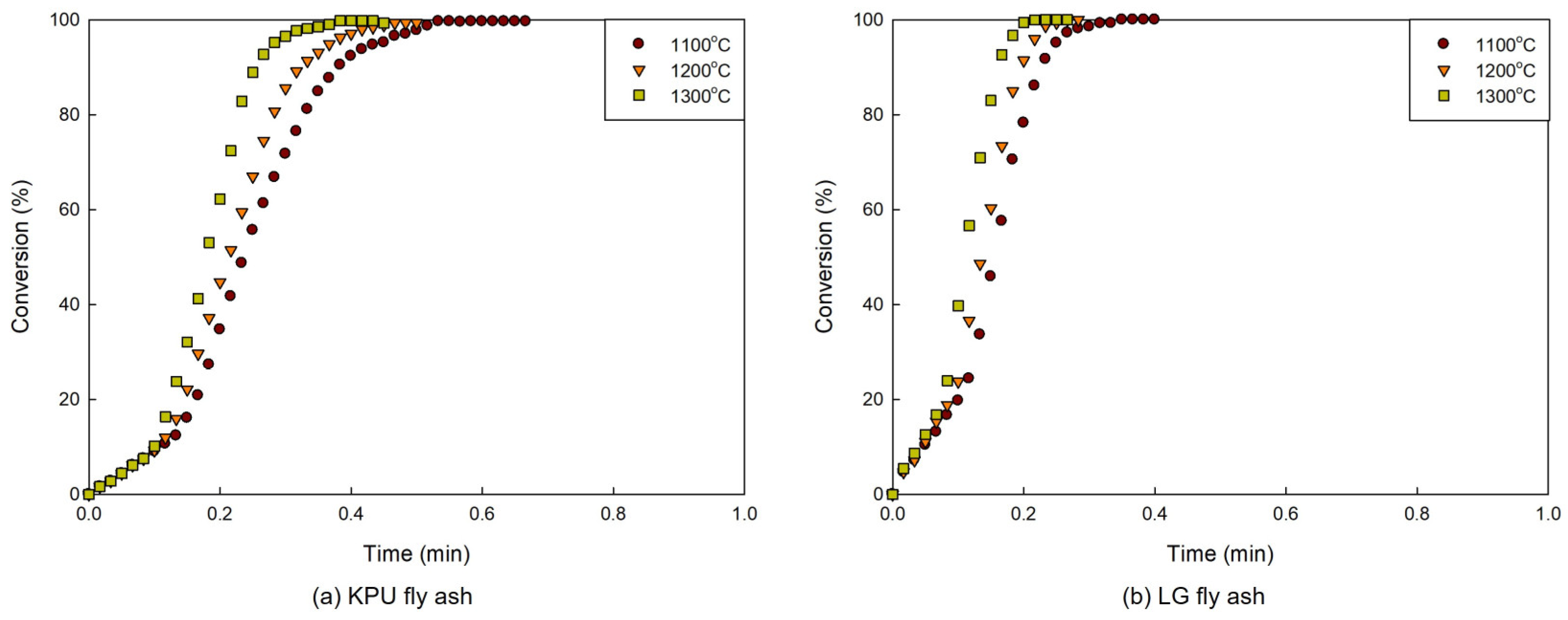
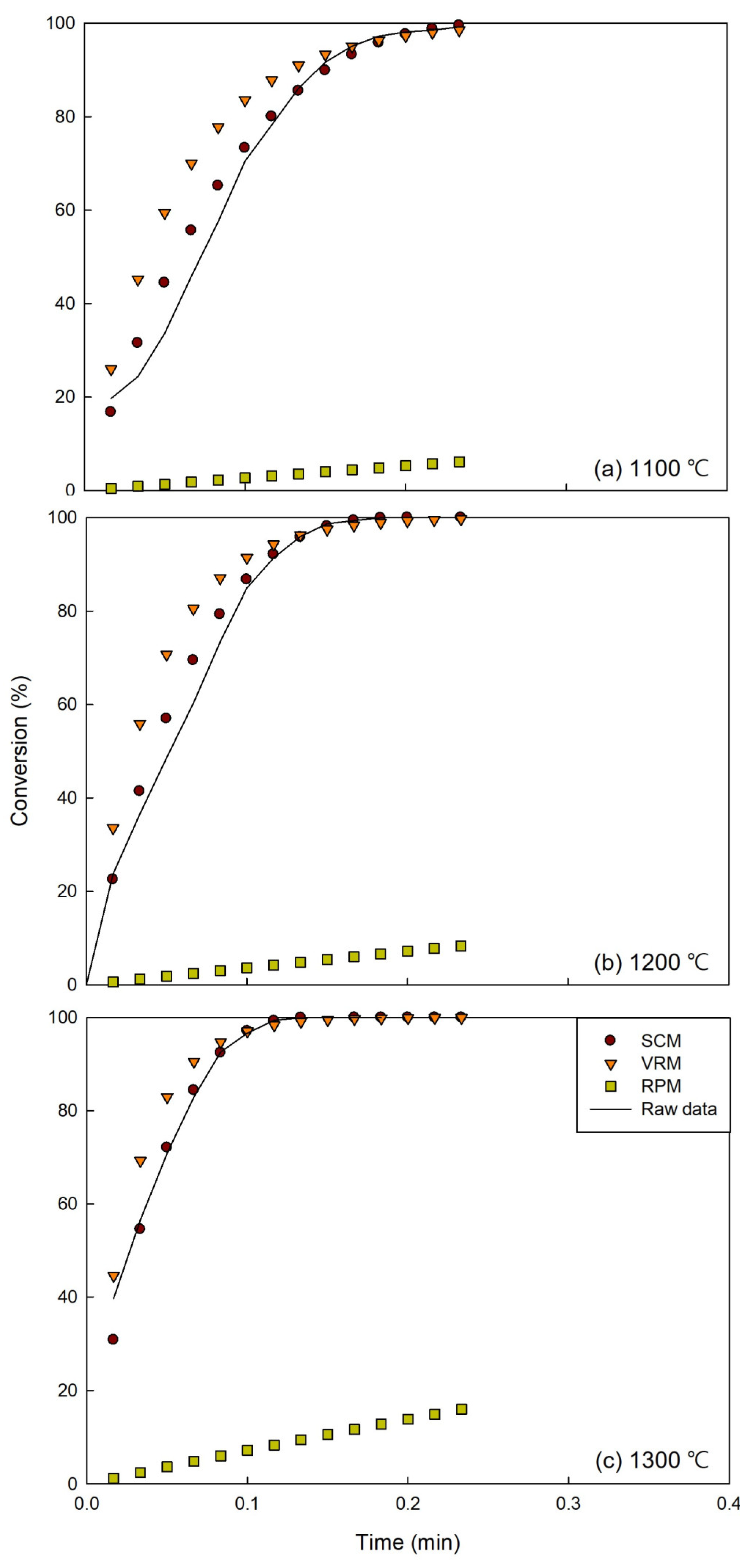
3.1. Temperature Effect on Conversion
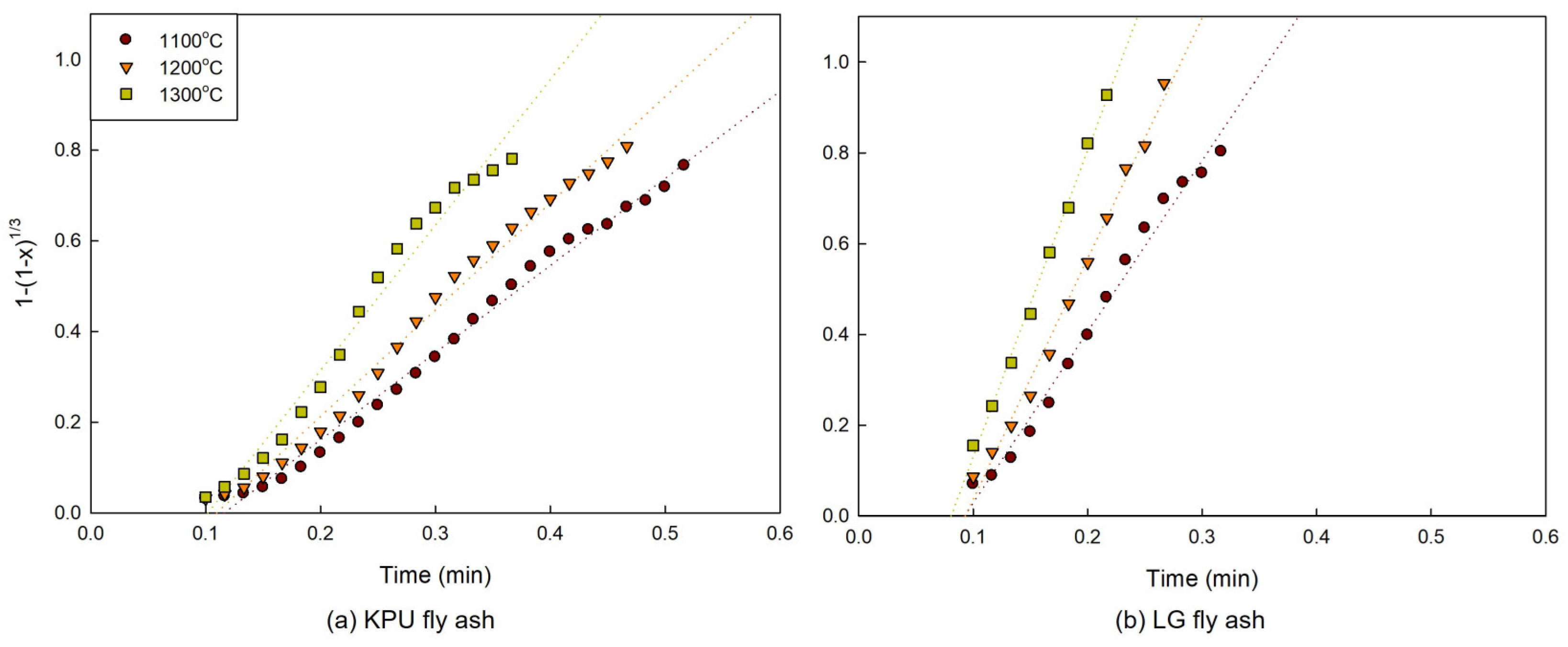
3.2. Model Plots
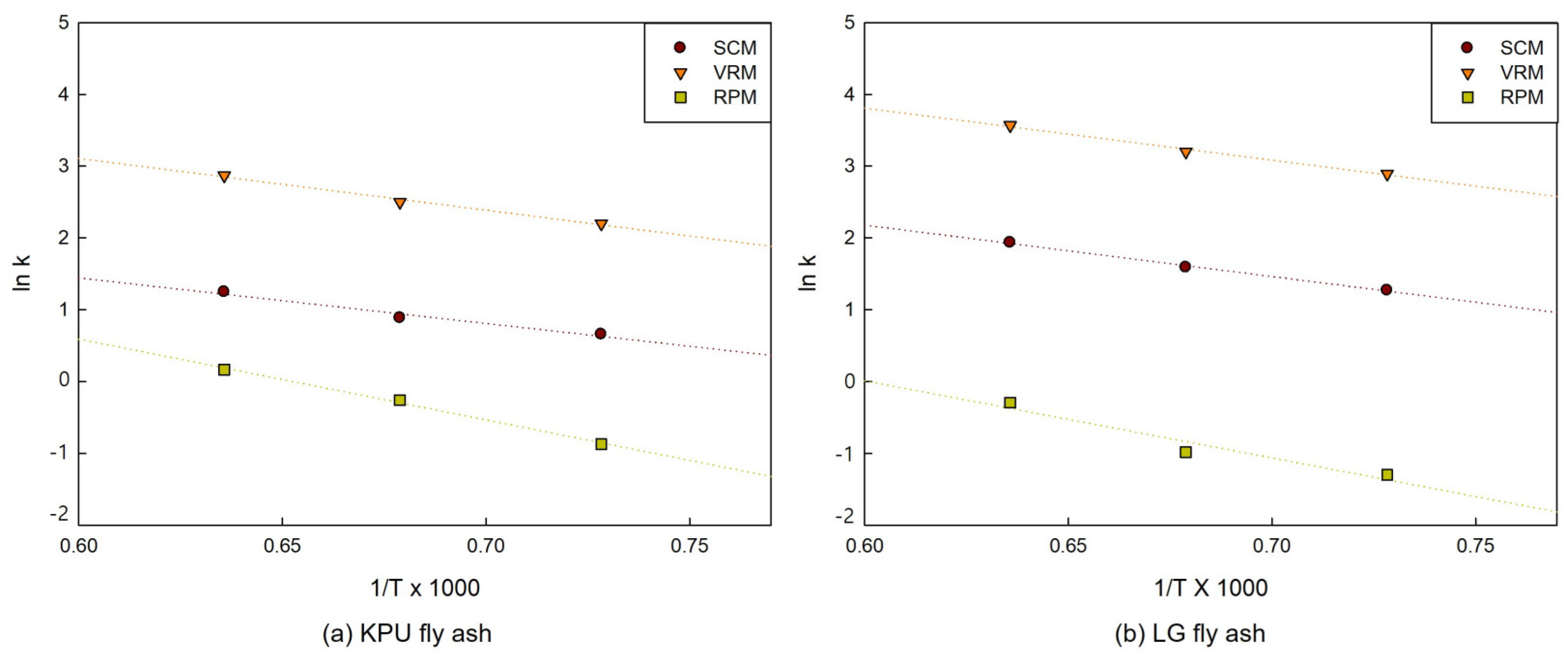
3.3. Activation Energy and Pre-Exponential Factor
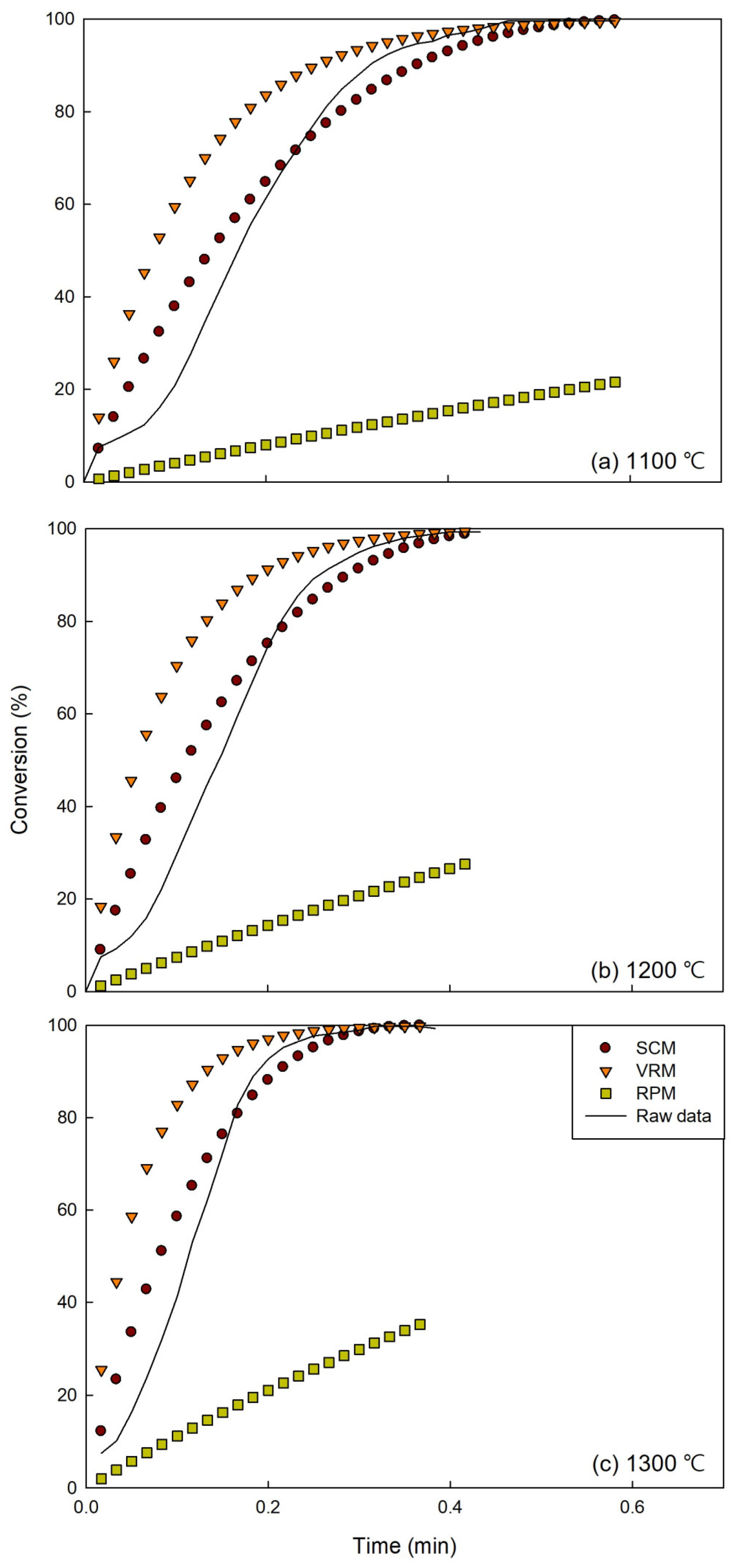
3.4. Experimental and Model Result Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| E | activation energy (kJ/mol) |
| A | pre-exponential factor (l/min) |
| R | gas constant (J/molK) |
| T | absolute temperature (K) |
| x | conversion degrees |
| t | reaction time (min) |
| k | rate constant associated with temperature |
| wi | mass of fly ash at i time (g) |
| w0 | initial mass of fly ash (g) |
References
- Kim, J.H.; Jeong, T.Y.; Yu, J.; Jeon, C.H. Influence of biomass pretreatment on co-combustion characteristics with coal and biomass blends. J. Mech. Sci. Technol. 2019, 33, 2493–2501. [Google Scholar] [CrossRef]
- Roncancio, R.; Gore, J.P. CO2 char gasification: A systematic review from 2014 to 2020. Energy Convers. Manag. X 2021, 10, 100060. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Kibria, M.A.; Bhattacharya, S. Evaluation of high-temperature pyrolysis and CO2 gasification performance of bituminous coal in an entrained flow gasifier. J. Energy Inst. 2021, 94, 294–309. [Google Scholar] [CrossRef]
- Tong, W.; Liu, Q.; Yang, C.; Cai, Z.; Wu, H.; Ren, S. Effect of pore structure on CO2 gasification reactivity of biomass chars under high-temperature pyrolysis. J. Energy Inst. 2020, 93, 962–976. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andrew, R.M.; Canadell, J.G.; Sitch, S.; Korsbakken, J.I.; Peters, G.P.; Manning, A.C.; Boden, T.A.; Tans, P.P.; Houghton, R.A.; et al. Global carbon budget 2016. Earth Syst. Sci. Data 2016, 8, 605–649. [Google Scholar] [CrossRef]
- Hong, D.; Si, T.; Li, X.; Guo, X. Reactive molecular dynamic simulations of the CO2 gasification effect on the oxy-fuel combustion of Zhundong coal char. Fuel Process. Technol. 2020, 199, 106305. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gacem, A.; Choudhary, N.; Rai, A.; Kumar, P.; Yadav, K.K.; Islam, S. Status of coal-based thermal power plants, coal fly ash production, utilization in India and their emerging applications. Minerals 2022, 12, 1503. [Google Scholar] [CrossRef]
- Marinina, O.; Nevskaya, M.; Jonek-Kowalska, I.; Wolniak, R.; Marinin, M. Recycling of coal fly ash as an example of an efficient circular economy: A stakeholder approach. Energies 2021, 14, 3597. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, B.; Sun, Z.; Zhang, Q.; Feng, H.; Hu, H.; Zhao, C. Reaction process and characteristics for coal char gasification under changed CO2/H2O atmosphere in various reaction stages. Energy 2021, 229, 120677. [Google Scholar] [CrossRef]
- Zdeb, J.; Howaniec, N.; Smoliński, A. Experimental study on combined valorization of bituminous coal derived fluidized bed fly ash and carbon dioxide from energy sector. Energy 2023, 265, 126367. [Google Scholar] [CrossRef]
- Bunn, D.W.; Redondo-Martin, J.; Muñoz-Hernandez, J.I.; Diaz-Cachinero, P. Analysis of coal conversion to biomass as a transitional technology. Renew. Energy 2019, 132, 752–760. [Google Scholar] [CrossRef]
- Proskurina, S.; Heinimö, J.; Schipfer, F.; Vakkilainen, E. Biomass for industrial applications: The role of torrefaction. Renew. Energy 2017, 111, 265–274. [Google Scholar] [CrossRef]
- Roni, M.S.; Chowdhury, S.; Mamun, S.; Marufuzzaman, M.; Lein, W.; Johnson, S. Biomass co-firing technology with policies, challenges, and opportunities: A global review. Renew. Sustain. Energy Rev. 2017, 78, 1089–1101. [Google Scholar] [CrossRef]
- Nunes, L.J. Torrefied biomass as an alternative in coal-fueled power Plants: A case study on grindability of agroforestry waste forms. Clean Technol. 2020, 2, 270–289. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Abdul Wahid, M.; Jamil, M.M.; Azli, A.A.; Misbah, M.F. A review on biomass-based hydrogen production for renewable energy supply. Int. J. Energy Res. 2015, 39, 1597–1615. [Google Scholar] [CrossRef]
- Das, D.; Rout, P.K. A Review of Coal Fly Ash Utilization to Save the Environment. Water Air Soil Pollut. 2023, 234, 128. [Google Scholar] [CrossRef]
- Gao, K.; Iliuta, M.C. Trends and advances in the development of coal fly ash-based materials for application in hydrogen-rich gas production: A review. J. Energy Chem. 2022, 73, 485–512. [Google Scholar] [CrossRef]
- Project Today. Available online: https://www.projectstoday.com/News/Coal-gasification-of-100-MT-to-be-set-up-by-2030-support-for-green-projects (accessed on 2 February 2024).
- Boom and Bust Coal 2024. Available online: https://globalenergymonitor.org/report/boom-and-bust-coal-2024/ (accessed on 30 April 2024).
- APERC. APERC Coal Report; Asia Pacific Energy Research Centre: Tokyo, Japan, 2023. [Google Scholar]
- Smith, L.V.; Tarui, N.; Yamagata, T. Assessing the impact of COVID-19 on global fossil fuel consumption and CO2 emissions. Energy Econ. 2021, 97, 105170. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, H.; Li, Y.; Charoensuppanimit, P.; Mohammad, S.A.; Gasem, K.A.; Wang, S. Sequestration of carbon dioxide in coal: Energetics and bonding from first-principles calculations. Comput. Mater. Sci. 2017, 133, 145–151. [Google Scholar] [CrossRef]
- Spiegl, N.; Berrueco, C.; Long, X.; Paterson, N.; Millan, M. Production of a fuel gas by fluidised bed coal gasification compatible with CO2 capture. Fuel 2020, 259, 116242. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, J.H.; Park, S.N.; Byun, Y.S.; Seo, S.J.; Yun, Y.S.; Park, S.R. Kinetic studies of pyrolysis and Char-CO2 gasification on low rank coals. Korean Chem. Eng. Res. 2011, 49, 114–119. [Google Scholar] [CrossRef]
- Xu, X.Q.; Wang, Y.G.; Chen, Z.D.; Chen, X.J.; Zhang, H.Y.; Bai, L.; Zhang, S. Variations in char structure and reactivity due to the pyrolysis and in-situ gasification using Shengli brown coal. J. Anal. Appl. Pyrolysis 2015, 115, 233–241. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, H.; Wang, M.; Wei, B.; Hu, H. Effect of temperature and simulated coal gas composition on tar production during pyrolysis of a subbituminous coal. Fuel 2019, 241, 1129–1137. [Google Scholar] [CrossRef]
- Naidu, V.S.; Aghalayam, P.; Jayanti, S. Synergetic and inhibition effects in carbon dioxide gasification of blends of coals and biomass fuels of Indian origin. Bioresour. Technol. 2016, 209, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, C.; He, X.; Dai, J.; Xu, D.; Wang, Y. Study on the mechanism of calcium catalyzed CO2 gasification of lignite. Fuel Process. Technol. 2021, 213, 106689. [Google Scholar] [CrossRef]
- Kamble, A.D.; Saxena, V.K.; Chavan, P.D.; Mendhe, V.A. Co-gasification of coal and biomass an emerging clean energy technology: Status and prospects of development in Indian context. Int. J. Min. Sci. Technol. 2019, 29, 171–186. [Google Scholar] [CrossRef]
- Dingcheng, L.; Qiang, X.; Guangsheng, L.; Junya, C.; Jun, Z. Influence of heating rate on reactivity and surface chemistry of chars derived from pyrolysis of two Chinese low rank coals. Int. J. Min. Sci. Technol. 2018, 28, 613–619. [Google Scholar] [CrossRef]
- Nanou, P.; Murillo HE, G.; van Swaaij, W.P.; van Rossum, G.; Kersten, S.R. Intrinsic reactivity of biomass-derived char under steam gasification conditions-potential of wood ash as catalyst. Chem. Eng. J. 2013, 217, 289–299. [Google Scholar] [CrossRef]
- Abdalazeez, A.; Li, T.; Wang, W.; Abuelgasim, S. A brief review of CO2 utilization for alkali carbonate gasification and biomass/coal co-gasification: Reactivity, products and process. J. CO2 Util. 2021, 43, 101370. [Google Scholar] [CrossRef]
- Islam, M.W. Effect of different gasifying agents (steam, H2O2, oxygen, CO2, and air) on gasification parameters. Int. J. Hydrogen Energy 2020, 45, 31760–31774. [Google Scholar] [CrossRef]
- Wood, D.A.; Nwaoha, C.; Towler, B.F. Gas-to-liquids (GTL): A review of an industry offering several routes for monetizing natural gas. J. Nat. Gas Sci. Eng. 2012, 9, 196–208. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Production of synthetic natural gas (SNG) from coal and dry biomass–A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, J.H.; Lee, J.W.; Yun, Y.; Kim, G.T.; Kim, Y. Kinetic Studies of CO2 Gasification by Non-isothermal Method on Fly Ash Char. Korean Chem. Eng. Res. 2013, 51, 493–499. [Google Scholar] [CrossRef]
- Kibria, M.A.; Sripada, P.; Bhattacharya, S. Steady state kinetic model for entrained flow CO2 gasification of biomass at high temperature. Energy 2020, 196, 117073. [Google Scholar] [CrossRef]
- Tian, H.; He, Z.; Wang, J.; Jiao, H.; Hu, Z.; Yang, Y. Density functional theory study on the mechanism of biochar gasification in CO2 environment. Ind. Eng. Chem. Res. 2020, 59, 19972–19981. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, H.; Zeng, K.; Zhu, Y.; Hu, J.; Mao, Q.; Chen, H. Study on CO2 gasification of biochar in molten salts: Reactivity and structure evolution. Fuel 2019, 254, 115614. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.P.; Yang, F.L.; Zhao, X.Y.; Tang, W.; Cui, X.; Wei, X.Y. Layered uniformly delocalized electronic structure of carbon supported Ni catalyst for catalytic reforming of toluene and biomass tar. Energy Convers. Manag. 2019, 183, 182–192. [Google Scholar] [CrossRef]
- Yang, F.L.; Cao, J.P.; Zhao, X.Y.; Ren, J.; Tang, W.; Huang, X.; Wei, X.Y. Acid washed lignite char supported bimetallic Ni-Co catalyst for low temperature catalytic reforming of corncob derived volatiles. Energy Convers. Manag. 2019, 196, 1257–1266. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Yao, Z.; Cui, X.; Wang, C.H. CO2 gasification of woody biomass: Experimental study from a lab-scale reactor to a small-scale autothermal gasifier. Energy 2019, 170, 497–506. [Google Scholar] [CrossRef]
- Qin, K.; Lin, W.; Jensen, P.A.; Jensen, A.D. High-temperature entrained flow gasification of biomass. Fuel 2012, 93, 589–600. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Wu, J.H.; Zhang, D. CO2 and H2O Gasification Kinetics of a Coal Char in the Presence of Methane. Energy Fuels 2008, 22, 2160–2165. [Google Scholar] [CrossRef]
- Xu, T.; Bhattacharya, S. Direct and two-step gasification behaviour of Victorian brown coals in an entrained flow reactor. Energy Convers. Manag. 2019, 195, 1044–1055. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, C.; Li, X.; Ding, H.; Shen, C.; Cao, D.; Zhang, L. Development of LaFeO3 modified with potassium as catalyst for coal char CO2 gasification. J. CO2 Util. 2019, 32, 163–169. [Google Scholar] [CrossRef]
- Detchusananard, T.; Im-orb, K.; Ponpesh, P.; Arpornwichanop, A. Biomass gasification integrated with CO2 capture processes for high-purity hydrogen production: Process performance and energy analysis. Energy Convers. Manag. 2018, 171, 1560–1572. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Acharya, B.; Heidari, M.; Arku, P.; Dutta, A. Numerical investigation of CO2 valorization via the steam gasification of biomass for producing syngas with flexible H2 to CO ratio. J. CO2 Util. 2018, 27, 32–41. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Zhu, S.; Bai, Y.; Hao, C.; Li, F.; Bao, W. Investigation into the structural features and gasification reactivity of coal chars formed in CO2 and N2 atmospheres. J. CO2 Util. 2017, 19, 9–15. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Bhattacharya, S. Effect of reactant types (steam, CO2 and steam + CO2) on the gasification performance of coal using entrained flow gasifier. Int. J. Energy Res. 2021, 45, 9492–9501. [Google Scholar] [CrossRef]
- Xu, B.; Cao, Q.; Kuang, D.; Gasem, K.A.; Adidharma, H.; Ding, D.; Fan, M. Kinetics and mechanism of CO2 gasification of coal catalyzed by Na2CO3, FeCO3 and Na2CO3–FeCO3. J. Energy Inst. 2020, 93, 922–933. [Google Scholar] [CrossRef]
- Matsunami, J.; Yoshida, S.; Oku, Y.; Yokota, O.; Tamaura, Y.; Kitamura, M. Coal gasification by CO2 gas bubbling in molten salt for solar/fossil energy hybridization. Sol. Energy 2020, 68, 257–261. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, P.; Gao, F.; Ren, Z.; Li, R.; Chen, H.; Hao, Q. Fuel gas production and char upgrading by catalytic CO2 gasification of pine sawdust char. Fuel 2020, 280, 118686. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Zhang, M.; Li, X.; He, X.; Lei, T.; Gupta, A.K. Syngas evolution and energy efficiency in CO2-assisted gasification of pine bark. Appl. Energy 2020, 269, 114996. [Google Scholar] [CrossRef]
- Li, J.; Burra, K.G.; Wang, Z.; Liu, X.; Gupta, A.K. Syngas evolution and energy efficiency in CO2 assisted gasification of ion-exchanged pine wood. Fuel 2022, 317, 123549. [Google Scholar] [CrossRef]
- Ordorica-Garcia, G.; Douglas, P.; Croiset, E.; Zheng, L. Technoeconomic evaluation of IGCC power plants for CO2 avoidance. Energy Convers. Manag. 2006, 47, 2250–2259. [Google Scholar] [CrossRef]
- Gnanapragasam, N.; Reddy, B.; Rosen, M. Reducing CO2 emissions for an IGCC power generation system: Effect of variations in gasifier and system operating conditions. Energy Convers. Manag. 2009, 50, 1915–1923. [Google Scholar] [CrossRef]
- Renganathan, T.; Yadav, M.V.; Pushpavanam, S.; Voolapalli, R.K.; Cho, Y.S. CO2 utilization for gasification of carbonaceous feedstocks: A thermodynamic analysis. Chem. Eng. Sci. 2012, 83, 159–170. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Li, M.; Fang, Y. Understanding fly-ash formation during fluidized-bed gasification of high-silicon-aluminum coal based on its characteristics. Energy 2018, 150, 142–152. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhao, W.; Hou, J.; Cui, L.; Zou, L.; Zhu, Y. Hierarchical porous nanosilica derived from coal gasification fly ash with excellent CO2 adsorption performance. Chem. Eng. J. 2023, 455, 140622. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Volli, V.; Shu, C.M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Rout, P.K. Synthesis, characterization and properties of fly ash based geopolymer materials. J. Mater. Eng. Perform. 2021, 30, 3213–3231. [Google Scholar] [CrossRef]
- Das, D.; Rout, P.K. Synthesis and characterization of fly ash and GBFS based geopolymer material. Biointerface Res. Appl. Chem. 2021, 11, 14506–14519. [Google Scholar] [CrossRef]
- Roy, R.; Das, D.; Rout, P.K. A review of advanced mullite ceramics. Eng. Sci. 2021, 18, 20–30. [Google Scholar] [CrossRef]
- Roy, R.; Das, D.; Rout, P.K. Mullite ceramics derived from fly ash powder by using albumin as an organic gelling agent. Biointerface Res. Appl. Chem. 2022, 13, 339. [Google Scholar] [CrossRef]
- Deb Barma, S.; Sathish, R.; Rao, D.S.; Prakash, R. Mechanistic investigation on the microwave-assisted leaching of low-grade coal for low-ash coal production. Sep. Sci. Technol. 2021, 56, 3151–3166. [Google Scholar] [CrossRef]
- Paul, T.R.; Nath, H.; Chauhan, V.; Sahoo, A. Gasification studies of high ash Indian coals using Aspen plus simulation. Mater. Today Proc. 2021, 46, 6149–6155. [Google Scholar] [CrossRef]
- Ding, J.; Ma, S.; Shen, S.; Xie, Z.; Zheng, S.; Zhang, Y. Research and industrialization progress of recovering alumina from fly ash: A concise review. Waste Manag. 2017, 60, 375–387. [Google Scholar] [CrossRef]
- Rao, A.V.; Haranath, D. Effect of methyltrimethoxysilane as a synthesis component on the hydrophobicity and some physical properties of silica aerogels. Microporous Mesoporous Mater. 1999, 30, 267–273. [Google Scholar] [CrossRef]
- Nadesan, M.S.; Dinakar, P. Structural concrete using sintered fly ash lightweight aggregate: A review. Constr. Build. Mater. 2017, 154, 928–944. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Khankhaje, E.; Rezania, S. Assessment of environmental and chemical properties of coal ashes including fly ash and bottom ash, and coal ash concrete. J. Build. Eng. 2022, 49, 104040. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Argiz, C. Fineness of coal fly ash for use in cement and concrete. Fuels 2021, 2, 471–486. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, Y.; Cai, W.; He, Y.; Chai, M.; Liu, B.; Cai, J. Theoretical analysis of double Logistic distributed activation energy model for thermal decomposition kinetics of solid fuels. Ind. Eng. Chem. Res. 2018, 57, 7817–7825. [Google Scholar] [CrossRef]
- Wu, G.; Wang, T.; Chen, G.; Shen, Z.; Pan, W.P. Coal fly ash activated by NaOH roasting: Rare earth elements recovery and harmful trace elements migration. Fuel 2022, 324, 124515. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.D. Gasification kinetics of waste tire-char with CO2 in a thermobalance reactor. Energy 1996, 21, 343–352. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, S.; Hu, X.; Song, W.; Cai, J.; Xu, Q. Restraining effect of nitrogen on coal oxidation in different stages: Non-isothermal TG-DSC and EPR research. Int. J. Min. Sci. Technol. 2020, 30, 387–395. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B.; Bada, S. Spontaneous combustion liability between coal seams: A thermogravimetric study. Int. J. Min. Sci. Technol. 2020, 30, 691–698. [Google Scholar] [CrossRef]
- Kibria, M.A.; Sripada, P.; Bhattacharya, S. Rational design of thermogravimetric experiments to determine intrinsic char gasification kinetics. Proc. Combust. Inst. 2019, 37, 3023–3031. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol. 2020, 30, 303–311. [Google Scholar] [CrossRef]
- Liu, X.; Tu, Y.; Xu, X.; Liu, Z. The development and research prospect on catalytic coal gasification. Chem. Eng. Trans. 2017, 61, 1165–1170. [Google Scholar] [CrossRef]
- Spiro, C.L.; McKee, D.W.; Kosky, P.G.; Lamby, E.J.; Maylotte, D.H. Significant parameters in the catalysed CO2 gasification of coal chars. Fuel 1983, 62, 323–330. [Google Scholar] [CrossRef]
- Öztürk, M.; Özek, N.; Yüksel, Y.E. Gasification of various types of tertiary coals: A sustainability approach. Energy Convers. Manag. 2012, 56, 157–165. [Google Scholar] [CrossRef]
- Jin, G.; Iwaki, H.; Arai, N.; Kitagawa, K. Study on the gasification of wastepaper/carbon dioxide catalyzed by molten carbonate salts. Energy 2005, 30, 1192–1203. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Seo, D.K.; Lee, S.K.; Kang, M.W.; Hwang, J.; Yu, T.U. Gasification reactivity of biomass chars with CO2. Biomass Bioenergy 2010, 34, 1946–1953. [Google Scholar] [CrossRef]
- Wang, G.; Ren, S.; Zhang, J.; Ning, X.; Liang, W.; Zhang, N.; Wang, C. Influence mechanism of alkali metals on CO2 gasification properties of metallurgical coke. Chem. Eng. J. 2020, 387, 124093. [Google Scholar] [CrossRef]
- Szekely, J.; Evans, J.W. A structural model for gas—Solid reactions with a moving boundary. Chem. Eng. Sci. 1970, 25, 1091–1107. [Google Scholar] [CrossRef]
- He, Q.; Ding, L.; Raheem, A.; Guo, Q.; Gong, Y.; Yu, G. Kinetics comparison and insight into structure-performance correlation for leached biochar gasification. Chem. Eng. J. 2021, 417, 129331. [Google Scholar] [CrossRef]
- Kajitani, S.; Hara, S.; Matsuda, H. Gasification rate analysis of coal char with a pressurized drop tube furnace. Fuel 2002, 81, 539–546. [Google Scholar] [CrossRef]
- Wen, C.Y. Noncatalytic heterogeneous solid-fluid reaction models. Ind. Eng. Chem. 1968, 60, 34–54. [Google Scholar] [CrossRef]
- Tanner, J.; Bhattacharya, S. Kinetics of CO2 and steam gasification of Victorian brown coal chars. Chem. Eng. J. 2016, 285, 331–340. [Google Scholar] [CrossRef]
- Fermoso, J.; Stevanov, C.; Moghtaderi, B.; Arias, B.; Pevida, C.; Plaza, M.G.; Pis, J.J. High-pressure gasification reactivity of biomass chars produced at different temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 287–293. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980, 26, 379–386. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: II. Diffusion and transport effects. AIChE J. 1981, 27, 247–254. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Gupta, A.K. Pyrolysis and gasification of food waste: Syngas characteristics and char gasification kinetics. Applied Energy 2010, 87, 101–108. [Google Scholar] [CrossRef]
- Liu, T.F.; Fang, Y.T.; Wang, Y. An experimental investigation into the gasification reactivity of chars prepared at high temperatures. Fuel 2008, 87, 460–466. [Google Scholar] [CrossRef]
- Liu, H.; Luo, C.; Kato, S.; Uemiya, S.; Kaneko, M.; Kojima, T. Kinetics of CO2/Char gasification at elevated temperatures: Part I: Experimental results. Fuel Process. Technol. 2006, 87, 775–781. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, X.; Li, X.; Hu, Z.; Huang, Y.; Xia, A.; Yao, H. Investigation of the combustion behaviors and kinetic modelling of municipal solid waste char under isothermal conditions using a micro-fluidized bed. J. Environ. Chem. Eng. 2021, 9, 105984. [Google Scholar] [CrossRef]
- Liu, M.; He, Q.; Bai, J.; Yu, J.; Kong, L.; Bai, Z.; Li, W. Char reactivity and kinetics based on the dynamic char structure during gasification by CO2. Fuel Process. Technol. 2021, 211, 106583. [Google Scholar] [CrossRef]
- Ochoa, J.; Cassanello, M.C.; Bonelli, P.R.; Cukierman, A.L. CO2 gasification of Argentinean coal chars: A kinetic characterization. Fuel Process. Technol. 2001, 74, 161–176. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Fang, Y.; Wang, Y. Gasification reactivity and kinetics of typical Chinese anthracite chars with steam and CO2. Energy Fuels 2006, 20, 1201–1210. [Google Scholar] [CrossRef]
- Ollero, P.; Serrera, A.; Arjona, R.; Alcantarilla, S. The CO2 gasification kinetics of olive residue. Biomass Bioenergy 2003, 24, 151–161. [Google Scholar] [CrossRef]
- Murillo, R.; Navarro, M.V.; López, J.M.; Aylón, E.; Callén, M.S.; García, T.; Mastral, A.M. Kinetic model comparison for waste tire char reaction with CO2. Ind. Eng. Chem. Res. 2004, 43, 7768–7773. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, D.; Wang, Y.; Argyle, M.D.; Fan, M. CO2 gasification of Powder River Basin coal catalyzed by a cost-effective and environmentally friendly iron catalyst. Appl. Energy 2015, 145, 295–305. [Google Scholar] [CrossRef]
- Tian, H.; Hu, Q.; Wang, J.; Chen, D.; Yang, Y.; Bridgwater, A.V. Kinetic study on the CO2 gasification of biochar derived from Miscanthus at different processing conditions. Energy 2021, 217, 119341. [Google Scholar] [CrossRef]
- Nath, S.K.; Mukherjee, S.; Maitra, S.; Kumar, S. Kinetics study of geopolymerization of fly ash using isothermal conduction calorimetry. J. Therm. Anal. Calorim. 2017, 127, 1953–1961. [Google Scholar] [CrossRef]
- Yang, L.; Peifang, F.; Kang, B.; Tianyao, X.; Muhammad, A. Non-isothermal kinetics of coal char oxyfuel combustion by isothermal model-fitting method. Energy Rep. 2022, 8, 2062–2071. [Google Scholar] [CrossRef]
- Park, S.T.; Choi, Y.T.; Sohn, J.M. The study of CO2 gasification of low rank coal impregnated by K2CO3, Mn(NO3)2, and Ce(NO3)3. Appl. Chem. Eng. 2011, 22, 312–318. [Google Scholar] [CrossRef]
- Trommer, D.; Steinfeld, A. Kinetic modeling for the combined pyrolysis and steam gasification of petroleum coke and experimental determination of the rate constants by dynamic thermogravimetry in the 500–1520 K range. Energy Fuels 2006, 20, 1250–1258. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Li, X.; Ge, Y.; Gao, F.; Chen, H.; Ma, X. Syngas production by integrating CO2 partial gasification of pine sawdust and methane pyrolysis over the gasification residue. Int. J. Hydrogen Energy 2019, 44, 19742–19754. [Google Scholar] [CrossRef]
- Chan, Y.H.; Rahman SN FS, A.; Lahuri, H.M.; Khalid, A. Recent progress on CO-rich syngas production via CO2 gasification of various wastes: A critical review on efficiency, challenges and outlook. Environ. Pollut. 2021, 278, 116843. [Google Scholar] [CrossRef]
- Porada, S.; Czerski, G.; Grzywacz, P.; Makowska, D.; Dziok, T. Comparison of the gasification of coals and their chars with CO2 based on the formation kinetics of gaseous products. Thermochim. Acta 2017, 653, 97–105. [Google Scholar] [CrossRef]
| Coal | KPU Coal Fly Ash | LG Coal Fly Ash | |
|---|---|---|---|
| Proximate analysis (wt.%, air-dry/dry) | Moisture | 5.4 (0.0) | 4.2 (0.0) |
| Volatile Matter | 3.0 (3.2) | 1.8 (1.9) | |
| Fixed Carbon | 51.0 (53.9) | 31.3 (32.7) | |
| Ash | 40.6 (42.9) | 62.7 (65.4) | |
| Ultimate analysis (wt.%, dry) | C | 55.4 | 32.5 |
| H | 0.4 | 0.3 | |
| O | 0.0 | 0.3 | |
| N | 0.4 | 0.5 | |
| S | 0.9 | 1.0 | |
| Ash | 42.9 | 65.4 | |
| HHV (kcal/kg, dry) | 4623 | 2755 |
| Ref. | Sample | Kinetic Model | Activation Energy (kJ/mol) | Pre-Exponential Factor (min−1) | Temperature (°C) | Comments |
|---|---|---|---|---|---|---|
| [36] | KPU coal fly ash char | VRM | 198.3 | 2.47 × 106 | 900~1200 | Non-isothermal |
| LG coal fly ash char | 200.8 | 8.11 × 104 | ||||
| [24] | ABK coal char | SCM | 172.6 | 4.47 × 104 | 900~1200 | Non-isothermal |
| Lignite char | 183.0 | 2.10 × 105 | ||||
| [44] | Bituminous coal char | SCM | 239.0 | |||
| [86] | Pinus densiflora for Multicaulis char | VRM SCM RPM | 172 142 134 | 850~1050 | ||
| [100] | Bituminous coal char | RPM | 178.38 | 2.01 × 107 | 950 | |
| [101] | Sub-bituminous coal char | Random capillary | 35.5 | n = 0.57 | ||
| RPM | 37.3 | N = 0.56 | ||||
| High volatile bituminous coal char | Random capillary | 37.3 | N = 0.56 | |||
| RPM | 39.4 | N = 0.58 | ||||
| [102] | Chinese anthracite char | SCM | 146.4 201.2 | 4.51 × 103 9.78 × 105 | ||
| [103] | Olive residue char | nth order model | 133 | 800~900 | N = 0.43 | |
| [104] | Waste tire char | VRM SCM RPM | 191.8 197.5 197.7 | |||
| [105] | Sub-bituminous coal | SCM | 92.7 | 700~900 | TGA (Isothermal) | |
| [106] | Bio-char | VRM | 78.09 | 330 | 1000 | Isothermal |
| This work | KPU coal fly ash | SCM VRM RPM | 52.7 59.9 93.6 | 1.90 × 102 1.68 × 103 1.87 × 103 | 1100~1300 | Isothermal |
| LG coal fly ash | SCM VRM RPM | 59.6 60.2 89.4 | 6.51 × 102 3.46 × 103 6.39 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.-J.; Lee, J.-H.; Lee, D.-H.; Kim, H.-S.; Kang, S.-H. Effect of High Temperature on CO2 Gasification Kinetics of Sub-Bituminous Coal Fly Ash. Sustainability 2025, 17, 1519. https://doi.org/10.3390/su17041519
Kang T-J, Lee J-H, Lee D-H, Kim H-S, Kang S-H. Effect of High Temperature on CO2 Gasification Kinetics of Sub-Bituminous Coal Fly Ash. Sustainability. 2025; 17(4):1519. https://doi.org/10.3390/su17041519
Chicago/Turabian StyleKang, Tae-Jin, Jin-Hee Lee, Da-Hye Lee, Hyo-Sik Kim, and Suk-Hwan Kang. 2025. "Effect of High Temperature on CO2 Gasification Kinetics of Sub-Bituminous Coal Fly Ash" Sustainability 17, no. 4: 1519. https://doi.org/10.3390/su17041519
APA StyleKang, T.-J., Lee, J.-H., Lee, D.-H., Kim, H.-S., & Kang, S.-H. (2025). Effect of High Temperature on CO2 Gasification Kinetics of Sub-Bituminous Coal Fly Ash. Sustainability, 17(4), 1519. https://doi.org/10.3390/su17041519






