Plant Diversity and Interspecific Interactions in Desert-Oasis Transition Zones: Insights from the Badain Jilin Desert
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Research Methodology
2.2.1. Vegetation Survey
2.2.2. Analysis of Species Importance Values
2.3. Patterns of Interspecific Spatial Distribution
2.4. Calculation of Interspecific Correlations
2.4.1. Significance Test for Association Between Multiple Species
2.4.2. Association Tests for Paired Species
2.4.3. Measurement of the Degree of Interspecific Association
2.5. Data Analysis and Processing
3. Results
3.1. Plant Species Importance Analysis
3.2. Community Species Diversity and Patterns of Spatial Distribution of Populations
3.2.1. Dioecious Plant Community
3.2.2. Herbaceous Plant Community
3.3. Analysis of Spatial Correlation of Populations
3.3.1. Overall Correlation Analysis
3.3.2. Determination and Analysis of Correlations Between Major Populations
3.3.3. Inter-Pair Linkage Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Fang, G.; Li, Z.; Zhang, X.; Gao, L.; Elbeltagi, A.; El Shaer, H.; Duan, W.; Wassif, O.M.A.; Li, Y.; et al. The Crisis in Oases: Research on Ecological Security and Sustainable Development in Arid Regions. Annu. Rev. Environ. Resour. 2024, 49, 1–20. [Google Scholar] [CrossRef]
- Ngabire, M.; Wang, T.; Xue, X.; Liao, J.; Sahbeni, G.; Huang, C.; Song, X.; Duan, H.; Nyiransengiyumva, C. Synergic effects of land-use management systems towards the reclamation of Aeolian Desertified Land in the Shiyang River Basin. Ecol. Indic. 2022, 139, 108882. [Google Scholar] [CrossRef]
- Shen, J.; Li, S.; Liu, L.; Liang, Z.; Wang, Y.; Wang, H.; Wu, S. Uncovering the relationships between ecosystem services and social-ecological drivers at different spatial scales in the Beijing-Tianjin-Hebei region. J. Clean. Prod. 2021, 290, 125193. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Marta, S.; Guerrieri, A.; Gobbi, M.; Ambrosini, R.; Fontaneto, D.; Zerboni, A.; Poulenard, J.; Caccianiga, M.; Thuiller, W. Dynamics of Ecological Communities Following Current Retreat of Glaciers. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 405–426. [Google Scholar] [CrossRef]
- Godoy, O.; Soler-Toscano, F.; Portillo, J.R.; Langa, J.A. The assembly and dynamics of ecological communities in an ever-changing world. Ecol. Monogr. 2024, 94, e1633. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, C.; Wu, D.; Guo, X. Different driving mechanisms of spatial heterogeneity at the community and landscape levels in the wetland ecosystem dominated by hydro-ecological processes. Hydrol. Process. 2023, 37, e14916. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Li, J.; Zhang, C.; Li, M.; Hu, Z.; Cui, X. Effects of grazing disturbance of spatial distribution pattern and interspecies relationship of two desert shrubs. J. For. Res. 2022, 33, 507–518. [Google Scholar] [CrossRef]
- Luo, Y.; Gong, Y. α Diversity of desert shrub communities and Its relationship with Climatic factors in Xin-jiang. Forests 2023, 14, 178. [Google Scholar] [CrossRef]
- Cui, D.; Wang, Q.; Ma, S.; Zhang, Y.; Xu, L.; Yan, L. Natural Regeneration Patterns of Juglans mandshurica in Different Habitats. Forests 2024, 15, 1201. [Google Scholar] [CrossRef]
- Wanishsakpong, W.; Sodrung, K.; Thongteeraparp, A. A Comparison of Nonparametric Statistics and Bootstrap Methods for Testing Two Independent Populations with Unequal Variance. Int. J. Anal. Appl. 2023, 21, 36. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Han, X.; Zhang, W.; Shao, C.; Li, L.; Chen, J.; Han, X.; Zhang, W.; Shao, C. Desert Rangeland Ecosystem. In Grassland Ecosystems of China: A Synthesis and Resume; Springer: Berlin/Heidelberg, Germany, 2020; pp. 401–454. [Google Scholar]
- Groner, E.; Babad, A.; Swiderski, N.B.; Shachak, M. Toward an extreme world: The hyper-arid ecosystem as a natural model. Ecosphere 2023, 14, e4586. [Google Scholar] [CrossRef]
- El-Bana, M.I.; Nijs, I.; Kockelbergh, F. Microenvironmental and vegetational heterogeneity induced by phytogenic nebkhas in an arid coastal ecosystem. Plant Soil 2002, 247, 283–293. [Google Scholar] [CrossRef]
- McGuire, L.P.; Fuller, N.W.; Dzal, Y.A.; Haase, C.G.; Klüg-Baerwald, B.J.; Silas, K.A.; Plowright, R.K.; Lausen, C.L.; Willis, C.K.R.; Olson, S.H. Interspecific variation in evaporative water loss and temperature response, but not metabolic rate, among hibernating bats. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lei, J.; Xu, X.; Gao, P.; Qiu, Y.; Xu, B.; Zhong, X.; Wang, Y.; Yan, J.; Wang, G. Spatial pattern for soil water and chemical properties in Gurbantunggut Desert. Acta Ecol. Sin. 2014, 34, 4380–4389. [Google Scholar] [CrossRef]
- Lortie, C.J.; Cushman, J.H. Effects of a directional abiotic gradient on plant community dynamics and invasion in a coastal dune system. J. Ecol. 2007, 95, 468–481. [Google Scholar] [CrossRef]
- Liu, C.; Jia, X.; Bai, X.; Shao, M. Analysis of soil water use by exotic and native vegetation in a semi-arid area and their associated interspecific competition. Sci. Total. Environ. 2023, 905, 167066. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; Garcia-Gomez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Soliveres, S.; Maestre, F.T.; Bowker, M.A.; Torices, R.; Quero, J.L.; García-Gómez, M.; Cabrera, O.; Cea, A.P.; Coaguila, D.; Eldridge, D.J.; et al. Functional traits determine plant co-occurrence more than environment or evolutionary relatedness in global drylands. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kingsford, R.T.; West, R.S.; Pedler, R.D.; Keith, D.A.; Moseby, K.E.; Read, J.L.; Letnic, M.; Leggett, K.E.A.; Ryall, S.R. Strategic adaptive management planning—Restoring a desert ecosystem by managing introduced species and native herbivores and reintroducing mammals. Conserv. Sci. Pr. 2020, 3, e268. [Google Scholar] [CrossRef]
- Rachkovskaya, E.I.; Bragina, T. Steppes of Kazakhstan: Diversity and Present State. In Eurasian Steppes: Ecological Problems and Livelihoods in a Changing World; Springer: Berlin/Heidelberg, Germany, 2012; pp. 35–54. [Google Scholar] [CrossRef]
- Calvo, L.; Tárrega, R.; De Luis, E. Secondary succession after perturbations in scrubland community. Acta Oecol. 2002, 23, 393–404. [Google Scholar] [CrossRef]
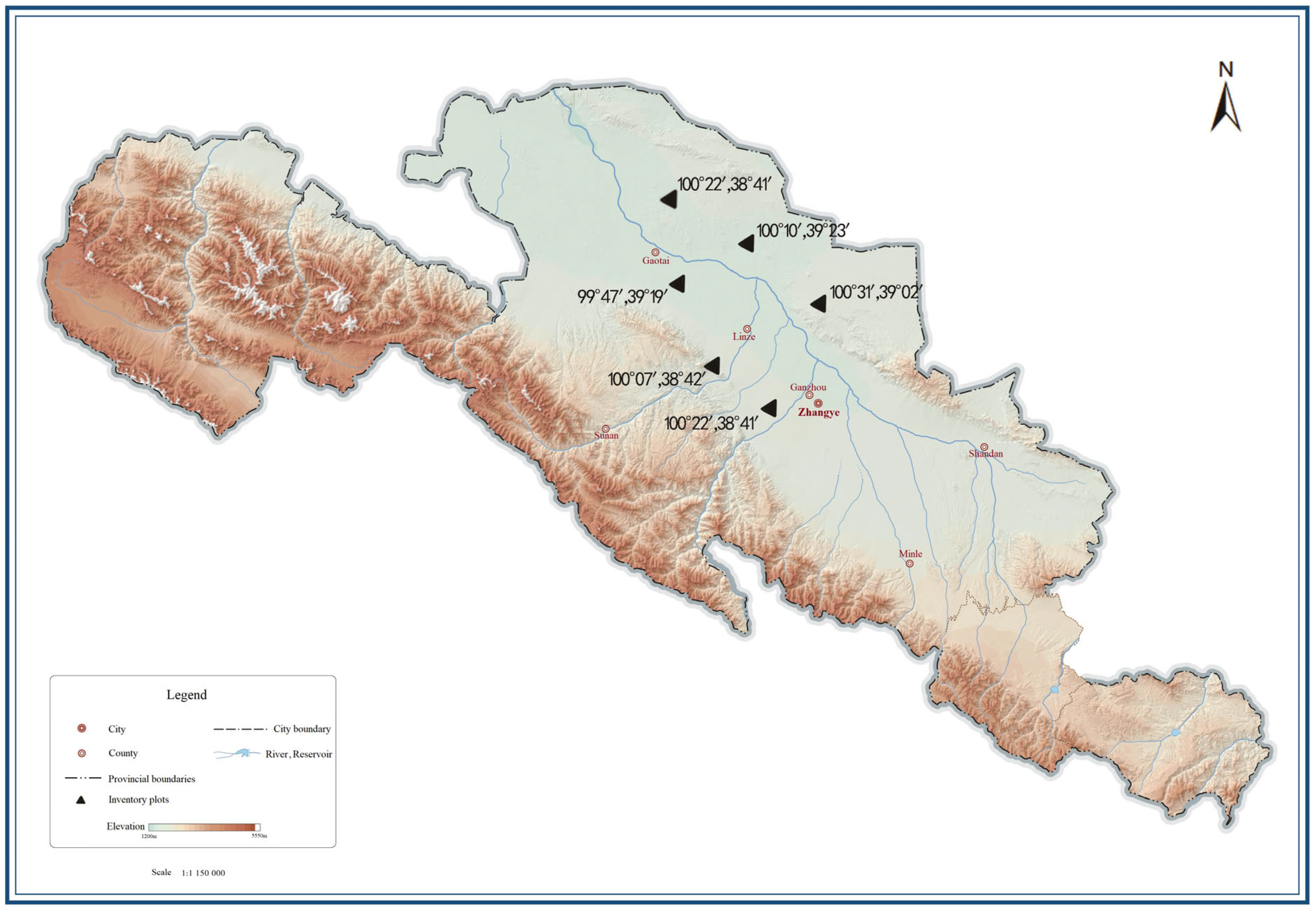
| Sample Plot | Location | Elevation (m) | Constriction (i.e., Degree of Depression) | Soil Type | Shrub Species | Herbaceous Species |
|---|---|---|---|---|---|---|
| South Beach, Ganzhou District | N 38°42′0.905″ E 100°21′46.673″ | 1848.8 | 65 | sandy soil | Pearl Pork Hair Vegetable | tundra |
| Ganshu District Rabbit Dam Beach | N 39°03′56.555″ E 100°31′2.185″ | 1485.7 | 45 | sandy loam and gravel | red sand | Semen euryales (botany) |
| Linze boardwalk | N 38°42′0.905″ E 100°21′46.673″ | 1395.8 | 30 | sandy soil | red sand | tarragon |
| Linzehpingchuan, town in Gansu | N 39°22′59.056″ E 100°09′11.440″ | 1385.6 | 70 | sandy soil | red sand | tarragon |
| Kurozumi, Kotai | N 39°27′20.455″ E 99°45′18.865″ | 1344.7 | 25 | desert soil | red sand | Artifacts of Inner Mongolia |
| Godai Nanhua | N 39°17′1.122″ E 99°46′12.064″ | 1426.6 | 20 | sandy loam and gravel | red sand | sargassum |
| Serial Number | Shrub | Herb | Important Value | |||||
|---|---|---|---|---|---|---|---|---|
| Species | Important Value | Species | Important Value | Species | Important Value | Species | ||
| 1 | Reaumuria songarica (Pall.) Maxim | 111.175 | Aristida adscensionis L. | 48.6174 | Eragrostis pilosa (L.) P. Beauv | 8.693 | Corispermum hyssopifolium L. | 1.785 |
| 2 | Caroxylon passerinum (Bunge) Akhani & Roalson | 54.896 | Carex tristachya Thunb. | 23.3892 | Neotrinia splendens (Trin.) M. Nobis | 4.817 | Peganum harmala L. | 1.716 |
| 3 | Nitraria tangutorum Bobrov | 24.422 | Chloris radiata (L.) Sw. | 18.2158 | Limonium aureum (L.) Chaz | 3.772 | Halogeton arachnoideus (Bunge) Moq. | 1.712 |
| 4 | Sympegma regelii Bunge | 20.111 | Grubovia dasyphylla (Fisch. & C.A. Mey) | 13.6116 | Arnebia fimbritum (Maxim) | 3.187 | Bothriospermum chinense Bunge | 1.672 |
| 5 | Calligonum mongolicum Turcz | 18.740 | Cleistogenes songorica (Roshev.) Ohwi | 13.6054 | Tragus racemosus (L.) All. | 3.176 | Arnebia szechenyi Kanitz | 1.608 |
| 6 | Caragana jubata (Pall.) Poir. | 18.677 | Salsola paulsenii Litv. | 11.8383 | Lepidium apetalum Willd. | 2.608 | Suaeda glauca (Bunge) Bunge | 1.593 |
| 7 | Artemisia frigida Willd. | 18.457 | Artemisia scoparia Waldst. & Kit. | 11.4009 | Corispermum squarrosum (L.) Moq. | 2.021 | Zygophyllum fabago L. | 1.587 |
| 8 | Artemisia xerophytica Krasch. | 16.578 | Stipa tianschanica Roshev | 10.3616 | Cynanchum thesioides (Freyn) | 1.989 | ||
| 9 | Asterothamnus centrali-asiaticus Novopokr | 8.134 | Salsola tragys L. | 10.0202 | Astragalus pseudotataricus Boriss | 1.812 | ||
| 10 | Hedysarum scoparium Fisch. & C.A. Mey. | 5.312 | ||||||
| H′ | 1.754 | 2.498 | ||||||
| R | 1.343 | 4.343 | ||||||
| C | 0.265 | 0.091 | ||||||
| J | 2.197 | 3.367 | ||||||
| Community Type | Sample Plot | Mean Value of Population Multiplicity | Population Multivariate Variance | Diffusion Coefficient | Clumping Indicator | Average Congestion Factor | Aggregation Index PAI | Negative Binomial Distribution Parameter | Cassie’s Indicator | t-Test | Green’s Coefficient GI | Distribution Pattern |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dioecious | 1 | 66 | 4050 | 61.36 | 60.36 | 126.36 | 1.91 | 1.09 | 0.91 | 49.92 | 60.36 | congregate |
| 2 | 15.25 | 132.92 | 8.72 | 7.72 | 22.97 | 1.51 | 1.98 | 0.51 | 5.65 | 2.57 | congregate | |
| 3 | 12.8 | 397.2 | 31.03 | 30.03 | 42.83 | 3.35 | 0.43 | 2.35 | 24.41 | 7.51 | congregate | |
| 4 | 8.2 | 16.2 | 1.98 | 0.98 | 9.18 | 1.12 | 8.41 | 0.12 | −0.02 | 0.24 | uniformly | |
| 5 | 23 | 428.5 | 18.63 | 17.63 | 40.63 | 1.77 | 1.30 | 0.77 | 13.98 | 4.41 | congregate | |
| 6 | 16.67 | 424.33 | 25.46 | 24.46 | 41.13 | 2.47 | 0.68 | 1.47 | 19.73 | 12.23 | congregate | |
| herbaceous | 1 | 20.5 | 1841.9 | 89.85 | 88.85 | 109.35 | 5.33 | 0.23 | 4.33 | 62.12 | 17.77 | congregate |
| 2 | 29.17 | 1134.17 | 38.89 | 37.89 | 67.05 | 2.30 | 0.77 | 1.30 | 31.02 | 7.58 | congregate | |
| 3 | 3.5 | 25.39 | 7.25 | 6.25 | 9.75 | 2.79 | 0.56 | 1.79 | 4.42 | 0.69 | congregate | |
| 4 | 11.2 | 183.29 | 16.37 | 15.37 | 26.57 | 2.37 | 1.37 | 1.37 | 12.08 | 1.71 | congregate | |
| 5 | 1.89 | 0.86 | 0.46 | −0.54 | 1.34 | 0.71 | −3.47 | −0.29 | −1.09 | −0.07 | uniformly | |
| 6 | 8.5 | 190.7 | 22.44 | 21.44 | 29.94 | 3.52 | 0.40 | 2.52 | 17.18 | 4.29 | congregate |
| Phase | Variance Ratio VR | Test Statistic w | Measurements | Logarithm of Highly Significant Correlation | Logarithm of Significant Correlation | Logarithm of Association (math.) | Unrelated Logarithm | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Greater Than Zero | Carry (On One’s Back) | Greater Than Zero | Carry (On One’s Back) | Greater Than Zero | Carry (On One’s Back) | |||||
| Dioecious | 0.864 | 186.4 | Non-significant positive linkage | 4 | 2 | 2 | 0 | 2 | 0 | 35 |
| Herbaceous | 1.686 | 168.6 | Significant negative linkage | 9 | 2 | 3 | 16 | 0 | 0 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, P.; Amoah, I.D. Plant Diversity and Interspecific Interactions in Desert-Oasis Transition Zones: Insights from the Badain Jilin Desert. Sustainability 2025, 17, 1259. https://doi.org/10.3390/su17031259
Chen J, Zhang P, Amoah ID. Plant Diversity and Interspecific Interactions in Desert-Oasis Transition Zones: Insights from the Badain Jilin Desert. Sustainability. 2025; 17(3):1259. https://doi.org/10.3390/su17031259
Chicago/Turabian StyleChen, Jinlong, Pengju Zhang, and Isaac Dennis Amoah. 2025. "Plant Diversity and Interspecific Interactions in Desert-Oasis Transition Zones: Insights from the Badain Jilin Desert" Sustainability 17, no. 3: 1259. https://doi.org/10.3390/su17031259
APA StyleChen, J., Zhang, P., & Amoah, I. D. (2025). Plant Diversity and Interspecific Interactions in Desert-Oasis Transition Zones: Insights from the Badain Jilin Desert. Sustainability, 17(3), 1259. https://doi.org/10.3390/su17031259






