Spatial Distribution Patterns, Eco-Environmental Risk Assessment, and Human Health Impacts of Uranium and Thorium in Beach Sediments in the Central Gulf of Gabes (Southern Mediterranean Sea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling, and Laboratory Analyses
2.2. Data Analysis
2.2.1. Environmental and Ecological Risk Assessment
2.2.2. Numerical Modeling
3. Results
3.1. U and Th Concentrations and Distribution
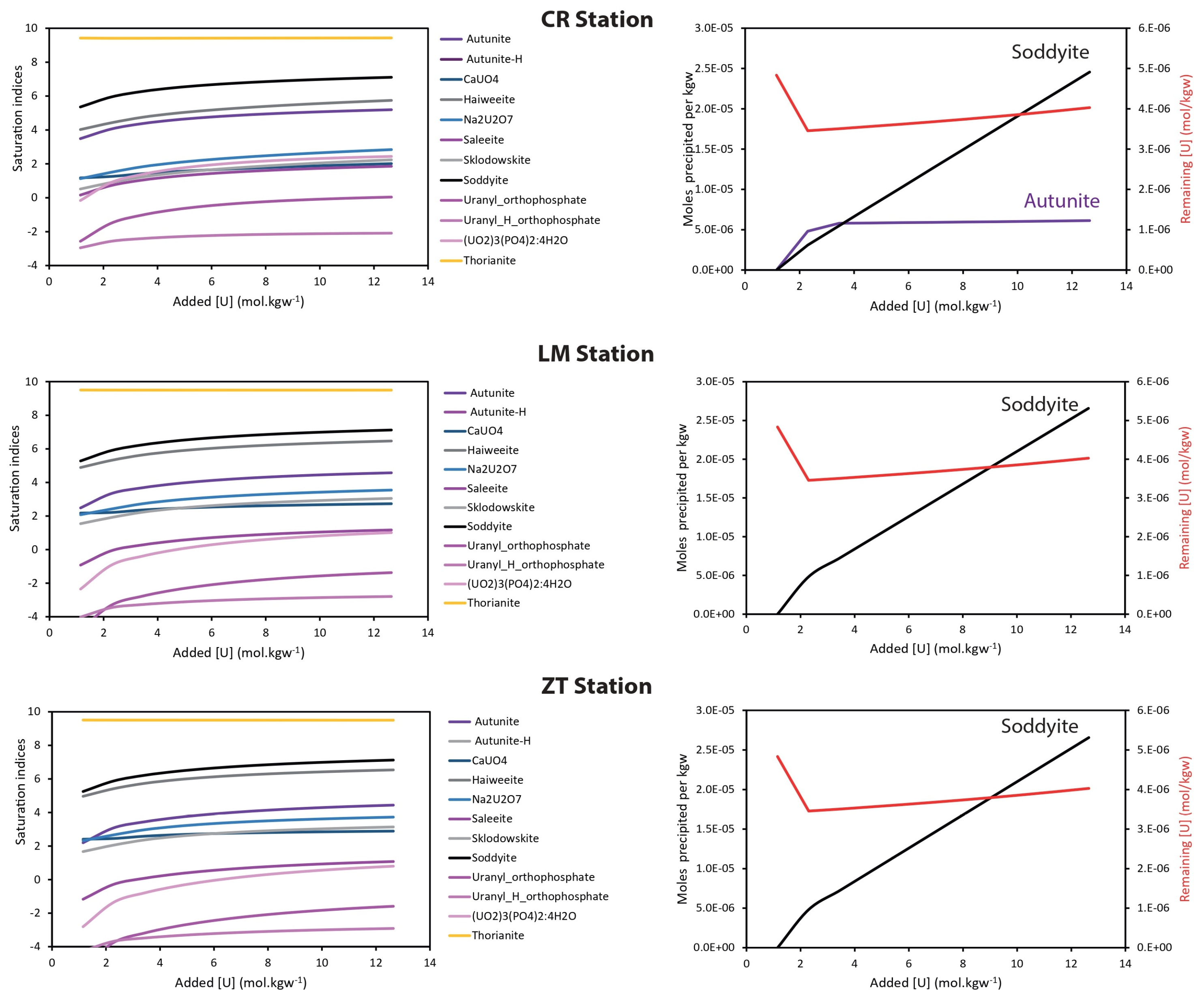
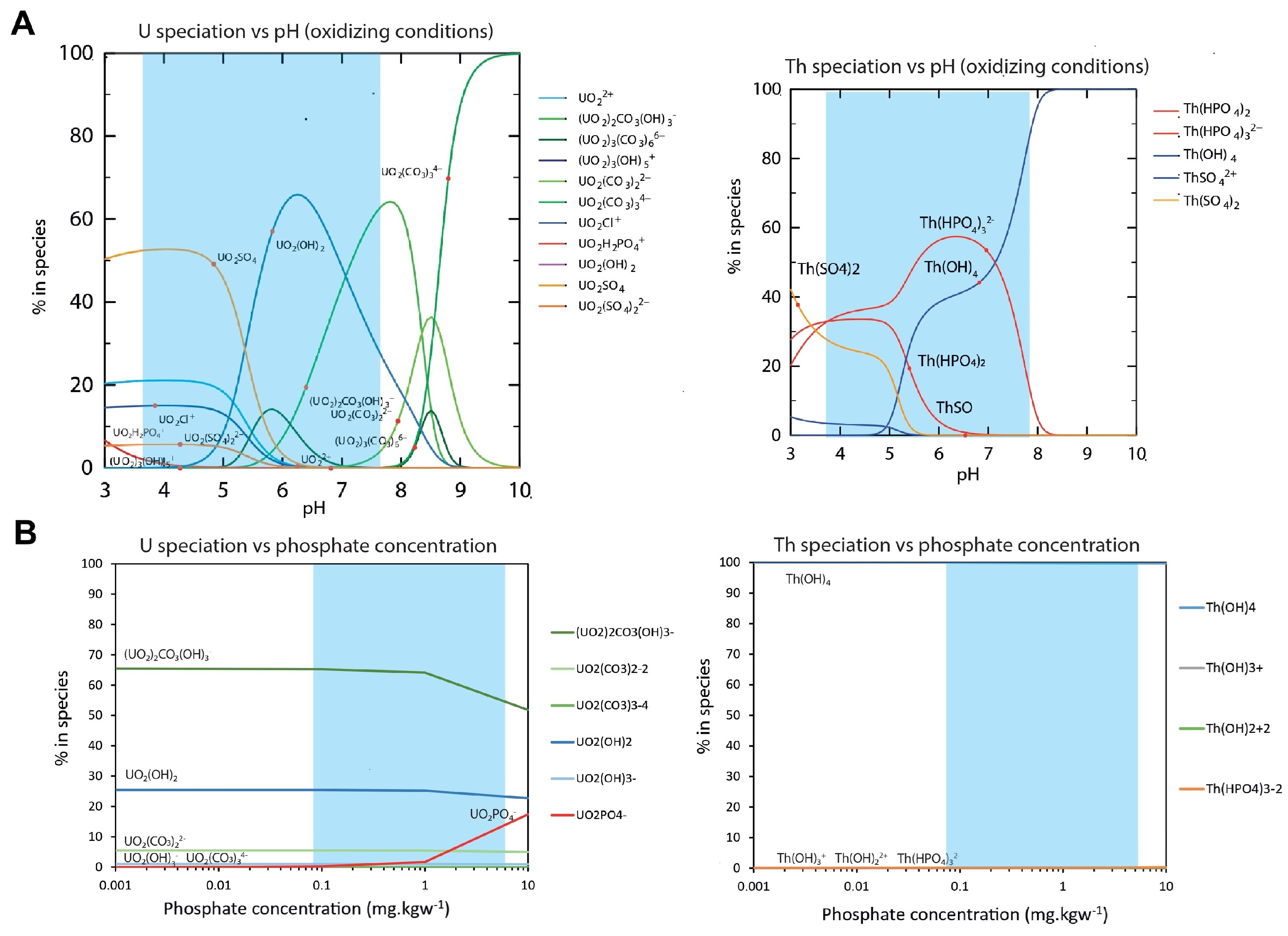
3.2. U and Th Behavior Explained by Geochemical Modeling
3.3. U and Th Fate in the Gulf of Gabes
3.4. Environmental and Ecological Risk Assessment of Coastal Sediment Contamination by U and Th
4. Discussion
4.1. Uranium and Thorium Geochemical Behaviors
4.2. Factors Influencing the Spatial Distribution of Uranium and Thorium
4.3. Environmental, Ecological, and Health Impacts of U and Th Contamination in Beach Sediments
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Indexes | Formulae/Description | Variables | Scales and Interpretation | References |
|---|---|---|---|---|
| Contamination factor (Cf) | Cf = Cs/Cb | • Cs: trace element concentration in the sample • Cb: trace element background concentration | • Cf < 1: low factor • 1 ≤ Cf < 3: moderate factor • 3 ≤ Cf < 6: considerable factor • Cf ≥ 6: very high factor | [130] |
| Pollution Load Index (PLI) | PLI = (Cf1 × Cf2 × Cfn)1/n | • Cf: contamination factor • n: number of trace elements analyzed | • PLI ≤ 1: non-polluted • PLI > 1: polluted | [131] |
| Geo-accumulation index (Igeo) | Igeo = log2 (Cs/1.5 × Cb) | • Cs: trace element concentration in the sample • Cb: trace element background concentration • 1.5: background matrix correction factor | • Igeo ≤ 0: uncontaminated • 0 < Igeo ≤1: uncontaminated to moderately contaminated • 1 < Igeo ≤ 2: moderately contaminated • 2 < Igeo ≤ 3: moderately to strongly contaminated • 3 < Igeo ≤ 4: strongly contaminated • 4 < Igeo ≤ 5: strongly to extremely contaminated • Igeo > 5: extremely contaminated | [132] |
| Potential Ecological Risk Index (PERI) | RI = ∑ Eir = ∑ Tir × Cif | • RI: sum of individual potential ecological risk for all trace elements • Eir: PERI of an individual trace element • Tir: toxic-response factor for a given trace element (TU = 40 [133]) • Cif: contamination factor | • RI < 150: low ecological risk • 150 ≤ RI < 300: moderate ecological risk • 300 ≤ RI < 600: considerable ecological risk • RI ≥ 600: very high ecological risk | [130] |
Appendix C
| Sectors | Stations (N → S) | pH | T (°C) | P (mg.kgw−1) | U (mg.kgw−1) * |
|---|---|---|---|---|---|
| Northern sector | ME | 7.84 | 26.7 | <0.14 | 1.11 |
| GH | 7.62 | 27.5 | 0.27 ± 0.01 | ||
| Central sector | LG | 3.61 | 31.2 | 5.61 ± 0.09 | |
| CE1 | 4.35 | 28.4 | 0.85 ± 0.14 | ||
| CE2 | |||||
| Southern sector | CR | 7.32 | 26.9 | 0.63 ± 0.12 | |
| LM | 7.89 | 25.4 | 0.36 ± 0.08 | ||
| ZT | 7.98 | 26.2 | 0.33 ± 0.10 |
Appendix D
| U | Th | TOC | FF | DILD | pH | P | |
|---|---|---|---|---|---|---|---|
| U | 1.00 | ||||||
| Th | 0.94 | 1.00 | |||||
| TOC | 0.81 | 0.91 | 1.00 | ||||
| FF | −0.15 | 0.08 | −0.11 | 1.00 | |||
| DILD | −0.52 | −0.29 | −0.36 | 0.85 | 1.00 | ||
| pH | −0.88 | −0.76 | −0.66 | 0.28 | 0.65 | 1.00 | |
| P | 0.76 | 0.87 | 0.99 | −0.14 | −0.37 | −0.67 | 1.00 |
| U | Th | TOC | FF | DILD | pH | P | |
|---|---|---|---|---|---|---|---|
| U | - | ||||||
| Th | 0.0006 | - | |||||
| TOC | 0.0144 | 0.0015 | - | ||||
| FF | 0.7186 | 0.8490 | 0.8399 | - | |||
| DILD | 0.1847 | 0.4831 | 0.3619 | 0.0080 | - | ||
| pH | 0.0037 | 0.0272 | 0.0675 | 0.5006 | 0.0786 | - | |
| P | 0.0236 | 0.0031 | 2.4 × 107 | 0.8304 | 0.3551 | 0.0685 | - |
Appendix E
| Calcium | 412.3 |
| Magnesium | 1241.8 |
| Sodium | 10,768.0 |
| Potassium | 399.1 |
| Iron | 0.002 |
| Manganese | 0.0002 |
| Silica as SiO2 | 4.28 |
| Chloride | 19,553.0 |
| Alkalinity as HCO3− | 141.682 |
| Sulfate as SO42− | 2712.0 |
| Nitrate as NO3− | 0.29 |
| Ammonium as NH4+ | 0.03 |
| Uranium | Var. |
| pH, standard units | 8.22 |
| pe, unitless | 8.451 |
| Temperature, °C | 25.0 |
| Density, kg/L | 1.023 |
| U+4 + 4 H2O = U(OH)4 + 4 H+ |
| log K = −8.538 |
| U+4 + 5 H2O = U(OH)5− + 5 H+ |
| log K = −13.147 |
| U+4 + 2 H2O = UO2+ + 4 H+ + e− |
| log K = −6.432 |
| U+4 + 2 H2O = UO2+2 + 4 H+ + 2 e− |
| log K = −9.217 |
| UO2+2 + H2O = UO2OH+ + H+ |
| log K = −5.782 |
| 2UO2+2 + 2H2O = (UO2)2(OH)2+2 + 2H+ |
| log K = −5.626 |
| 3UO2+2 + 5H2O = (UO2)3(OH)5+ + 5H+ |
| log K = −15.641 |
| UO2+2 + CO3−2 = UO2CO3 |
| log K = 10.064 |
| UO2+2 + 2CO3−2 = UO2(CO3)2−2 |
| log K = 16.977 |
| UO2+2 + 3CO3−2 = UO2(CO3)3−4 |
| log K = 21.397 |
| 2 HPO4−2 + 2 H+ + Th+4 = Th(H2PO4)2+2 |
| log K = 23.2070 |
| 2 HPO4−2 + Th+4 = Th(HPO4)2 |
| log K = 22.6 |
| 3 HPO4−2 + Th+4 = Th(HPO4)3−2 |
| log K = 31.1894 |
| 2 H2O + Th+4 = Th(OH)2+2 +2 H+ |
| log K = −7.1068 |
| 3 H2O + Th+4 = Th(OH)3+ +3 H+ |
| log K = −11.8623 |
| 4H2O + Th+4 = Th(OH)4 +4H+ |
| log K = −16.0315 |
| 2 SO4−2 + Th+4 = Th(SO4)2 |
| log K = 9.6170 |
| 3 SO4−2 + Th+4 = Th(SO4)3−2 |
| log K=10.4014 |
| 4 SO4−2 + Th+4 = Th(SO4)4−4 |
| log K=8.400 |
| 2 Th+4 + 2 H2O = Th2(OH)2+6 +2 H+ |
| log K = −6.4618 |
| 8 H2O + 4 Th+4 = Th4(OH)8+8 +8 H+ |
| log K = −21.7568 |
| 15 H2O + 6Th+4 = Th6(OH)15+9 +15 H+ |
| log K = −37.7027 |
| Th+4 + Cl− = ThCl+3 |
| log K = 0.9536 |
| 2 Cl− + Th+4 = ThCl2+2 |
| log K = 0.6758 |
| 3 Cl− + Th+4 = ThCl3+ |
| log K =1.4975 |
| 4 Cl− + Th+4 = ThCl4 |
| log K = 1.0731 |
| Th+4 + F− = ThF+3 |
| log K = 7.8725 |
| 2F− + Th+4 = ThF2+2 |
| log K = 14.0884 |
| 3F− + Th+4 = ThF3+ |
| log K = 18.7357 |
| 4 F− + Th+4 = ThF4 |
| log K = 22.1515 |
| Th+4 + HPO4−2 + H+ = ThH2PO4+3 |
| log K = 11.7061 |
| 2H+ + Th+4 + HPO4−2 = ThH3PO4+4 |
| log K = 11.1197 |
| Th+4 + HPO4−2 = ThHPO4+2 |
| log K = 10.6799 |
| Th+4 + H2O = ThOH+3 +H+ |
| log K = −3.8871 |
| Th+4 + SO4−2 = ThSO4+2 |
| log K = 5.3143 |
| Uraninite |
| UO2 + 4 H+ = U+4 + 2 H2O |
| log K = −3.490 |
| Autunite |
| Ca(UO2)2(PO4)2(H2O)3 = Ca+2 + 2UO2+2 + 2PO4−3 + 3H2O |
| log K = −48.36 |
| Uranyl_H_orthophosphate |
| UO2HPO4(H2O)3 = UO2+2 + HPO4−2 + 3H2O |
| log K = −13.17 |
| Uranyl_orthophosphate |
| (UO2)3(PO4)2(H2O)4 = 3UO2+2 + 2PO4−3 + 4H2O |
| log K = −49.36 |
| Thorianite |
| ThO2 +4 H+ = Th+4 + 2 H2O |
| log K =1.8624 |
References
- El Zrelli, R. Metallic Trace Element Transfer Modalities in the Central Part of Gabes Gulf, Tunisia: A Geochemical, Mineralogical, Sedimentological, and Biological Approach. Ph.D. Dissertation, University of Toulouse III-Paul Sabatier, Toulouse, France, 2017. [Google Scholar]
- Chaalal, O.; Madhuranthakam, C.M.R.; Moussa, B.; Hossain, M.M. Sustainable Approach for Recovery of Sulfur from Phophogypsum. ACS Omega 2020, 5, 8151–8157. [Google Scholar] [CrossRef] [PubMed]
- Arhouni, F.E.; Hakkar, M.; Ouakkas, S.; Haneklaus, N.; Boukhair, A.; Nourreddine, A.; Benjelloun, M. Evaluation of the physicochemical, heavy metal and radiological contamination from phosphogypsum discharges of the phosphoric acid production unit on the coast of El Jadida Province in Morocco. J. Radioanal. Nucl. Chem. 2023, 332, 4019–4028. [Google Scholar] [CrossRef]
- Cheggour, M.; Langston, W.J.; Chafik, A.; Texier, H.; Idrissi, H.; Boumezzough, A. Phosphate industry discharges and their impact on metal contamination and intertidal macrobenthos: Jorf Lasfar and Safi coastline (Morocco). Toxicol. Environ. Chem. 1999, 70, 159–179. [Google Scholar] [CrossRef]
- Fakhri, M.; Abboud-Abi Saab, M.; Romano, J.-C. The use of sediments to assess the impact of Selaata phosphate plant on Batroun coastal area (Lebanon, Levantine Basin). Leban. Sci. J. 2008, 9, 29–42. [Google Scholar]
- El Zrelli, R.; Rabaoui, L.; Daghbouj, N.; Abda, H.; Castet, S.; Josse, C.; van Beek, P.; Souhaut, M.; Michel, S.; Bejaoui, N.; et al. Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories (SE Tunisia): High mining potential and implications for environmental protection, Environ. Sci. Pollut. Res. 2018, 25, 14690–14702. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Samek, R.A. Environmental impacts of phosphogypsum. Sci. Total Environ. 1994, 149, 1–38. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Arocena, J.M. Radioactivity and elemental composition of phosphogypsum produced from three phosphate rock sources. Waste Manag. Res. 1995, 13, 407–423. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Arocena, J.M. Heterogeneous distribution of radionuclides, barium and strontium in phosphogypsum by-product. Sci. Total Environ. 1996, 180, 201–209. [Google Scholar] [CrossRef]
- Papastefanou, C.; Stoulos, S.; Ioannidou, A.; Manolopoulou, M. The application of phosphogypsum in agriculture and the radiological impact. J. Environ. Radioact. 2006, 89, 188–198. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolivar, J.P.; Santisteban, M. Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Appl. Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Van Beek, P.; Castet, S.; Souhaut, M.; Grégoire, M.; Courjault-Radé, P. Natural radioactivity and radiation hazard assessment of industrial wastes from the coastal phosphate treatment plants of Gabes (Tunisia, Southern Mediterranean Sea). Mar. Pollut. Bull. 2019, 146, 454–461. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.; Baliteau, J.Y.; Yacoubi, L.; Castet, S.; Grégoire, M.; Fabre, S.; Sarazin, V.; Daconceicao, L.; Courjault-Radé, P.; Rabaoui, L. Rare earth elements characterization associated to the phosphate fertilizer plants of Gabes (Tunisia, Central Mediterranean Sea): Geochemical properties and behavior, related economic losses, and potential hazards. Sci. Total Environ. 2021, 791, 148268. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Oliveira, M.L.S.; Crissien, T.J.; Santosh, M.; Bolivar, J.; Shao, L.; Dotto, G.L.; Gasparotto, G.; Schindler, M. A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere 2022, 286, 131513. [Google Scholar] [CrossRef] [PubMed]
- Zairi, M.; Rouis, M.J. Impacts environnementaux du stockage du phosphogypse à Sfax (Tunisie). Bull. Lab. Ponts Chaussées 1999, 219, 29–40. [Google Scholar]
- Jalali, J.; Gaudin, P.; Capiaux, H.; Ammar, E.; Lebeau, T. Fate and transport of metal trace elements from phosphogypsum piles in Tunisia and their impact on soil bacteria and wild plants. Ecotoxicol. Environ. Saf. 2019, 174, 12–25. [Google Scholar] [CrossRef]
- Ben Garali, A.; Salah, S.; Henchiri, M.; Srarfi, F. Assessment of heavy metals contamination/pollution of phosphogypsum waste of the Mdhilla region (Gafsa, southern Tunisia). Environ. Monit. Assess. 2024, 196, 1204. [Google Scholar] [CrossRef]
- Pérez-López, R.; Macías, F.; Cánovas, C.R.; Sarmiento, A.M.; Pérez-Moreno, S.M. Pollutant flows from a phosphogypsum disposal area to an estuarine environment: An insight from geochemical signatures. Sci. Total Environ. 2016, 553, 42–51. [Google Scholar] [CrossRef]
- González, F. InSAR-based mapping of ground deformation caused by industrial waste disposals: The case study of the Huelva phosphogypsum stack, SW Spain. Bull. Eng. Geol. Environ. 2022, 81, 304. [Google Scholar] [CrossRef]
- Pérez-López, R.; Álvarez-Valero, A.M.; Nieto, J.M. Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelva (SW Spain) and environmental impact of phosphogypsum wastes. J. Hazard. Mater. 2007, 148, 745–750. [Google Scholar] [CrossRef]
- Zou, C.; Shi, Z.; Yang, Y.; Zhang, J.; Hou, Y.; Zhang, N. The Characteristics, Enrichment, and Migration Mechanism of Cadmium in Phosphate Rock and Phosphogypsum of the Qingping Phosphate Deposit, Southwest China. Minerals 2023, 13, 107. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, A.; Duan, H.; Zhang, H.; Yang, J. Status and development trends of phosphogypsum utilization in China. Circ. Econ. 2024, 3, 100116. [Google Scholar] [CrossRef]
- Ke, H.; Zheng, S.N.; Zhang, P.Z.; Xiao, B.; Lan, J.W.; Zhang, S.; Hu, J. Leaching behavior and release mechanism of pollutants from different depths in a phosphogypsum stockpile. Waste Manag. 2024, 189, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.D.; Burnett, W.C. Radiochemistry of Florida phosphogypsum. J. Environ. Radioact. 1996, 32, 213–238. [Google Scholar] [CrossRef]
- Adeoye, C.; Gupta, J.; Demers, N.; Adhikari, A. Variations of radon and airborne particulate matter near three large phosphogypsum stacks in Florida. Environ. Monit. Assess. 2021, 193, 284. [Google Scholar] [CrossRef]
- Burnett, W.C.; Elzerman, A.W. Nuclide migration and the environmental radiochemistry of Florida phosphogypsum. J. Environ. Radioact. 2001, 54, 27–51. [Google Scholar] [CrossRef]
- Mazzilli, B.; Palmiro, V.; Saueia, C.; Nisti, M.B. Radiochemical characterization of Brazilian phosphogypsum. J. Environ. Radioact. 2000, 49, 113–122. [Google Scholar] [CrossRef]
- Borges, R.C.; Ribeiro, F.C.A.; Lauria, D.d.C.; Bernedo, A.V.B. Radioactive characterization of phosphogypsum from Imbituba, Brazil. J. Environ. Radioact. 2013, 126, 188–195. [Google Scholar] [CrossRef]
- Reis, R.G.; Lauria, D.C. The potential radiological impact from a Brazilian phosphate facility. J. Environ. Radioact. 2014, 136, 188–194. [Google Scholar] [CrossRef]
- Hallin, I.L.; Naeth, M.A.; Chanasyk, D.S.; Nichol, C.K. Assessment of a Reclamation Cover System for Phosphogypsum Stacks in Central Alberta, Canada. J. Environ. Qual. 2010, 39, 2160–2169. [Google Scholar] [CrossRef]
- Turner, L.E.; Dhar, A.; Naeth, M.A.; Chanasyk, D.S.; Nichol, C.K. Effect of soil capping depth on phosphogypsum stack revegetation. Environ. Sci. Pollut. Res. 2022, 29, 50166–50176. [Google Scholar] [CrossRef]
- Robinson, M.J.C.; Dhar, A.; Naeth, M.A.; Nichol, C.K. Phosphogypsum impacts on soil chemical properties and vegetation tissue following reclamation. Environ. Monit. Assess. 2023, 195, 769. [Google Scholar] [CrossRef] [PubMed]
- Msila, X.; Labuschagne, F.; Barnard, W.; Billing, D.G. Radioactive nuclides in phosphogypsum from the lowveld region of South Africa. S. Afr. J. Sci. 2016, 112, 5. [Google Scholar] [CrossRef] [PubMed]
- Wildenboer, R.A.; Sandenbergh, R.F. Extraction of Rare Earth Elements from Phalaborwa phosphogypsum. J. S. Afr. Inst. Min. Metall. 2024, 124, 575–582. [Google Scholar] [CrossRef]
- Louw, I. Potential radiological impact of the phosphate industry in South Africa on the public and the environment (Paper 1). J. Environ. Radioact. 2020, 217, 106214. [Google Scholar] [CrossRef]
- Lysandrou, M.; Pashalidis, I. Uranium chemistry in stack solutions and leachates of phosphogypsum disposed at a coastal area in Cyprus. J. Environ. Radioact. 2008, 99, 359–366. [Google Scholar] [CrossRef]
- Lysandrou, M.; Charalambides, A.; Pashalidis, I. Radon emanation from phosphogypsum and related mineral samples in Cyprus. Radiat. Meas. 2007, 42, 1583–1585. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, P. Radio-environmental impacts and uranium radiochemistry of phosphogypsum disposed at a coastal area in Cyprus. In Proceedings of the 4th International Conference on Sustainable Solid Waste Management 2016, Limassol, Cyprus, 23–25 June 2016. [Google Scholar]
- Al-Hwaiti, M.S.; Ranville, J.F. Distribution of potentially toxic metal and radionuclide contamination in soils related to phosphogypsum waste stockpiling in the Eshidiya Mine, Jordan. Geochem. Explor. Environ. Anal. 2010, 10, 419–433. [Google Scholar] [CrossRef]
- Al-Hwaiti, M.S.; Ranville, J.F.; Ross, P.E. Bioavailability and mobility of trace metals in phosphogypsum from Aqaba and Eshidiya, Jordan. Geochemistry 2010, 70, 283–291. [Google Scholar] [CrossRef]
- Zielinski, R.A.; Al-Hwaiti, M.S.; Budahn, J.R.; Ranville, J.F. Radionuclides, trace elements, and radium residence in phosphogypsum of Jordan. Environ. Geochem. Health 2011, 33, 149–165. [Google Scholar] [CrossRef]
- El Zrelli, R.; Yacoubi, L.; Castet, S.; Grégoire, M.; Josse, C.; Olive, J.-F.; Courjault-Radé, P.; van Beek, P.; Zambardi, T.; Souhaut, M.; et al. PET plastics as a Trojan horse for radionuclides. J. Hazard. Mater. 2023, 441, 129886. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Abda, H.; Daghbouj, N.; Pérez-López, R.; Castet, S.; Aigouy, T.; Bejaoui, N.; Courjault-Radé, P. Characterization of the role of phosphogypsum foam in the transport of metals and radionuclides in the Southern Mediterranean Sea. J. Hazard. Mater. 2019, 363, 258–267. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.; Fabre, S.; Castet, S.; Grégoire, M.; Fersi, O.; Josse, C.; Cousin, A.-M.; Courjault-Radé, P. Unveiling the organic nature of phosphogypsum foam: Insights into formation dynamics, pollution load, and contribution to marine pollution in the Southern Mediterranean Sea. J. Hazard. Mater. 2024, 480, 135732. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.; Rabaoui, L.; Ben Alaya, M.; Daghbouj, N.; Castet, S.; Besson, P.; Michel, S.; Bejaoui, N.; Courjault-Radé, P. Seawater quality assessment and identification of pollution sources along the central coastal area of Gabes Gulf (SE Tunisia): Evidence of industrial impact and implications for marine environment protection. Mar. Pollut. Bull. 2018, 127, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Darmoul, B.; Vitiello, P. Recherches expérimentales sur la toxicité aiguë des rejets de phosphogypse sur quelques organismes benthiques marins. Bull. Inst. Nat. Sci. Technol. Mer. Salammbô 1980, 7, 63–89. [Google Scholar]
- Darmoul, B.; Hadj Ali Salem, M.; Vitiello, P. Effets des rejets industriels de la région de Gabès (Tunisie) sur le milieu récepteur. Bull. Inst. Nat. Sci. Technol. Mer. Salammbô 1980, 7, 5–61. [Google Scholar]
- Darmoul, B. Pollution dans le Golfe de Gabès (Tunisie): Bilan des six années de surveillance (1976–1981). Bull. Inst. Nat. Sci. Technol. Mer. Salammbô 1988, 15, 61–84. [Google Scholar]
- Soussi, N.; Ben Mammou, A. Les rejets de phosphogypse dans le Golfe de Gabès et leur impact sur l’environnement marin. Rapp. Comm. Int. Mer. Médit. 1992, 33. Available online: https://www.ciesm.org/online/archives/abstracts/pdf/33/CIESM_Congress_1992_Trieste_article_l_0150.pdf (accessed on 3 October 2024).
- Guillaumont, B.; Ben Mustapha, S.; Ben Moussa, H.; Zaouali, J.; Soussi, N.; Ben Mammou, A.; Cariou, C. Pollution impact study in Gabes Gulf (Tunisia) using remote sensing data. Mar. Technol. Soc. J. 1995, 29, 46–58. [Google Scholar]
- Ben Mammou, A.; Soussi, N.; Added, A. Répartition et évolution du phosphogypse dans le Golfe de Gabès. Rev. Méditerr.Environ. 2009, 3, 544–555. [Google Scholar]
- El Zrelli, R.; Courjault-Radé, P.; Rabaoui, L.; Daghbouj, N.; Mansour, L.; Balti, R.; Castet, S.; Attia, F.; Michel, S.; Bejaoui, N. Biomonitoring of coastal pollution in the Gulf of Gabes (SE. Tunisia): Use of Posidonia oceanica seagrass as a bioindicator and its mat as an archive of coastal metallic contamination. Environ. Sci. Pollut. Res. 2017, 24, 22214–22225. [Google Scholar] [CrossRef]
- El Kateb, A.; Stalder, C.; Rüggeberg, A.; Neururer, C.; Spangenberg, J.E.; Spezzaferri, S. Impact of industrial phosphate waste discharge on the marine environment in the Gulf of Gabes (Tunisia). PLoS ONE 2018, 13, e0197731. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.; Yacoubi, L.; Castet, S.; Grégoire, M.; Lin, Y.-J.; Attia, F.; Ayranci, K.; Abdel Baki, Z.; Courjault-Radé, P.; Rabaoui, L. Compartmentation of trace metals in Cymodocea nodosa from a heavily polluted area (Central Gulf of Gabes; Southern Mediterranean Sea): Potential use of the seagrass as environmental monitoring and bioremediation tool. Reg. Study Mar. Sci. 2023, 65, 103056. [Google Scholar] [CrossRef]
- Bejaoui, B.; Raïs, S.; Koutitonsky, V. Modélisation de la dispersion du phosphogypse dans le golfe de Gabès. Bull. Inst. Nat. Sci. Technol. Mer. Salammbô 2004, 31, 103–109. [Google Scholar]
- Rabaoui, L.; El Zrelli, R.; Ben Mansour, M.; Balti, R.; Mansour, L.; Tlig-Zouari, S.; Guerfel, M. On the relationship between the diversity and structure of benthic macroinvertebrate communities and sediment enrichment with heavy metals in Gabes gulf Tunisia. J. Mar. Biol. Assoc. UK 2015, 95, 233–245. [Google Scholar] [CrossRef]
- Rabaoui, L.; El Zrelli, R.; Balti, R.; Mansour, L.; Courjault-Radé, P.; Daghbouj, N.; Tlig Zouari, S. Metal bioaccumulation in two edible cephalopods in the Gulf of Gabes, south-eastern Tunisia: Environmental and human health risk assessment. Environ. Sci. Pollut. Res. 2017, 24, 1686–1699. [Google Scholar] [CrossRef]
- El Kateb, A.; Stalder, C.; Neururer, C.; Pisapia, C.; Spezzaferri, S. Correlation between pollution and decline of Scleractinian Cladocora caespitosa (Linnaeus. 1758) in the Gulf of Gabes. Heliyon 2016, 2, e00195. [Google Scholar] [CrossRef]
- El Kateb, A.; Stalder, C.; Martínez-Colón, M.; Mateu-Vicens, G.; Francescangeli, F.; Coletti, G.; Stainbank, S.; Spezzaferri, S. Foraminiferal-based biotic indices to assess the ecological quality status of the Gulf of Gabes (Tunisia): Present limitations and future perspectives. Ecol. Indic. 2020, 111, 105962. [Google Scholar] [CrossRef]
- Oudi, A.; Chokri, M.A.; Hammouda, A.; Chaabane, R.; Badraoui, R.; Besnard, A.; Santos, R. Physiological impacts of pollution exposure in seabird’s progeny nesting in a Mediterranean contaminated area. Mar. Pollut. Bull. 2019, 142, 196–205. [Google Scholar] [CrossRef]
- Ghemari, C.; Waterlot, C.; Ayari, A.; Douay, F.; Nasri-Ammar, K. Bioaccumulation of heavy metals in the terrestrial isopod Porcellionides pruinosus in the vicinity of Gabes-Ghannouch industrial complex. Hum. Ecol. Risk Assess. Int. J. 2019, 26, 1270–1284. [Google Scholar] [CrossRef]
- Hammouda, A.; Ayadi, T.; Selmi, S. Long-term Exposure to Industrial Chemical Contamination Affects the Magnitude of Predator-induced Immunosuppression in a Free-living Passerine. Bull. Environ. Contam. Toxicol. 2024, 112, 42. [Google Scholar] [CrossRef]
- Hattab, S.; Boughattas, I.; Cappello, T.; Zitouni, N.; Touil, G.; Romdhani, I.; Livet, A.; Bousserrhine, N.; Banni, M. Heavy metal accumulation, biochemical and transcriptomic biomarkers in earthworms Eisenia andrei exposed to industrially contaminated soils from south-eastern Tunisia (Gabes Governorate). Sci. Total Environ. 2023, 887, 163950. [Google Scholar] [CrossRef] [PubMed]
- Nasri, I.; Hammouda, A.; Hamza, F.; Zrig, A.; Selmi, S. Heavy metal accumulation in lizards living near a phosphate treatment plant: Possible transfer of contaminants from aquatic to terrestrial food webs. Environ. Sci. Pollut. Res. 2017, 24, 12009–12014. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, D.; Annabi-Trabelsi, N.; Karam, Q.; Ali, M.; Ayadi, H. Assessment of metallic pollution in the waters, suspended particulate matter, and surface sediments of the central coastal area of the Gulf of Gabès, Mediterranean Sea. J. Mater. Environ. Sci. 2021, 12, 584–594. [Google Scholar]
- El Zrelli, R.; Courjault-Radé, P.; Rabaoui, L.; Castet, S.; Michel, S.; Bejaoui, N. Heavy metal contamination and ecological risk assessment in the surface sediments of the coastal area surrounding the industrial complex of Gabes city Gulf of Gabes, SE Tunisia. Mar. Pollut. Bull. 2015, 101, 922–929. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Ben Alaya, M.; Castet, S.; Zouiten, C.; Bejaoui, N.; Courjault-Radé, P. Decadal effects of solid industrial wastes on the coast: Gulf of Gabes (Tunisia, Southern Mediterranean Sea) as an example. Estuar. Coast. Shelf Sci. 2019, 224, 281–288. [Google Scholar] [CrossRef]
- Gargouri, D.; Gzam, M.; Kharroubi, A.; Jedoui, Y. Use of sediment quality indicators for heavy metals contamination and ecological risk assessment in urbanized coastal zones. Environ. Earth Sci. 2018, 77, 381. [Google Scholar] [CrossRef]
- Mansouri, B.; Gzam, M.; Souid, F.; Telahigue, F.; Chahlaoui, A.; Ouarrak, K.; Kharroubi, A. Assessment of heavy metal contamination in Gulf of Gabès coastland (south-eastern Tunisia): Impact of chemical industries and drift currents. Arab. J. Geosci. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Zaouali, J. Les peuplements benthiques de la petite Syrte, golfe de Gabès-Tunisie. Résultats de la campagne de prospection du mois de juillet 1996. Étude préliminaire: Biocénoses et thanatocénoses récentes. Mar. Life 1993, 3, 47–60. [Google Scholar]
- El Zrelli, R.; Rabaoui, L.; Roa-Ureta, R.H.; Gallai, N.; Castet, S.; Grégoire, M.; Bejaoui, N.; Courjault-Radé, P. Economic impact of human-induced shrinkage of Posidonia oceanica meadows on coastal fisheries in the Gabes Gulf (Tunisia, Southern Mediterranean Sea). Mar. Pollut. Bull. 2020, 155, 111124. [Google Scholar] [CrossRef]
- El Zrelli, R.; Hcine, A.; Yacoubi, L.; Roa-Ureta, R.H.; Gallai, N.; Castet, S.; Grégoire, M.; Courjault-Radé, P.; Rabaoui, L. Economic losses related to the reduction of Posidonia ecosystem services in the Gulf of Gabes (Southern Mediterranean Sea). Mar. Pollut. Bull. 2023, 186, 114418. [Google Scholar] [CrossRef]
- Crocetta, F.; Agius, D.; Balistreri, P.; Bariche, M.; Bayhan, Y.; Çakir, M.; Ciriaco, S.; Corsini-Foka, M.; Deidun, A.; EL Zrelli, R.; et al. New Mediterranean Biodiversity Records (October 2015). Mediterr. Mar. Sci. 2015, 16, 682–702. [Google Scholar] [CrossRef]
- Rabaoui, L.; Arculeo, M.; Mansour, L.; Tlig-Zouari, S. Occurrence of lepsessian Portunus segnis (Crustacea: Decapoda) in the Gulf of Gabes (Tunisia): First record and new information on its biology and ecology. Cah. Biol. Mar. 2015, 56, 159–175. [Google Scholar]
- Shaiek, M.; El Zrelli, R.; Crocetta, F.; Rabaoui, L. On the occurrence of three exotic decapods, Callinectes sapidus (Portunidae), Portunus segnis (Portunidae), and Trachysalambria palaestinensis (Penaeidae), in northern Tunisia, with updates on the distribution of the two invasive portunids in the Mediterranean Sea. BioInvasions Rec. 2021, 1, 158–169. [Google Scholar] [CrossRef]
- EL Zrelli, R.; Mansour, L.; Crocetta, F.; Rabaoui, L. The macroalgae Lophocladia lallemandii and Sarconema filiforme and the spaghetti bryozoan amathia verticillate in native seagrass beds in the Gulf of Gabes (Southeastern Tunisia, Mediterranean Sea). BioInvasions Rec. 2021, 10, 103–108. [Google Scholar] [CrossRef]
- Kousteni, V.; Anastasiadis, A.; Bariche, M.; Battaglia, P.; Bonifazi, A.; Ćetković, I.; Chimienti, G.; Colombo, M.; Constantinou, C.; Corsini-Foka, M.; et al. New records of rare species in the Mediterranean Sea (May 2022). Mediterr. Mar. Sci. 2022, 23, 417–446. [Google Scholar]
- Jabeur, C.; Gobert, B.; Missaoui, H. Typology of the small-scale fishing fleet in the Gulf of Gabès (Tunisia). Aquat. Living Resour. 2000, 13, 421–428. [Google Scholar] [CrossRef]
- Papaconstantinou, C.; Farrugio, H. Fisheries in the Mediterranean. Mediterr. Mar. Sci. 2000, 1, 5–18. [Google Scholar] [CrossRef]
- Hattab, T.; Ben Rais Lasram, F.; Sammari, C. Modélisation de l’habitat des ressources halieutiques dans le Golfe de Gabès et projections selon un scénario de changement global. Bull. Inst. Nat. Sci. Technol. Mer. Salammbô 2011, 38, 65–71. [Google Scholar]
- Rabaoui, L.; Balti, R.; El Zrelli, R.; Tlig-Zouari, S. Assessment of heavy metals pollution in the Gulf of Gabes (Tunisia) using four mollusk species. Mediterr. Mar. Sci. 2014, 15, 45–58. [Google Scholar] [CrossRef]
- The United States Environmental Protection Agency. SW-846 Reference Methodology: Method 3050B. Standard Operating Procedure for the Digestion of Soil/Sediment Samples Using a Hotplate/Beaker Digestion Technique; The United States Environmental Protection Agency: Chicago, IL, USA, 1999. [Google Scholar]
- Elmasri, K.; Shetwi, A. Natural Radioactivity levels along the Mediterranean sand beach between Tajoura and Misrata, Libya. J. Eng. Res. 2023, 35, 91–100. [Google Scholar]
- Kritsananuwat, R.; Sahoo, S.K.; Fukushi, M.; Pangza, K.; Chanyotha, S. Radiological risk assessment of 238U, 232Th and 40K in Thailand coastal sediments at selected areas proposed for nuclear power plant sites. J Radioanal. Nucl. Chem. 2014, 1, 325–334. [Google Scholar] [CrossRef]
- Roy-Barman, M.; Lemaître, C.; Ayrault, S.; Jeandel, C.; Souhaut, M.; Miquel, J.-C. The influence of particle composition on Thorium scavenging in the Mediterranean Sea. Earth Planet. Sci. Lett. 2009, 286, 526–534. [Google Scholar] [CrossRef]
- Roy-Barman, M.; Coppola, L.; Souhaut, M. Thorium isotopes in the western Mediterranean Sea: An insight into the marine particle dynamics. Earth Planet. Sci. Lett. 2002, 196, 161–174. [Google Scholar] [CrossRef]
- Quinby-Hunt, M.S.; Turekian, K.K. Distribution of elements in sea water. Eos Trans. Am. Geophys. Union 1983, 64, 130. [Google Scholar] [CrossRef]
- Erel, Y.; Morgan, J.J. The effect of surface reactions on the relative abundances of trace metals in deep-ocean water. Geochim. Cosmochim. Acta 1991, 55, 1807–1813. [Google Scholar] [CrossRef]
- Broecker, W.; Peng, T.H. Tracers in the Sea; Eldigio Press: New York, NY, USA, 1982. [Google Scholar]
- Parkhurst, D.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations, Water-Ressources Investigations Report 1999; U.S. Department of the Interior, U.S. Geological Survey: Denver, CO, USA, 1999. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRS Press: London, UK, 2005. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Plummer, L.N.; Wigley, T.M.L.; Wolery, T.J.; Ball, J.W.; Jenne, E.A.; Basset, R.L.; Crerar, D.A.; Florence, T.M.; Fritz, B.; et al. A comparison of computerized chemical models for equilibrium calculations in aqueous species, in: Chemical Modeling in Aqueous Systems, Speciation, Sorption, Solubility and Kinetics. Am. Chem. Soc. 1979, 38, 857–892. [Google Scholar] [CrossRef]
- Delany, J.M.; Wolery, T.J. The Lawrence Livermore National Laboratory (LLNL) Thermochemical Database; Report UCRL-21658; LLNL, CA., Department of Energy: Washington, DC, USA, 1989. [Google Scholar]
- Kinniburgh, D.G.; Cooper, D.M. Predominance and Mineral Stability Diagrams Revisited. Environ. Sci. Technol. 2004, 38, 3641–3648. [Google Scholar] [CrossRef]
- Choppin, G.R.; Wong, P.J. The chemistry of actinide behavior in marine systems. Aquat. Geochem. 1998, 4, 77–101. [Google Scholar] [CrossRef]
- Langmuir, D.; Herman, J.S. The mobility of thorium in natural waters at low temperatures. Geochim. Cosmochim. Acta 1980, 44, 1753–1766. [Google Scholar] [CrossRef]
- Vassas, C.; Pourcelot, L.; Vella, C.; Carpena, J.; Pupin, J.P.; Bouisset, P.; Gulliot, L. Mechanisms of enrichment of natural radioactivity along the beaches of Camargue, France. J. Environ. Radioact. 2006, 91, 146–159. [Google Scholar] [CrossRef]
- Akyil, S.; Yusof, A.M. The distribution of uranium and thorium in samples taken from different polluted marine environments. J. Hazard. Mater. 2007, 144, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Kannan, V.; Rajan, M.P.; Iyengar, M.A.R.; Ramesh, R. Distribution of natural and anthropogenic radionuclides in soil and beach sand samples of Kalpakkam (India) using hyper pure germanium (HPGe) gamma ray spectrometry. Appl. Radiat. Isot. 2002, 57, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Harb, S. Natural radioactivity and external gamma radiation exposure at the coastal Red Sea in Egypt. Radiat. Prot. Dosim. 2008, 130, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.R.; Faghihian, H.; Kamal, M.; Mostajaboddavati, M.; Hasanzadeh, A. Distribution of natural radionuclides on coasts of Bushahr, Persian Gulf, Iran. Iran. J. Sci. Technol. Trans. A Sci. 2006, 30, 259–269. [Google Scholar]
- Mahdavi, A. The thorium, uranium, and potassium contents of Atlantic and Gulf Coast beach sands. In The Natural Radiation Environment; Adames, J.A.S., Lowder, W.M., Eds.; University of Chicago Press: Chicago, IL, USA, 1964; p. 87. [Google Scholar]
- Rudnick, R.L.; Gao, S. The Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; The Crust, Elsevier-Pergamon: Oxford, UK, 2003; Volume 3, pp. 1–64. [Google Scholar] [CrossRef]
- White, W.M. Geochemistry; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Cumberland, S.A.; Douglas, G.; Grice, K.; Moreau, J.W. Uranium mobility in organic matter-rich sediments: A review of geological and geochemical processes. Earth-Sci. Rev. 2016, 159, 160–185. [Google Scholar] [CrossRef]
- Bacon, M.P.; Anderson, R.F. Distribution of thorium isotopes between dissolved and particulate forms in the deep sea. J. Geophys. Res. 1982, 87, 2045. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Oceanic stratified euphotic zone as elucidated by 234Th:238U disequilibria. Limnol. Oceanogr. 1987, 32, 189–200. [Google Scholar] [CrossRef]
- Tipping, E. Humic Ion-Binding Model VI: An Improved Description of the Interactions of Protons and Metal Ions with Humic Substances. Aquat. Geochem. 1998, 4, 3–47. [Google Scholar] [CrossRef]
- Davis, J.A.; Meece, D.E.; Kohler, M.; Curtis, G.P. Approaches to surface complexation modeling of Uranium (VI) adsorption on aquifer sediments. Geochim. Cosmochim. Acta 2004, 68, 3621–3641. [Google Scholar] [CrossRef]
- Quigley, M.S.; Santschi, P.H.; Guo, L.; Honeyman, B.D. Sorption irreversibility and coagulation behavior of 234Th with marine organic matter. Mar. Chem. 2001, 76, 27–45. [Google Scholar] [CrossRef]
- Kantar, C. Heterogeneous processes affecting metal ion transport in the presence of organic ligands: Reactive transport modeling. Earth-Sci. Rev. 2007, 81, 175–198. [Google Scholar] [CrossRef]
- Bryan, N.D.; Abrahamsen, L.; Evans, N.; Warwick, P.; Buckau, G.; Weng, L.; Van Riemsdijk, W.H. The effects of humic substances on the transport of radionuclides: Recent improvements in the prediction of behaviour and the understanding of mechanisms. Appl. Geochem. 2012, 27, 378–389. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Santschi, P.H.; Wen, L.S.; Guo, L.; Xu, C.; Zhang, S.; Jiang, Y.; Ho, Y.F.; Schwehr, K.A.; Quigg, A.; et al. Binding of Th. Pa. Pb. Po and Be radionuclides to marine colloidal macromolecular organic matter. Mar. Chem. 2015, 173, 320–329. [Google Scholar] [CrossRef]
- The International Union for Conservation of Nature. The IUCN Red List Assessment, Loggerhead Turtle, Caretta Caretta. Available online: https://www.iucnredlist.org/species/3897/119333622 (accessed on 9 February 2023).
- Abdel-Gawad, I.I.; Mohammad, M.H.M. Congenital Malformations in Neonates After Irradiation of Rats During Pregnancy. In Proceedings of the Seventh Conference of Nuclear Sciences and Applications 2000, Cairo, Egypt, 6–10 February 2000; Aly, H.F., Ed.; International Atomic Energy Agency: Vienna, Austria, 2000. [Google Scholar]
- Copplestone, D.; Bielby, S.; Jones, S.R.; Patton, D.; Daniel, P.; Gize, I. Impact Assessment of Ionising Radiation on Wildlife; R&D Publication: Bristol, UK, 2001; p. 128. [Google Scholar]
- De Santis, M.; Cesari, E.; Nobili, E.; Straface, G.; Cavaliere, A.F.; Caruso, A. Radiation effects on development. Birth Defects Res. C Embryo Today 2007, 81, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yablokov, A.V. 10. Chernobyl’s radioactive impact on fauna. Ann. N. Y. Acad. Sci. 2009, 1181, 255–280. [Google Scholar] [CrossRef]
- The Agency for Toxic Substances and Diseases Registry. Toxicological Profile of Uranium. 2013. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp150.pdf (accessed on 1 November 2024).
- The World Health Organization. Health Effects of Depleted Uranium. 2001. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA54/ea5419a1.pdf (accessed on 1 November 2024).
- The National Cancer Institute. Thorium, Cancer Causing Substances. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/thorium (accessed on 1 November 2024).
- The United States Environmental Protection Agency. Radionuclide Basics: Uranium. 2023. Available online: https://www.epa.gov/radiation/radionuclide-basics-uranium (accessed on 1 November 2024).
- The United States Environmental Protection Agency. Radionuclide Basics: Thorium. 2023. Available online: https://www.epa.gov/radiation/radionuclide-basics-thorium (accessed on 1 November 2024).
- Savabieasfahani, M.; Ahamadani, F.; Damghani, A. Living near an active U.S. military base in Iraq is associated with significantly higher hair thorium and increased likelihood of congenital anomalies in infants and children. Environ. Pollut. 2020, 256, 113070. [Google Scholar] [CrossRef]
- Yin, S.; Tian, T.; Wang, C.; Wang, D.; Pi, X.; Liu, M.; Jin, L.; Liu, J.; Wang, L.; Li, Z. Prenatal uranium exposure and risk for fetal neural tube defects: A case-control study in women living in a rural area of northern China. J. Hazard. Mater. 2022, 424, 127466. [Google Scholar] [CrossRef]
- Yu, L.; Lin, Z.; Cheng, X.; Chu, J.; Li, X.; Chen, C.; Zhu, T.; Li, W.; Lin, W.; Tang, W. Thorium inhibits human respiratory chain complex IV (cytochrome c oxidase). J. Hazard. Mater. 2022, 424, 127546. [Google Scholar] [CrossRef]
- The European Union. Etude D’impact de la Pollution Industrielle Sur L’économie de la Région de Gabès; Requête n°2016/372829/1, Rapport final; The European Union: Luxembourg, 2017; 140p. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control. a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Tomlinson, D.C.; Wilson, J.G.; Harris, C.R.; Jeffery, D.W. Problems in the Assessment of Heavy Metals Levels in Estuaries and the Formation of a Pollution Index. Environ. Eval. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in the sediments of the Rhine River. Geol. J. 1969, 2, 108–118. [Google Scholar]
- Xu, Z.Q.; Ni, S.J.; Tuo, X.G.; Zhang, C.J. Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ. Sci. Technol. 2008, 31, 112–115. [Google Scholar]
- Gorman-Lewis, D.; Shvareva, T.; Kubatko, K.-A.; Burns, P.C.; Wellman, D.M.; McNamara, B.; Szymanowski, J.E.; Navrotsky, A.; Fein, J.B. Thermodynamic properties of autunite, uranyl hydrogen phosphate, and uranyl orthophosphate from solubility and calorimetric measurements. Environ. Sci. Technol. 2009, 43, 7416–7422. [Google Scholar] [CrossRef]
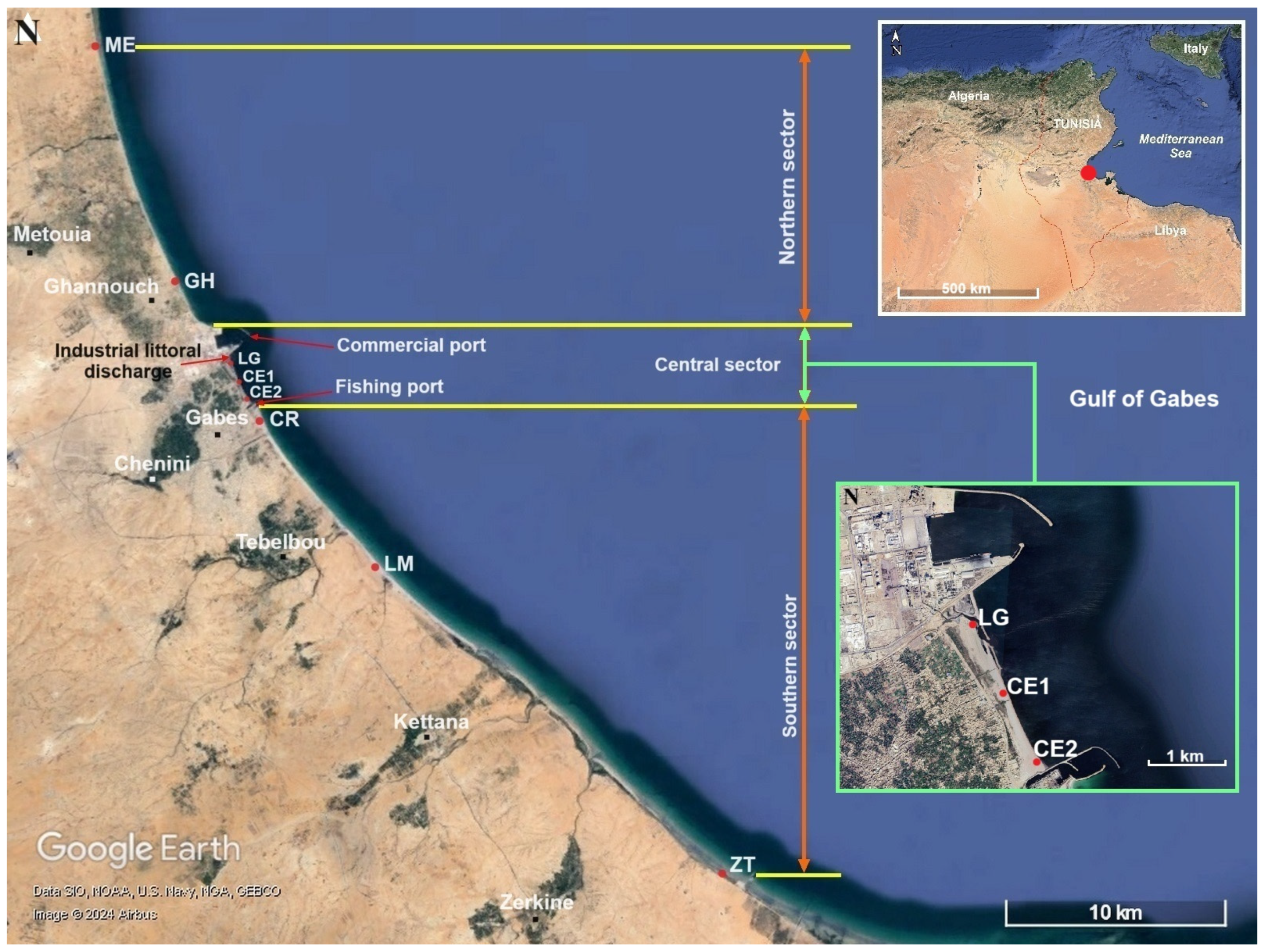




| Sectors | Stations (N → S) | Area Description | Latitude (°N) | Longitude (°E) |
|---|---|---|---|---|
| Northern sector | ME | No anthropogenic source of pollution; intensive coastal fishing activity; presumed as a non-polluted site. | 34.046564 | 10.034136 |
| GH | Some industrial (ICF) and domestic pollution sources; intensive coastal fishing activity; presumed as a moderately polluted site. | 33.946650 | 10.075997 | |
| Central sector | LG | In the vicinity of the industrial (GCT fertilizer factories) and domestic waste (water and solids) littoral discharge points, which are considered to be the main sources of marine pollution in the Gulf of Gabes. | 33.912483 | 10.103112 |
| CE1 | Located in the inter-harbor area (Chatt Sidi Abd Essalam beach); important mix of industrial (PG, PGF, PR, fluoridated wastewater, etc.) and urban wastewater marine discharges; low coastal fishing activity; considered to be the most polluted site in the Gulf of Gabes. | 33.904828 | 10.108053 | |
| CE2 | 33.897073 | 10.113337 | ||
| Southern sector | CR | In front of Corniche beach; rare coastal fishing activity; presumed as a non-polluted site. | 33.888606 | 10.119686 |
| LM | No anthropogenic source of pollution; average coastal fishing activity; presumed as a non-polluted site. | 33.829291 | 10.177279 | |
| ZT | 33.702642 | 10.345200 |
| ME | GH | LG | CE1 | CE2 | CR | LM | ZT | |
|---|---|---|---|---|---|---|---|---|
| U | 1.62 ± 0.08 | 1.25 ± 0.06 | 38.0 ± 1.9 | 33.0 ± 1.6 | 9.6 ± 0.5 | 0.71 ± 0.04 | 0.77 ± 0.04 | 1.14 ± 0.06 |
| Th | 1.38 ± 0.07 | 1.22 ± 0.06 | 10.6 ± 0.5 | 6.6 ± 0.3 | 2.43 ± 0.12 | 1.00 ± 0.05 | 2.14 ± 0.11 | 3.46 ± 0.17 |
| TOC | 0.040 ± 0.002 | 0.020 ± 0.001 | 6.1 ± 0.3 | 1.06 ± 0.05 | 0.120 ± 0.006 | 0.010 ± 0.001 | <0.010 | 0.070 ± 0.004 |
| FF | 0.23 | 0.09 | 0.35 | 0.36 | 0.11 | 0.13 | 0.21 | 2.65 |
| DILD | 16.20 | 4.54 | 0.005 | 1.02 | 1.98 | 3.12 | 11.54 | 32.36 |
| Stations (N → S) | Cf | PLI | Igeo | PERI | |||
|---|---|---|---|---|---|---|---|
| U | Th | Value | U | Th | Total | EU | |
| MT | 2.3 | 1.4 | 1.8 | −0.4 | −0.1 | −0.5 | 91.3 |
| GH | 1.8 | 1.2 | 1.5 | −0.8 | −0.3 | −1.1 | 70.4 |
| LG | 53.5 | 10.6 | 23.9 | 4.2 | 2.8 | 7.0 | 2141 |
| CE1 | 46.5 | 6.6 | 17.5 | 4.0 | 2.1 | 6.1 | 1859 |
| CE2 | 13.6 | 2.4 | 5.7 | 2.2 | 0.7 | 2.9 | 542 |
| CR | 1.0 | 1.0 | 1.0 | −1.6 | −0.6 | −2.2 | 40.0 |
| LM | 1.1 | 2.1 | 1.5 | −1.5 | 0.5 | −0.9 | 43.4 |
| ZT | 1.6 | 3.5 | 2.4 | −0.9 | 1.2 | 0.3 | 64.2 |
| Locations | Range/Mean | U | Th | References |
|---|---|---|---|---|
| Gulf of Gabes (Tunisia) | Range | 0.71–38.00 | 1.00–10.60 | Present study |
| Mean | 10.77 | 3.60 | ||
| Camargue (France) | Range | 7.64–180.21 (95–2240 Bq.kg−1) * | 33.17–852.58 (135–3470 Bq.kg−1) * | [97] |
| Mean | - | - | ||
| Coastal areas of Malaysia | Range | 3.00–6.60 | 0.01–0.68 | [98] |
| Mean | 4.49 | 0.26 | ||
| Kalpakkam (India) | Range | 2.90–20.76 (36–258 Bq.kg−1) * | 86.49–951.35 (352–3872 Bq.kg−1) * | [99] |
| Mean | 9.98 (124 Bq.kg−1) * | 396.31 (1613 Bq.kg−1) * | ||
| The Red Sea (Egypt) | Range | 0.58–3.29 (7.2–40.9 Bq.kg−1) * | 0.91–9.31 (3.7–37.9 Bq.kg−1) * | [100] |
| Mean | - | - | ||
| Bushahr (Iran) | Range | 0.97–6.03 (12–75 Bq.kg−1) * | 1.97–8.11 (8–33 Bq.kg−1) * | [101] |
| Mean | 1.83 (22.7 Bq.kg−1) * | 3.05 (12.4 Bq.kg−1) * | ||
| Atlantic and Gulf Coasts (USA) | Range | 0.3–0.6 | 1–2 | [102] |
| Mean | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Zrelli, R.B.; Klar, J.K.; Castet, S.; Grégoire, M.; Courjault-Radé, P.; Fabre, S. Spatial Distribution Patterns, Eco-Environmental Risk Assessment, and Human Health Impacts of Uranium and Thorium in Beach Sediments in the Central Gulf of Gabes (Southern Mediterranean Sea). Sustainability 2025, 17, 1283. https://doi.org/10.3390/su17031283
El Zrelli RB, Klar JK, Castet S, Grégoire M, Courjault-Radé P, Fabre S. Spatial Distribution Patterns, Eco-Environmental Risk Assessment, and Human Health Impacts of Uranium and Thorium in Beach Sediments in the Central Gulf of Gabes (Southern Mediterranean Sea). Sustainability. 2025; 17(3):1283. https://doi.org/10.3390/su17031283
Chicago/Turabian StyleEl Zrelli, Radhouan Belgacem, Jessica K. Klar, Sylvie Castet, Michel Grégoire, Pierre Courjault-Radé, and Sébastien Fabre. 2025. "Spatial Distribution Patterns, Eco-Environmental Risk Assessment, and Human Health Impacts of Uranium and Thorium in Beach Sediments in the Central Gulf of Gabes (Southern Mediterranean Sea)" Sustainability 17, no. 3: 1283. https://doi.org/10.3390/su17031283
APA StyleEl Zrelli, R. B., Klar, J. K., Castet, S., Grégoire, M., Courjault-Radé, P., & Fabre, S. (2025). Spatial Distribution Patterns, Eco-Environmental Risk Assessment, and Human Health Impacts of Uranium and Thorium in Beach Sediments in the Central Gulf of Gabes (Southern Mediterranean Sea). Sustainability, 17(3), 1283. https://doi.org/10.3390/su17031283








