Tetracyanoquinodimethane and Its Derivatives as Promising Sustainable Materials for Clean Energy Storage and Conversion Technologies: A Review
Abstract
1. Introduction
- Modern sustainable TCNQ organic molecular solids for efficient stable photovoltaics;
- Cathode TCNQ-based materials in sustainable high-capacity and long-lifetime batteries;
- TCNQ-based triboelectric nanogenerators as an efficient and high-performance wearable electronic power source.
2. Using TCNQ and Its Derivatives for High-Efficiency Stable Photovoltaics
3. TCNQ-Based Materials in Sustainable Green Energy Devices
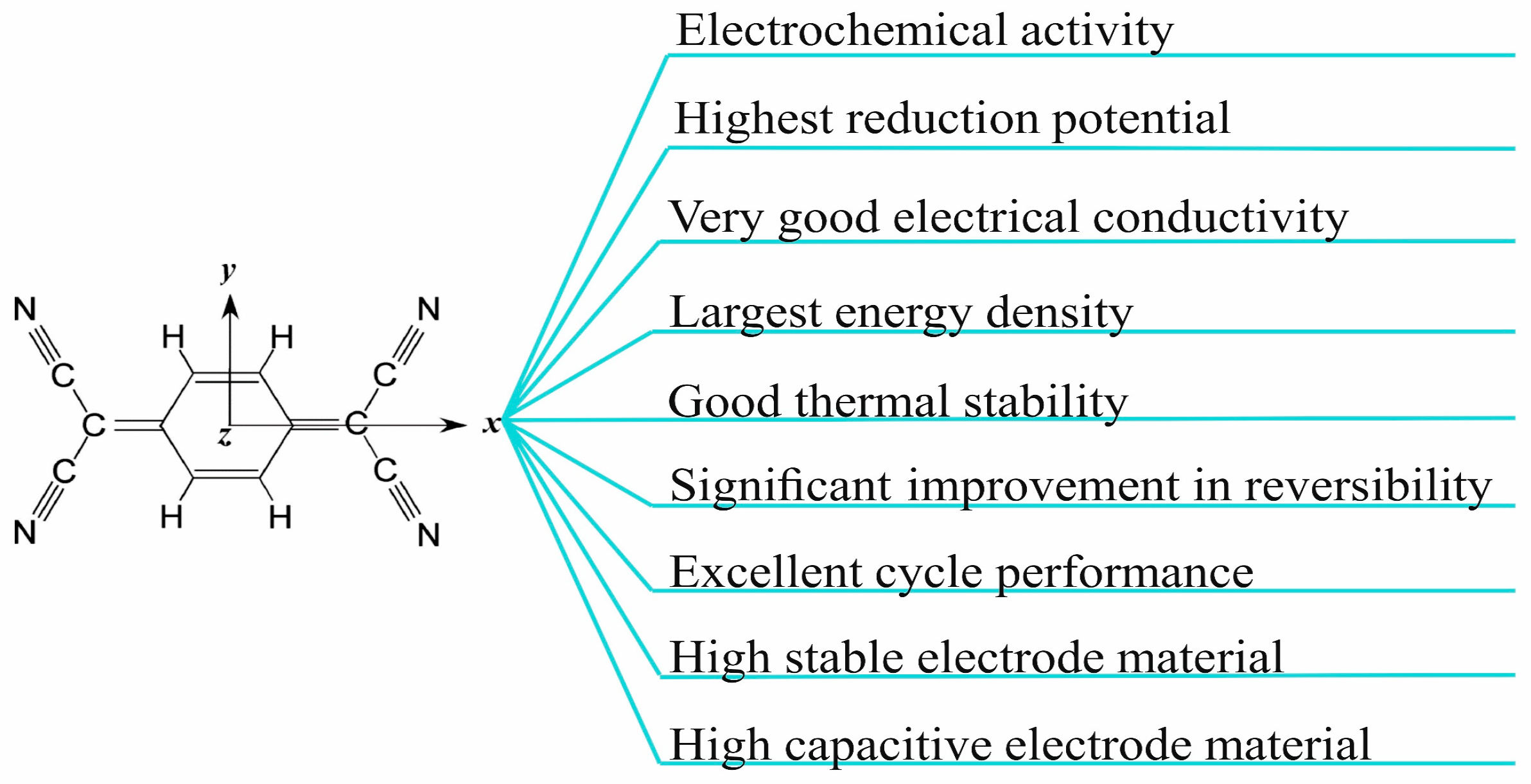
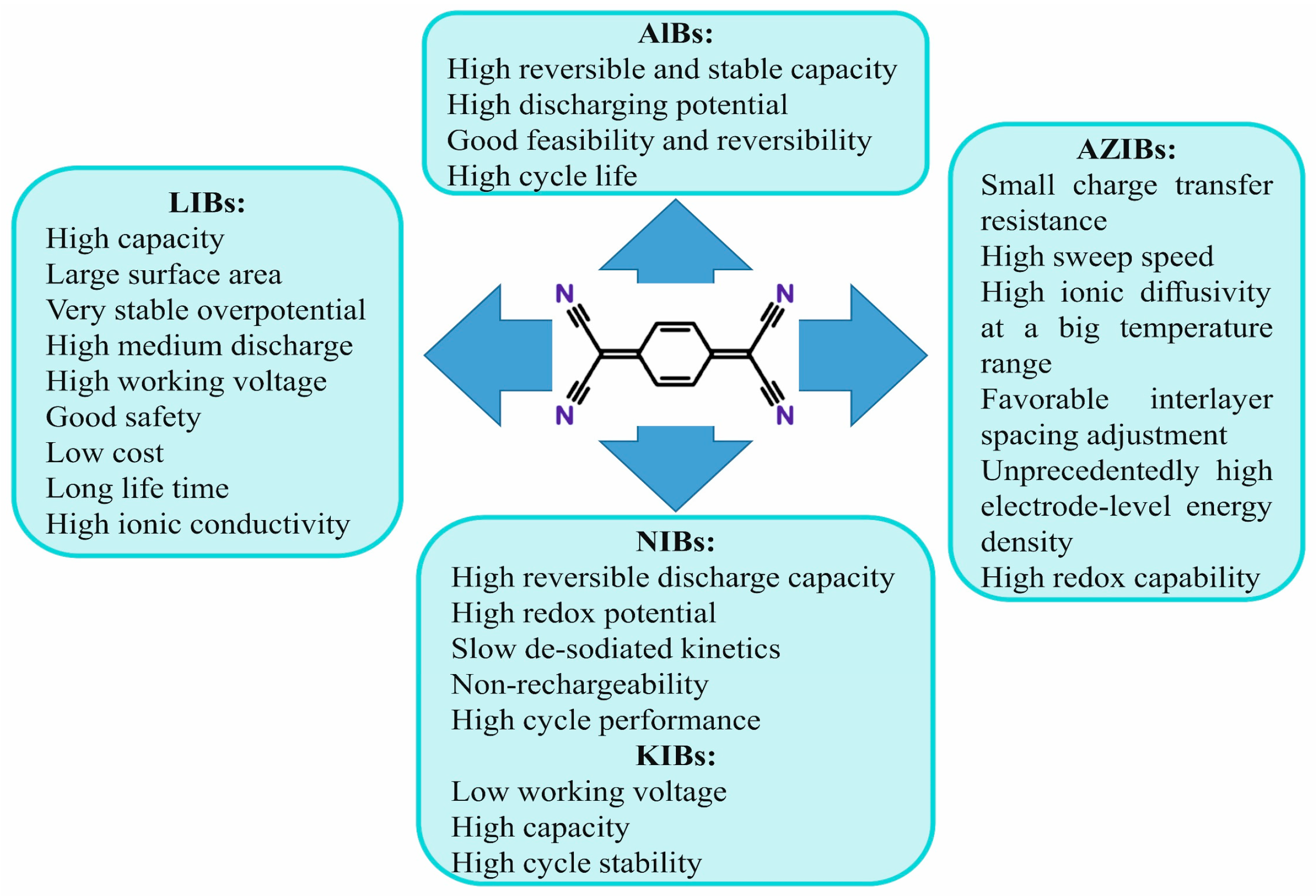
4. Cathode TCNQ-Based Materials in Sustainable Batteries
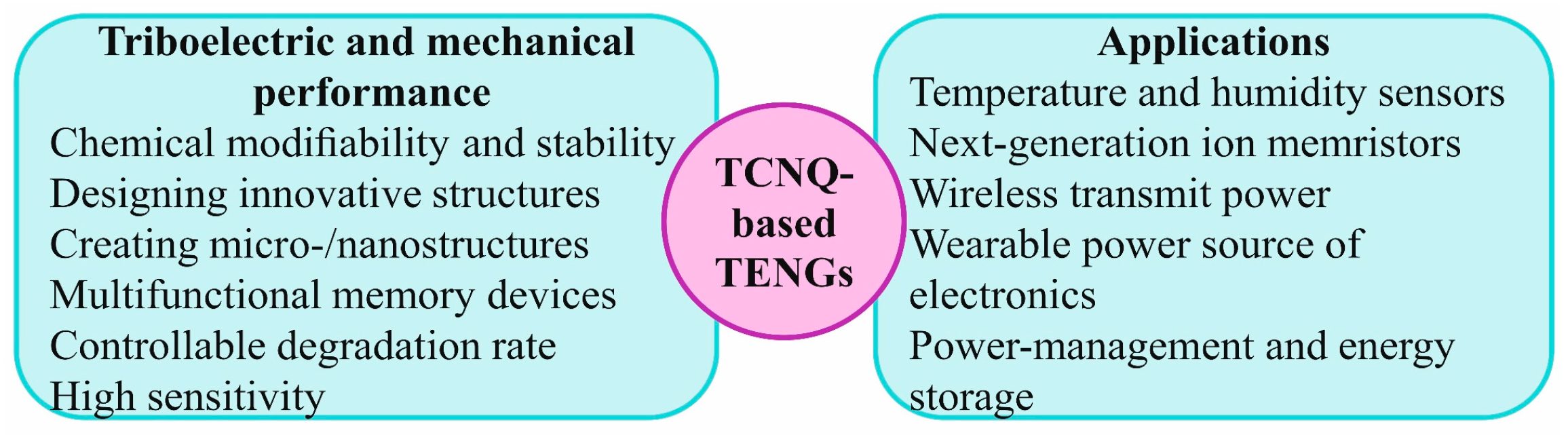
5. TCNQ-Based Triboelectric Nanogenerators as Sustainable Wearable Power Sources for Energy Conversion
6. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AZIB | aqueous zinc ion batteries |
| CABI | n-type lead-free Cu2AgBiI6 perovskite |
| CCP | cyanuric chloride and pyrene |
| COF | covalent organic framework |
| CTC | charge transfer complex |
| CVD | chemical vapor deposition |
| DADQ | diaminodicyanoquinodimethane |
| DIB | dual-ion battery |
| DPPS | decaphenylpentacyclosilane |
| DSSC | dye-sensitized solar cell |
| FF | fill factor |
| HTL | hole transport layer |
| ITO | indium tin oxide |
| LIB | lithium-ion battery |
| MOF | metal–organic framework |
| NIB | sodium-ion battery |
| OCTC | organic charge transfer complex |
| OIHP | organic–inorganic halide perovskite |
| OSC | organic solar cell |
| PANI | polyaniline |
| Pc | phthalocyanine |
| PCE | power conversion efficiency |
| PEAI | phenylethylammonium iodide |
| PEO | polyethylene oxide |
| P3HT | poly(3-hexylthiophene) |
| PMMA | polymethyl methacrylate |
| PNZ | phenazine |
| PPy | polypyrrole |
| PSC | perovskite solar cell |
| PTh | polythiophene |
| PTAA | poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] |
| PTTA | poly(triarylamine) |
| PVA | polyvinyl alcohol |
| PVK | poly(9-vinylcarbazole) |
| SHE | standard hydrogen electrode |
| STSC | solar thermal selective coating |
| TCNQ | 7,7′,8,8′-tetracyanoquinodimethane |
| TENG | triboelectric nanogenerator |
| THF | tetrahydrofuran |
| TL | thin layer |
| TTF | tetrathiafulvalene |
References
- Melby, L.R.; Harder, R.J.; Hertler, W.R.; Mahler, W.; Benson, R.E.; Mochel, W.E. Substituted quinodimethans. II. Anion-radical derivatives and complexes of 7,7,8,8-tetracyanoquinodimethan. J. Am. Chem. Soc. 1962, 84, 3374–3387. [Google Scholar] [CrossRef]
- Starodub, V.A.; Starodub, T.N. Radical anion salts and charge transfer complexes based on tetracyanoquinodimethane and other strong π-electron acceptors. Rus. Chem. Rev. 2014, 83, 391–438. [Google Scholar] [CrossRef]
- Starodub, W.; Starodub, T.; Chojnacki, J. Physicochemistry of Materials of Modern Electronics and Spintronics; Scientific Publishing House PWN SA: Warsaw, Poland, 2019; p. 360. ISBN 978-83-01-20734-2. (In Polish) [Google Scholar]
- Starodub, T.; Michalkiewicz, S. TCNQ and its Derivatives as Electrode Materials in Electrochemical Investigations—Achievement and Prospects: A Review. Materials 2024, 17, 5864. [Google Scholar] [CrossRef] [PubMed]
- Acker, D.S.; Hertler, W.R. Substituted quinodimethans. I. Preparation and chemistry of 7,7,8,8-tetracyanoquinodimethan. J. Am. Chem. Soc. 1962, 84, 3370–3374. [Google Scholar] [CrossRef]
- Ballester, L.; Gutierrez, A.; Perpinan, M.F.; Azcondo, M.T. Supramolecular architectures in low dimensional TCNQ compounds containing nickel and copper polyamine fragments. Coord. Chem. Rev. 1999, 190–192, 447–470. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Rios, C.; Jiménez-Sandoval, O.; Salcedo, R. A Comparative Study of the Semiconductor Behavior of Organic Thin Films: TCNQ-Doped Cobalt Phthalocyanine and Cobalt Octaethylporphyrin. Molecules 2020, 25, 5800. [Google Scholar] [CrossRef]
- O’Mullane, A.P.; Fay, N.; Nafady, A.; Bond, A.M. Preparation of metal-TCNQ charge-transfer complexes on conducting and insulating surfaces by photocrystallization. J. Am. Chem. Soc. 2007, 129, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Starodub, T.N.; Barszcz, B.; Bednarski, W.; Mizera, A.; Starodub, V.A. Optical properties of RAS (N-CH3-2-NH2-5Cl-Py)(TCNQ)(CH3CN) solvate. J. Mol. Struct. 2020, 1201, 127121. [Google Scholar] [CrossRef]
- Tseng, T.C.; Urban, C.; Wang, Y.; Otero, R.; Tait, S.L.; Alcamí, M.; Ecija, D.; Trelka, M.; Gallego, J.M.; Lin, N.; et al. Charge-transfer-induced structural rearrangements at both sides of organic/metal interfaces. Nat. Chem. 2010, 2, 374–379. [Google Scholar] [CrossRef]
- Fernandez, C.A.; Martin, P.C.; Schaef, T.; Bowden, M.E.; Thallapally, P.K.; Dang, L.; Xu, W.; Chen, X.; McGrail, B.P. An electrically switchable metal-organic framework. Sci. Rep. 2014, 4, 6114–6120. [Google Scholar] [CrossRef]
- Tan, C.-H.; Ma, X.; Zhu, Q.-L.; Huang, Y.-H.; Wen, Y.-H.; Hu, S.-M.; Sheng, T.-L.; Wu, X.-T. Synthesis and characterization of cobalt(III) cyanide complexes: Cobalt participation in the decomposition of radical anion of TCNQ. CrystEngComm 2012, 14, 8708–8713. [Google Scholar] [CrossRef]
- Kaim, W.; Moscherosch, M. The coordination chemistry of TCNE, TCNQ and related polynitrile π acceptors. Coord. Chem. Rev. 1994, 129, 157–193. [Google Scholar] [CrossRef]
- Starodub, T.; Starodub, V. Sole anionorodnikowe TCNQ z kationami kompleksowymi metali przejściowych. Wiad. Chem. 2020, 74, 761–796. [Google Scholar]
- Ungor, O.; Shatruk, M. Transition metal complexes with fractionally charges TCNQ radical anions as structural templates for multifunctional molecular conductors. Polyhedron 2020, 177, 114254–114271. [Google Scholar] [CrossRef]
- Mao, G.; Kilani, M.; Ahmed, M. Review—Micro/Nanoelectrodes and Their Use in Electrocrystallization: Historical Perspective and current trend. J. Electrochem. Soc. 2022, 169, 022505–022517. [Google Scholar] [CrossRef]
- Saito, G.; Yoshida, Y. Frontiers of Organic Conductors and Superconductors. Top. Curr. Chem. 2012, 312, 67–126. [Google Scholar] [CrossRef]
- Jerome, D. Organic conductors: From charge density wave TTF-TCNQ to superconducting (TMTSF)2PF6. Chem. Rev. 2004, 104, 5565–5591. [Google Scholar] [CrossRef]
- Horiuchi, S.; Tokura, Y. Organic ferroelectrics. Nat. Mater. 2008, 7, 357–366. [Google Scholar] [CrossRef]
- Li, Q.-S.; Ye, B.-C.; Liu, B.-X.; Zhong, J.-J. Inprovement of the performance of H2O2 oxidation at low working potential by incorporating TTF-TCNQ into a platinum wire electrode for glucose determination. Biosens. Bioelectron. 1999, 14, 327–334. [Google Scholar] [CrossRef]
- Palmisano, F.; Zambonin, P.; Centonze, D.; Quinto, M. A disposable, reagentless, third-generation glucose biosensor based on overoxidized poly(pyrrole)/tetrathiafulvalene-tetracyanoquinodimethane composite. Anal. Chem. 2002, 74, 5913–5918. [Google Scholar] [CrossRef]
- Hernández-Cruz, M.; Galán-Vidal, C.A.; Álvarez-Romero, G.A.; Ramírez-Silva, M.T.; Páez-Hernández, M.E.; González-Vidal, J.L. Behavior of Two and Three Electrode Configuration and Different Mediators in Working Electrode on Development of Disposable Screen-Printing Biosensors for Determination of Free Cholesterol. J. Mex. Chem. Soc. 2013, 57, 47–53. [Google Scholar] [CrossRef]
- Yuge, R.; Yudasaka, M.; Maigne, A.; Tomonari, M.; Miyawaki, J.; Kubo, Y.; Imai, H.; Ichihashi, T.; Iijima, S. Adsorption Phenomena of Tetracyano-p-quinodimethane on Single-Wall Carbon Nanohorns. J. Phys. Chem. C 2008, 112, 5416–5422. [Google Scholar] [CrossRef]
- Khanra, P.; Lee, C.-N.; Kuila, T.; Kim, N.H.; Park, M.J.; Lee, J.H. 7,7,8,8-tetracyanoquinodimethane-assisted one-step electrochemical exfoliation of graphite and its performance as an electrode material. Nanoscale 2014, 6, 4864–4873. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, C.; Zhang, R.; Lv, D.; Li, S.; Zhang, D.; Liu, L.; Fernandez, C. Glassy carbon electrode modified with 7,7,8,8-tetracyanoquinodimethane and graphene oxide triggered a synergistic effect: Low-potential amperometric detection of reduced glutathione. Biosens. Bioelectron. 2017, 96, 1–7. [Google Scholar] [CrossRef]
- Sivasankaran, R.P.; Das, P.K.; Arunachalam, M.; Kanase, R.S.; Il Park, Y.; Seo, J.; Kang, S.H. TiO2 Nanotube Arrays Decorated with Reduced Graphene Oxide and Cu–Tetracyanoquinodimethane as Anode Materials for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2021, 4, 13218–13233. [Google Scholar] [CrossRef]
- Shimomura, S.; Kitagawa, S. Soft porous crystal meets TCNQ: Charge transfer-type porous coordination polymers. J. Mater. Chem. 2011, 21, 5537–5546. [Google Scholar] [CrossRef]
- Miyasaka, H.; Izawa, T.; Takahashi, N.; Yamashita, M.; Dunbar, K.R. Long-Range Ordered Magnet of a Charge-Transfer Ru24+/TCNQ Two-Dimensional Network Compound. J. Am. Chem. Soc. 2006, 128, 11358–11359. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Matsunaga, S.; Yamashita, M.; Sugimoto, K.; Mori, T.; Toyota, N.; Dunbar, K.R. Control of Charge Transfer in a Series of Ru2II,II/TCNQ Two-Dimensional Networks by Tuning the Electron Affinity of TCNQ Units: A Route to Synergistic Magnetic/Conducting Materials. J. Am. Chem. Soc. 2010, 132, 1532–1544. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Yan, P.; Hou, G.; Li, G. Two 7,7,8,8-tetracyanoquinodimethane lead and zinc complexes featuring 3D and 0D structure: Synthesis, structure and electrochemical properties. Inorg. Chim. Acta 2014, 413, 32–37. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Ren, Z.; Luo, J.; Zhang, C.; Cao, C.; Yuan, H.; Pang, Y. Marine biomaterial-based triboelectric nanogenerators: Insights and applications. Nano Energy 2024, 119, 109046–109068. [Google Scholar] [CrossRef]
- Sarma, S.J.; Brar, S.K.; Sydney, E.B.; Le Bihan, Y.; Buelna, G.; Soccol, C.R. Microbial hydrogen production by bioconversion of crude glycerol: A review. Int. J. Hydrogen Energy 2012, 37, 6473–6490. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.; Luo, C. Organic Electrode Materials for Metal Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5361–5380. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Q. Recent Progress in Multivalent Metal (Mg, Zn, Ca, and Al) and Metal-Ion Rechargeable Batteries with Organic Materials as Promising Electrodes. Small 2019, 15, 1805061–1805080. [Google Scholar] [CrossRef]
- Huang, J.; Dong, X.; Guo, Z.; Wang, Y. Progress of Organic Electrodesing Aqueous Electrolyte for Energy Storage and Conversion. Angew. Chem. Int. Ed. 2020, 59, 18322–18333. [Google Scholar] [CrossRef]
- Gannett, C.N.; Kim, J.; Tirtariyadi, D.; Milner, P.J.; Abruna, H.D. Investigation of ion-electrode interactions of linear polyimides and alkali metal ions for next generation alternative-ion batteries. Chem. Sci. 2022, 13, 9191–9201. [Google Scholar] [CrossRef]
- Cha, H.; Kim, H.N.; An, T.K.; Kang, M.S.; Kwon, S.K.; Kim, Y.H.; Park, C.E. Effects of Cyano-Substituents on the Molecular Packing Structures of Conjugated Polymers for Bulk-Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 15774–15782. [Google Scholar] [CrossRef]
- Häupler, B.; Burges, R.; Janoschka, T.; Jähnert, T.; Wild, A.; Schubert, U.S. PolyTCNQ in organic batteries: Enhanced capacity at constant cell potential using two-electron-redox-reactions. J. Mater. Chem. A 2014, 2, 8999–9001. [Google Scholar] [CrossRef]
- Neelamraju, B.; Watts, K.E.; Pemberton, J.E.; Ratcliff, E.L. Correlation of Coexistent Charge Transfer States in F4TCNQ-Doped P3HT with Microstructure. J. Phys. Chem. Lett. 2018, 9, 6871–6877. [Google Scholar] [CrossRef]
- Bavdane, P.P.; Dave, V.; Sreenath, S.; Madiyan, P.; Nagarale, R.K. Redox-active organic molecule encapsulated MWCNT catholyte for aqueous zinc flow battery. Next Energy 2025, 9, 100379. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y.; Meng, J.; Lin, X.; Huang, Z.; Shen, Y.; Huang, Y. Boosting the cyclability of tetracyanoquinodimethane (TCNQ) as cathode material in aqueous battery with high valent cation. Energy Storage Mater. 2021, 43, 492–498. [Google Scholar] [CrossRef]
- Meng, J.; Tang, Q.; Zhou, L.; Zhao, C.; Chen, M.; Shen, Y.; Zhou, J.; Feng, G.; Shen, Y.; Huang, Y. A Stirred Self-Stratified Battery for Large-Scale Energy Storage. Joule 2020, 4, 953–966. [Google Scholar] [CrossRef]
- Wang, S.; Hu, N.; Huang, Y.; Deng, W. Charge-transfer complex promoted energy storage performance of single-moiety organic electrode materials in aqueous zinc-ion battery at low temperatures. Appl. Surf. Sci. 2023, 619, 156725–156733. [Google Scholar] [CrossRef]
- Yang, M.; Leon, N.; Pan, B.; Yu, Z.; Cheng, L.; Liao, C. Mechanistic insights in quinone-based zinc batteries with nonaqueous electrolytes. J. Electrochem. Soc. 2020, 167, 100536. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, Q.; Tang, W.; Cheng, M.; Wang, X.; Liu, S.; Gao, J.; Wang, M.; Xiong, M.; Hu, J.; et al. Ultra-Stable, Ultra-Long-Lifespan and Ultra-High-Rate Na-Ion Batteries Using Small-Molecule Organic Cathodes. Energy Storage Mater. 2021, 41, 738–747. [Google Scholar] [CrossRef]
- Mike, J.F.; Lutkenhaus, J.L. Recent advances in conjugated polymer energy storage. J. Polym. Sci. B Polym. Phys. 2013, 51, 468–480. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Meng, C.; Chen, T.; Fang, C.; Huang, Y.; Hu, P.; Tong, Y.; Bian, T.; Zhang, J.; Wang, Z.; Yuan, A. Multiple Active Sites: Lithium Storage Mechanism of Cu-TCNQ as an Anode Material for Lithium-Ion Batteries. Chem. Asian J. 2019, 14, 4289–4295. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Zhang, Z.; Zhao, C.; Sun, P.; Bai, P.; Yang, J.; Zhou, Z.; Xu, Y. Electrolyte regulated solid-electrolyte interphase enables long cycle life performance in organic cathodes for potassium-ion batteries. Adv. Funct. Mater. 2019, 29, 1807137. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, C.; Zeng, R.; Liu, Y.; Zhang, W.; Wang, Y.; Liu, Q.; Huang, Y. In Situ-Formed Hierarchical Metal–Organic Flexible Cathode for High-Energy Sodium-Ion Batteries. ChemSusChem 2017, 10, 4704–4708. [Google Scholar] [CrossRef]
- Fang, C.; Ye, Z.; Wang, Y.; Zhao, X.; Huang, Y.; Zhao, R.; Liu, J.; Han, J.; Huang, Y. Immobilizing an organic electrode material through p-p interaction for high performance Li-organic batteries. J. Mater. Chem. A 2019, 7, 22398–22404. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Wang, Y.; Lv, X.; Wang, H.-G. A redox-active metal–organic compound for lithium/sodium-base dual-ion batteries. J. Colloid Interface Sci. 2022, 606, 1024–1030. [Google Scholar] [CrossRef]
- Geng, Z.; Shi, W.; Wang, J.; Zhang, X.; Hu, N.; Wang, S.; Deng, W. A high-voltage solid-state organic lithium battery based on 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane cathode. Int. J. Energy Res. 2022, 46, 12316–12322. [Google Scholar] [CrossRef]
- Bitenc, J.; Pirnat, K.; Luzanin, O.; Dominko, R. Organic Cathodes, a Path toward Future Sustainable Batteries: Mirage or realistic Future? Chem. Mater. 2024, 36, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Abicho, S.; Hailegnaw, B.; Mayr, F.; Cobet, M.; Yumusak, C.; Sergawi, A.; Yohannes, T.; Kaltenbrunner, M.; Scharber, M.C.; Workneh, G.A. The role of TCNQ for surface and interface passivation in inverted perovskite solar cells. Mater. Ren. Sustain. Energy 2025, 14, 11. [Google Scholar] [CrossRef]
- Schloemer, T.H.; Christians, J.A.; Luther, J.M.; Sellinger, A. Doping strategies for small molecule organic hole-transport materials: Impacts on perovskite solar cell performance and stability. Chem. Sci. 2019, 10, 1904–1935. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dahlström, S.; Ahläng, C.; Wilken, S.; Degterev, A.; Matuhina, A.; Hadadian, M.; Markkanen, M.; Aitola, K.; Kamppinen, A.; et al. Beyond hydrophobicity: How F4-TCNQ doping of the hole transport material improves stability of mesoporous triple-cation perovskite solar cells. J. Mater. Chem. A 2022, 10, 11721–11731. [Google Scholar] [CrossRef]
- Mohitkar, A.; Renuka, H.; Goel, S.; Jayanty, S. Efficient Standalone Flexible Small Molecule Organic Solar Cell Devices: Structure-Performance Relation Among Tetracyanoquinodimethane Derivatives. ACS Omega 2023, 8, 40836–40847. [Google Scholar] [CrossRef]
- Mi, B.X.; Gao, Z.Q.; Cheah, K.W.; Chen, C.H. Organic Light-Emitting Diodes Using 3,6-Difluoro-2,5,7,7,8,8-Hexacyanoquinodimethane as p-Type Dopant. Appl. Phys. Lett. 2009, 94, 073507–073510. [Google Scholar] [CrossRef]
- Song, T.; Chen, Q.; Zhou, H.; Jiang, C.; Wang, H.; Yang, Y.M.; Liu, Y.; You, J.; Yang, Y. Perovskite solar cells: Film formation and properties. J. Mater. Chem. A 2015, 3, 9032–9050. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lin, Z.; Xu, Y.; Zhang, B.; Guo, X.; Hu, Z.; Zhao, X.; Hao, Y.; Chang, J. Dynamic p-n junction direct current-generating triboelectric nanogenerators based on lead-free perovskite. Nano Energy 2025, 138, 110857. [Google Scholar] [CrossRef]
- Li, Q.X.; Shi, C.; Huang, M.L.; Wei, X.; Yan, H.; Yang, C.L.; Yuan, A.H. B- and N-embedded color-tunable phosphorescent iridium complexes and B-N Lewis adducts with intriguing structural and optical changes. Chem. Sci. 2019, 10, 3257–3263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.K.; Zhou, J.P.; Gai, L.Z.; Yuan, A.H.; Shen, Z. Low-cost CuNi@MIL-101 as an excellent catalyst toward cascade reaction: Integration of ammonia borane dehydrogenation with nitroarene hydrogenation. Chem. Commun. 2017, 53, 6621–6624. [Google Scholar] [CrossRef]
- Njoh, A.J.; Etta, S.; Essia, U.; Ngyah-Etchutambe, I.; Enomah, L.E.; Tabrey, H.T.; Tarke, O.M. Implications of institutional frameworks for renewable energy policy administration: Case study of the Esaghem, Cameroon community PV solar electrification project. Energy Pol. 2019, 128, 17–24. [Google Scholar] [CrossRef]
- Talaat, M.; Farahat, M.A.; Elkholy, M.H. Renewable power integration: Experimental and simulation study to investigate the ability of integrating wave, solar and wind energies. Energy 2019, 170, 668–682. [Google Scholar] [CrossRef]
- Ajayan, J.; Nirmal, D.; Mohankumar, P.; Saravanan, M.; Jagadesh, M.; Arivazhagan, L. A review of photovoltaic performance of organic/inorganic solar cells for future renewable and sustainable energy technologies. Superlattices Microstruct. 2020, 143, 106549–106602. [Google Scholar] [CrossRef]
- Alferov, Z.I.; Andreev, V.M.; Kagan, M.B.; Protasov, I.I.; Trofim, V.G. Solar-energy converters based on p-n AlxGa12xAs-GaAs heterojunctions. Fiz. Tekh. Poluprovodn. 1970, 4, 2378–2379. [Google Scholar]
- Hawkins, H.M. Concepts of Digital Electronics; Tab Books: Blue Ridge Summit, PA, USA, 1983; p. 231. ISBN 0830615318. [Google Scholar]
- Merritt, V.Y. Organic Photovoltaic Materials: Squarylium and Cyanine-TCNQ Dyes. IBM J. Res. Dev. 1978, 22, 353–371. [Google Scholar] [CrossRef]
- Weber, D. CH3NH3SnBrxI3-x (x = 0-3), ein Sn(II)-System mit kubischer Perowskitstruktur/CH3NH3SnBrxI3-x(x = 0-3), a Sn(II)-System with Cubic Perovskite Structure. Z. Naturforschung 1978, 33, 862–865. [Google Scholar] [CrossRef]
- Zweibel, K. Thin Films: Past, Present and Future; NREL/TP-413-7486; National Renewable Energy Laboratory: Golden, CO, USA, 1995. [CrossRef]
- Zhang, Y.; Elawad, M.; Yu, Z.; Jiang, X.; Lai, J.; Sun, L. Enhanced performance of perovskite solar cells with P3HT hole-transporting materials via molecular p-type doping. RSC Adv. 2016, 110, 108888–108895. [Google Scholar] [CrossRef]
- Cao, H.; He, W.; Mao, Y.; Lin, X.; Ishikawa, K.; Dickerson, J.H.; Hess, W.P. Recent progress in degradation and stabilization of organic solar cell. J. Power Sources 2014, 264, 168–183. [Google Scholar] [CrossRef]
- Zayed, M.E.; Elsheikh, A.H.; Essa, F.A.; Elbanna, A.M.; Li, W.; Zhao, J. High-temperature solar selective absorbing coatings for concentrated solar power systems. Sustain. Mater. Green Proc. Energy Conv. 2022, 12, 361–398. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and Semitransparent Organic Solar Cells. Adv. Energy Mater. 2017, 8, 1701791–1701819. [Google Scholar] [CrossRef]
- Yan, L.L.; Han, C.; Shi, B.; Zhao, Y.; Zhang, X.D. A review on the crystalline silicon bottom cell for monolithic perovskite/silicon tandem solar cells. Mater. Today Nano 2019, 7, 100045–100058. [Google Scholar] [CrossRef]
- Cho, D.H.; Jo, H.S.; Lee, W.J.; Kim, T.G.; Shin, B.; Yoon, S.S.; Chung, Y.D. Enhanced electrical conductivity of transparent electrode using metal microfiber networks for griddles thin-film solar cells. Sol. Energy Mater. Sol. Cells 2019, 200, 109998. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Sharma, S.; Siwach, B.; Ghoshal, S.K.; Mohan, D. Dye sensitized solar cells: From genesis to recent drifts. Renew. Sustain. Energy Rev. 2017, 70, 529–537. [Google Scholar] [CrossRef]
- Ikpesu, J.E.; Iyuke, S.E.; Daramola, M.; Okewale, A.O. Synthesis of improved dye-sensitized solar cell for renewable energy power generation. Sol. Energy 2020, 206, 918–934. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Feng, S.; Ding, W.L. Integrated probing the influence of dye acceptor with several electron withdrawing groups for dye-sensitized solar cells. Sol. Energy 2020, 195, 491–498. [Google Scholar] [CrossRef]
- Heng, P.; Mao, L.; Guo, X.; Wang, L.; Zhang, J. Accurate estimation of the photoelectric conversion efficiency of a series of anthracene-based organic dyes for dye-sensitized solar cells. J. Mater. Chem. 2020, 8, 2388–2399. [Google Scholar] [CrossRef]
- Zayed, M.E. Recent Advances in Solar Thermal Selective Coatings for Solar power Applications: Technology Categorization, Preparation methods, and Induced aging mechanisms. Appl. Sci. 2024, 14, 8438. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Liu, Z.; Huang, J.; Ma, X.; Liu, Y.; Sun, Q.; Dai, L.; Ahmad, S.; Shen, Y.; et al. Progress and Challenges Toward Effective Flexible Perovskite Solar Cells. Nano-Micro Lett. 2023, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.R.; Zhang, Y.; Li, X.Y.; Wang, W.; Gong, J.J.; Liu, Z.J.; Chen, H.S. The bis-dimethylfluoreneaniline organic dye sensitizers for solar cells: A theoretical study and design. J. Mol. Graph. 2019, 88, 23–31. [Google Scholar] [CrossRef]
- Walki, S.; Naik, L.; Savanur, H.M.; Yogananda, K.C.; Naik, S.; Ravindra, M.K.; Malimat, G.H.; Mahadevan, K.M. Design of new imidazole-derivative dye having donor-π-acceptor moieties for highly efficient organic-dye-sensitized solar cells. Optik 2019, 208, 164074. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Duan, Y.C.; Pan, Q.Q.; Wu, Y.; Geng, Y.; Su, Z.M. Theoretical insights on the rigidified dithiophene effects on the performance of near-infrared cis-squaraine-based dye-sensitized solar cells with panchromatic absorption. J. Photochem. Photobiol. A 2019, 369, 150–158. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.J.; Baena, J.P.C.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhu, C.; Chen, M.; Jiang, C.; Guo, J.; Feng, Y.; Dai, Z.; Yadavalli, S.K.; Hu, M.; Cao, X.; et al. Interpenetrating interfaces for efficient perovskite solar cells with high operational stability and mechanical robustness. Nat. Commun. 2021, 12, 973. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.; Agarwal, S.; Dhaka, M.S. Stability and efficiency issues, solutions and advancements in perovskite solar cells: A review. Sol. Energy 2022, 244, 516–535. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Dong, H.; Li, J.; Zhu, X.; Xu, J.; Pan, F.; Yuan, F.; Dai, J.; Jiao, B.; et al. Highly efficient and stable perovskite solar cells enabled by lowdimensional perovskitoids. Sci. Adv. 2022, 8, eabk2722. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Sutty, S.; Aziz, H. Interplay between efficiency and device architecture for small molecule organic solar cells. Phys. Chem. Chem. Phys. 2014, 16, 11398–11408. [Google Scholar] [CrossRef]
- Vohra, V.; Kawashima, K.; Kakara, T.; Koganezawa, T.; Osaka, I.; Takimiya, K.; Murata, H. Efficient inverted polymer solar cells employing favourable molecular orientation. Nat. Photonics 2015, 9, 403–408. [Google Scholar] [CrossRef]
- Alves, H.; Molinari, A.S.; Xie, H.; Alberto, F.M. Metallic Conduction at Organic Charge-Transfer Interfaces. Nat. Mater. 2008, 7, 574–580. [Google Scholar] [CrossRef]
- Kirtley, J.R.; Mannhart, J. Organic electronics: When TTF met TCNQ. Nat. Mater. 2008, 7, 520–521. [Google Scholar] [CrossRef]
- Suzuki, A.; Ohtsuki, T.; Oku, T.; Akiyama, T. Fabrication and characterization of tetracyanoquinodimethane/phthalocyanine solar cells. Mater. Sci. Engin. B 2012, 177, 877–881. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Lin, C.-T.; Huang, J.-H.; Chu, C.-W.; Wei, K.-H.; Li, L.-J. Layer-by-Layer Graphene/TCNQ Stacked Films as Conducting Anodes for Organic Solar Cells. Asc Nano 2012, 6, 5031–5039. [Google Scholar] [CrossRef]
- Krebs, F.C. All Solution Roll-to-Roll Processed Polymer Solar Cells Free from Indium-Tin-Oxide and Vacuum Coating Steps. Org. Electron. 2009, 10, 761–768. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, Z.; Zhang, X.; Shen, L.; Guo, W.; Zhang, L.; Long, Y.; Ruan, S. Improving the charge carrier transport of organic solar cells by incorporating a deep energy level molecule. Phys. Chem. Chem. Phys. 2017, 19, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Yim, K.H.; Whiting, G.L.; Murphy, C.E.; Halls, J.J.M.; Burroughes, J.H.; Friend, R.H.; Kim, J.S. Controlling Electrical properties of Conjugated polymers via a Solution-Based p-Type Doping. Adv. Mater. 2008, 20, 3319–3324. [Google Scholar] [CrossRef]
- Hu, P.; Li, H.; Li, Y.; Jiang, H.; Kloc, C. Single-crystal growth, structures, charge transfer and transport properties of anthracene-F4TCNQ and tetracene-F4TCNQ charge-transfer compounds. CrystEngComm 2017, 19, 618–624. [Google Scholar] [CrossRef]
- Untilova, V.; Zeng, H.; Durand, P.; Herrmann, L.; Leclerc, N.; Brinkmann, M. Intercalation and Ordering of F6TCNNQ and F4TCNQ Dopants in Regioregular Poly(3-hexylthiophene) Crystals: Impact on Anisotropic Thermoelectric Properties of Oriented Thin Films. Macromolecules 2021, 54, 6073–6084. [Google Scholar] [CrossRef]
- Gao, W.; Kahn, A. Electronic structure and current injection in zinc phthalocyanine doped with tetrafluorotetracyanoquinodimethane: Interface versus bulk effects. Org. Electron. 2002, 3, 53–63. [Google Scholar] [CrossRef]
- Su, Y.H.; Ke, Y.F.; Cai, S.L.; Yao, Q.Y. Surface plasmon resonance of layer-by-layer gold nanoparticles induced photoelectric current in environmentally-friendly plasmon-sensitized solar cell. Light Sci. Appl. 2012, 1, e14–e19. [Google Scholar] [CrossRef]
- Chen, X.; Jia, B.H.; Zhang, Y.A.; Gu, M. Exceeding the limit of plasmonic light trapping in textured screen-printed solar cells using Al nanoparticles and wrinkle-like graphene sheets. Light Sci. Appl. 2013, 2, e92–e98. [Google Scholar] [CrossRef]
- Holman, Z.C.; Wolf, S.D.; Ballif, C. Improving metal reflectors by suppressing surface plasmon polaritons: A priori calculation of the internal reflectance of a solar cell. Light Sci. Appl. 2013, 2, e106–e122. [Google Scholar] [CrossRef]
- Chandaluri, C.G.; Radhakrishnan, T.P. Zwitterionic Diaminodicyanoquinodimethanes with Enhanced Blue-Green Emission in the Solid State. Opt. Mater. 2011, 34, 119–125. [Google Scholar] [CrossRef]
- Boyineni, A.; Jayanty, S. Supramolecular Helical Self-Assemblies and Large Stokes Shift in 1-(2-Cyanophenyl)Piperazine and 4-Piperidinopiperidine Bis-Substituted Tetracyanoquinodimethane Fluorophores. Dye. Pigm. 2014, 101, 303–311. [Google Scholar] [CrossRef]
- Radhakrishnan, T.P. Molecular Structure, Symmetry, and Shape as Design Elements in the Fabrication of Molecular Crystals for Second Harmonic Generation and the Role of Molecules-in-Materials. Acc. Chem. Res. 2008, 41, 367–376. [Google Scholar] [CrossRef]
- Mohammadtaheri, M.; Ramanathan, R.; Bansal, V. Emerging Applications of Metal-TCNQ Based Organic Semiconductor Charge Transfer Complexes for Catalysis. Catal. Today 2016, 278, 319–329. [Google Scholar] [CrossRef]
- Zhang, Z.; Gou, G.; Wan, J.; Li, H.; Wang, M.; Li, L. Synthesis, Structure, and Significant Energy Gap Modulation of Symmetrical Silafluorene-Cored Tetracyanobutadiene and Tetracyanoquinodimethane Derivatives. J. Org. Chem. 2022, 87, 2470–2479. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ogawa, K.; Ono, R.; Nikaido, K.; Inoue, S.; Higashino, T.; Tanaka, M.; Tsutsumi, J.; Kondo, R.; Kumai, R.; et al. Highly Stable and Isomorphic Donor-Acceptor Stacking in a Family of n-Type Organic Semiconductors of BTBT-TCNQ Derivatives. J. Mater. Chem. C 2022, 10, 16471–16479. [Google Scholar] [CrossRef]
- Syed, A.; Battula, H.; Mishra, S.; Jayanty, S. Distinct Tetracyanoquinodimethane Derivatives: Enhanced Fluorescence in Solutions and Unprecedented Cation Recognition in the Solid State. ACS Omega 2021, 6, 3090–3105. [Google Scholar] [CrossRef]
- Pakravesh, F.; Izadyar, M.; Arkan, F. Effect of electron donor and acceptor on the photovoltaic properties of organic dyes for efficient dye-sensitized solar cells. Physica B 2021, 609, 412815–412851. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, D.; Li, X.; Xu, Y. D-A-π-A based organic dyes for efficient DSSCs: A theoretical study on the role of π-spacer. Comput. Mater. Sci. 2019, 161, 163–176. [Google Scholar] [CrossRef]
- Fu, Y.; Li, B.; Liu, H.; Xue, B.; Liu, E. High efficiency dye-sensitized solar cells based on a series of small dye molecules with N-methylcarbazole derivatives as donors. Mater. Chem. Phys. 2020, 239, 121970–121976. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Xu, Y. Theoretical screening of high-efficiency sensitizers with D-π-A framework for DSSCs by altering promising donor group. Sol. Energy 2020, 196, 146–156. [Google Scholar] [CrossRef]
- Kivala, M.; Boudon, C.; Gisselbrecht, J.P.; Seiler, P.; Gross, M.; Diederich, F. A novel reaction of 7,7,8,8-tetracyanoquinodimethane (TCNQ): Charge-transfer chromophores by [2 + 2] cycloaddition with alkynes. Chem. Commun. 2007, 7, 4731–4733. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Graetzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The main progress of perovskite solar cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, T.; Schulz, P.; Li, Z.; Li, G.; Kim, D.H.; Guo, N.; Berry, J.J.; Zhu, K.; Zhao, Y. Facile fabrication of large-grain CH3NH3PbI3−xBrx films for high-efficiency solar cells via CH3NH3Br-selective Ostwald ripening. Nat. Commun. 2016, 7, 12305–12314. [Google Scholar] [CrossRef]
- Koh, T.M.; Wang, H.; Ng, Y.; Bruno, A.; Mhaisalkar, S.; Mathews, N. Halide perovskite solar cells for building integrated photovoltaics: Transforming building facades into power generators. Adv. Mater. 2022, 34, 2104661–2104699. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Tong, J.; Xian, Y.; Kerner, R.; Dunfield, S.; Xiao, H.; Scheidt, R.A.; Kuciauskas, D.; Wang, X.; Hautzinger, M.P.; et al. Surface reaction for efficient and stable inverted perovskite solar cells. Nature 2022, 611, 278–283. [Google Scholar] [CrossRef]
- Wagner, P. Silicon solar cells. Microelectron. J. 1988, 19, 37–50. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, K.K.; Sharma, A. Solar cells: In Research and Applications. Mater. Sci. Appl. 2015, 6, 1145–1155. [Google Scholar] [CrossRef]
- De Wolf, S.; Holovsky, J.; Moon, S.J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic halide perovskites: Sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Comin, R.; Walters, G.; Thibau, E.S.; Voznyy, O.; Lu, Z.H.; Sargent, E.H. Structural, optical, and electronic studies of wide-bandgap lead halide perovskites. J. Mater. Chem. C. 2015, 3, 8839–8843. [Google Scholar] [CrossRef]
- Herz, L.M. Charge-carrier mobilities in Metal Halide perovskites: Fundamental mechanisms and limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- Wolff, C.M.; Bourelle, S.A.; Phuong, L.Q.; Kurpiers, J.; Feldmann, S.; Caprioglio, P.; Marquez, J.A.; Wolansky, J.; Unold, T.; Stolterfoht, M.; et al. Orders of recombination in complete Perovskite Solar cells—Linking time-resolved and steady-state measurements. Adv. Energy Mater. 2021, 11, 2101823. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, J.; Zeng, P.; Liu, M. Recent progress in Interfacial Dipole Engineering for Perovskite Solar cells. Nano-Micro Lett. 2023, 15, 173. [Google Scholar] [CrossRef]
- Zhang, C.; Arumugam, G.M.; Liu, C.; Hu, J.; Yang, Y.; Schropp, R.E.J.; Mai, Y. Inorganic halide perovskite materials and solar cells. APL Mater. 2019, 7, 120702. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Dong, Q.; Xiao, Z.; Yuan, Y.; Huang, J. Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process. Energy Environ. Sci. 2014, 7, 2359–2365. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Yuan, J.; Hong, Q.; Shi, G.; Yuan, D.; Wei, J.; Huang, C.; Tang, J.; Fung, M.-K. Improved performance of inverted planar perovskite solar cells with F4TCNQ doped PEDOT:PSS hole transport layers. J. Mater. Chem. A 2017, 5, 5701–5708. [Google Scholar] [CrossRef]
- Huang, L.; Hu, Z.; Xu, J.; Zhang, K.; Zhang, J.; Zhang, J.; Zhu, Y. Efficient and Stable Planar Perovskite Solar Cells with a Non-Hygroscopic Small Molecule Oxidant Doped Hole. Transport Layer. Electrochim. Acta 2016, 196, 328–336. [Google Scholar] [CrossRef]
- Song, D.; Wei, D.; Cui, P.; Li, M.; Duan, Z.; Wang, T.; Ji, J.; Li, Y.; Michel Mbengue, J.; Li, Y.; et al. Dual Function Interfacial Layer for Highly Efficient and Stable Lead Halide Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 6091–6097. [Google Scholar] [CrossRef]
- Momblona, C.; Gil-Escrig, L.; Bandiello, E.; Hutter, E.M.; Sessolo, M.; Lederer, K.; Blochwitz-Nimoth, J.; Bolink, H.J. Efficient Vacuum Deposited p–i–n and n–i–p Perovskite Solar Cells Employing Doped Charge Transport Layers. Energy Environ. Sci. 2016, 9, 3456–3463. [Google Scholar] [CrossRef]
- Zhao, S.; Zhuang, J.; Liu, X.; Zhang, H.; Zheng, R.; Peng, X.; Gong, X.; Guo, H.; Wang, H.; Li, H. F4TCNQ doped strategy of nickel oxide as high-efficient hole transporting materials for invert perovskite solar cell. Mater. Sci. Semicond. Proc. 2001, 121, 105458. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Q.; Saidaminov, M.I.; Wang, F.; Johnston, A.; Hou, Y.; Peng, Z.; Xu, K.; Zhou, W.; Liu, Z. Efficient and stable inverted perovskite solar cells incorporating secondary amines. Adv. Mater. 2019, 31, 1903559. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Jin, J.; Liu, W.; Dong, B.; Bai, X.; Song, H.; Reiss, P. Inverted perovskite solar cells employing doped NiO hole transport layers: A review. Nano Energy 2019, 63, 103860–103874. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, L.; Ren, W.; Zhang, C.; Wu, Y.; Liu, Y.; Sun, Q.; Dai, Z.; Cui, Y.; Cai, L.; et al. High Efficiency Perovskite Solar Cells with PTAA Hole Transport Layer enabled by PMMA:F4TCNQ Buried Interface Layer. J. Mater. Chem. C 2022, 10, 9714–9722. [Google Scholar] [CrossRef]
- Jung, M.-C.; Raga, S.R.; Ono, L.K.; Qi, Y. Substantial improvement of perovskite solar cells stability by pinhole-free hole transport layer with doping engineering. Sci. Rep. 2015, 5, 9863. [Google Scholar] [CrossRef]
- Calado, P.; Telford, A.M.; Bryant, D.; Li, X.; Nelson, J.; O’Regan, B.C.; Barnes, P.R.F. Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis. Nat. Commun. 2016, 7, 13831–13841. [Google Scholar] [CrossRef]
- Luo, J.; Jia, C.; Wan, Z.; Han, F.; Zhao, B.; Wang, R. The novel dopant for hole-transporting material opens a new processing route to efficiently reduce hysteresis and improve stability of planar perovskite solar cells. J. Power Sources 2017, 342, 886–895. [Google Scholar] [CrossRef]
- Trifiletti, V.; Degousée, T.; Manfredi, N.; Fenwick, O.; Colella, S.; Rizzo, A. Molecular Doping for Hole Transporting Materials in Hybrid Perovskite Solar Cells. Metals 2020, 10, 14. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Gonçales, V.R.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. A high performance and low-cost hole transporting layer for efficient and stable perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 21033–21045. [Google Scholar] [CrossRef]
- Zhang, F.; Song, J.; Hu, R.; Xiang, Y.; He, J.; Hao, Y.; Lian, J.; Zhang, B.; Zeng, P.; Qu, J. Interfacial Passivation of the p-Doped Hole-Transporting Layer Using General Insulating Polymers for High-Performance Inverted Perovskite Solar Cells. Small 2018, 14, 1704007–1704017. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rochester, C.W.; Jacobs, I.E.; Friedrich, S.; Stroeve, P.; Riede, M.; Moule, A.J. Measurement of Small Molecular Dopant F4TCNQ and C60F36 Diffusion in Organic Bilayer Architectures. ACS Appl. Mater. Interfaces 2015, 7, 28420–28428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, M.; Yang, W.; Judin, L.; Hukka, T.I.; Priimagi, A.; Deng, Z.; Vivo, P. Thionation Enhances the Performance of Polymeric Dopant-Free Hole-Transporting Materials for Perovskite Solar Cells. Adv. Mater. Interfaces 2019, 18, 1901036. [Google Scholar] [CrossRef]
- Tran, H.C.V.; Jiang, W.; Lyu, M.; Chae, H. Tetrahydrofuran as solvent for P3HT/F4TCNQ hole-transporting layer to increase the efficiency and stability of FAPbi3-based perovskite solar cell. J. Phys. Chem. C 2020, 124, 14099–14104. [Google Scholar] [CrossRef]
- Yao, H.; Hou, J. Recent Advances in Single-Junction Organic Solar Cells. Ang. Chem. Int. Ed. 2022, 61, e202209021–e202209030. [Google Scholar] [CrossRef]
- Esqueda, M.L.; Vergara, M.E.S.; Bada, J.R.Á.; Salcedo, R. CuPc: Effects of its doping and a study of its organic-semiconducting properties for application in flexible devices. Materials 2019, 12, 434. [Google Scholar] [CrossRef]
- Capitan, M.J.; Alvarez, J.; Naviod, C. Study of the electronic structure of electron accepting cyano-films: TCNQ versus TCNE. Phys. Chem. Chem. Phys. 2018, 20, 10450–10459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hasegawa, R.; Funayama, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Additive effects of CuPcX4-TCNQ on CH3NH3PbI3 perovskite solar cells. J. Mater. Sci. Mater. Electron. 2023, 34, 588. [Google Scholar] [CrossRef]
- Boix, P.P.; Nonomura, K.; Mathews, N.; Mhaisalkar, S.G. Current Progress and Future Perspectives for Organic/Inorganic Perovskite Solar Cells. Mater. Today 2014, 17, 16–23. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The Emergence of Perovskite Solar Cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.-L.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid Organic–Inorganic Perovskites (HOIPs): Opportunities and Challenges. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar] [CrossRef] [PubMed]
- Tiep, N.H.; Ku, Z.; Fan, H.J. Recent Advances in Improving the Stability of Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1501420. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; McMeekin, D.P.; Snaith, H.J.; Nicholas, R.J. Research Update: Strategies for Improving the Stability of Perovskite Solar Cells. APL Mater. 2016, 4, 091503–091518. [Google Scholar] [CrossRef]
- Hörantner, M.T.; Leijtens, T.; Ziffer, M.E.; Eperon, G.E.; Christoforo, M.G.; McGehee, M.D.; Snaith, H.J. The Potential of Multijunction Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2506–2513. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Deng, D.; Wang, Z.; Zhang, Z.; Li, Y.; Zhang, J.; Lv, K.; Liu, L.; Zhang, X.; et al. High Miscibility Compatible with Ordered Molecular Packing Enables an Excellent Efficiency of 16.2% in All-Small-Molecule Organic Solar Cells. Adv. Mater. 2022, 34, 2106316. [Google Scholar] [CrossRef]
- Ge, J.; Hong, L.; Ma, H.; Ye, Q.; Chen, Y.; Xie, L.; Song, W.; Li, D.; Chen, Z.; Yu, K.; et al. Asymmetric Substitution of End-Groups Triggers 16.34% Efficiency for All-Small- Molecule Organic Solar Cells. Adv. Mater. 2022, 34, 2202752. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Manion, J.G.; Yuan, M.; Pelayo García de Arquer, F.; McKeown, G.R.; Beaupré, S.; Leclerc, M.; Sargent, E.H.; Seferos, D.S. Increasing Polymer Solar Cell Fill Factor by Trap-filling with F4TCNQ at Parts Per Thousand Concentration. Adv. Mater. 2016, 28, 6491–6496. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, L.; Gadisa, A.; Zhang, Q.; Rech, J.J.; You, W.; Ade, H. Revealing the Impact of F4-TCNQ as Additive on Morphology and Performance of High-Efficiency Nonfullerene Organic Solar Cells. Adv. Funct. Mater. 2019, 29, 1806262–1806271. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, H.; Wu, M.; Yuan, S.; Han, Y.; Liu, Z.; Guo, K.; Liu, S.; Yang, S.; Zhao, H.; et al. Highly efficient and stable planar CsPbI2Br perovskite solar cell with a new sensitive-dopant-free hole transport layer obtained via an effective Surface passivation. Sol. Energy Mater. Sol. Cells 2019, 201, 110052. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, W.; Wang, W.; Wang, H.; Zhong, Y.; Zhao, K. Controlling phase transition toward future low-cost and eco-friendly printing of perovskite solar cells. J. Phys. Chem. Lett. 2022, 13, 6503–6513. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, C.; Ma, Y.; Li, W.; Wang, Y.; Wu, S.; Liu, C.; Mai, Y. Efficient and Stable Perovskite Solar Cells and Modules Enabled by Tailoring Additive Distribution According to the Film Growth Dynamics. Nano-Micro Lett. 2025, 17, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Y.; Rauf, S.; Liu, S.; Chen, W.; Shen, Y.; Kumar, M. Revolutionizing the solar photovoltaic efficiency: A comprehensive review on the cutting-edge thermal management methods for advanced and conventional solar photovoltaics. Energy Environ. Sci. 2025, 18, 1130–1175. [Google Scholar] [CrossRef]
- Yan, J.; Savenije, T.J.; Mazzarella, L.; Isabella, O. Progress and challenges on scaling up of perovskite solar cell technology. Sustain. Energy Fuels 2022, 6, 243–266. [Google Scholar] [CrossRef]
- Shen, W.; Zhao, Y.; Liu, F. Highlights of mainstream solar cell efficiencies in 2023. Front. Energy 2024, 18, 8–15. [Google Scholar] [CrossRef]
- Peng, C.; Ning, G.-H.; Su, J.; Zhong, G.; Tang, W.; Tian, B.; Su, C.; Yu, D.; Zu, L.; Yang, J.; et al. Reversible multi-electron re- dox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat. Energy 2017, 2, 17074. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Park, S.; Wang, H. Recent progress on metal-organic framework derived carbon and its composites as anode materials for potassium-ion batteries. Energy Mater. 2023, 3, 300042–300074. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Bak, C.; Hong, Y.; Joung, D.; Ko, J.B.; Lee, Y.M.; Kim, C. Enhancing hydrophilicity of thick electrodes for high energy density aqueous batteries. Nano-Micro Lett. 2023, 15, 97–108. [Google Scholar] [CrossRef]
- Lv, C.; Xu, W.; Liu, H.; Zhang, L.; Chen, S.; Yang, X.; Xu, X.; Yang, D. 3D Sulfur and Nitrogen Co doped Carbon Nanofiber Aerogels with Optimized Electronic Structure and Enlarged Interlayer Spacing Boost Potassium-Ion Storage. Small 2019, 15, 1900816–1900824. [Google Scholar] [CrossRef]
- Li, D.; Guo, Y.; Zhang, C.; Chen, X.; Zhang, W.; Mei, S.; Yao, C.-J. Unveiling Organic Electrode Materials in Aqueous Zinc-Ion Batteries: From Structural Design to Electrochemical Performance. Nano-Micro Lett. 2024, 16, 194–228. [Google Scholar] [CrossRef]
- Hanyu, Y.; Honma, I. Rechargeable quasi-solid state lithium battery with organic crystalline cathode. Sci. Rep. 2012, 2, 453–458. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.; Jung, S.K.; Ku, K.; Kwon, G.; Seong, W.M.; Kim, H.; Yoon, G.; Kang, I.; Hong, K.; et al. Charge-transfer complexes for high-power organic rechargeable batteries. Energy Storage Mater. 2019, 20, 462–469. [Google Scholar] [CrossRef]
- Kye, H.; Kang, Y.; Jang, D.; Kwon, J.E.; Kim, B.-G. p-Type Redox-Active Organic Electrode Materials for Next-Generation Rechargeable Batteries. Adv. Energy Sustain. Res. 2022, 3, 2200030–2200055. [Google Scholar] [CrossRef]
- Shi, H.J.; Ye, Y.J.; Liu, K.; Song, Y.; Sun, X. A Long-Cycle-Life Self-Doped Polyaniline Cathode for Rechargeable Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2018, 57, 16359–16363. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Liang, P.; Liu, X.; Wu, K.; Liu, Y.; Wang, Y. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc–air batteries. Energy Environ. Sci. 2018, 11, 3075–3095. [Google Scholar] [CrossRef]
- Li, H.; Fang, M.; Hou, Y.; Tang, R.; Yang, Y.; Zhong, C.; Li, Q.; Li, Z. Different Effect of the Additional Electron-Withdrawing Cyano Group in Different Conjugation Bridge: The Adjusted Molecular Energy Levels and Largely Improved Photovoltaic Performance. ACS Appl. Mater. Interface 2016, 8, 12134–12140. [Google Scholar] [CrossRef]
- Casey, A.; Dimitrov, S.D.; Shakya-Tuladhar, P.; Fei, Z.; Nguyen, M.; Han, Y.; Anthopoulos, T.D.; Durrant, J.R.; Heeney, M. Effect of Systematically Tuning Conjugated Donor Polymer Lowest Unoccupied Molecular Orbital Levels via Cyano Substitution on organic Photovoltaic Device Performance. Chem. Mater. 2016, 28, 5110–5120. [Google Scholar] [CrossRef]
- Ji, Z.; Dong, H.; Liu, M.; Hu, W. Water-controlled synthesis of low-dimentuinal Molecular crystals and the fabrication of a new water and moisture indicator. Nano Res. 2009, 11, 857–864. [Google Scholar] [CrossRef]
- Kundu, D.; Oberholzer, P.; Glaros, C.; Bouzid, A.; Tervoort, E.; Pasquarello, A.; Niederberger, M. Organic cathode for aqueous Zn-ion batteries: Taming a unique phase evolution toward stable electrochemical cycling. Chem. Mater. 2018, 30, 3874–3881. [Google Scholar] [CrossRef]
- Chola, N.M.; Nagarale, R.K. TCNQ confined in porous organic structure as cathode for aqueous zinc battery. J. Electrochem. Soc. 2020, 167, 100552–100561. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Geng, Z.; Li, P.; Hu, N.; Zhu, L.; Sun, W.; Li, C.M. Significantly raising tetracyanoquinodimethane electrode performance in zinc-ion battery at low temperatures by eliminating impurities. Battery Energy 2023, 2, 20220050–20220073. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Zhou, Y.; Deng, W. Effect of Fluoride Atoms on the Electrochemical Performance of Tetracyanoquinodimethane (TCNQ) Electrodes in Zinc-ion Batteries. Chem. Phys. Chem. 2023, 24, e202300436–e202300441. [Google Scholar] [CrossRef]
- Canevet, D.; Sallé, M.; Zhang, G.; Zhang, D.; Zhu, D. Tetrathiafulvalene (TTF) derivatives: Key building-blocks for switchable processes. Chem. Commun. 2009, 17, 2245–2269. [Google Scholar] [CrossRef]
- Ma, W.; Lei, C.; Liu, T.; Liang, X. A high-voltage tetracyanoquinodimethane (TCNQ) organic cathode enabled by Na+ and I− synergy for durable aqueous batteries. Sci. China Chem. 2025, 68, 4478–4485. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Hong, J.; Kang, K. Unlocking the Full Redox Capability of Organic Charge-Transfer Complex in High-Loading Electrodes for Organic Rechargeable Batteries. Adv. Energy Mater. 2025, 15, 2404116–2404124. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Wu, Z.; Luo, Y.; Qiu, W.; Fan, X.; Long, B.; Huang, M.; Liu, P.; Tong, Y. High power density nitridated hematite (α-Fe2O3) nanorods as anode for high-performance flexible lithium ion batteries. J. Power Sources 2016, 308, 7–17. [Google Scholar] [CrossRef]
- Huang, A.; Ma, Y.; Peng, J.; Li, L.; Chou, S.-L.; Ramakrishna, S.; Peng, S. Tailoring the structure of silicon-based materials for lithium-ion batteries via electrospinning technology. eScience 2021, 1, 141–162. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte design for lithium metal anode-based batteries toward extreme temperature application. Adv. Sci. 2021, 8, 2101051–2101070. [Google Scholar] [CrossRef]
- Gou, X.; Hao, Z.; Hao, Z.; Yang, G.; Yang, Z.; Zhang, X.; Yan, Z.; Zhao, Q.; Chen, J. In situ surface self-reconstruction strategies in Li-rich Mn-based layered cathodes for energy-dense Li-ion batteries. Adv. Funct. Mater. 2022, 32, 2112088–2112103. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, F.; Wang, T.; Yang, Q.; Huang, Z.; Wang, D.; Liang, G.; Chen, A.; Li, Q.; Guo, Y.; et al. Zinc/selenium conversion battery: A system highly compatible with both organic and aqueous electrolytes. Energy Environ. Sci. 2021, 14, 2441–2450. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, J.; Wang, Z.; Zhou, Y.; He, X.; Zhou, X.; Liu, J.; Yan, C.; Qian, T. Phase diagrams guided design of low-temperature aqueous electrolyte for Zn metal batteries. Chem. Eng. J. 2023, 454, 140413–140419. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Y.; Wang, Y.; Cong, J.; Ji, H.; Huang, Z.; Huang, Y. A co-solvent in aqueous electrolyte towards ultra long-life rechargeable zinc ion batteries. Energy Storage Mater. 2023, 56, 174–182. [Google Scholar] [CrossRef]
- Fang, C.; Huang, Y.; Yuan, L.; Liu, Y.; Chen, W.; Huang, Y.; Chen, K.; Han, J.; Liu, Q.; Huang, Y. A metal-organic compound as cathode material with super high capacity achieved by reversible cationic and anionic redox chemistry for high-energy sodium-ion batteries. Ang. Chem. Int. Ed. 2017, 56, 6793–6797. [Google Scholar] [CrossRef] [PubMed]
- Precht, R.; Hausbrand, R.; Jaegermann, W. Electronic structure and electrode properties of tetracyanoquinodimethane (TCNQ): A surface science investigation of lithium intercalation into TCNQ. Phys. Chem. Chem. Phys. 2015, 17, 6588–6596. [Google Scholar] [CrossRef]
- Ma, E.; Zhou, C.; Fan, B.; Wu, C.; Li, Z.; Lu, H.; Li, J. Endowing CuTCNQ with a new role: A high-capacity cathode for K-ion batteries. Chem. Commun. 2018, 54, 5578–5581. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, C.; Zhang, W.; Liu, Q.J.; Huang, Y.H. Room temperature N-heterocyclic carbene manganese catalyzed selective N-alkylation of anilines with alcohols. Chem. Commun. 2019, 55, 608–611. [Google Scholar] [CrossRef]
- Fujihara, Y.; Kobayashi, H.; Takaishi, S.; Tomai, S.; Yamashita, M.; Honma, I. Electrical Conductivity-Relay between Organic Charge-Transfer and radical Salts toward Conductive Additive-Free rechargeable Battery. ACS Appl. Mater. Interfaces 2020, 12, 25748–25755. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium Batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hibino, M.; Ogasawara, Y.; Yamaguchi, K.; Kudo, T.; Okuoka, S.-I.; Yonehara, K.; Ono, H.; Sumida, Y.; Oshima, M. Improved performance of Co-doped Li2O cathodes for lithium-peroxide batteries using LiCoO2 as a dopant source. J. Power Sources 2016, 306, 567–572. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef]
- Heintz, R.A.; Zhao, H.H.; Ouyang, X.; Grandinetti, G.; Cowen, J.; Dunbar, K.R. New Insight into the Nature of Cu(TCNQ): Solution Routes to Two Distinct Polymorphs and Their Relationship to Crystalline Films That Display Bistable Switching Behavior. Inorg. Chem. 1999, 38, 144–156. [Google Scholar] [CrossRef]
- Song, H.W.; Gong, Y.; Su, J.; Li, W.Y.; Li, Y.; Gu, L.; Wang, C.H. Surfaces/Interfaces Modification for Vacancies Enhancing Lithium Storage Capability of Cu2O Ultrasmall Nanocrystals. ACS Appl. Mater. Interfaces 2018, 10, 35137–35144. [Google Scholar] [CrossRef]
- Leong, C.F.; Usov, P.M.; D′Alessandro, D.M. Intrinsically conducting metal–organic frameworks. MRS Bull. 2016, 41, 858–864. [Google Scholar] [CrossRef]
- Yin, D.; Wang, Z.; Li, Q.; Xue, H.; Cheng, Y.; Wang, L.; Huang, G. In Situ Growth of Lithiophilic MOF Layer Enabling Dendrite-free Lithium Deposition. iScience 2020, 23, 101869–101879. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal-Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Commercial metal–organic frameworks as heterogeneous catalysts. Chem. Commun. 2012, 48, 11275–11288. [Google Scholar] [CrossRef] [PubMed]
- Placke, T.; Heckmann, A.; Schmuch, R.; Meister, P.; Beltrop, K.; Winter, M. Perspective on Performance, Cost, and Technical Challenges for Practical Dual-Ion Batteries. Joule 2018, 2, 2528–2550. [Google Scholar] [CrossRef]
- Rodriguez-Perez, I.A.; Ji, X.L. Anion Hosting Cathodes in Dual-Ion Batteries. ACS Energy Lett. 2017, 2, 1762–1770. [Google Scholar] [CrossRef]
- Wang, M.; Tang, Y.B. A review on the features and progress of dual-ion batteries. Adv. Energy Mater. 2018, 8, 1703320–1703339. [Google Scholar] [CrossRef]
- Dühnen, S.; Nölle, R.; Wrogemann, J.; Winter, M.; Placke, T. Reversible Anion Storage in a Metal-Organic Framework for Dual-Ion Battery Systems. J. Electrochem. Soc. 2019, 166, A5474–A5482. [Google Scholar] [CrossRef]
- Wu, F.X.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Jia, H.P.; Kloepsch, R.; He, X.; Evertz, M.; Nowak, S.; Li, J.; Winter, M.; Placke, T. Nanostructured ZnFe2O4 as Anode Material for Lithium-Ion Batteries: Ionic Liquid-Assisted Synthesis and Performance Evaluation with Special Emphasis on Comparative Metal Dissolution. Acta Chim. Slov. 2016, 63, 470–483. [Google Scholar] [CrossRef]
- Qian, Y.X.; Niehoff, P.; Zhou, D.; Adam, R.; Mikhailova, D.; Pyschik, M.; Borner, M.; Kloepsch, R.; Rafaja, D.; Schumacher, G.; et al. Investigation of Nano-Sized Cu(II)O As a High Capacity Conversion Material for Li Metal Cells and Lithium Ion Full Cells. J. Mater. Chem. A 2017, 5, 6556–6568. [Google Scholar] [CrossRef]
- Wei, W.; Li, L.; Zhang, L.; Hong, J.; He, G. An all-solid-state Li organic battery with Quinone-based polymer cathode and composite polymer electrolyte. Electrochem. Commun. 2018, 90, 21–25. [Google Scholar] [CrossRef]
- Huang, W.; Zheng, S.; Zhang, X.; Zhou, W.; Xiong, W.; Chen, J. Synthesis and application of Calix[6]quinone as a High-Capacity Organic Cathode for Plastic Crystal Electrolyte-Based Lithium-Ion Batteries. Energy Storage Mater. 2020, 26, 465–471. [Google Scholar] [CrossRef]
- Hao, F.; Liang, Y.; Zhang, Y.; Chen, Z.; Zhang, J.; Ai, Q.; Guo, H.; Fan, Z.; Lou, J.; Yao, Y. High-energy all-solid-state organic–lithium batteries based on ceramic electrolytes. ACS Energy Lett. 2021, 6, 201–207. [Google Scholar] [CrossRef]
- Bonilla, M.R.; Daza, F.A.G.; Ranque, P.; Aguesse, F.; Carrasco, J.; Akhmatskaya, E. Unveiling interfacial Li-ion dynamics in Li7La3Zr2O12/PEO(LiTFSI) composite polymer-ceramic solid electrolytes for all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2021, 13, 30653–30667. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, 2103617–2103652. [Google Scholar] [CrossRef]
- Canals-Riclot, J.; Irié-Bi, B.; Dolhem, F.; Becuwe, M.; Gautron, E.; Seznec, V.; Dedryvère, R. Organic All-Solid-State Lithium Metal Battery Using Polymer/Covalent Organic Framework Electrolyte. Batter. Supercaps 2024, 8, e202400357–e202400364. [Google Scholar] [CrossRef]
- Xia, J.; Wang, R.; Qian, C.; Sun, K.; Liu, H.; Guo, C.; Li, J.; Yu, F.; Bao, W. Supercapacitors of Nanocrystalline Covalent Organic Frameworks—A Review. Crystals 2022, 12, 1350. [Google Scholar] [CrossRef]
- Sasmal, H.S.; Mahato, A.K.; Majumder, P.; Banerjee, R. Landscaping Covalent Organic Framework Nanomorphologies. Am. Chem. Soc. 2022, 144, 11482–11498. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, D.; Zhang, Z.; Matsushita, M.M.; Awaga, K. Electron Highways into Nanochannels of Covalent Organic Frameworks for High Electrical Conductivity and Energy Storage. ACS Appl. Mater. Interfaces 2019, 11, 7661–7665. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Qi, H.; Chen, Y.; Guo, W.; Yu, H.; Chen, H.; Ying, Y. Humidity-Independent Artificial Olfactory Array Enabled by Hydrophobic Core-Shell Dye/MOFs@COFs. Composites for Plant Disease Diagnosis. ACS Nano 2022, 16, 14297–14307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Bai, Y.; Feng, F. Applications of covalent organic frameworks (COFs)-based sensors for food safety: Synthetic strategies, characteristics and current state-of-art. Food Chem. 2025, 469, 142495. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mallick, S.; Izquierdo-Ruiz, F.; Schäfer, C.; Xing, X.; Rahm, M.; Börjesson, K. A Highly Conductive All-Carbon Linked 3D Covalent Organic Framework Film. Small 2021, 17, 2103152–2103157. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Dhir, S.; Wheeler, S.; Capone, I.; Pasta, M. Outlook on K-Ion Batteries. Chem 2020, 6, 2442–2460. [Google Scholar] [CrossRef]
- Roberts, S.; Kendrick, E. The reemergence of sodium ion batteries: Testing, processing, and manufacturability. Nanotechnol. Sci. Appl. 2018, 11, 23–33. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Cheng, X.; Xia, M.; Zheng, R.; Peng, N.; Yu, H.; Shui, M.; Shu, J. Development status and future prospect of non-aqueous potassium ion batteries for large scale energy storage. Nano Energy 2019, 60, 340–361. [Google Scholar] [CrossRef]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research development on K-ion batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Fan, Q.; Zhang, C.; Zhou, T.; Kalantar-Zadeh, K.; Guo, Z. Liquid metal batteries for future energy storage. Energy Environ. Sci. 2021, 14, 4177–4202. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Liu, Y.; Xi, S.; Liu, X.; Wu, Z.; Hao, J.; Pang, W.K.; Zhou, T.; Guo, Z. Dehydration-Triggered Ionic Channel Engineering in Potassium Niobate for Li/K-Ion Storage. Adv. Mater. 2020, 32, 2000380–2000389. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Huang, Z.; Wang, M.; Song, W.-L.; Lv, A.; Han, X.; Tu, J.; Jiao, S. Active cyano groups to coordinate AlCl2+ cation for rechargeable aluminum batteries. Energy Storage Mater. 2020, 33, 250–257. [Google Scholar] [CrossRef]
- Lin, M.C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 325–328. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.M.; Li, F. The rechargeable aluminum battery: Opportunities and challenges. Ang. Chem. Int. Ed. 2019, 58, 11978–11996. [Google Scholar] [CrossRef]
- Sun, H.; Wang, W.; Yu, Z.; Yuan, Y.; Wang, S.; Jiao, S. A new aluminium-ion battery with high voltage, high safety and low cost. Chem. Commun. 2015, 51, 11892–11895. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Wang, S.; Piveteau, L.; Kovalenko, M.V. Efficient aluminum chloride—Natural graphite battery. Chem. Mater. 2017, 29, 4484–4492. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wei, C.Y.; Lin, M.C.; Pan, C.J.; Chou, H.L.; Chen, H.A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M.; et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283–14289. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, B.; Wang, J.; Ding, H.; Zhang, S.; Duan, H.; Yu, X.; Lu, B. Rapidly synthesizing interconnected carbon nano cage by microwave toward high-performance aluminum batteries. Chem. Eng. J. 2020, 389, 124407–124414. [Google Scholar] [CrossRef]
- Moriyama, K.; Kuramochi, M.; Fujii, K.; Morita, T.; Togo, H. Nitroxyl-radical-catalyzed oxidative coupling of amides with silylated nucleophiles through N-halogenation. Ang. Chem. Int. Ed. 2016, 55, 14546–14551. [Google Scholar] [CrossRef]
- Precht, R.; Stolz, S.; Mankel, E.; Mayer, T.; Jaegermann, W.; Hausbrand, R. Investigation of sodium insertion into tetracyanoquinodimethane (TCNQ): Results for a TCNQ thin film obtained by a surface science approach. Phys. Chem. Chem. Phys. 2016, 18, 3056–3064. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, B.; Guo, H.; Wub, Z.; Zoub, H.; Yangc, J.; Wang, Z.L. Super-robust and frequency multiplied triboelectric nanogenerator for efficient harvesting water and wind energy. Nano Energy 2019, 64, 103908–103914. [Google Scholar] [CrossRef]
- Shi, B.; Liu, Z.; Zheng, Q.; Meng, J.; Ouyang, H.; Zou, Y.; Jiang, D.; Qu, X.; Yu, M.; Zhao, L.; et al. Body-integrated self-powered system for wearable and implantable applications. ACS Nano 2019, 13, 6017–6024. [Google Scholar] [CrossRef]
- Zhou, Q.; Pan, J.; Deng, S.; Xia, F.; Kim, T. Triboelectric Nanogenerator-Based Sensor Systems for Chemical or Biological Detection. Adv. Mater. 2021, 33, 2008276–2008296. [Google Scholar] [CrossRef]
- Song, Y.; Wang, N.; Hu, C.; Wang, Z.L.; Yang, Y. Soft triboelectric nanogenerators for mechanical energy scavenging and self-powered sensors. Nano Energy 2021, 84, 105919–105936. [Google Scholar] [CrossRef]
- Pu, X.; An, S.; Tang, Q.; Guo, H.; Hu, C. Wearable triboelectric sensors for biomedical monitoring and human-machine interface. iScience 2021, 24, 102027–102047. [Google Scholar] [CrossRef]
- Ning, C.; Cheng, R.; Jiang, Y.; Sheng, F.; Yi, J.; Shen, S.; Zhang, Y.; Peng, X.; Dong, K.; Wang, Z.L. Helical fiber strain sensors based on triboelectric nanogenerators for self-powered human respiratory monitoring. ACS Nano 2022, 16, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhang, C.; Wang, Z.L. Triboelectric nanogenerators as wearable power sources and self-powered sensors. Nat. Sci. Rev. 2023, 10, 170–190. [Google Scholar] [CrossRef]
- Fu, Q.; Cui, C.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Emerging cellulose-derived materials: A promising platform for the design of flexible wearable sensors toward health and environment monitoring. Mater. Chem. Front. 2021, 5, 2051–2091. [Google Scholar] [CrossRef]
- Wang, P.; Hu, M.; Wang, H.; Chen, Z.; Feng, Y.; Wang, J.; Ling, W.; Huang, Y. The evolution of flexible electronics: From nature, beyond nature, and to nature. Adv. Sci. 2020, 7, 2001116–2001144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wang, A.C. On the origin of contact-electrification. Mater Today 2019, 30, 34–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, D.; Zhao, J.; Liu, G.; Bu, T.; Zhang, C.; Wang, Z.L. Tribovoltaic effect on metal–semiconductor interface for direct-current low-impedance triboelectric nanogenerators. Adv. Energy Mater. 2020, 10, 1903713–1903720. [Google Scholar] [CrossRef]
- Slabov, V.; Kopyl, S.; Soares dos Santos, M.P.; Kholkin, A.L. Natural and eco-friendly materials for triboelectric energy harvesting. Nano-Micro Lett. 2020, 12, 42–59. [Google Scholar] [CrossRef]
- Jiang, W.; Li, H.; Liu, Z.; Li, Z.; Tian, J.; Shi, B.; Zou, Y.; Ouyang, H.; Zhao, C.; Zhao, L.; et al. Fully bioabsorbable natural-materials-based triboelectric nanogenerators. Adv. Mater. 2018, 30, 1801895–1801904. [Google Scholar] [CrossRef]
- Jie, Y.; Jia, X.; Zou, J.; Chen, Y.; Wang, N.; Wang, Z.L.; Cao, X. Natural leaf made triboelectric nanogenerator for harvesting environmental mechanical energy. Adv. Energy Mater. 2018, 8, 1703133–1703139. [Google Scholar] [CrossRef]
- Chao, S.; Ouyang, H.; Jiang, D.; Fan, Y.; Li, Z. Triboelectric nanogenerator based on degradable materials. EcoMat 2020, 3, e12072–e12090. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Zhang, Y.; Wang, L.; Wei, C.; Lv, T.; Wang, Z.L.; Wu, Z. Sweat-Permeable, Biodegradable, Transparent and Self-powered Chitosan-Based Electronic Skin with Ultrathin Elastic Gold Nanofibers. Adv. Funct. Mater. 2022, 32, 2112241–2112250. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Bo, X.; Wang, H.; Yang, S.; Tao, X.; Zi, Y.; Yu, W.W.; Li, W.J.; Daoud, W.A. High-Performance Biomechanical Energy Harvester Enabled by Switching Interfacial Adhesion via Hydrogen Bonding and Phase Separation. Adv. Funct. Mater. 2022, 32, 2204304–2204314. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Ding, W.; Zhu, Y.; Qian, H.; Zhou, J.; Chen, Y.; Li, J.; Li, W.; Huang, L.; et al. Rationally Designing High-Performance Versatile Organic Memristors through Molecule-Mediated Ion Movements. Adv. Mater. 2023, 35, 2302863–2302874. [Google Scholar] [CrossRef]
- Talin, A.A.; Stavila, V.; Allendorf, M.D.; Foster, M.E.; He, Y.; Leonard, F.; Spataru, C.D.; Jones, R.E. Molecule@MOF: A New Class of Opto-electronic Materials. Sandia Rep. 2017, 1, 1–27. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; Gabaly, F.E.; Yoon, H.P.; et al. Tunable Electrical Conductivity in metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef]
- Choi, S.; Tan, S.H.; Li, Z.; Kim, Y.; Choi, C.; Chen, P.Y.; Yeon, H.; Yu, S.; Kim, J. SiGe epitaxial memory for neuromorphic computing with reproducible high performance based on engineered dislocations. Nat. Mater. 2018, 17, 335. [Google Scholar] [CrossRef] [PubMed]







| TCNQ-Based Organic Solar Cell | Voc (V) | Jsc (mA/cm) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ZnPc/TCNQ CuPc/TCNQ | 0.58 0.48 | 0.27·10−3 0.37·10−3 | 18.0 16.0 | 1.6·10−5 2.8·10−5 | [100] |
| Graphene/TCNQ/graphene | 0.60 | 8.90 | 48.0 | 2.58 | [101] |
| Spiro-MeOTAD/DMC/F4TCNQ | 0.98 | 24.00 | 62.4 | 14.40 | [145] |
| P3HT/F4TCNQ | 0.87 | 9.85 | 63.2 | 5.83 | [103] |
| DADQs/TCNQ/TiO2 | 3.00 | 9.12 | 59.0 | 11.75 | [60] |
| Spiro-MeOTAD/F4TCNQ | 1.04 | 19.40 | 69.9 | 14.30 | [139] |
| Spiro-MeOTAD/F4TCNQ | 0.95 | 18.72 | 56.8 | 10.59 | [154] |
| CH3NH3PbI3/F4TCNQ | 1.06 | 20.30 | 75.4 | 18.10 | [140] |
| PBDTTT-EFT/F4TCNQ | 0.80 | 17.39 | 61.8 | 8.60 | [164] |
| PEDOT:PSS/F4TCNQ | 1.02 | 21.93 | 77.0 | 17.22 | [137] |
| PTAA/F4TCNQ/Bphen/Al | 1.12 | 23.38 | 77.1 | 20.16 | [150] |
| FTAZ:IT-M/F4TCNQ | 0.95 | 18.70 | 71.0 | 12.40 | [165] |
| 2mF-X59/spiro-OMeTAD/TCNQ/CsPbI2Br | 1.20 | 14.78 | 75.85 | 13.42 | [166] |
| F4TCNQ HTL/NiOx | 1.02 | 20.07 | 74.5 | 15.70 | [141] |
| AQ/TCNQ/TiO2 TQ/TCNQ/TiO2 | 3.25 2.72 | 10.63 11.86 | 95.0 94.0 | 18.92 18.90 | [118] |
| PMMA/F4TCNQ | 1.06 | 21.81 | 76.3 | 17.90 | [144] |
| CuPc(NH2)4/TCNQ/CH3NH3PbI3 | 0.77 | 23.1 | 61.84 | 10.90 | [157] |
| Cathode Material | Type of Battery | Average Voltage (V) | Cycling Stability (%)/Cycles | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| CuTCNQ | Li+ | 0.01–3.20 | 75/500 | 280.9 | [50] |
| CuTCNQ | Li/Na DIBs | 4.26 | 75/200 | 195.4 | [54] |
| CuTCNQ-MOF | Li+ DIBs | 3.75 | 70/50 | 157.0 | [226] |
| F4TCNQ/ LLZTO-PEO | Li+ | 3.40 | 77/50 | 80.00 | [55] |
| CuTCNQ | Na+ | 2.00–4.10 | 84/50 | 255.0 | [202] |
| TCNQ | Na+ | 2.20–3.00 | 70/50 | 233.0 | [239] |
| CuTCNQ | K+ | 2.80 | 75/50 | 244.0 | [204] |
| TCNQ/CCP | Zn2+ | 1.10 | 78.54/1000 | 171.0 | [186] |
| TCNQ/PPy | Zn2+ | 0.45 | 75.40/1000 | 245.8 | [50] |
| p-TCNQ | Zn2+ | 1.10 | 55.20/50 | 250.0 | [189] |
| TCNQ | Zn2+ | 1.02 | 98.90/50 | 244.4 | [190] |
| FTCNQ | Zn2+ | 1.20 | 93.40/50 | 168.5 | [190] |
| F4TCNQ | Zn2+ | 1.40 | 75.30/50 | 135.1 | [190] |
| TCNQ/PNZ | Zn2+ | 1.00–3.80 | 88/100 | 150.0 | [193] |
| TCNQ | Al3+ | 1.60 | 75/2000 | 180.0 | [245] |
| TCNQ/PPy | Al3+ | 0.45–0.85 | 76/100 | 245.8 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starodub, T. Tetracyanoquinodimethane and Its Derivatives as Promising Sustainable Materials for Clean Energy Storage and Conversion Technologies: A Review. Sustainability 2025, 17, 10612. https://doi.org/10.3390/su172310612
Starodub T. Tetracyanoquinodimethane and Its Derivatives as Promising Sustainable Materials for Clean Energy Storage and Conversion Technologies: A Review. Sustainability. 2025; 17(23):10612. https://doi.org/10.3390/su172310612
Chicago/Turabian StyleStarodub, Tetiana. 2025. "Tetracyanoquinodimethane and Its Derivatives as Promising Sustainable Materials for Clean Energy Storage and Conversion Technologies: A Review" Sustainability 17, no. 23: 10612. https://doi.org/10.3390/su172310612
APA StyleStarodub, T. (2025). Tetracyanoquinodimethane and Its Derivatives as Promising Sustainable Materials for Clean Energy Storage and Conversion Technologies: A Review. Sustainability, 17(23), 10612. https://doi.org/10.3390/su172310612







