Abstract
This paper focuses on identifying the human health risks as a result of the presence of polycyclic aromatic hydrocarbons (PAHs) in groundwater due to the Bucharest landfill leakages. The main subjects were neighboring areas as the main receptors. The functional landfill located near the capital of Romania was selected as a case study. Fluorene (Pf), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu) and pyrene (Pyr) were detected using gas chromatography-tandem mass spectrometry (GC-MS/MS) analysis. The health risks for receptors via ingestion and dermal exposure scenarios were developed considering dermal contact once per day during showering for 20 min and regular ingestion of the groundwater most contaminated with PAHs at a rate of 2 L per day. The concentration ranges of PAHs in groundwater wer <0.0010–0.0037 μg L−1 for Pf, 0.0014–0.0065 μg L−1 for Phe, <0.0010–0.0013 μg L−1 for Ant, <0.0010–0.0011 μg L−1 for Flu, and 0.0030–0.0032 μg L−1 for Pyr. The rest of the PAHs were under the limit of detection. Both the cumulative hazard and risk quotient are well below the value of 1, which indicates a significant low risk for the ingestion of PAH-contaminated groundwater. However, the risk due to daily groundwater consumption and skin contact is minimal.
1. Introduction
One of the most common methods used in the world today for waste management is still waste storage in landfills [1]. Industrial by-products, household waste, and construction debris are all among the discarded materials that municipal landfills serve as repositories for, contributing to the production of complex environmental contaminants [2]. Among these contaminants, polycyclic aromatic hydrocarbons (PAHs) are of particular concern due to their bioaccumulation potential, persistence, and associated health risks. PAHs are listed as persistent organic pollutants (POPs).
When released into the environment from landfills, PAHs can leach into groundwater and migrate into surrounding soils, contaminating ecosystems and potentially exposing nearby populations to significant health risks like cancers, respiratory ailments, reproductive and developmental toxicity, endocrine disruption, and neurocognitive impairments [3]. PAHs can compromise the immune system, increasing susceptibility to infections and other illnesses, and can also impact the skin, leading to conditions such as dermatitis, rashes, and irritation [4]. Human exposure to PAHs through soil and groundwater contamination occurs via multiple pathways, including ingestion of contaminated water, direct dermal contact with polluted soil, and, to a lesser extent, inhalation of volatilized PAHs. The primary sources of PAHs were identified as the incomplete combustion of fossil fuels, vehicle emissions, and petroleum effluents discharged from a nearby depot. Chronic exposure to PAHs has been shown to increase the risk of cancers, particularly of the skin, lungs, and bladder, due to their ability to form DNA adducts and induce mutations [5,6]. Higher concentrations of benzo(a)pyrene (BaP) were observed in individuals with chronic obstructive pulmonary disease (COPD) compared to the control group in both urban (0.90 vs. 0.47 μg L−1) and rural areas (0.73 vs. 0.44 μg L−1) of Iasi, Romania [7]. These results were associated with traffic and vehicle emissions. Instead, the total concentrations of Σ16 PAHs ranged from 0.00017 to 0.17671 μg L−1, with an average of 0.05649 ± 0.03858 μg L−1, based on the analysis of 70 water samples collected from centralized drinking water sources across mainland China [8]. For vulnerable populations, such as children and pregnant women, these risks are particularly pronounced, with studies linking prenatal and early childhood exposure to adverse developmental and behavioral outcomes.
PAHs are a class of chemical substances, consisting of carbon and hydrogen only, which have multiple fused aromatic ring systems [9,10]. PAHs are generated through steel mills [11], the thermal degradation of organic compounds, from fossil fuel used for power generation, and from motor vehicles to biomass [12,13]. PAHs in the sediment samples suggest that biomass combustion and the burning of fossil fuels, such as coal and petroleum, are the primary sources of these compounds [4]. High concentrations of PAHs were reported for groundwater, lakes and reservoirs, and air environments. For instance, the highest concentration of naphthalene, of 36.4 μg L−1, was detected in groundwater samples from the Marco neighborhood in northern Brazil, using GC-FID analysis, indicating potential contamination from oil-derived fuels [14]. Additionally, a total of 15 PAHs ranging from 10 to 3450 μg L−1 (mean of 420 μg L−1) were found in ten groundwater samples in Ibadan, southwestern Nigeria [15]. Also, concentrations higher than 0.5 ng g−1 of benzo(a)pyrene, known as PAHs, have been detected in food products, such as smoked meat, well-cooked red meat, hamburgers, grilled chicken, popcorn and pumpkin pies [16,17,18]. PAHs and other trace POPs were reported in topsoil in Chengdu, China (Σ16 PAHs ranged between 88.56 to 4448.34 ng g−1) [12], leachates from landfills in the USA (concentrations of DEET and sucralose ranging from 6900 to 143,000 ng L−1 and <10 to 621,000 ng L−1, respectively) [19], Norway (perfluorinated compounds (PFCs) at concentrations up to 6.231 µg L−1) [20], and also in groundwater located adjacent to industrial sites [14,21].
PAHs infiltrate water resources through multiple pathways, including urban runoff, which carries pollutants from roads, roofs, and paved surfaces into nearby water bodies [22]. Additionally, wastewater treatment plants may release residual PAHs if they are not adequately removed during treatment processes. Industrial activities, such as the discharge of untreated effluents, spills, or emissions from factories, also contribute significantly to PAH contamination. In general, groundwater contamination was primarily observed within 1000 m of a landfill, with the most severe cases occurring within 200 m [21], influenced by factors such as seasonal rainfall, landfill age, and natural attenuation processes. It has been demonstrated that PAHs represent a class of pollutants with a high frequency in the composition of soils and groundwater present near landfills.
Minimizing the environmental impact of PAHs is crucial, and various strategies are available to mitigate their adverse effects [4,23]. Common strategies for removing PAHs from the environment include physical techniques like adsorption, electrokinetic remediation, chemical processes, and phytoremediation [23]. Among these, adsorption stands out for its effectiveness and simplicity, with various adsorbents under investigation, such as minerals recovered from acid mine water [24], wood waste-derived biochar [25], and a magnetic chitosan/molybdenum disulfide (CS/MoS2/Fe3O4) nanocomposite [26]. Advanced oxidation processes (AOPs) are recognized as the most effective methods for the removal of organic contaminants, wherein the in situ-generated reactive species play a key role in the degradation [27]. Examples of AOPs include UV/oxidants, such as H2O2, O3, peroxy disulfate (S2O82−), and peroxy monosulfate (HSO5−), Fenton and photo-Fenton processes, and photocatalysis including TiO2-based materials, as well as electrochemical advanced oxidation processes [28]. However, studies on PAHs in groundwater are limited in the literature, primarily due to their significantly lower concentrations in this medium compared to the levels found in litter, contaminated soils, sewage, or marine sediments [14]. According to the current legislation in Romania, the monitoring of pollutants by landfill operators is not mandatory, in contrast to in other countries. In the case of maximum limits for pollutants in groundwater, these are not established for PAHs, with only threshold values on the ground. Under these conditions, the risks regarding the impact on human health as a result of contact with groundwater are not monitored either.
The current study represents a continuation of the investigations started in 2020 regarding the assessment of the risk to human health of the inhabitants of the three areas bordering Bucharest, Glina, Chiajna, and Vidra, who are exposed to the risk of contamination with PAHs through the consumption of water and agricultural products from contaminated soils as a result of the landfills [29]. In the present study, the Vidra area is investigated, with the main aspects pursued in the study being the establishment of the PAH concentrations in the groundwater near the municipal landfill and the assessment of the health risks for subjects exposed to PAHs from the groundwater through ingestion and dermal contact. There are no previous related studies about the Vidra landfill. As a result, the historical exposure could not be addressed. However, the current study should be regarded as a baseline for a screening phase in exposure assessments, which may trigger additional investigations and studies to establish the historical exposure to the contaminants. The novelty of this study pertains to the assessment of the PAH contamination of groundwater and its impact on human health, especially in areas where groundwater is an important source of drinking water. This paper could serve as a well-defined methodology for risk assessment, including water sample collection, laboratory analysis, the identification of risk-based screening levels (RBSLs), the quantification of potential hazards, and recommendations for future risk mitigation. The approach is based on the international risk assessment methodologies associated with contaminated sites, but it focuses on an assessment starting from the receptor rather than the the source of contamination. The broader implications of this study are related to both ingestion and dermal contact as routes of exposure to PAHs, recognizing the importance of dermal contact, especially through the use of contaminated water for showering. Furthermore, it provides a simple and basic approach which may be further studied for establishing methodologies to confirm causality for environmental liabilities.
2. Materials and Methods
2.1. Health Assessment Methodology
Health assessment is defined as “the evaluation of data and information on the release of hazardous substances into the environment in order to assess any current or future impact on public health, develop health advisories or other recommendations, and identify studies or actions needed to evaluate and mitigate or prevent human health effects” [30]. As a result, the following steps were carried out to assess the potential health effects of PAHs near the Bucharest landfill:
- Collection of groundwater samples and analyses in an ISO 17025 [31]-certified laboratory.
- Identification of risk-based screening levels (RBSLs) in accordance with the ASTM E2081-22 [32].
- Comparison of the RBSLs to the actual concentrations on the site and quantification of the potential health hazards.
- Providing recommendations for the mitigation of future health hazards.
2.2. General Information Regarding the Bucharest Landfill
Vidra, one municipal landfill, is located in the southeastern part of Romania, around the capital of the country—Bucharest City. This location is depicted in Figure 1.
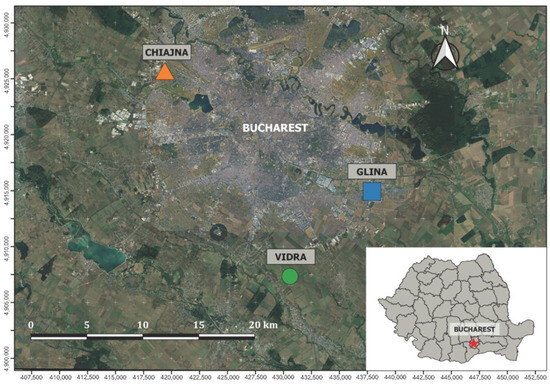
Figure 1.
Location of the Vidra landfill, near the Chiajna and Glina landfills, around Bucharest city (Source: Aerial imagery from Google Earth).
The current research focuses on exposure to contaminated groundwater around the Vidra landfill, which is the only active landfill near Bucharest. The shallow aquifer is known as Colentina Gravels of the upper Pleistocene (), and the groundwater is located at approximately 6 m below ground level, based on regional hydrogeological maps and manual soil augering [33].
2.3. Groundwater Sampling
According to evaluations derived from the hydrogeological map of Bucharest [34], the landfill is situated in a hydrogeological high-point zone. The regional aquifer in the Vidra landfill vicinity is channeled by the Sabar River, exhibiting a diverging local flow pattern from north to south within the area under consideration. The closest sensitive receptors are the residents of Sintești village, part of the Vidra commune, positioned to the south and southwest of the landfill.
According to estimations from the hydrogeological map of Bucharest [34], the landfill is located in a hydrogeological peak area. The regional aquifer in the Vidra landfill vicinity is drained by the Sabar River, exhibiting a diverging local flow pattern from north to south within the area under consideration.
The closest sensitive receptors are the residents of the Sintești village, part of the Vidra commune, positioned to the south and southwest of the landfill. Any potential contamination generated by the site would reach the southern part of the Sintești village and the agricultural land located south and east of the landfill. The groundwater table has a local elevation of 59–60 m above the Black Sea level and the groundwater velocities are between 0.08 m/day and 0.27 m/day.
To identify the concentration of PAHs in the groundwater, the groundwater samples were collected from two wells near the Vidra landfill. One of the samples was collected from the kitchen tap of a household and the other sample was collected from a garden tap of a second household. To protect the identity of the two households, the location of the sampling points was placed within a 100 m radius around the actual sampling point.
The location of the sampling points, following the reasoning behind the sampling approach, is depicted in Figure 2.
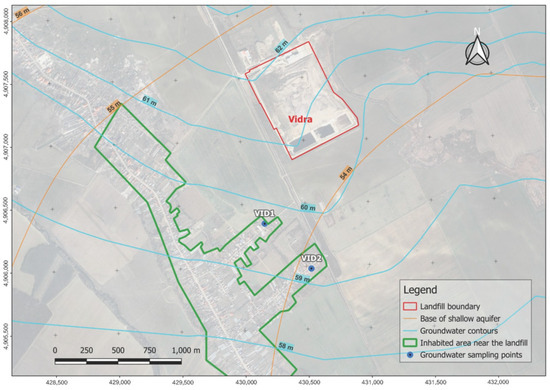
Figure 2.
Groundwater samples, coded VID1 and VID2, collected near the Vidra landfill.
The groundwater samples were dispatched to a laboratory accredited to the ISO 17025 standard [31] for PAH analysis using gas chromatography-tandem mass spectrometry (GC-MS/MS).
2.4. Potential Exposure Routes and Qualitative Risk Assessment
Potential receptors were identified based on the general groundwater flow direction. The groundwater velocity was calculated based on the average hydraulic gradient, as provided by the hydrogeological maps, and based on the hydraulic conductivity reported for Bucharest City of between 39 and 135 m/day [35].
Based on visual observations and discussions with local people, all the residents around the landfill use groundwater for sanitary, drinking, and food preparation purposes. It was inferred that, in the investigated area, most of the inhabitants were not connected to the municipal water supply.
Inhalation can take place both outdoors and indoors, though it is predominantly linked to cooling activities. Since PAH ingestion is more likely to result from food preparation involving contaminated water, the inhalation risks can be neglected in the preliminary phase. However, exposure to naphthalene should be regarded as significant, due to its ability to sublimate. Nonetheless, the naphthalene concentrations in the two groundwater samples collected were below the detection limit. Consequently, the risk of PAH inhalation is presumed to be significantly low, and no further assessment is considered necessary for this study.
Consumption of contaminated groundwater can occur via two primary routes:
1. Direct consumption of groundwater, which is almost inevitable in the Vidra landfill region, where groundwater serves as a source for drinking and sanitary uses.
2. Indirect intake through foods that are washed or prepared using groundwater containing PAHs. It was inferred that residents around the Vidra landfill use groundwater not only for gardening or sanitary purposes but also for food preparation.
The risk is presumed to be average as there is a high-frequency rate in the ingestion of contaminated groundwater at low concentrations of PAHs. Further assessment of the risks linked to groundwater ingestion focuses solely on direct consumption, as it inherently encompasses indirect ingestion.
Dermal exposure is assumed to be considerably high, given that many residents rely on groundwater for sanitary activities, such as bathing. As a result, further assessment was deemed necessary in the current research.
To achieve a more precise estimation of the health risks, the following categories of exposed people were taken into consideration:
Category 1: individuals residing near the landfill for their entire 70-year lifespan, consistently using contaminated water.
Category 2: individuals living near the landfill until the age of 20, then relocating to an uncontaminated area.
Category 3: individuals relocating to the vicinity of the landfill at the age of 30, and remaining there for the rest of their 70-year life expectancy.
Category 4: infants under 2 years old.
Category 5: children and young adults aged between 2 and 20 years.
The International Agency for Research on Cancer (IARC) classifies agents in four categories of carcinogens [36]:
- Group 1: carcinogenic to humans, when there is sufficient evidence of carcinogenicity in humans and animals.
- Group 2A: probably carcinogenic to humans, when two of the following evaluations were made by the working group and at least one of them involves humans or human cells/tissues—there is limited evidence of carcinogenicity in humans, sufficient evidence of carcinogenicity in animals, and strong evidence that the agent exhibits key characteristics of carcinogens.
- Group 2B: possibly carcinogenic to humans, when only one evaluation was performed by the working group in conformity with the requirements under group 2A.
- Group 3: not classifiable as to its carcinogenicity to humans, when the agent may not be classified in the previous categories.
2.5. Equations for Calculating the Health Risk Level
2.5.1. Equations for Ingestion Exposure
Risk-based screening levels (RBSLs) represent general, non-site-specific thresholds for chemicals of concern, designed to safeguard human health for defined exposure routes [32]. Equation (1) was employed to determine RBSLs for non-carcinogenic PAHs in groundwater, specifically for exposure via the ingestion of contaminated water [32].
where denotes the target hazard quotient for individual constituents, is the oral chronic reference dose , represents body weight , refers to the averaging time for non-carcinogens , is the daily water intake rate , signifies the exposure frequency , and is the exposure duration .
The target hazard quotient for individual constituents was set as 1. The body weight was assumed to be a standard 80 kg for adults, whereas for young adults and infants, the body weight was estimated as follows:
- Infants—9 kg as an average, based on the World Health Organization (WHO) growth charts for boys and girls, with 50% confidence.
- Children and young adults—38 kg as an average, based on the Centers for Disease Control and Prevention (CDC) growth charts for boys and girls, with 50% confidence.
The outcomes for groundwater ingestion were presented using two quotients as follows
where is the chemical concentration in water .
The hazard quotient for groundwater ingestion was expressed as the ratio between the intake of the non-carcinogenic chemical and the oral reference dose, which is specific for each chemical. The dermal hazard quotient (DHQ) was expressed as a division between the dermally absorbed dose (DADw) and the absorbed reference dose (RfDABS). As a result, a hazard quotient lower than 1 indicates that the intake of chemicals (ingestion or dermal absorption) is lower than the dose generating negative effects, while a value equal to or higher than 1 indicates that significant health effects may be expected. The risk quotient for groundwater ingestion was expressed as the ratio between the existing concentration in groundwater and the risk-based screening levels (RBSLs) identified for each chemical. As a result, a risk quotient lower than 1 indicates that the groundwater contamination is below levels of concern, while a value equal to or higher than 1 indicates that a significant groundwater contamination occurred. An HQW or RQW value exceeding 1 signifies a considerable hazard or risk for the specified group of receptors. By adding all HQ and RQ values, the cumulative hazard and risk quotients were determined for each category of receptors, with a total value greater than 1 indicating a substantial health risk.
2.5.2. Equations for Dermal Contact Exposure
Equation (4) was employed to compute the anticipated dermally absorbed dose of PAHs in groundwater due to dermal contact exposure [37].
where represents the dermally absorbed dose , is the absorbed dose per event , denotes the event frequency , is the exposure duration , is the exposure frequency , is the skin surface area available for contact , is the body weight and is the averaging time .
When calculating DADW, leap years were excluded, so one year was assumed to consist of 365 days when applying the averaging time.
The absorbed dose per event was determined based on the event duration and the time required for the specific chemical to reach steady-state, using Equations (5) and (6) [38,39].
where represents the fraction of absorbed water, is the dermal permeability coefficient of the compound in water , is the chemical concentration in water , is the time lag per event , is the event duration , is the time required to reach steady-state and is a dimensionless ratio of the permeability coefficient of a compound through the stratum corneum compared to its permeability across the viable epidermis.
The time lag refers to the duration required during an event for the concentration across the skin to attain a linear gradient after exposure. It can be determined graphically by plotting the cumulative concentration penetrating the skin over time, with the time lag represented as the intercept on the time axis. Alternatively, it can be expressed analytically using Equation (6) [40].
where represent the skin thickness and is the diffusion coefficient of the chemical component in the skin .
While several models have been created to estimate the diffusion coefficient of substances through the skin [41], the most possible model was chosen, in which the skin is treated as a uniform membrane [42].
where is the skin-water partition coefficient.
The dermal permeability coefficient was determined using Equation (9) [43], applicable to chemicals with a molecular weight (MW) ranging from 18 to over 750 and a log Kow value between −3 and +6, calculated as Equation (8).
where is expressed in and is the molecular weight . Thus,
The thickness of human skin ranges from 1.5 mm in the thigh region to 2.5 mm in the suprascapular area, with greater values for males compared to females. It is composed of three layers: the dermis, the epidermis, and the stratum corneum [44]. The stratum corneum serves as the primary barrier, protecting the body from physical and chemical stressors while preventing an excessive loss of water. This thin layer, approximately 10–15 mm in thickness, is typically described as having a brick (corneocytes) and mortar (intercellular lipids) structure, with corneocytes acting as the bricks and intercellular lipids as the mortar. The key lipid components include ceramide, cholesterol, and fatty acids [45]. A relationship between the stratum corneum lipid phase/water partition coefficient () and the octanol/water partition coefficient () has been established, calculated as Equation (10) [46].
where and represent the Abraham solvation parameters, specifically the hydrogen bond acidity () and the hydrogen bond basicity (. In the literature, these parameters are commonly denoted as A and B, but Greek letters are used to avoid confusion with B in Equation (11). The Abraham solvation parameters for the chemicals of concern were obtained from the UFZ-LSER database [47].
The time to reach steady-state (t) refers to the duration required for a contaminant to enter and exit the skin at an equal rate, and it is influenced by the half-life (t1/2) of the specific chemical. A reliable estimate of t can be derived from Equation (11) [48]:
The dermal half-life was assumed to be 3 h for all chemicals, as indicated for benzo(a)pyrene in the toxicological profile of PAHs [49].
The skin area was calculated as a weighted average based on the mean values for both sexes at the 50th percentile, categorized by age group [50].
The dermal hazard quotient (DHQ) was calculated as the ratio of DADw to the absorbed reference dose (RfDABS). The RfDABS was derived from the oral reference dose (RfDo) specific to a particular chemical, adjusted by the fraction of the contaminant absorbed in the gastrointestinal tract (ABSGI), using Equation (12) [37]:
The values for the RfDO were sourced from the Risk Assessment Information System [51], except for phenanthrene, which was assumed to be the same as anthracene due to their comparable chemical structure. The ABSGI was set at 89%, as advised by the US Environmental Protection Agency for PAHs [37].
3. Results
3.1. PAH Concentrations in Groundwater near Vidra Landfill
Table 1 includes the PAHs detected in each sample along with their respective concentrations.

Table 1.
Concentrations of PAHs identified in groundwater near the analyzed landfill (n = 3).
Table 2 includes the relevant physical and chemical properties of the PAHs identified through laboratory analysis.

Table 2.
Physical and chemical properties of identified PAHs in the Vidra landfill.
After entering the groundwater, certain PAHs may either remain near the surface or settle at the bottom of the aquifer, depending on their density. Others may dissolve in the water, influenced by their octanol-water partition coefficient (Kow) and solubility. Additionally, some PAHs may volatilize from the water, driven by their Henry’s law constant (H) and solubility [33].
Based on the PAH concentrations recorded in the collected groundwater samples, the scenarios were developed considering dermal contact once per day during showering for 20 min and regular ingestion of Pf-, Phe-, Ant-, Flu-, and Pyr-contaminated groundwater at a rate of 2 L per day.
3.2. Health Risks for Receptors via Ingestion
The results of the quantitative risk assessment for exposure of the receptors through the ingestion of contaminated Vidra groundwater are included in Table 3. The HQW and RQW were expressed as maximum values based on the maximum concentration of contaminants recorded near each landfill.

Table 3.
Risk quotient for exposure to PAH-contaminated groundwater through ingestion.
As seen in Figure 3 and Figure 4, both the cumulative hazard and risk quotient are well below one, suggesting a significantly low risk for the ingestion of PAH-contaminated groundwater. Similar results were obtained by Ganiyu [15], which investigated the value of the hazard index for the PAH-contaminated groundwater in Ibadan, southwestern Nigeria.
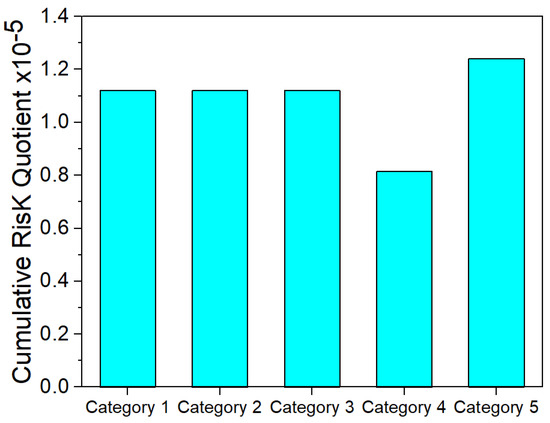
Figure 3.
The cumulative HQw for the ingestion of PAH-contaminated groundwater.
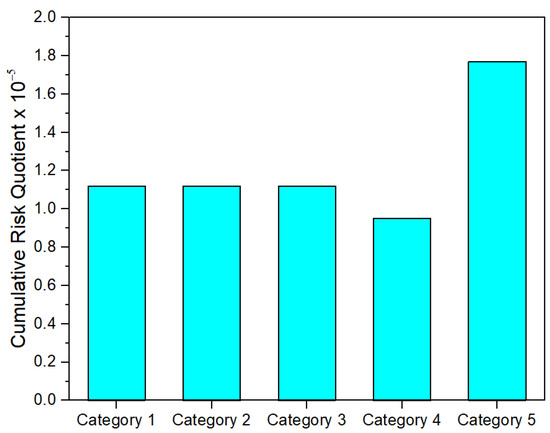
Figure 4.
The cumulative RQw for the ingestion of PAH-contaminated groundwater.
3.3. Health Risks for Receptors via Dermal Exposure
The results of the dermal exposure assessment are included in Table 4.

Table 4.
Results of the Dermal Exposure Assessment to PAH-Contaminated Groundwater.
The cumulative hazard quotient was shown in Figure 5 after using a multiplication factor of 107 to highlight the hazard quotient for the Vidra landfill.
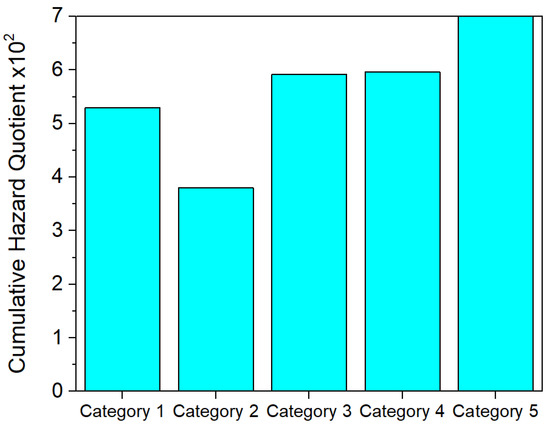
Figure 5.
The cumulative HQw for exposure of the receptors through dermal contact to PAH-contaminated groundwater.
The cumulative hazard quotient for dermal exposure to groundwater is greater in the Vidra region, primarily due to increased exposure of the receptors during showering, especially for children and young adults (category 5). No significant difference was observed between the five categories of receptors.
4. Discussion
There are more than 100 compounds classified as PAHs [56], although, from a toxicological point of view, only 16 PAHs are frequently monitored in Europe and the USA [57]. The PAHs with detectable concentrations in the Vidra groundwater samples were fluorene (Pf), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), and pyrene (Pyr). The rest of the PAHs were under the detection limit of the analytical instrument. Table 1 specifies the limit of detection, ranging from 0.0003 to 0.001 µg L−1. According to Ziyaei et al. [22], PAH pollution in water can be categorized as low pollution (0.010–0.050 µg L−1), slight pollution (0.050–0.250 µg L−1), moderate pollution (0.250–1 µg L−1), and severe pollution (>1 µg L−1). However, our results indicated very low levels of PAH pollution for groundwater samples collected from the Vidra landfill.
The physical and chemical properties in Table 2 were expressed only for the relevant compounds identified in the groundwater and only in the context of household activities (especially food preparation). Based on the physical and chemical properties of the PAHs included in Table 2, it can be concluded that the PAHs are highly hydrophobic and insoluble in water, given the value of the octanol-water partition coefficient. All the identified compounds have a higher density than water, except phenanthrene (Phe), so almost all the PAHs tend to accumulate at the base of the aquifer after reaching the groundwater. Also, the melting point of Phe indicates that it typically exists in a solid state in the natural environment but can transition to a liquid state when heated to around 100 °C. From a toxicological perspective, all compounds have a boiling point above 200 °C, indicating that boiling groundwater would not eliminate the contaminants if the groundwater is used for the preparation of food or hot beverages.
The exposure scenarios were developed starting from the exposed receptors. Since the source of potential contamination (i.e., the Vidra landfill) could not be assessed without the consent of the company operating the landfill, the source was considered a “black box”. As a result, the entire landfill was considered a source of contamination and not the specific activities conducted. All chemicals identified in the Vidra groundwater are classified as non-carcinogens. The approach is deemed relevant for exposure assessments in the context of environmental liabilities. The current exposure assessment should be regarded as the first screening step for establishing a correlation between the cause and effect (i.e., the source and the receptor).
Table 3 shows the results of the exposure to contaminated groundwater through ingestion. The body weight was considered the same for all categories (80 kg). The difference is in the exposure duration (ED), which is 70 years for category 1, 20 years for category 2, and 40 years for category 3. Since the contaminants are non-carcinogens, the averaging time for non-carcinogens (ATn) was considered equal to the exposure duration, meaning that their harmful impact stops when exposure stops. As a result, their division yields one, meaning that the exposure duration is irrelevant compared to the body weight (BW) and ingestion rate (IR), although their variation along the exposure time is relevant.
The results shown in Table 4 and Figure 5 indicated that the dermal contact is a more significant pathway for PAH exposure than ingestion. Another study reported an inherited lifetime carcinogenic risk (ILCR) of 1.72 × 10−5 [12], indicating a non-negligible potential threat from PAHs to the residents of Chengdu, China. However, although the cancer risks estimated were below the guideline values set by the United States Environmental Protection Agency (USEPA), the toxicity and carcinogenicity of many PAHs can still pose adverse biological effects on human health, even at lower concentrations [22]. A study by Ziyaei et al. [22] indicated that the highest cancer risk due to exposure to PAHs was identified in children and adults living near the Atlas Cove jetty (seawater) and various Nigerian rivers. Children are more susceptible to cancer compared to adults [15], and their increased vulnerability to environmental PAHs, particularly from dermal exposure to contaminated water, poses a significant public health concern.
There are several limitations of the study. For instance, the study does not consider all types of potential receptors around the landfill, such as potential allergies and conditions (e.g., liver or skin disease). However, most potential situations have been considered due to the wide range of age and exposure duration included in the exposure scenarios. There are no publicly available hydrogeological studies around the Vidra landfill. As a result, future potential exposure could not be addressed. If hydrogeological studies would be publicly available, they might represent a basis for the future potential exposure of receptors and they might provide information for also establishing the historical exposure of receptors. However, the presence of such contaminants even below the relevant risk levels should raise significant concerns and trigger additional investigations and studies. The public is reluctant to disclose any information and allow samples to be collected due to fear of repercussions from the landfill operators.
As the legislation in Romania does not contain a legal requirement on specific parameters which have to be analyzed around landfills, the current process should be regarded as a first screening phase for establishing the potential exposure of the public as follows: (1) exposure of receptors to harmful contaminants which are not monitored by the potential source of contamination around the receptors due to a lack of or light legal requirements, (2) areas where clean water cannot be provided by the authorities, public, or private companies for all households, and (3) areas where complaints from the public or private companies are expressed and which require expertise to establish the potential associated risk.
The study emphasized the need for better legislation in Romania and similar regions to regulate private wells accessing shallow aquifers. It recommended localized groundwater monitoring at receptor sites to detect overlooked contamination and urged the integration of monitoring results and risk assessments into urban and rural planning for sustainable development. Promoting alternatives to contaminated water sources (such as providing clean water), reducing coal consumption, strengthening regulations around industrial activities such as oil exploration, as well as educating local communities about the risks of PAH exposure would be vital in reducing cancer risk.
Future directions will envisage increasing the number of samples collected, sampling the samples at different times to ensure the objectivity and authenticity of data analysis, and assessing the source of contamination, the extent of contamination near the landfill, as well as the fate and transport of the contaminants.
5. Conclusions
Based on the findings of the current study, it can be concluded that there is no significant risk linked to the consumption of PAH-contaminated groundwater around the existing landfill near Bucharest city. However, the study does not indicate that the shallow groundwater is safe for human consumption or household use, owing to the potential presence of other chemical and biological contaminants.
The current study serves as an example of a general preliminary risk assessment, which should form the foundation for designing a more detailed groundwater risk evaluation to assess public health, particularly in areas where groundwater used by the population may be affected by human activities. Since the assessment primarily focused on the receptors rather than the contamination source, the extent of contamination near the landfill, as well as the fate and movement of the contaminants, remains unclear.
A future in-depth evaluation of the contamination source, along with a groundwater flow model, would provide clarity on whether higher concentrations are likely to reach the receptors in the future. Therefore, it is evident that an assessment focused solely on the receptors, without specific details about the contamination source, may only be valid for a particular moment in time, rather than offering a comprehensive understanding of the risks associated with groundwater contamination.
The study also highlighted the need for enhanced legislation in Romania and other countries facing similar situations, where private wells are used to supply groundwater from shallow aquifers. The groundwater sampling may be conducted based on a local groundwater monitoring program at the receptors’ location to identify any potential contamination that was not intercepted by source monitoring. Additionally, the legislation should take into account the findings from groundwater monitoring and risk assessments when planning urban and rural development for the future.
Author Contributions
Conceptualization, A.I.B.; Data Curation, M.R.; Formal Analysis, A.-A.Ș.; Funding Acquisition, E.M.; Investigation, A.I.B.; Methodology, A.I.B.; Resources, E.M. and I.M.M.; Supervision, E.M.; Validation, E.M. and I.M.M.; Writing—Original Draft, A.I.B.; Writing—Review & Editing, M.R., A.-A.Ș. and I.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request due to privacy.
Acknowledgments
Author Balint Alexandru Ioan thank the company Enviroland SRL of Romania for funding the research.
Conflicts of Interest
Author Balint Alexandru Ioan was employed by the company Enviroland SRL. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest Enviroland SRL of Romania for funding the research..
References
- Przydatek, G.; Kanownik, W. Impact of small municipal solid waste landfill on groundwater quality. Environ. Monit. Assess. 2019, 191, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tenodi, S.; Krcmar, D.; Agbaba, J.; Zrnic, K.; Radenovic, M.; Ubavin, D.; Dalmacija, B. Assessment of the environmental impact of sanitary and unsanitary parts of a municipal solid waste landfill. J. Environ. Manag. 2020, 258, 110019. [Google Scholar] [CrossRef]
- Mohammadi, S.; Lorestani, B.; Ardakani, S.; Cheraghi, M.; Sadr, M. Source identification and health risk assessment of PAHs in surface soils from the vicinity of Arad-Kouh processing and disposal complex, Tehran, Iran. Int. J. Environ. Anal. Chem. 2023, 103, 9647–9660. [Google Scholar] [CrossRef]
- Barathi, S.; Gitanjali, J.; Rathinasamy, G.; Sabapathi, N.; Aruljothi, K.N.; Lee, J.; Kandasamy, S. Recent trends in polycyclic aromatic hydrocarbons pollution distribution and counteracting bio-remediation strategies. Chemosphere 2023, 337, 139396. [Google Scholar] [CrossRef] [PubMed]
- Takam, P.; Schäffer, A.; Laovitthayanggoon, S.; Charerntantanakul, W.; Sillapawattana, P. Toxic effect of polycyclic aromatic hydrocarbons (PAHs) on co-culture model of human alveolar epithelial cells (A549) and macrophages (THP-1). Environ. Sci. Eur. 2024, 36, 176. [Google Scholar] [CrossRef]
- Saad-Hussein, A.; Beshir, S.; Shaheen, W.; Saleh, I.; Elhamshary, M.; Mohammed, A. Integrated evaluation of workplace exposures and biomarkers of bladder cancer among textile dyeing workers. J. Egypt. Public Health Assoc. 2024, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Buculei, I.; Dobrin, M.; Matei, D.; Onu, I.; Cioroiu, I.; Caba, B.; Postelnicu, M.; Buhociu, D.; Musat, C.; Crisan-Dabija, R.; et al. HPLC Analysis and Risk Assessment of 15 Priority PAHs in Human Blood Serum of COPD Patient from Urban and Rural Areas, Iasi (Romania). J. Pers. Med. 2023, 13, 1290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chang, S.; Zhang, Q.; Bai, Y.; Wang, E.; Fan, Y.; Tu, X.; Fu, Q.; Wei, L.; Yu, Y. Occurrence, source modeling, influencing factors and exposure assessment of polycyclic aromatic hydrocarbons in water sources: A mega-study from mainland China. Environ. Technol. Innov. 2024, 35, 103634. [Google Scholar] [CrossRef]
- Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names; The Royal Society of Chemistry: London, UK, 2013. [Google Scholar] [CrossRef]
- IUPAC. Compendium of chemical terminology. Int. Union Pure Appl. Chem. 2014, 528. [Google Scholar]
- You, Q.; Yan, K.; Yuan, Z.; Feng, D.; Wang, H.; Wu, L.; Xu, J. Polycyclic aromatic hydrocarbons (PAHs) pollution and risk assessment of soils at contaminated sites in China over the past two decades. J. Clean. Prod. 2024, 450, 141876. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Yang, Y.; Wang, R.; Liao, J.; Zhang, P.; Liu, L.; Zhao, Y.; Deng, Y. Distribution and source identification of polycyclic aromatic hydrocarbons (PAHs) with PCA-MLR and PMF methods in the topsoil of Chengdu at SW, China. Sci. Total Environ. 2024, 908, 168263. [Google Scholar] [CrossRef] [PubMed]
- Noro, K.; Omagari, R.; Ito, K.; Wang, Q.; Sei, K.; Miyake, Y.; Amagai, T. Sampling, pretreatment, instrumental analysis, and observed concentrations of polycyclic aromatic hydrocarbons, polychlorinated naphthalenes, and halogenated polycyclic aromatic hydrocarbons: A review. TrAC-Trends Anal. Chem. 2023, 169, 117384. [Google Scholar] [CrossRef]
- Carvalho, F.; Dantas, H.; Dantas, K. Simultaneous determination of 16 polycyclic aromatic hydrocarbons in groundwater by GC-FID after solid-phase extraction. SN Appl. Sci. 2019, 1, 804. [Google Scholar] [CrossRef]
- Ganiyu, S.; Komolafe, A.; Basheeru, K.; Lasisi, R.; Adeyemi, A. Levels, distribution, origins, and human health risk evaluation of polycyclic aromatic hydrocarbons in groundwater around a petroleum depot wastewater discharge point. Environ. Chem. Ecotoxicol. 2024, 6, 303–314. [Google Scholar] [CrossRef]
- Grimmer, G. Environmental Carcinogens–Polycyclic Aromatic Hydrocarbons: Chemistry, Occurrence, Biochemistry, Carcinogenicity; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Adlard, E.R. Amy, J. Forsgren (Ed): Wastewater Treatment: Occurrence and Fate of Polycyclic Aromatic Hydrocarbons (PAHs). Chromatographia 2018, 81, 729. [Google Scholar] [CrossRef][Green Version]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.; Anumol, T.; Barlaz, M.; Snyder, S. Investigating landfill leachate as a source of trace organic pollutants. Chemosphere 2015, 127, 269–275. [Google Scholar] [CrossRef]
- Eggen, T.; Moeder, M.; Arukwe, A. Municipal landfill leachates: A significant source for new and emerging pollutants. Sci. Total Environ. 2010, 408, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ma, H.; Shi, G.; He, L.; Wei, L.; Shi, Q. A review of groundwater contamination near municipal solid waste landfill sites in China. Sci. Total Environ. 2016, 569, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Ziyaei, K.; Mokhtari, M.; Hashemi, M.; Rezaei, K.; Abdi, F. Association between exposure to water sources contaminated with polycyclic aromatic hydrocarbons and cancer risk: A systematic review. Sci. Total Environ. 2024, 924, 171261. [Google Scholar] [CrossRef]
- Qu, X.; Niu, Q.; Sheng, C.; Xia, M.; Zhang, C.; Qu, X.; Yang, C. Co-toxicity and co-contamination remediation of polycyclic aromatic hydrocarbons and heavy metals: Research progress and future perspectives. Environ. Res. 2024, 263, 120211. [Google Scholar] [CrossRef] [PubMed]
- Mogashane, T.; Maree, J.; Mokoena, L. Adsorption of Polycyclic Aromatic Hydrocarbons from Wastewater Using Iron Oxide Nanomaterials Recovered from Acid Mine Water: A Review. Minerals 2024, 14, 826. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, L.; Moghaddam, T.; Chen, M.; Wu, S.; Yuan, X. Adsorption mechanism of polycyclic aromatic hydrocarbons using wood waste-derived biochar. J. Hazard. Mater. 2022, 425, 128003. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Mohammadi, M.; Mazaheri, Y.; Fallahizadeh, S.; Ghorbani, H.; Shokri, S.; Shariatifar, N.; Darroudi, M.; Shamloo, E. Employing a magnetic chitosan/molybdenum disulfide nanocomposite for efficiently removing polycyclic aromatic hydrocarbons from milk samples. Sci. Rep. 2024, 14, 15054. [Google Scholar] [CrossRef]
- Peluffo, M.; Rosso, J.; Morelli, I.; Mora, V. Strategies for oxidation of PAHs in aged contaminated soil by batch reactors. Ecotoxicol. Environ. Saf. 2018, 151, 76–82. [Google Scholar] [CrossRef]
- Rayaroth, M.; Marchel, M.; Boczkaj, G. Advanced oxidation processes for the removal of mono and polycyclic aromatic hydrocarbons—A review. Sci. Total Environ. 2023, 857, 159043. [Google Scholar] [CrossRef] [PubMed]
- Balint, A. Assessment of Human Exposure to Polycyclic Aromatic Hydrocarbons in Groundwater near Municipal Landfills. 2021. Available online: https://www.matec-conferences.org/articles/matecconf/pdf/2021/11/matecconf_simpro21_03016.pdf (accessed on 12 December 2024).
- Title 42 US Code of Federal Regulations Part 90. Available online: https://www.ecfr.gov/current/title-42/chapter-I/subchapter-H/part-90/section-90.2 (accessed on 11 December 2024).
- ISO/IEC 17025:2018; Testing and Calibration Laboratories. ISO: ISO: Geneva, Switzerland. Available online: https://www.asro.ro/sr-en-iso-170242018/ (accessed on 15 January 2025).
- ASTM-E2081-22; Standard Guide for Risk-Based Corrective Action. ASTM International: West Conshohocken, PA, USA, 2022.
- Balint, A. Geological and hydrogeological characterization of the landfill areas located around Bucharest city in the context of environmental management. MATEC Web Conf. 2021, 342, 03015. [Google Scholar] [CrossRef]
- Bandrabur, T.; Petrescu, I.; Enea, G. Hydrogeological Map of Romania, Scale 1:100,000, Sheet 44c Vidra; Geological Institute of Romania: Bucharest, Romania, 1970. [Google Scholar]
- Boukhemacha, M.A.; Gogu, R.; Serpescu, I.; Gaitanaru, S.; Bica, I.; Diaconescu, A.; Brusten, A. Hydraulic characterizing of tunnel’s barrier effect for groundwater flow modeling-application for Bucharest city. In Proceedings of the International Multidisciplinary Scientific GeoConference, Albena, Bulgaria, June 16–22 2013; Volume 2, p. 179. [Google Scholar]
- Plumb, R.H., Jr. The Occurrence of Appendix IX Organic Constituents in Disposal Site Ground Water. Groundwater Monitoring & Remediation. Groundw. Monit. Remediat. 1991, 11, 157–164. [Google Scholar] [CrossRef]
- USEPA. EPA/540/R/99/005 Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); Report EPA/540/R/99/005; United States Environmental Protection Agency: Washington, DC, USA, 2004; p. 20460.
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1979. [Google Scholar]
- Frasch, H.F.; Barbero, A.M. The transient dermal exposure: Theory and experimental examples using skin and silicone membranes. J. Pharm. Sci. 2008, 97, 1578–1592. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusion in and Through Solids; The University Press: Cambridge, UK, 1941. [Google Scholar]
- Mitragotri, S.; Anissimov, Y.; Bunge, A.; Frasch, H.; Guy, R.; Hadgraft, J.; Kasting, G.; Lane, M.; Roberts, M. Mathematical models of skin permeability: An overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef]
- Stein, W.D. Transport and Diffusion Across Cell Membranes; Academic Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Potts, R.; Guy, R. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Laurent, A.; Mistretta, F.; Bottigioli, D.; Dahel, K.; Goujon, C.; Nicolas, J.; Hennino, A.; Laurent, P. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 2007, 25, 6423–6430. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Nitsche, J.M.; Kasting, G.B. How Predictable Are Human Stratum Corneum Lipid/Water Partition Coefficients? Assessment and Useful Correlations for Dermal Absorption. J. Pharm. Sci. 2018, 107, 727–738. [Google Scholar] [CrossRef]
- Ulrich, N.; Endo, S.; Brown, T.N.; Watanabe, N.; Bronner, G.; Abraham, M.H.; Goss, K.U.; UFZ-LSER database v 3.2 [Internet]. 2017, Helmholtz Centre for Environmental Research-UFZ, Leipzig, Germany. Available online: http://www.ufz.de/lserd (accessed on 15 November 2024).
- Gupta, P.K. Fundamentals of Toxicology: Essential Concepts and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 1995.
- US EPA. Regional Screening Tables for Chemical Contaminants Ate Superfund Sites; US Environmental Protection Agency: Washington, DC, USA, 2011.
- The Risk Assessment Information System. RAIS Toxicity Values and Physical Parameters Search. Available online: https://rais.ornl.gov/index.html (accessed on 11 December 2024).
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 92, pp. 1–853. [Google Scholar] [PubMed] [PubMed Central]
- Ma, Y.-G.; Lei, Y.; Xiao, H.; Wania, F.; Wang, W.-H. Critical Review and Recommended Values for the Physical-Chemical Property Data of 15 Polycyclic Aromatic Hydrocarbons at 25 °C. J. Chem. Eng. Data 2009, 55, 819–825. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–315. [Google Scholar] [CrossRef]
- Lerda, D. Polycyclic Aromatic Hydrocarbons (PAHs) Factsheet, 4th ed.; Technical notes Joint Research Centre; European Commission: Brussels, Belgium, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).