Abstract
The concentrations of estrogens and xenoestrogens in the environment are rising rapidly, posing significant and multifaceted risks to human health and ecosystems. It is imperative for governments to develop policies that leverage sustainable technologies to mitigate the presence of pharmaceutical estrogenic compounds in the environment. This review examines the global environmental and human health risks associated with indigenous estrogens and synthetic pharmaceutical xenoestrogens, while critically evaluating sustainable approaches to their management. A total of 28 studies, published between December 2013 and 18 January 2024, and sourced from PubMed and Scopus, were systematically reviewed. Most of these studies focused on estrogenic compounds in aquatic environments where they contribute to reproductive and developmental abnormalities in fish and may enter the human food chain, primarily through fish consumption. Sustainable methods for removing or neutralizing estrogenic compounds include adsorption, filtration, and enzymatic degradation. Additionally, technologies such as activated sludge processes and high-rate algal ponds demonstrate promise for large-scale applications; however, further research and standardized operational guidelines are needed to optimize their efficiency and sustainability. This review has concluded that ECs can have severe consequences on the environment, most notably, impairment of reproductive functions in fish and humans, underscoring the urgent need for governments to implement drug take-back programs, establish evidence-based guidelines for wastewater and pharmaceutical waste treatment, and set enforceable thresholds for estrogenic compounds in surface and drinking water. Existing regulations such as the UK’s Regulation on the registration, evaluation, authorization, and restriction of chemicals and the United States’ National Primary Drinking Water Regulations can be modified to include ECs as dangerous chemicals to aid in maintaining safe EC levels”. Such measures are critical for reducing the environmental concentrations of pharmaceutical estrogenic compounds and safeguarding both public health and ecological integrity.
1. Introduction
Estrogen is a steroid hormone involved in regulating various bodily functions, including those of the neuroendocrine, vascular, skeletal, immune, central nervous system, and reproductive systems [1,2]. The three main naturally occurring estrogens in the human body are estrone (E1), estradiol (E2), and estriol (E3). E1 and E2 are produced in the ovaries and adrenal glands, while E3, a metabolite of E1 and E2, is primarily produced by the placenta during pregnancy [3,4]. E1 is the primary form of estrogen after menopause, while E2 is predominant before menopause. Estrogens are also produced in the male body from androgens such as testosterone [5]. What is unique about steroids in comparison to other cell-signaling molecules is their ability to permeate the cell membrane and enter the nucleus to alter gene expression [6]. However, naturally occurring estrogens are not the only compounds that can be estrogenic. Molecules that are foreign to the body and have a similar enough structure to bind to estrogen receptors are considered xenoestrogens [7].
Estrogenic compounds (ECs) are molecules that interact with estrogen receptors on cell membranes or in the nuclei to induce biological responses [8]. This may include indigenous estrogens produced in the human body, phytoestrogens from plants, mycotoxins from fungi, and xenoestrogens, which are synthetic molecules that mimic estrogen [9]. Xenoestrogens have a wide range of industrial applications such as in plastics production, preservatives, and personal care products [10]. Common categories of synthetic xenoestrogens include bisphenols, parabens, and phthalates [10]. Xenoestrogens are also produced for therapeutic purposes. For example, Ethinyl estradiol (EE2) is a very common xenoestrogen found in hormonal birth control and hormone replacement therapy that is 200 times more potent than E2 [11].
Estrogenic compounds can be easily released into the natural environment and may have unintended negative consequences [12]. Estrogens that are naturally produced in the body can be released through excrement and urine, particularly by pregnant animals [13]. The largest source of naturally occurring estrogen is livestock in the agricultural industry [13]. Their excrement is distributed in the environment as fertilizer and contaminates the waterways through runoff [14]. Estrogenic compounds can also be released through landfills and general plastic pollution, which can leach chemicals such as bisphenol A (BPA) [15].
Once these estrogens enter the environment, their half-life can range from 12 h to 180 days, depending on the presence of UV light, microorganisms, oxygen, and other environmental factors [16]. During aerobic microorganism degradation in aerated soil, the half-lives of E1, E2, and E3 were 2.8–4.9, 0.8–1.1, and 0.7–1.7 days, respectively. In river water, however, E1, E2, and EE2 were 2–3, 2–3, and 4–6 days, respectively [13,17]. Generally, EE2 is the most resilient steroidal estrogen to decompose, and it is the most biologically active. Various microbes can metabolize estrogen, but they can also convert one form of estrogen into another. For example, nitrifying bacteria can convert E1 into E3 and Sphingobacterium sp. can convert EE2 to E1. This transformation between different types of estrogen may also vary depending on other compounds present in the system such as methanogenic, sulfate, iron, and nitrate-reducing agents [13].
The majority of EC management processes focus on aquatic environments, primarily wastewater treatment plants. Wastewater treatment plants have different methods of processing water due to a lack of standardized regulations regarding wastewater treatment and various factors such as cost, wastewater composition, and environmental impacts [18]. Generally, treatment involves a four-step process: preliminary debris removal followed by primary, secondary, and tertiary treatment [19]. Primary treatment removes solids, oils, and fats by settling, sedimentation, and filtration [19]. Secondary treatment removes remaining suspended solids and microbes through methods such as aerobic treatment, anaerobic treatment, bioreactors, activated sludge, and biological filters [19]. Lastly, tertiary treatment is the advanced removal of chemical contaminants such as detergents and toxic compounds through chemical precipitation, neutralization, adsorption, disinfection, and ion exchange [20]. Advanced treatments like nanofiltration (NF), membrane bioreactors (MBRs), reverse osmosis (RO) ultrafiltration (UF), and advanced oxidation processes (AOPs) are efficient in removing estrogens [20]. However, these processes often come with high costs and are not ubiquitous [21].
If ECs are improperly managed, they can be released into the environment where they can severely impact a wide range of human bodily systems and disrupt ecosystems. In the human body specifically, exposure to estrogenic compounds can increase the risk of certain cancers such as breast, skin, liver, and testicular cancers [22]. They may also cause other conditions such as diabetes, autism spectrum disorder, prostate hyperplasia, reproductive abnormalities, and thyroid disease [23,24]. Estrogen is also important in neurological development, especially during fetal and perinatal life, where it is involved in brain development and controlling sexual behavior such as gender identity [25]. In animals, ECs can contaminate the ecosystems and disrupt the sex ratios of vulnerable animal populations through the feminization of males [26]. Their effects have been documented in both wild and domestic animals with significant interruptions in aquatic biomass, impaired vision in cats, infertility in sheep, and morphological abnormalities in cows [13].
Estrogens and xenoestrogens are becoming increasingly concerning as their concentrations rapidly increase in the environment [13]. The impacts that estrogenic compounds can have on human health and the environment are vast and complex as they can easily spread between water, soil, and air [26]. It is more critical than ever for governments to create policies and industries to utilize sustainable technologies to treat or reduce the amount of pharmaceutical estrogenic compounds in the environment, which to date remain opaque. Current reviews on this topic focus broadly on the effects of ECs on the environment and health [13,21]. However, there is a gap in the literature focusing on pharmaceutical estrogens specifically, the sustainability of estrogen management, and the role that federal and regional government can play in regulating pharmaceutical estrogen management. This review, therefore, aims to investigate the global environmental and human health risks of indigenous estrogens and synthetic pharmaceutical xenoestrogens in the environment and to critically appraise the sustainable methods of processing them.
2. Materials and Methods
The methodology in this review follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Figure 1 and Supplementary Materials). Table 1 is a Population Exposure Control Outcome (PECO) table used to formulate the research question and search terms. Our population is people who live near areas that contain high levels of estrogenic compounds (Table 1). The exposure is pharmaceutical xenoestrogens and native estrogens. The control group would include individuals who experience little to no exposure to estrogenic compounds from the environment. Lastly, the outcome is the adverse health effects associated with increased estrogen exposure (Table 1).
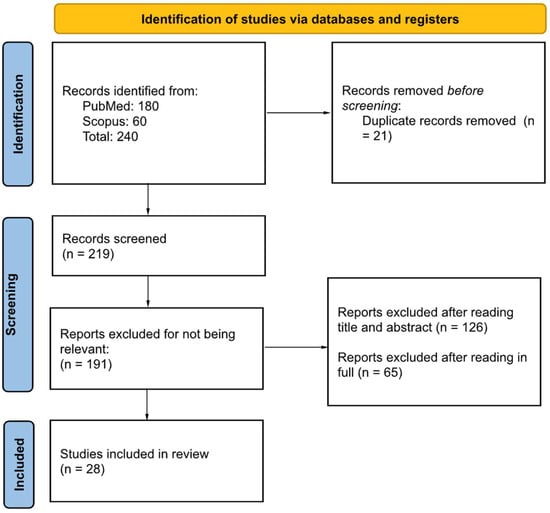
Figure 1.
PRISMA flow diagram outlining search strategy.

Table 1.
PECO table used to formulate the research question.
2.1. Eligibility Criteria
This review examined published primary research discussing the impact of estrogens and ECs from pharmaceutical sources on humans and our environment (food, soil, air, or water) as well as the management practices of these pharmaceutical ECs.
2.2. Search Strategy
Articles published from December 2013 to 18 January 2024 were searched using PubMed and Scopus databases with the following keywords and Boolean operators: (healthcare OR drug OR medical OR pharmaceutical) AND (estrogen OR oestrogen OR estrogenic OR oestrogenic) AND (environment OR food OR soil OR water OR air) AND (waste management OR waste processing OR environment management). After the initial search, the terms pollution and contamination were included, but significant differences were not found.
All articles written in English were selected. Search results were exported to Zotero (https://www.zotero.org/ accessed on 20 November 2024) citation manager, where results were de-duplicated. The titles and abstracts of the studies were analyzed to ensure relevance to the topic.
Papers were sorted into “yes”, “no”, and “maybe” categories. Papers in the “maybe” category were read in full to determine whether they were appropriate. The majority of excluded papers were removed due to irrelevance as many did not directly discuss pharmaceutical estrogenic compounds or natural estrogens. Relevance was determined using the PECO Table (Table 1). The included papers were then studied in full.
2.3. Quality Appraisal
Selected articles were assessed using the Mixed Methods Appraisal tool [27]. The questions that were used in this tool were dependent on the type of research method used in the paper (Table 2). These acted as guidelines to critically appraise the studies.

Table 2.
Risk of bias checklist formatted off of the Mixed Methods Appraisal Tool Version 2018.
2.4. Risk of Bias
The risk of bias for each of the studies included in this paper was assessed with a list of 6 questions derived from the Mixed Methods Appraisal Tool Version 2018, a set of questions that are used to evaluate the quality of empirical research. Questions were answered with “yes” or “no” answers. Each “yes” answer is worth 1 point and each “no” answer is worth 0 points. Points were summed to create a score that was used to compare each study’s validity (Table 2).
3. Results
3.1. Search Results
There were 219 articles from the initial filtered search. After reviewing the papers for relevance, 191 studies were removed, leaving 28 papers (see Figure 1). The majority of excluded papers were removed due to irrelevance. These studies were then assessed for risk of bias and none were removed in this process.
3.2. Study Characteristics
The studies included in this review came from 13 countries and used many different study designs to analyze pharmaceutical xenoestrogens and naturally occurring estrogens in water and soil. Table 3 provides an outline of the characteristics of each study. Studies were published between 2014 to 2023 and were carried out in the United Kingdom, France, Italy, China, Taiwan, the United States, Spain, Switzerland, Australia, Brazil, Finland, Portugal, and Canada (Table 3). The different study designs included laboratory experimental studies, field studies, and secondary analyses (Table 3). Three of the studies investigated estrogenic compounds in soil while 24 were in water, and one discussed both (Table 3). Four studies utilized 24 h composite samples when studying estrogens and xenoestrogens in water. Nine studies utilized cross-sectional sampling, while 10 studies sampled longitudinally. Details about sampling including sample size and duration of sampling are included in Table 4. A range of estrogenic compounds were included in these studies with E1, E2, and EE2 being the most widely discussed with 13, 14, and 17 studies directly referencing them, respectively (Table 3). An in-depth description of each of the studies, including aims and outcomes can be found in Table 4.

Table 3.
This table displays the specific papers that fall into various categories of study characteristics.

Table 4.
This table shows the characteristics of each study including their main objectives, study design or data source, and general findings.
3.3. Risk of Bias
4. Discussion
4.1. Evaluation of Available Literature
In this paper, 28 papers were reviewed. Each study had a relatively low risk of bias, with points only being deducted for addressing and minimizing bias. Many of the studies that did not receive a “yes” in this category did not discuss the limitations of the study or possible sources of bias (Table 2). This does not indicate that the study is inherently biased or invalid, but the lack of transparency on the topic requires readers to be more discerning when assessing the research. All researchers declared the sources of funding and/or any conflicts of interest. The range of topics discussed in the literature was extensive with an emphasis on the variety of wastewater treatment methods to remove E1, E2, E3, and EE2.
As many studies investigated ECs in aqueous environments, this left a gap in the material regarding ECs in soil and air. Only 4 studies in this review discuss the presence of ECs in soil and none include ECs found in the air [28,29,33,45]. Though there is little to no research on pharmaceutical ECs in the air, there is evidence that particulate matter can have estrogenic properties and the management of these compounds, and their health risks must be further investigated [56]. Also, although there was extensive research conducted on aquatic ECs and how to remove them from contaminated waters, discussion about management practices to reduce the introduction of pharmaceutical ECs into water systems and the general environment outside of a wastewater treatment plant was lacking.
In five studies, wastewater treatment plants were compared for efficacy in removing estrogenic compounds by measuring the estrogenicity of water or the concentrations of specific xenoestrogens along different steps of the treatment process. This research method is particularly useful in assessing what methods are the most effective and can help shape regulations and recommendations to create standardized wastewater treatment systems. However, these studies were only conducted in Europe, Asia, and Australia. This may be due to a variety of factors including funding availability, research infrastructure, and political stability. The contaminants found in the wastewater and each country’s environment and capacity to invest in different removal methods vary significantly, requiring further investigation.
4.2. Characterizing Estrogenic Compounds in the Environment
The estrogens and xenoestrogens included in these studies were E1, E2, E3, EE2, BPA, 4-nonylphenol, t-nonylphenol, 4-tert-octylphenol, nonylphenol mono-ethoxylate, nonylphenol di-ethoxylate, 4-t-butylphenol, diethylstilbestrol, diethyl phthalate, di(2-ethylhexyl)phthalate, 2-hydroxybiphenyl, alachlor, methyl paraben, ethyl paraben, propyl paraben, butyl paraben, tris(2-butoxyethyl)phosphate, tris(2-chloroethyl) phosphate, triphenyl phosphate, equilin, mestranol, dienestrol, hexestrol, levonorgestrel, 17b-estradiol-3-sulfate, 19-norethindrone, tonalide, triclosan, triclocarban, vinclozolin, oxybenzone, 4-nonylphenol-monoethoxylate, 4-nonylphenol-diethoxylate, and dienogest and drospirenone transformation products.
Due to the wide range of ECs and their potential to transform into different compounds with varying levels of ecological impact, several studies measured estrogenicity using biological assays such as the chemical-activated luciferase expression (ERα-CALUX) assay, which measures estrogenic and anti-estrogenic activity using a reporter protein that glows when it comes in contact with estrogenic substances, rather than measuring specific compound concentrations [30,48,50,57]. Although biological assays are useful for understanding the presence of ECs on a larger scale, studies assessing individual ECs were able to characterize specific compounds that may pose a larger ecological risk. Many found that EE2 and BPA were harder to remove through traditional wastewater treatment processes and can have higher levels of estrogenicity [29,32,33,34,46,51,53]. Both forms of measuring EC presence in the environment are necessary to better understand their potential implications and design removal guidelines that more effectively address pollutants that are the most prevalent.
Creating these guidelines can be difficult due to the variety of ways that ECs can enter the environment, including leaching from landfills, runoff from agricultural practices, and sewage sludge [28,29]. Some of these ECs are free estrogens, like E1, E2, E3, and EE2, which are biologically active. There may also be conjugated estrogens that are generally non-toxic, but they can be naturally degraded into free estrogens, which have the potential for long-term harm. For example, 17β-estradiol-3-sulfate is a naturally produced compound that acts as an E3 storage pool and it can be deconjugated by bacteria found in river sediments, converting it to E3 [45]. The presence of conjugated estrogens was not widely discussed in studies evaluated here, but many did attempt to measure the presence of free estrogens.
EE2 was frequently detected, which is expected given its more stable structure, but the specific source was not identified. EE2 and E3 were measured at much higher concentrations in forest lands compared to urban, grassland, and agricultural areas in France [29]. However, the origins and fate of these compounds were not determined and are not widely discussed in other literature. Another study by Arya et al. in the United States analyzing water and sediment in a section of the Potomac River receiving wastewater effluent observed high concentrations of EE2, norethindrone, and mestranol in water and EE2 and 19-norethindrone in sediment [33]. Wastewater treatment plants contributed to these ECs, but these emissions were not associated with the compounds found in sediment indicating that there must be another source of ECs that remains unspecified but perhaps due to the large presence of ECs in waters upstream from the wastewater treatment plants [33]. It is crucial that studies such as these are repeated in other regions and settings as the duration in which ECs can persist and the concentrations in which they are released strongly depend on environmental conditions. For example, hormones excreted by humans and animals have a half-life in soil ranging from 1 to 10 days and may degrade at faster rates in higher temperatures, UV exposure and aerobic conditions [29,32].
4.3. Consequences of Pharmaceutical Estrogenic Compounds in the Environment
Exposure to ECs in nature can lead to several adverse effects observed in both humans and wildlife. These effects can be quite extensive as ECs travel from one system to another, such as leaching from soil to water [22]. Once ECs enter the water system, they can more easily spread and reach shallow groundwater [22]. The most common sources of these contaminating ECs are biosolids or sewage sludge, contaminated soil, sediments, surface waters, influent and effluent of wastewater treatment plants (WWTPs), and landfill leachate [22]. Despite ECs’ potential to interact with the environment in several different forms, literature available on this topic greatly centers around the effects of ECs in water.
In lakes, rivers, and reservoirs in China, Dai et al. found that the primary exposure pathway for aquatic estrogenic compounds to enter the human body was through fish consumption rather than drinking water [31]. However, exposure pathways can be much more complex including ingestion, absorption, and inhalation [56]. This identifies a large gap in the research conducted on ECs and room for future developments in our understanding of these compounds on the environment and health. Of the compounds studied, E1, E3, and EE2 had the highest health risks for people of all age categories, with the highest risk for children from 2 to 6 years of age [31]. These exposures may lead to an increased risk of reproductive impairments, dysfunctions, cancers, and diseases despite being present in low concentrations [31]. These findings are consistent with previous research as children often spend more time in the environment where they can be exposed to ECs and their skin and blood–brain barriers are more permeable, making them more sensitive to pollutants [58]. Of note, they also have a larger body surface area to volume compared to adults.
Meyer et al. studied the effects of xenoestrogens on the gallbladder and liver and found that they may have negative effects on the development and function of bile ducts, leading to the development of cholestatic and primary biliary cholangitis, a chronic autoimmune liver disease due to the presence of antibodies to mitochondrial proteins [28]. The research relating estrogens and liver disease is somewhat disputed. It is widely accepted that native estrogens have a protective role in chronic liver disease, particularly in premenopausal women [59]. However, some clinical studies have documented increased estrogen levels in those who suffer from chronic liver disease [59]. More research must be conducted to understand the differences in the way that natural and synthetic estrogens can impact liver and other organ functions.
The effects of ECs may also be observed in wildlife, most notably fish and amphibians. It is widely recognized that feminization of male fish can occur due to xenoestrogen exposure [51]. In female fish, however, pharmaceutical estrogens such as levonorgestrel, found in emergency contraceptive pills, have been associated with impaired oogenesis, oocyte maturation, and ovulation or the masculinization of female fish [60,61]. There have also been links between EC exposure and higher rates of mortality and decreased body length among fish embryos [34,50]. Osachoff et al. found that concentrations of plasma vitellogenin protein, an egg yolk precursor used as a marker for environmental estrogen contamination, were lower in fish that lived in activated sludge effluent compared to those in influent [55]. However, the changes in plasma vitellogenin protein and other estrogen-dependent proteins were not proportional to the decrease in estrogen levels in the treated water, indicating that the changes in estrogenicity were not enough to eliminate ecological risk [55]. Because estrogen receptors have been phylogenetically conserved among vertebrates, the harmful effects of these compounds can span across numerous organisms in the ecosystem [60]. This is especially true when considering the potential for bioaccumulation of pharmaceuticals as observed by Arya et al. and the risks of human consumption of fish that lived in estrogenically active waters [31,33].
4.4. Evaluation of Estrogen Management Methods
The efficiency of wastewater treatment plants in removing ECs was analyzed in 3 studies from Italy, Hong Kong, and Finland, and they found that estrogenic activity can be detected in wastewater effluent of receiving waters, indicating that the wastewater treatment plant was not effective in removing estrogen from the water [30,34,50]. However, other studies in Finland, Sweden, the United Kingdom, and Hong Kong did find that the wastewater treatment plants studied were effective in removing estrogens and xenoestrogens [34,35,46,54]. This demonstrates the need to create standardized regulations for wastewater management and develop technologies that are effective, cost-efficient, and environmentally sustainable.
The methods used to treat wastewater and the alternatives that are currently being developed will be examined in this section. The specific wastewater treatment processes to be discussed can be grouped into four categories: activated sludge [40,44,55], estrogen adsorption and filtration [37,43,47], algal and enzymatic degradation [36,38,41,48], and other methods [39,42,49,52,53].
4.4.1. Activated Sludge
Activated sludge is often inefficient at removing estrogens and xenoestrogens, such as E1 and EE2, and biotransformation of dienogest and drospirenone into estrogenic products has been documented (Table 5) [40,44,55]. However, by optimizing conditions including solid and hydraulic retention time, efficiency can increase [40]. Activated sludge processes should also be modified to become more sustainable as the current methods typically require large amounts of energy, often from fossil fuels [62]. This may be achieved by utilizing renewable sources of energy and increasing the concentration of organic matter to improve the efficiency of energy recovery in the form of biogas [62].

Table 5.
SWO/AT analysis of EC management methods.
4.4.2. Adsorption and Filtration
Adsorption and filtration mechanisms are reported to be a good option for sustainable and cost-effective wastewater treatment (Table 5) [63]. Magnetic MXene composite Fe3O4@Ti3C2, Pinus elliotti bark, nanofiltration, and reverse osmosis were all discussed in the studies in this review [37,43,47]. Fe3O4@Ti3C2 was very effective at adsorbing EE2 with an efficiency of 95.34% after 6.7 h of treatment [57]. Pinus elliotti bark is a natural biosorbent that has a similar or greater removal capacity for E2 and EE2 than granulated activated charcoal, which can be derived from nonrenewable resources and suffer from high production cost [47]. There were also high success rates for the removal of estrogens by NF90, a nanofiltration membrane, and XLE, a reverse osmosis membrane, which were both over 92% [43].
4.4.3. Algal and Enzymatic Degradation
High-rate algal ponds can be used to treat wastewaters and in constructed wetlands, and are an affordable, low-energy method to remove xenoestrogens, such as oxybenzone, methylparaben, tris(2-chloroethyl) phosphate, triphenyl phosphate, and BPA from industrial, municipal, and agricultural sources, but strong evidence supporting its effectiveness on pharmaceutical ECs and natural estrogens has yet to be gathered (Table 5) [38,64,65]. Hom-Diaz et al. studied S. capricornutum and C. reinhardtii and found that after 7 days, S. capricornutum removed 88–100% of E2 and 60–95% of EE2, while C. reinhardtii completely removed both E2 and EE2 except for EE2 in the presence of an anaerobic digester centrate, which decreased removal to 76% [36]. Though these findings are promising, further research is needed to understand the full implementation of high-rate algal ponds in large-scale systems.
Enzymatic degradation is promising, particularly for bioremediation, because it is an eco-friendly, economical, and effective way of eliminating harmful pollutants (Table 5) [66]. Biological laccase enzymes from Trametes pubescens MUT 2400 were used to treat municipal wastewater with an average reduction in estrogenic activity of 93.7% in a sample that treated wastewater collected after primary sedimentation (W1) and 92.0% in a sample that had already undergone secondary treatment [48]. Laccases should be used at the end of wastewater treatment for higher efficacy due to the loss of laccase activity in W1 samples due to the potential for enzymatic inhibition by chemicals and microbes present in wastewater. Tetra-amido macrocyclic ligand (TAML) activators are functional peroxidase enzyme replicas that have been paired with peroxide to degrade EE2. Mills et al. found that 10 ppm of EE2 can be degraded with 80 nM TAML analog 2 and 11.46 ppm H2O2 within 25 min. Estrogenic intermediates were produced through this process and had an estrogenicity of 35–57% of EE2.
4.4.4. Other Methods
Ma et al. utilized ultraviolet-C radiation (UVC) and hydrogen peroxide to degrade E1, E2, and EE2 and found that when UVC exposure was paired with 15 mg/L of hydrogen peroxide, removal efficiencies reached 99.7, 76.4, and 77.6%, respectively. However, when multiple estrogens are degraded at once, removal efficiency decreases (Table 5) [42]. In practice, Ribeiro et al. observed that wastewater effluent from a plant that uses UV treatment contained EE2 concentrations between 584 and 776 ng/L while E1 and E2 were measured at very low concentrations, indicating that EE2 was poorly removed by UV treatment [53].
Manganese oxide (MnOx) coated coir fibers have been used to treat hospital wastewaters before dilution into larger WWTPs through oxidation by the MnOx and sorption by the coir fibers [49]. Hexestrol, a pharmaceutical xenoestrogen used for hormone replacement therapy, was removed by 54% after 24 h, and by the end of MnOx treatment, the organic compounds that were reactive with Estrogen Receptor β were removed below detection rates [49]. Deng et al. conducted trials pairing potassium permanganate and ultrasound and found that in natural water, E1, E2, and EE2 were removed at 60.8, 60.8, and 56.5% efficiency, respectively [39].
Biogenic platinum (Bio-Pt) nanoparticles can be used as reductive catalysts to break down estrogens in environmental remediation projects [52]. When activated by hydrogen gas, Bio-Pt removed 94% of E2 after 24 h of treatment, ultimately reducing estrogenic activity by 71% after taking into account the toxicity of the byproducts from the reaction of 17β-estradiol with Bio-Pt [52]. Bio-Pt is reported to be a sustainable option for removing ECs because it is cost-effective, has low toxicity, and is eco-friendly [67]. However, it must be combined with another treatment modality, such as nanofiltration to reach ecologically safe levels of estrogenicity.
There are several approaches to removing ECs from water. Some effective and environmentally sustainable options are adsorption and filtration using pinus elliotti bark or nanofiltration and degradation with laccase enzymes or TAML activators. Activated sludge processes and high-rate algal ponds also have the potential for large-scale applications but require further investigation and standardized guidelines to reach maximum efficiency and sustainability.
4.5. Implementation of Management Practices
Reducing pharmaceutical ECs found in the environment begins with proper disposal of waste and unused medications. U.S. guidelines for the disposal of pharmaceutical estrogens and xenoestrogens were not found at the time of this study. In the EU, the Regulation on the registration, evaluation, authorization, and restriction of chemicals (REACH) attempts to minimize the effects of harmful substances on human health and the environment by requiring reports from EU member states and the European Economic Area (EEA) countries every 5 years and registering chemical substances, their risks, and their management methods to limit or ban dangerous compounds [68]. Though ECs have not specifically been regulated through REACH, a similar approach may be recommended for overseeing their production, management, and disposal. The Healthcare Environmental Resource Center and Practice Greenhealth recommends treating ECs as hazardous waste or incinerating them using a municipal or regulated medical waste incinerator and although the U.S. Food and Drug Administration does not discuss pharmaceutical ECs specifically, there are drug-take-back facilities, as in the UK, that can aid in the proper disposal of unused or expired medications [68,69]. These may be a good starting point for reducing the introduction of ECs into the environment, but clearer guidelines that specifically address pharmaceutical xenoestrogens and infrastructure for accessible drug-take-back programs are necessary in every country to eliminate the problem.
Regulations about acceptable EC levels entering the environment vary from country to country. In the United States, for example, estrogenic compounds are not included in the National Primary Drinking Water Regulations [70]. However, the only pharmaceutical or natural estrogen, EE2, is on the Contaminant Candidate List, meaning that it does not have any legal regulation in public water systems [71]. The European Union has taken steps to advise monitoring estrogens found in surface water by proposing maximum detection limits of 0.035 ng/L for EE2 and 0.4 ng/L for E1 and E2, as well as adding E2 to the drinking water watch list [72,73]. However, attaining these limits in practice has been difficult despite modern methods of water treatment [72]. The UK Environmental Agency also monitors E1, E2, and EE2 in surface waters to protect marine life with maximum thresholds of 3 ng/L, 1 ng/L, and 0.1 ng/L, respectively. Because there are many potential sources of environmental ECs and they can easily disperse and contaminate surrounding areas, it is important that all countries create regulations on acceptable levels for ECs in wastewater effluent without causing ecological harm, similar to the guidelines set by the UK. Because there are no international guidelines on pharmaceutical estrogen management, further research is required to inform policies specific to each country’s needs followed by stringent inspection policies of water for estrogenicity. To ensure such regulations are enforced, very precise and rapid methods to measure EC concentrations are required. Such methods using liquid chromatography–mass spectrometry have been validated for E1, E2, E3, and EE2 as they are some of the most prevalent and widely researched, but as novel EC research is conducted, the speed in which testing can be performed and the diversity of ECs measured should be improved upon [73].
4.6. Strengths and Limitations
The strengths of this review include synthesizing and comparing several wastewater treatment processes to remove ECs with an emphasis on sustainability and investigating national guidelines to create actionable recommendations to reduce EC concentrations in the environment. On the other hand, some limitations include a lack of data about EC management practices, particularly in air and soil. None of the studies included in the study were conducted in Africa, and only one was conducted in South America. This may be due to low- and middle-income countries in South America and Africa being mostly developing with limited resources diverting governmental priorities to issues other than environmental and human health. It is also possible that research published in languages other than English, and not reported here, could enrich the discussion in this review. Because management practices of ECs are dependent on environmental factors and regional differences, it is crucial that further research is conducted to identify methods of treating ECs found in other substrates and regional solutions informed by region-specific research.
5. Conclusions
This review highlights the need for comprehensive research and management strategies for pharmaceutical xenoestrogens and naturally occurring estrogens in the environment. These compounds have extensive effects on the health of animals and humans, including endocrinological, vascular, immunological, and neuronal functions, and so require efficient and sustainable management strategies, particularly in aquatic ecosystems. Using approaches such as adsorption, filtration, and enzymatic degradation can help reduce the presence of ECs in water, but further research is required to inform policies standardizing wastewater treatment, address the gap in removing ECs from soil and air, and refine large-scale water treatment technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17020491/s1. PRISMA 2020 Checklist. Reference [74] is cited in supplementary materials.
Author Contributions
Conceptualization, R.Z., M.A. and N.L.; methodology, N.L., R.Z. and M.A.; validation, R.Z. and M.A.; formal analysis, N.L.; investigation, N.L., R.Z. and M.A.; data curation, N.L., R.Z. and M.A.; writing—original draft preparation, N.L.; writing—review and editing, R.Z. and M.A.; visualization, N.L.; supervision, R.Z. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No additional data is available.
Acknowledgments
The authors would like to acknowledge support from the Medical School and the University of Exeter for publication funding. ‘For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission’.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar]
- Zamani, M.R.; Desmond, N.L.; Levy, W.B. Estradiol Modulates Long-Term Synaptic Depression in Female Rat Hippocampus. J. Neurophysiol. 2000, 84, 1800–1808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falah, N.; Torday, J.; Quinney, S.K.; Haas, D.M. Estriol Review: Clinical Applications and Potential Biomedical Importance. Clin. Res. Trials. 2015, 1, 29–33. Available online: http://oatext.com/Estriol-review-Clinical-applications-and-potential-biomedical-importance.php (accessed on 23 September 2024). [CrossRef]
- Oyelowo, T. Chapter 2—Estrogen Concepts. In Mosby’s Guide to Women’s Health; Oyelowo, T., Ed.; Mosby: Saint Louis, MO, USA, 2007; pp. 8–10. Available online: https://www.sciencedirect.com/science/article/pii/B9780323046015500034 (accessed on 23 September 2024).
- Ishikawa, T.; Glidewell-Kenney, C.; Jameson, J.L. Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5α-reductase inhibitor. J. Steroid Biochem. Mol. Biol. 2006, 98, 133–138. [Google Scholar] [CrossRef]
- Cooper, G.M. Signaling Molecules and Their Receptors. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9924/ (accessed on 23 September 2024).
- Paterni, I.; Granchi, C.; Minutolo, F. Risks and benefits related to alimentary exposure to xenoestrogens. Crit. Rev. Food Sci. Nutr. 2017, 57, 3384–3404. [Google Scholar] [CrossRef] [PubMed]
- Swart, J.C.; Pool, E.J. Estrogenic Endocrine-Disrupting Chemicals. In Encyclopedia of Aquatic Ecotoxicology; Férard, J.F., Blaise, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 477–490. [Google Scholar] [CrossRef]
- Inadera, H. The immune system as a target for environmental chemicals: Xenoestrogens and other compounds. Toxicol. Lett. 2006, 164, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Kumar, D.; Singh, D.; Singh, R.K. Endocrine Disruptor Activity of Xenobiotics in Carcinogenesis. In Xenobiotics in Chemical Carcinogenesis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–196. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323905602000042 (accessed on 23 September 2024).
- Lobo, R.A.; Stanczyk, F.Z. New knowledge in the physiology of hormonal contraceptives. Am. J. Obstet. Gynecol. 1994, 170 Pt 2, 1499–1507. [Google Scholar] [CrossRef]
- Ciślak, M.; Kruszelnicka, I.; Zembrzuska, J.; Ginter-Kramarczyk, D. Estrogen pollution of the European aquatic environment: A critical review. Water Res. 2023, 229, 119413. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, X.; Xu, D.; Xiang, Y.; Ling, W.; Chen, M. Contamination and Risk Assessment of Estrogens in Livestock Manure: A Case Study in Jiangsu Province, China. Int. J. Environ. Res. Public Health 2018, 15, 125. [Google Scholar] [CrossRef]
- Yang, C.Z.; Yaniger, S.I.; Jordan, V.C.; Klein, D.J.; Bittner, G.D. Most Plastic Products Release Estrogenic Chemicals: A Potential Health Problem That Can Be Solved. Environ. Health Perspect. 2011, 119, 989. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowski, K.; Podobiński, P.; Cholewińska, P.; Smoliński, J.; Dorobisz, K. Impact of Estrogens Present in Environment on Health and Welfare of Animals. Animals 2021, 11, 2152. [Google Scholar] [CrossRef]
- Hung, H.S.; Yeh, K.J.C.; Hsieh, C.Y.; Chen, T.C. Occurrence and Degradation of Free and Conjugated Estrogens in a River Receiving Feedlot Animal Discharge. Appl. Sci. 2022, 12, 11961. [Google Scholar] [CrossRef]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manage. 2023, 348, 119230. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Verma, N.; Lugani, Y.; Kumar, S.; Asadnia, M. Conventional and Advanced Techniques of Wastewater Monitoring and Treatment. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–48. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128218839000096 (accessed on 23 September 2024).
- Awang, N.; Radzi, A.R.M.; Mahat, R.; Fatihhi, S.J.; Johari, A.; Abd Wahid, K.A.; Yajid, M.A.M. Microbial Fuel Cell in Industrial Wastewater: Treatment Processes and Resource Recovery. In Resource Recovery in Industrial Waste Waters; Elsevier: Amsterdam, The Netherlands, 2023; pp. 353–363. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323953276000324 (accessed on 23 September 2024).
- Bilal, M.; Rizwan, K.; Adeel, M.; Barceló, D.; Awad, Y.A.; Iqbal, H.M.N. Robust strategies to eliminate endocrine disruptive estrogens in water resources. Environ. Pollut. 2022, 306, 119373. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Moreno, J.; Mora, A.; Cervantes-Avilés, P.; Mahlknecht, J. Groundwater contamination pathways of phthalates and bisphenol A: Origin, characteristics, transport, and fate—A review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef]
- De Falco, M.; Laforgia, V. Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland. Int. J. Environ. Res. Public Health 2021, 18, 9772. [Google Scholar] [CrossRef]
- Kim, J.J.; Kumar, S.; Kumar, V.; Lee, Y.M.; Kim, Y.S.; Kumar, V. Bisphenols as a Legacy Pollutant, and Their Effects on Organ Vulnerability. Int. J. Environ. Res. Public Health 2020, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Rochira, V.; Carani, C. Estrogens, Male Reproduction and Beyond. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK278933/ (accessed on 23 September 2024).
- Marlatt, V.; Bayen, S.; Castaneda-Cortès, D.; Delbès, G.; Grigorova, P.; Langlois, V.; Martyniuk, C.; Metcalfe, C.; Parent, L.; Rwigemera, A.; et al. Impacts of endocrine disrupting chemicals on reproduction in wildlife and humans. Environ. Res. 2022, 208, 112584. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Meyer, S.K.; Probert, P.M.E.; Lakey, A.K.; Leitch, A.C.; Blake, L.; Jowsey, P.A.; Cooke, M.P.; Blain, P.G.; Wright, M.C. Environmental Xenoestrogens Super-Activate a Variant Murine ER Beta in Cholangiocytes. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 156, 54–71. [Google Scholar] [CrossRef][Green Version]
- Wakim, L.; Occelli, F.; Paumelle, M.; Brousmiche, D.; Bouhadj, L.; Cuny, D.; Descat, A.; Lanier, C.; Deram, A. Unveiling the presence of endocrine disrupting chemicals in northern French soils: Land cover variability and implications. Sci. Total Environ. 2024, 913, 169617. [Google Scholar] [CrossRef] [PubMed]
- Sanseverino, I.; Gómez, L.; Navarro, A.; Cappelli, F.; Niegowska, M.; Lahm, A.; Barbiere, M.; Porcel-Rodríguez, E.; Valsecchi, S.; Pedraccini, R.; et al. Holistic approach to chemical and microbiological quality of aquatic ecosystems impacted by wastewater effluent discharges. Sci. Total Environ. 2022, 835, 155388. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Li, S.; Duan, Y.; Leong, K.H.; Tu, Y.; Zhou, L. Human health risk assessment of selected pharmaceuticals in the five major river basins, China. Sci. Total Environ. 2021, 801, 149730. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Lin, Y.-S.; Yen, C.-H.; Miaw, C.-L.; Chen, T.-C.; Wu, M.-C.; Hsieh, C.-Y. Identification, contribution, and estrogenic activity of potential EDCs in a river receiving concentrated livestock effluent in Southern Taiwan. Sci. Total Environ. 2018, 636, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Tadayon, S.; Sadighian, J.; Jones, J.; de Mutsert, K.; Huff, T.B.; Foster, G.D. Pharmaceutical chemicals, steroids and xenoestrogens in water, sediments and fish from the tidal freshwater Potomac River (Virginia, USA). J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2017, 52, 686–696. [Google Scholar] [CrossRef]
- Xu, E.G.B.; Liu, S.; Ying, G.G.; Zheng, G.J.S.; Lee, J.H.W.; Leung, K.M.Y. The occurrence and ecological risks of endocrine disrupting chemicals in sewage effluents from three different sewage treatment plants, and in natural seawater from a marine reserve of Hong Kong. Mar. Pollut. Bull. 2014, 85, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Comber, S.; Gardner, M.; Sörme, P.; Leverett, D.; Ellor, B. Active pharmaceutical ingredients entering the aquatic environment from wastewater treatment works: A cause for concern? Sci. Total. Environ. 2018, 613–614, 538–547. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Llorca, M.; Rodríguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Microalgae cultivation on wastewater digestate: β-estradiol and 17α-ethynylestradiol degradation and transformation products identification. J. Environ. Manag. 2015, 155, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Huang, C.; Lu, J.; Wu, Z.; Zhu, X.; Li, H.; Xiao, L.; Luo, Z. Optimizing Adsorption of 17α-Ethinylestradiol from Water by Magnetic MXene Using Response Surface Methodology and Adsorption Kinetics, Isotherm, and Thermodynamics Studies. Molecules 2021, 26, 3150. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Tang, K.; Zhu, S.; Ma, X.; Zhang, K.; Song, Y.; Li, X.; Li, Q.; Liu, Z.; Zhou, K. Competitive Degradation of Steroid Estrogens by Potassium Permanganate Combined with Ultrasound. Int. J. Environ. Res. Public Health 2015, 12, 15434–15448. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; McAdam, E.J.; Lester, J.N.; Cartmell, E. Assessing potential modifications to the activated sludge process to improve simultaneous removal of a diverse range of micropollutants. Water Res. 2014, 62, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.R.; Arias-Salazar, K.; Baynes, A.; Shen, L.Q.; Churchley, J.; Beresford, N.; Gayathri, C.; Gil, R.R.; Kanda, R.; Jobling, S.; et al. Removal of ecotoxicity of 17α-ethinylestradiol using TAML/peroxide water treatment. Sci. Rep. 2015, 5, 10511. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, C.; Deng, J.; Song, Y.; Li, Q.; Guo, Y.; Li, C. Simultaneous Degradation of Estrone, 17β-Estradiol and 17α-Ethinyl Estradiol in an Aqueous UV/H2O2 System. Int. J. Environ. Res. Public Health 2015, 12, 12016–12029. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xia, C.; Jiang, J.; Chen, X.; Zhou, Y.; Yuan, C.; Bai, L.; Meng, S.; Cao, G. Removal of antibiotics and estrogens by nanofiltration and reverse osmosis membranes. J. Hazard. Mater. 2024, 461, 132628. [Google Scholar] [CrossRef]
- Zhao, H.N.; Tian, Z.; Kim, K.E.; Wang, R.; Lam, K.; Kolodziej, E.P. Biotransformation of Current-Use Progestin Dienogest and Drospirenone in Laboratory-Scale Activated Sludge Systems Forms High-Yield Products with Altered Endocrine Activity. Environ. Sci. Technol. 2021, 55, 13869–13880. [Google Scholar] [CrossRef]
- Mainetti, T.; Palmisano, M.; Rezzonico, F.; Stres, B.; Kern, S.; Smits, T.H.M. Broad diversity of bacteria degrading 17ß-estradiol-3-sulfate isolated from river sediment and biofilm at a wastewater treatment plant discharge. Arch. Microbiol. 2021, 203, 4209–4219. [Google Scholar] [CrossRef]
- Islam, R.; Yu, R.M.K.; Andrew-Priestley, M.; Smith, N.; Rahman, M.M.; Tran, T.K.A.; Connor, W.A.O.; MacFarlane, G.R. Secondary treatment phase of tertiary wastewater treatment works significantly reduces estrogenic load. Water Res. 2021, 200, 117257. [Google Scholar] [CrossRef]
- Alves, T.C.; Mota, J.A.X.; Pinheiro, A. Biosorption of organic micropollutants onto lignocellulosic-based material. Water Sci. Technol. 2020, 82, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Gea, M.; Bicchi, C.; Cordero, C.; Schilirò, T.; Varese, G.C. Ecofriendly laccases treatment to challenge micropollutants issue in municipal wastewaters. Environ. Pollut. 2020, 257, 113579. [Google Scholar] [CrossRef] [PubMed]
- Meza, L.C.; Piotrowski, P.; Farnan, J.; Tasker, T.L.; Xiong, B.; Weggler, B.; Murrell, K.; Dorman, F.L.; Heuvel, J.P.V.; Burgos, W.D. Detection and removal of biologically active organic micropollutants from hospital wastewater. Sci. Total Environ. 2020, 700, 134469. [Google Scholar] [CrossRef]
- Välitalo, P.; Massei, R.; Heiskanen, I.; Behnisch, P.; Brack, W.; Tindall, A.J.; Du Pasquier, D.; Küster, E.; Mikola, A.; Schulze, T.; et al. Effect-based assessment of toxicity removal during wastewater treatment. Water Res. 2017, 126, 153–163. [Google Scholar] [CrossRef]
- King, O.C.; van de Merwe, J.P.; McDonald, J.A.; Leusch, F.D.L. Concentrations of levonorgestrel and ethinylestradiol in wastewater effluents: Is the progestin also cause for concern? Environ. Toxicol. Chem. 2016, 35, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.T.B.; Pereira, I.A.C. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017, 108, 160–168. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Pedrosa, M.; Moreira, N.F.F.; Pereira, M.F.R.; Silva, A.M.T. Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU. J. Chromatogr. A 2015, 1418, 140–149. [Google Scholar] [CrossRef]
- Schindler Wildhaber, Y.; Mestankova, H.; Schärer, M.; Schirmer, K.; Salhi, E.; Von Gunten, U. Novel test procedure to evaluate the treatability of wastewater with ozone. Water Res. 2015, 75, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Osachoff, H.L.; Mohammadali, M.; Skirrow, R.C.; Hall, E.R.; Brown, L.L.; van Aggelen, G.C.; Kennedy, C.J.; Helbing, C.C. Evaluating the treatment of a synthetic wastewater containing a pharmaceutical and personal care product chemical cocktail: Compound removal efficiency and effects on juvenile rainbow trout. Water Res. 2014, 62, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wittliff, J.L.; Andres, S.A. Estrogens I: Estrogens and Their Conjugates. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 462–466. Available online: https://www.sciencedirect.com/science/article/pii/B9780123864543010149 (accessed on 29 September 2024).
- van der Burg, B.; Winter, R.; Weimer, M.; Berckmans, P.; Suzuki, G.; Gijsbers, L.; Jonas, A.; van der Linden, S.; Witters, H.; Aarts, J.; et al. Optimization and prevalidation of the in vitro ERα CALUX method to test estrogenic and antiestrogenic activity of compounds. Reprod. Toxicol. 2010, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, G.; Forcucci, F.; Chiarelli, F. Endocrine Disruptor Chemicals and Children’s Health. Int. J. Mol. Sci. 2023, 24, 2671. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Critical role of estrogen in the progression of chronic liver diseases. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 429–434. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Baynes, A.; Nicol, E.; Harris, G.; Webster, T.M.U.; Beresford, N.; Straszkiewicz, M.; Jobling, S.; Tyler, C.R. Feminizing effects of ethinylestradiol in roach (Rutilus rutilus) populations with different estrogenic pollution exposure histories. Aquat. Toxicol. 2022, 249, 106229. [Google Scholar] [CrossRef]
- Vrettakos, C.; Bajaj, T. Levonorgestrel. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539737/ (accessed on 29 September 2024).
- Guven, H.; Dereli, R.K.; Ozgun, H.; Ersahin, M.E.; Ozturk, I. Towards sustainable and energy efficient municipal wastewater treatment by up-concentration of organics. Prog. Energy Combust. Sci. 2019, 70, 145–168. [Google Scholar] [CrossRef]
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, L.; García-Galán, M.J.; Aquino, S.F.; de Cássia Franco Afonso, R.J.; Ferrer, I.; Passos, F.; Mota, C.R. Can high rate algal ponds be used as post-treatment of UASB reactors to remove micropollutants? Chemosphere 2020, 248, 125969. [Google Scholar] [CrossRef]
- Young, P.; Taylor, M.; Fallowfield, H.J. Mini-review: High rate algal ponds, flexible systems for sustainable wastewater treatment. World J. Microbiol. Biotechnol. 2017, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.M.; Dahiya, P. Chapter 14—Enzyme-Based Biodegradation of Toxic Environmental Pollutants. In Development in Wastewater Treatment Research and Processes; Rodriguez-Couto, S., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 311–333. Available online: https://www.sciencedirect.com/science/article/pii/B9780323858397000037 (accessed on 8 October 2024).
- Malode, U.; Patil, Y.S.; Selokar, Y.N.; Yadav, P.R.; Bhagat, R.P.; Nikose, V.M.; Thakare, R.U.; Nimbarte, S. Sustainable approaches for the synthesis of biogenic platinum nanoparticles. Bull. Natl. Res. Cent. 2023, 47, 130. [Google Scholar] [CrossRef]
- REACH Regulation—European Commission. 2024. Available online: https://environment.ec.europa.eu/topics/chemicals/reach-regulation_en (accessed on 20 November 2024).
- Catherine, Z. Managing Pharmaceutical Waste A 10-Step Blueprint for Healthcare Facilities in the United States. 2008. Available online: https://www.epa.gov/system/files/documents/2022-10/10_step_blueprint_guide_final_9-22.pdf (accessed on 30 October 2024).
- The Center for Drug Evaluation and Research (CDER); FDA. Disposal of Unused Medicines: What You Should Know. 2024. Available online: https://www.fda.gov/drugs/safe-disposal-medicines/disposal-unused-medicines-what-you-should-know (accessed on 30 October 2024).
- US EPA, O. National Primary Drinking Water Regulations. 2015. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 22 October 2024).
- US EPA, O. Basic Information on the CCL and Regulatory Determination. 2014. Available online: https://www.epa.gov/ccl/basic-information-ccl-and-regulatory-determination (accessed on 22 October 2024).
- Glineur, A.; Nott, K.; Carbonnelle, P.; Ronkart, S.; Purcaro, G. Development and Validation of a Method for Determining Estrogenic Compounds in Surface Water at the Ultra-Trace Level Required by the EU Water Framework Directive Watch List. J. Chromatogr. A 2020, 1624, 461242. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).