Evaluation of Capsaicin as a Selector for Growth Promotional Bacteria Isolated from Capsicum Peppers
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Fruits and Quantifying Capsaicin Levels with High Performance Liquid Chromatography
2.2. Bacterial Isolation
2.3. Capsaicin Broth Microdilution Assay
2.4. Assessing Bacterial Isolates: Plant Growth Promotion Assays
2.4.1. Phosphate and Potassium Solubilization
2.4.2. Protease Activity
2.4.3. IAA Production
2.4.4. Urease Activity
2.4.5. Siderophore Production
2.5. Identifying Bacteria Through 16S rRNA Sequencing
- 27F (5′-AGA GTT TGA TCC TGG CTC AG);
- 1492R (5′-CGG TTA CCT TGT TAC GAC TT).
- Denaturation: 94 °C for 30 s;
- Annealing: 55 °C for 30 s;
- Extension: 72 °C for 60 s.
2.6. Dual Culture Assays with Common Fungal Pathogens
2.7. Inoculation Tests with Trifolium pratense and Poa pratensis
3. Results
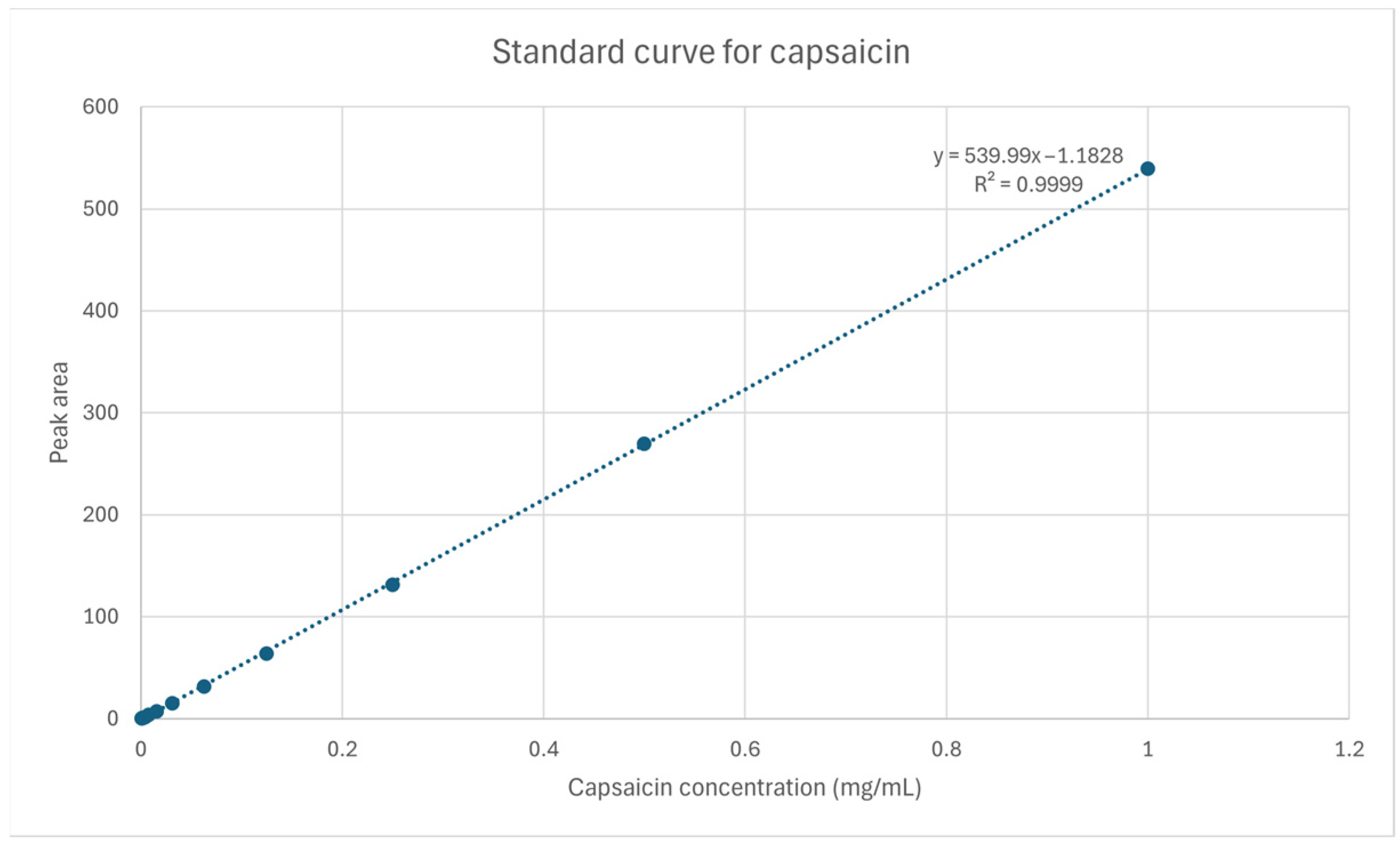
3.1. HPLC-UV Determination of Capsaicin Concentrations
3.2. Bacterial Isolates Recovered from Peppers and Their Capsaicin Resistances
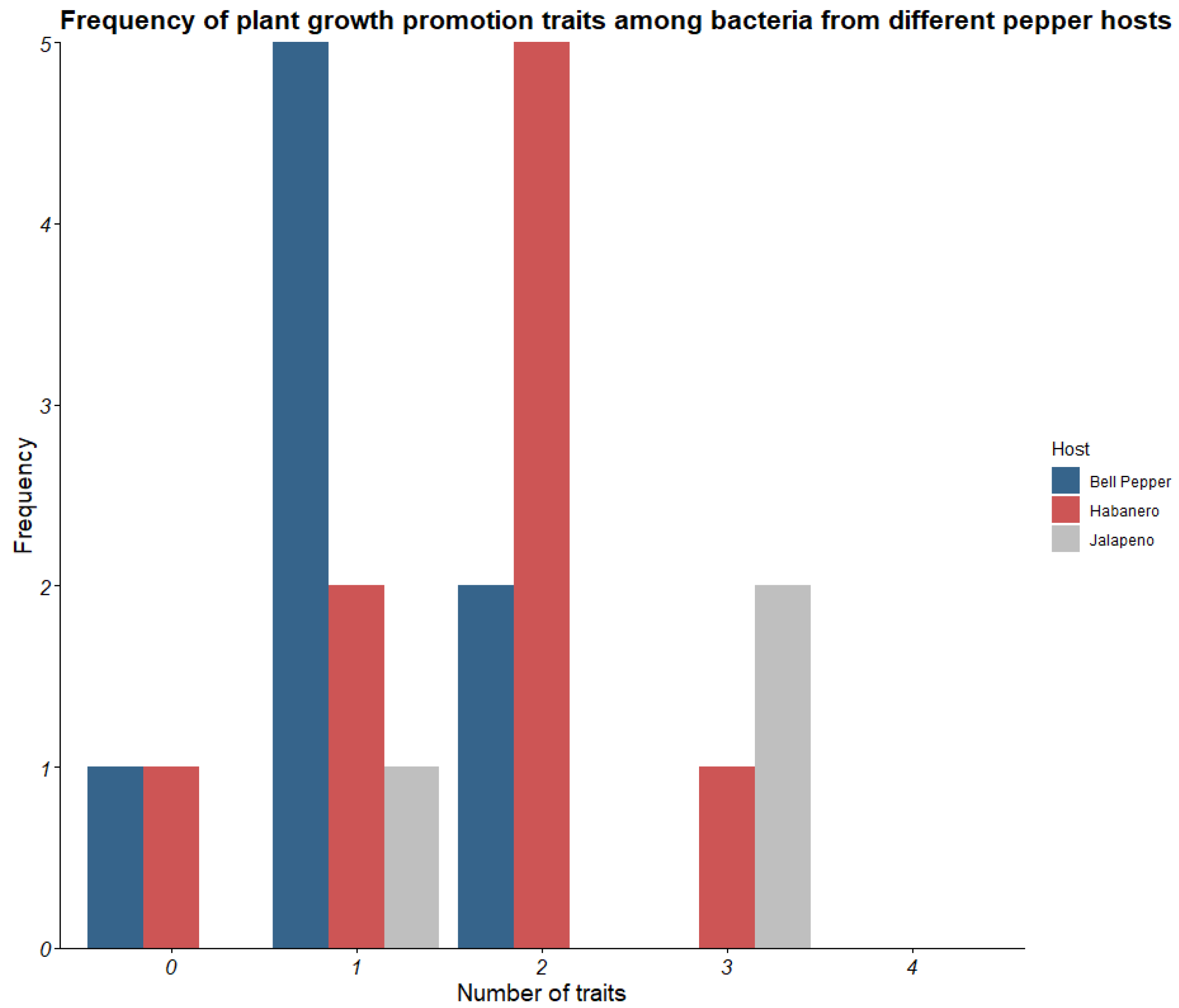
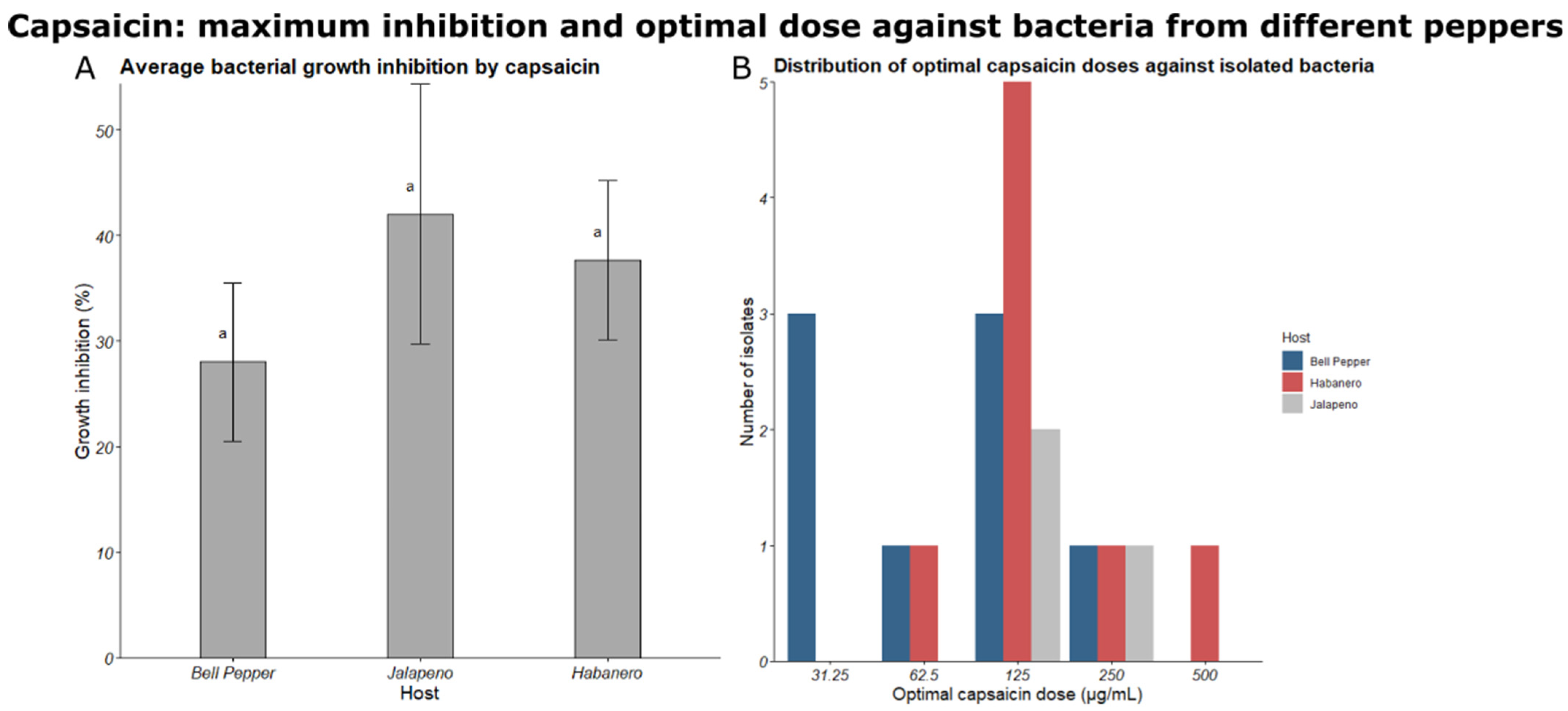
3.3. Growth Promotion Trait Testing
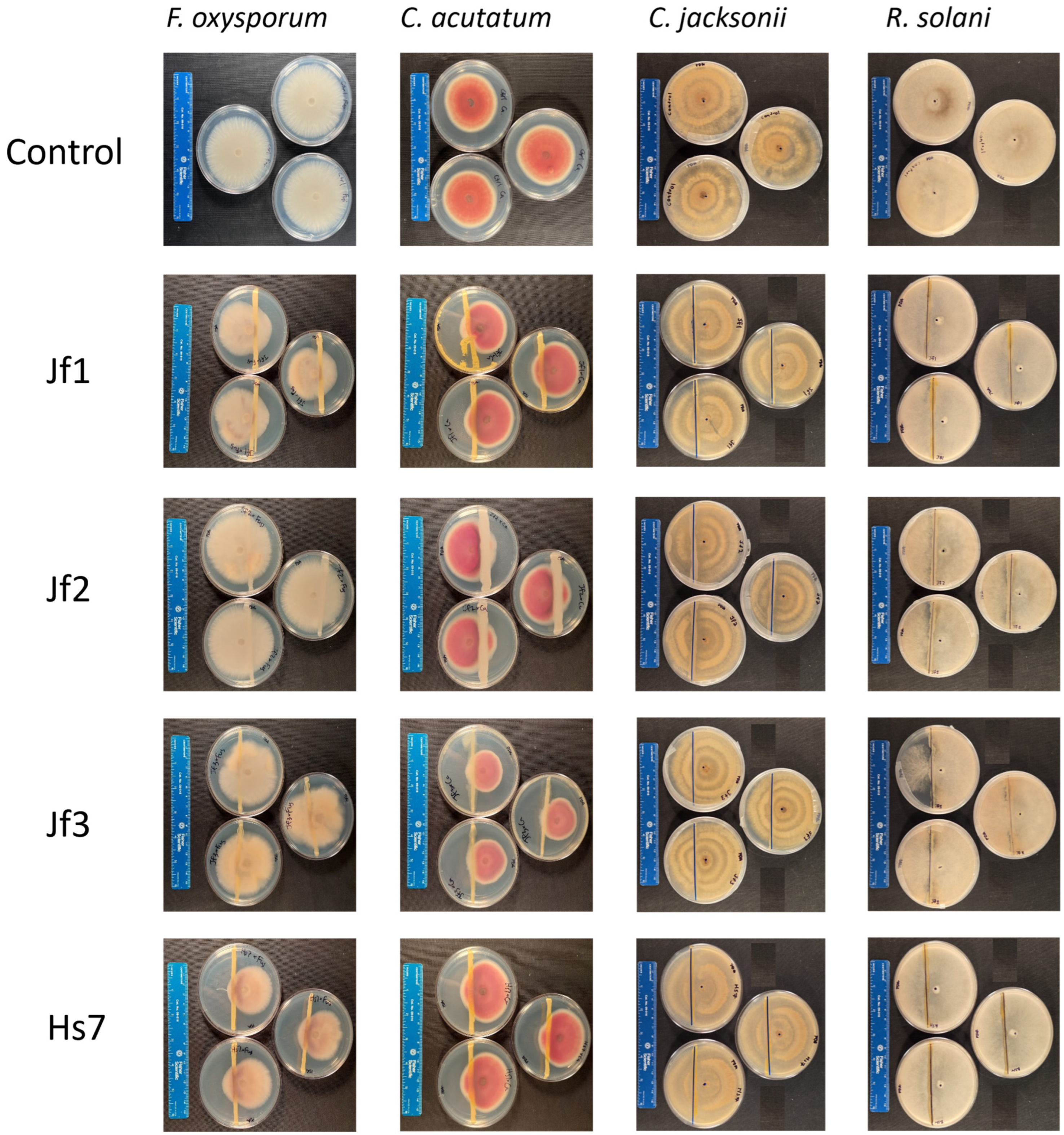
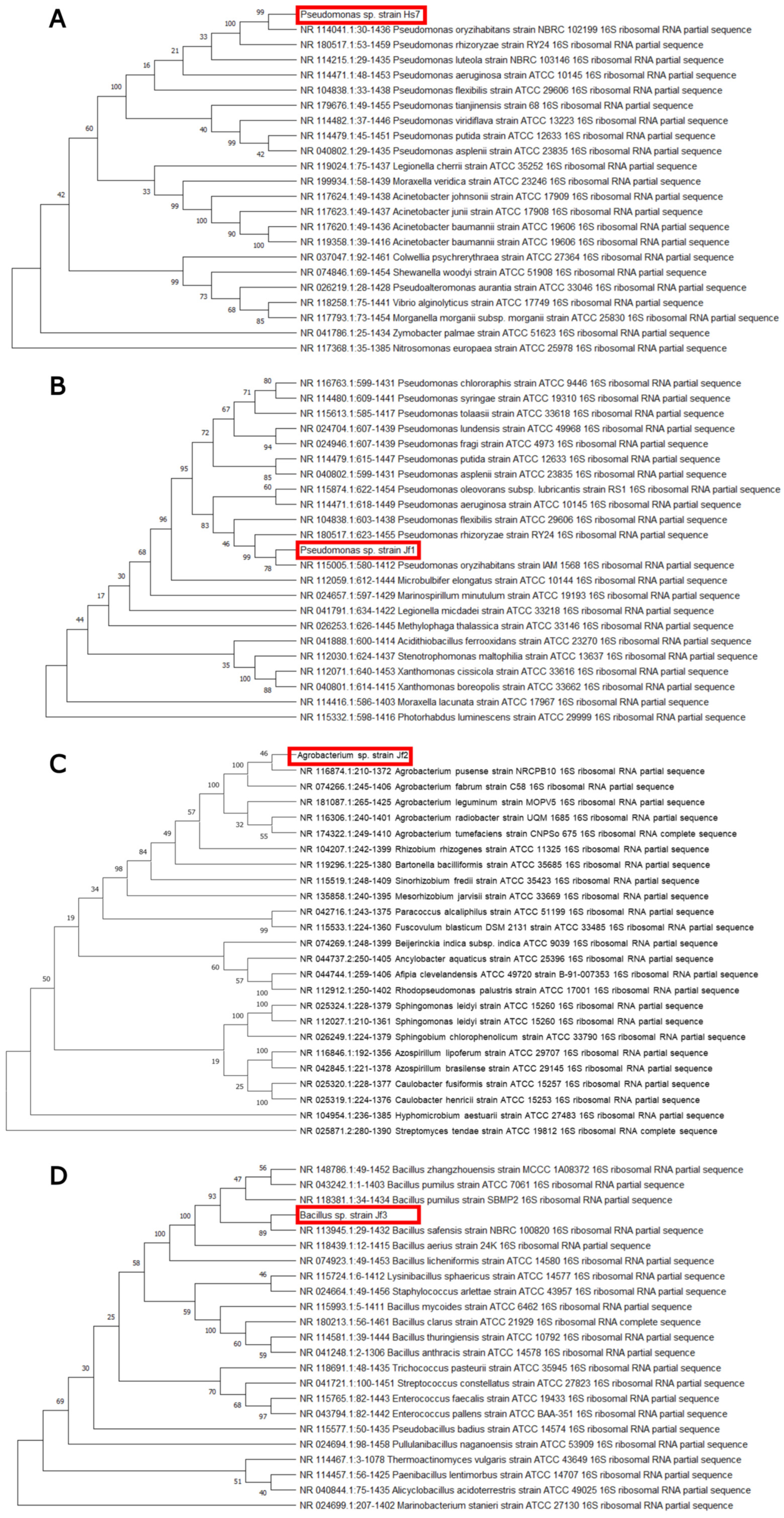
3.4. Choosing the Best Bacteria: Additional Tests and Identification
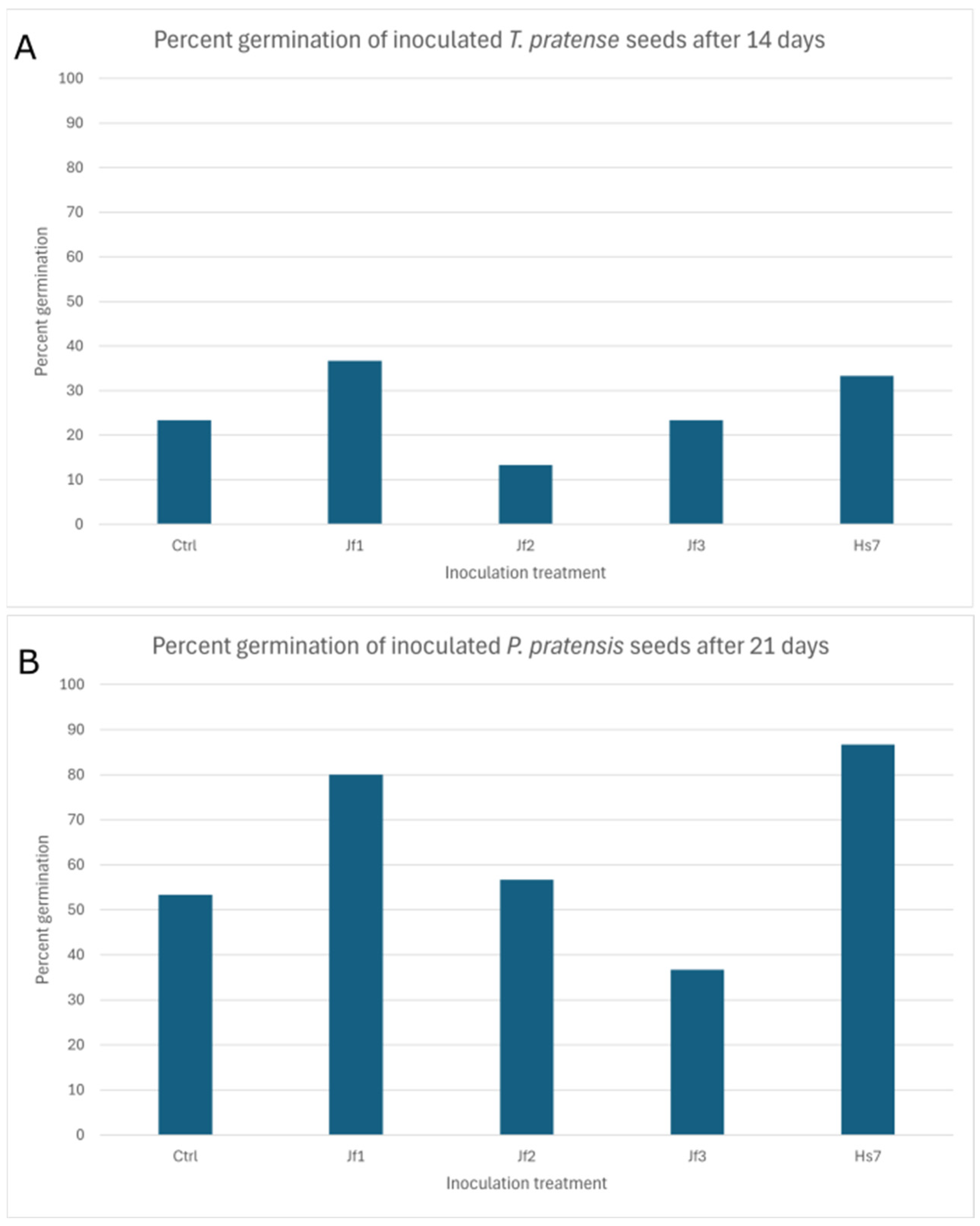
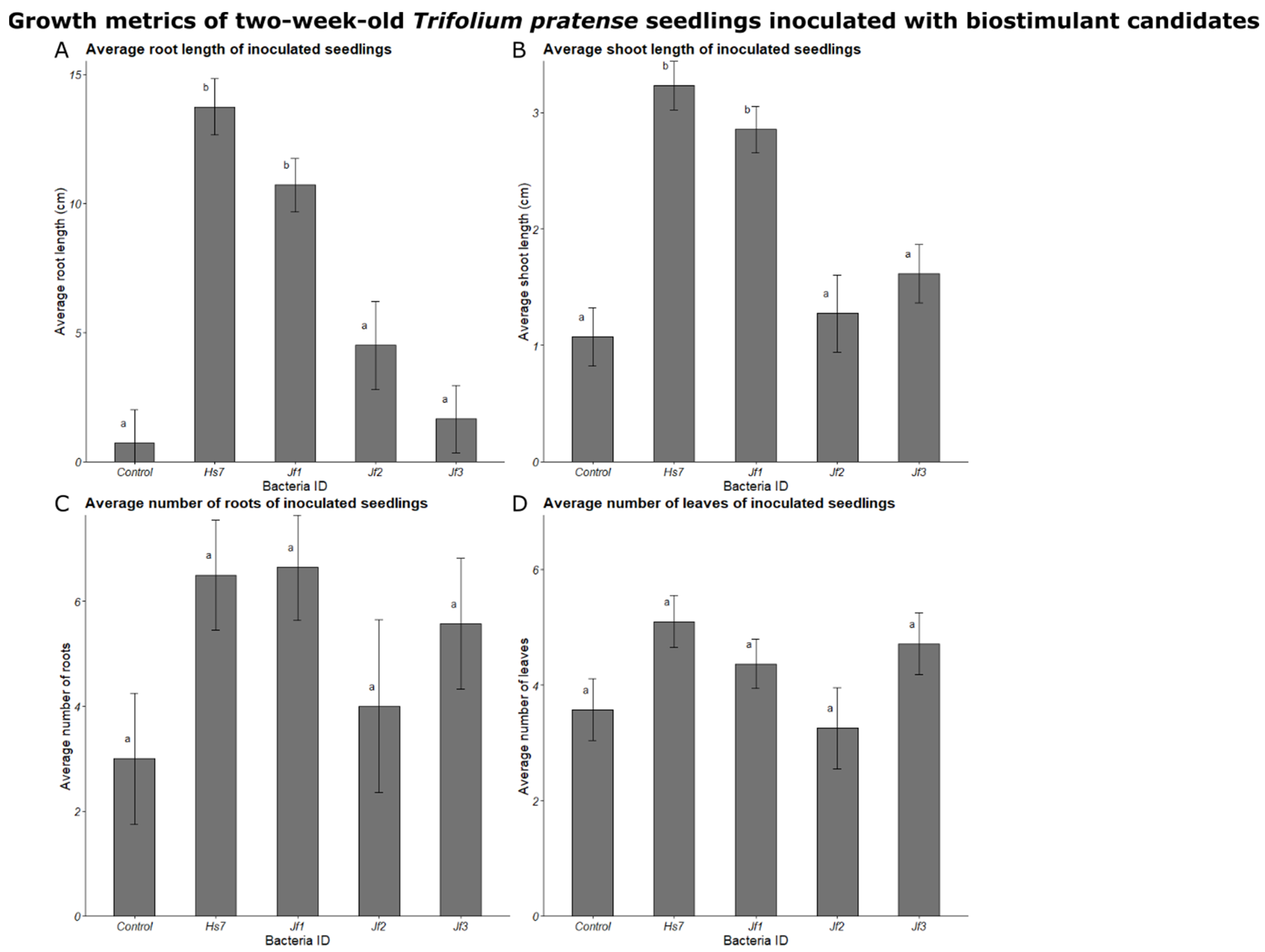
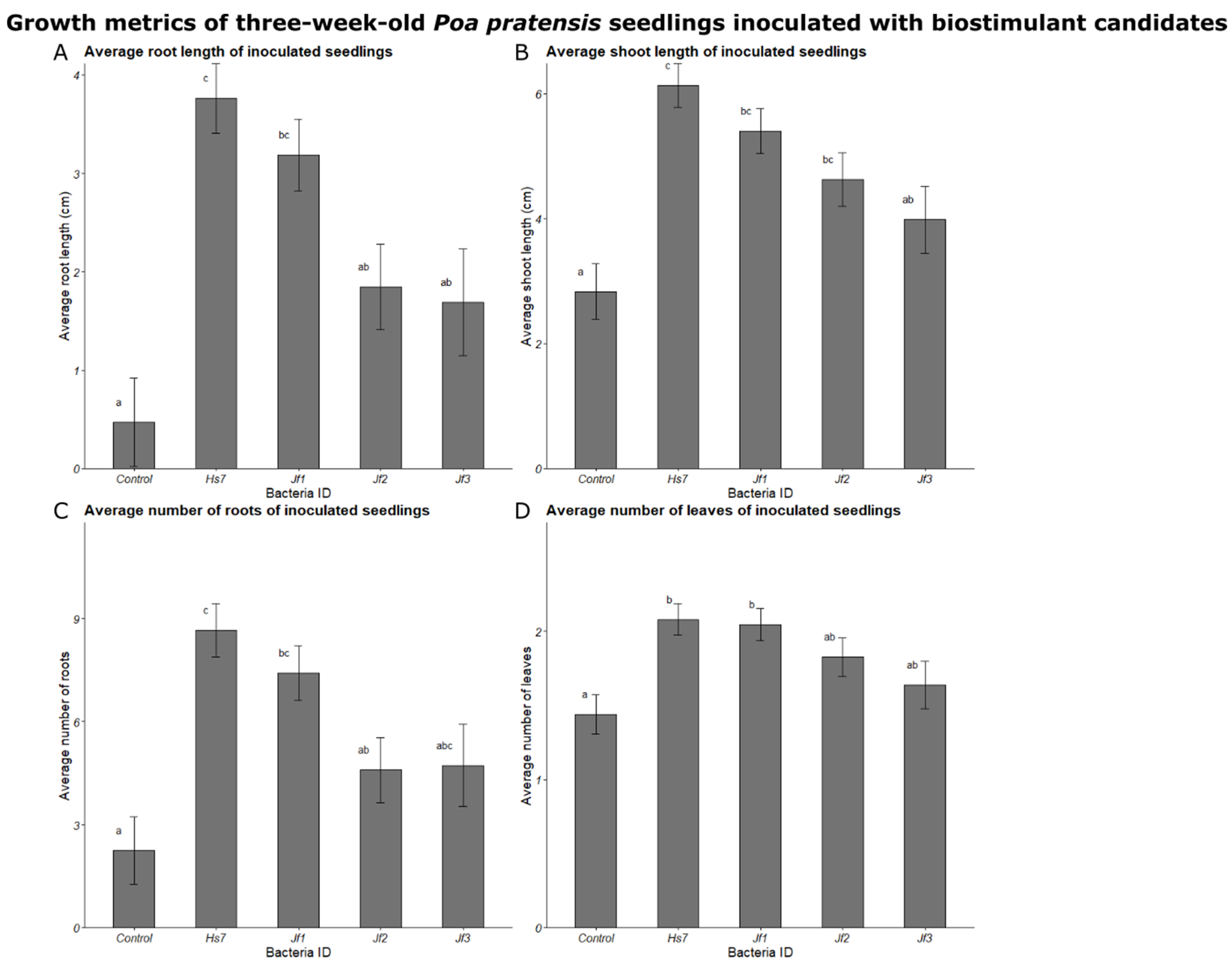
3.5. Growth Promotion Testing in Inoculation Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGPB | Plant growth-promoting bacteria |
| LB | Luria–Bertani |
| IAA | Indole-3-acetic acid |
| HPLC-UV | High-performance liquid chromatography with ultraviolet detection |
| MIC | Minimum inhibitory concentration |
| ANOVA | Analysis of variance |
| CAS | Chrome azurol S |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| SHU | Scoville heat unit |
Appendix A
| Time | Channel A: 100.0% Water 0.1% Formic Acid | Channel B 100.0% Acetonitrile 0.1% Formic Acid | |
|---|---|---|---|
| 1 | 0.70 min | 55.00% | 45.00% |
| 2 | 3.50 min | 55.00% | 45.00% |
| 3 | 3.60 min | 0.00% | 100.00% |
| 4 | 3.70 min | 0.00% | 100.00% |
| 5 | 4.00 min | 60.00% | 40.00% |
| 6 | 5.00 min | 60.00% | 40.00% |
References
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and Crop Responses: A Review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- Babaniyi, G.G.; Akor, U.J.; Odeseye, A.A. Pesticide Contributions to Greenhouse Gas Emissions. In The Interplay of Pesticides and Climate Change: Environmental Dynamics and Challenges; Babaniyi, B.R., Babaniyi, E.E., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 173–230. ISBN 978-3-031-81669-7. [Google Scholar]
- Audsley, E.; Stacey, K.F.; Parsons, D.J.; Williams, A.G. Estimation of the Greenhouse Gas Emissions from Agricultural Pesticide Manufacture and Use; Cranfield University: Bedford, UK, 2009. [Google Scholar]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, e963401. [Google Scholar] [CrossRef]
- Paul, N.B.; Sundara Rao, W.V.B. Phosphate-Dissolving Bacteria in the Rhizosphere of Some Cultivated Legumes. Plant Soil 1971, 35, 127–132. [Google Scholar] [CrossRef]
- Sheng, X.F. Growth Promotion and Increased Potassium Uptake of Cotton and Rape by a Potassium Releasing Strain of Bacillus edaphicus. Soil Biol. Biochem. 2005, 37, 1918–1922. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-Acetic Acid (IAA) Production in Klebsiellaby LC-MS/MS and the Salkowski Method. Bio Protoc. 2019, 9, e3230. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified Microplate Method for Rapid and Efficient Estimation of Siderophore Produced by Bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Hamonts, K.; Trivedi, P.; Garg, A.; Janitz, C.; Grinyer, J.; Holford, P.; Botha, F.C.; Anderson, I.C.; Singh, B.K. Field Study Reveals Core Plant Microbiota and Relative Importance of Their Drivers. Environ. Microbiol. 2018, 20, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Vannier, N.; Mony, C.; Bittebiere, A.-K.; Michon-Coudouel, S.; Biget, M.; Vandenkoornhuyse, P. A Microorganisms’ Journey Between Plant Generations. Microbiome 2018, 6, 79. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, J.; Lee, K.K.; Lee, Y.-H. Longitudinal Transmission of Bacterial and Fungal Communities from Seed to Seed in Rice. Commun. Biol. 2022, 5, 772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; White, J.F. Bioprospecting Desert Plants for Endophytic and Biostimulant Microbes: A Strategy for Enhancing Agricultural Production in a Hotter, Drier Future. Biology 2021, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic Potential of Endophytic Bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and Anti-Virulence Activity of Capsaicin Against Erythromycin-Resistant, Cell-Invasive Group A Streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef]
- Notashfard, A.; Nojavan Asghari, M. Inhibitory and Bactericidal Effect of Aqueous Pepper Extract (Capsicum annum L.), Capsaicin, and Capsaicin Combination with Amoxicillin Against Streptococcus Pyogenes. J. Med. Microbiol. Infect. Dis. 2024, 12, 259–269. [Google Scholar] [CrossRef]
- Periferakis, A.-T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef]
- Guo, T.; Li, M.; Sun, X.; Wang, Y.; Yang, L.; Jiao, H.; Li, G. Synergistic Activity of Capsaicin and Colistin Against Colistin-Resistant Acinetobacter baumannii: In Vitro/Vivo Efficacy and Mode of Action. Front. Pharmacol. 2021, 12, 744494. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Mujtaba, M.; Ilk, S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Islek, C. Supplementing Capsaicin with Chitosan-Based Films Enhanced the Anti-Quorum Sensing, Antimicrobial, Antioxidant, Transparency, Elasticity and Hydrophobicity. Int. J. Biol. Macromol. 2018, 115, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine Kinase Homologs That Act as Cytokinin Receptors Possess Overlapping Functions in the Regulation of Shoot and Root Growth in Arabidopsis. Plant Cell 2004, 16, 1365–1377. [Google Scholar] [CrossRef]
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.G.; Falcão, R.E.A.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434–5447. [Google Scholar] [CrossRef]
- Chatterjee, S.; Asakura, M.; Chowdhury, N.; Neogi, S.B.; Sugimoto, N.; Haldar, S.; Awasthi, S.P.; Hinenoya, A.; Aoki, S.; Yamasaki, S. Capsaicin, a Potential Inhibitor of Cholera Toxin Production in Vibrio cholerae. FEMS Microbiol. Lett. 2010, 306, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Pandhair, V.; Sharma, S. Accumulation of Capsaicin in Seed, Pericarp and Placenta of Capsicum annuum L Fruit. J. Plant Biochem. Biotechnol. 2008, 17, 23–27. [Google Scholar] [CrossRef]
- Guo, X.-M.; Yu, Y.-Y.; Bai, L.; Gao, R.-F. Dianthus chinensis L.: The Structural Difference Between Vascular Bundles in the Placenta and Ovary Wall Suggests Their Different Origin. Front. Plant Sci. 2017, 8, 1986. [Google Scholar] [CrossRef]
- Guo, X.-M.; Xiao, X.; Wang, G.-X.; Gao, R.-F. Vascular Anatomy of Kiwi Fruit and Its Implications for the Origin of Carpels. Front. Plant Sci. 2013, 4, 391. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Findlay, B.L. Fitness Costs of Antibiotic Resistance Impede the Evolution of Resistance to Other Antibiotics. ACS Infect. Dis. 2023, 9, 1834–1845. [Google Scholar] [CrossRef]
- Melnyk, A.H.; Wong, A.; Kassen, R. The Fitness Costs of Antibiotic Resistance Mutations. Evol. Appl. 2015, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Vogwill, T.; MacLean, R.C. The Genetic Basis of the Fitness Costs of Antimicrobial Resistance: A Meta-Analysis Approach. Evol. Appl. 2015, 8, 284–295. [Google Scholar] [CrossRef]
- Füchtbauer, S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Antibacterial Properties of Capsaicin and Its Derivatives and Their Potential to Fight Antibiotic Resistance—A Literature Survey. Eur. J. Microbiol. Immunol. 2021, 11, 10–17. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.; Ahemad, M.; Oves, M. Plant Growth Promotion by Phosphate Solubilizing Bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium Solubilizing Bacteria (KSB): Mechanisms, Promotion of Plant Growth, and Future Prospects—A Review. J. Soil. Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Dogan, G.; Taskin, B. Hydrolytic Enzymes Producing Bacterial Endophytes of Some Poaceae Plants. Pol. J. Microbiol. 2021, 70, 297–304. [Google Scholar] [CrossRef]
- Bunsangiam, S.; Thongpae, N.; Limtong, S.; Srisuk, N. Large Scale Production of Indole-3-Acetic Acid and Evaluation of the Inhibitory Effect of Indole-3-Acetic Acid on Weed Growth. Sci. Rep. 2021, 11, 13094. [Google Scholar] [CrossRef]
- Skoog, F. Experiments on Bud Inhibition with Indole-3-Acetic Acid. Am. J. Bot. 1939, 26, 702–707. [Google Scholar] [CrossRef]
- Park, J.-M.; Radhakrishnan, R.; Kang, S.-M.; Lee, I.-J. IAA Producing enterobacter Sp. I-3 as a Potent Bio-Herbicide Candidate for Weed Control: A Special Reference with Lettuce Growth Inhibition. Indian J. Microbiol. 2015, 55, 207–212. [Google Scholar] [CrossRef]
- Joseph, P.S.; Musa, D.A.; Egwim, E.C.; Uthman, A.; Joseph, P.S.; Musa, D.A.; Egwim, E.C.; Uthman, A. Function of Urease in Plants with Reference to Legumes: A Review. In Legumes Research-Volume 2; IntechOpen: London, UK, 2022; ISBN 978-1-80356-915-4. [Google Scholar]
- Witte, C.-P. Urea Metabolism in Plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Christensen, W.B. Urea Decomposition as a Means of Differentiating Proteus and Paracolon Cultures from Each Other and from Salmonella and Shigella Types. J. Bacteriol. 1946, 52, 461–466. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Zhang, Q.; Kingsley, K.L.; White, J.F. Endophytic pseudomonas Sp. from Agave Palmeri Participate in the Rhizophagy Cycle and Act as Biostimulants in Crop Plants. Biology 2022, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Arie, T. Fusarium Diseases of Cultivated Plants, Control, Diagnosis, and Molecular and Genetic Studies. J. Pestic. Sci. 2019, 44, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum Species Complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia Solani: Taxonomy, Population Biology and Management of Rhizoctonia Seedling Disease of Soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Bergen, M.; Kowalski, K.; White, J. Fungal Disease Prevention in Seedlings of Rice (Oryza sativa) and Other Grasses by Growth-Promoting Seed-Associated Endophytic Bacteria from Invasive Phragmites australis. Microorganisms 2018, 6, 21. [Google Scholar] [CrossRef]
- Othman, Z.A.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruit Samples Using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, I.; Bosland, P.W. Sensory Properties of Chile Pepper Heat–and Its Importance to Food Quality and Cultural Preference. Appetite 2017, 117, 186–190. [Google Scholar] [CrossRef]
- Eagle, H.; Musselman, A.D. The Rate of Bactericidal Action of Penicillin In Vitro as a Function of Its Concentration, and Its Paradoxically Reduced Activity at High Concentrations Against Certain Organisms. J. Exp. Med. 1948, 88, 99–131. [Google Scholar] [CrossRef] [PubMed]
- Prasetyoputri, A.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A.T. The Eagle Effect and Antibiotic-Induced Persistence: Two Sides of the Same Coin? Trends Microbiol. 2019, 27, 339–354. [Google Scholar] [CrossRef]
- Popelka, P.; Jevinová, P.; Šmejkal, K.; Roba, P. Determination of Capsaicin Content and Pungency Level of Different Fresh and Dried Chilli Peppers. Folia Vet. 2017, 61, 11–16. [Google Scholar] [CrossRef]
- Ekwere, M.; Udoh, E.D. Extraction and Comparative Analysis of Moisture and Capsaicin Contents of Capsicum Peppers. J. Pain. Relief 2016, 5, 2167-0846. [Google Scholar] [CrossRef]
- Palma-Orozco, G.; Orozco-Álvarez, C.; Chávez-Villeda, A.A.; Mixtega-Martínez, A.; Castro-Muñoz, R. Capsaicin Content in Red Habanero Chilli (Capsicum chinense Jacq.) and Its Preservation after Drying Process. Future Foods 2021, 4, 100070. [Google Scholar] [CrossRef]
- Youseif, S.H.; Abd El-Megeed, F.H.; Humm, E.A.; Maymon, M.; Mohamed, A.H.; Saleh, S.A.; Hirsch, A.M. Comparative Analysis of the Cultured and Total Bacterial Community in the Wheat Rhizosphere Microbiome Using Culture-Dependent and Culture-Independent Approaches. Microbiol. Spectr. 2021, 9, e00678-21. [Google Scholar] [CrossRef] [PubMed]
- Hinsu, A.; Dumadiya, A.; Joshi, A.; Kotadiya, R.; Andharia, K.; Koringa, P.; Kothari, R. To Culture or Not to Culture: A Snapshot of Culture-Dependent and Culture-Independent Bacterial Diversity from Peanut Rhizosphere. PeerJ 2021, 9, e12035. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Ruppel, S. Progress in Cultivation-Independent Phyllosphere Microbiology. FEMS Microbiol. Ecol. 2014, 87, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; He, X.; Tao, Y.; Zhou, C.; Li, X. The Changes of the Endophytic Bacterial Community from Pepper Varieties with Different Capsaicinoids. Microorganisms 2025, 13, 596. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhou, C.; Li, X.; Zou, X.; Ou, L.; Tao, Y. The Changes of Rhizosphere Microbial Communities in Pepper Varieties with Different Capsaicinoids. Front. Microbiol. 2024, 15, 1430682. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Adoko, M.Y.; Babalola, O.O.; Amogou, O.; Badé, F.T.; Noumavo, P.A.; Adjanohoun, A.; Baba-Moussa, L. Efficacy of Biostimulants Formulated With Pseudomonas putida and Clay, Peat, Clay-Peat Binders on Maize Productivity in a Farming Environment in Southern Benin. Front. Sustain. Food Syst. 2021, 5, 666718. [Google Scholar] [CrossRef]
- del-Canto, A.; Sanz-Saez, Á.; Sillero-Martínez, A.; Mintegi, E.; Lacuesta, M. Selected Indigenous Drought Tolerant Rhizobium Strains as Promising Biostimulants for Common Bean in Northern Spain. Front. Plant Sci. 2023, 14, 1046397. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for Plant Growth Promotion and Stress Resilience: What Have We Learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Costa, J.; Sepúlveda, M.; Gallardo, V.; Cayún, Y.; Santander, C.; Ruíz, A.; Reyes, M.; Santos, C.; Cornejo, P.; Lima, N.; et al. Antifungal Potential of Capsaicinoids and Capsinoids from the Capsicum Genus for the Safeguarding of Agrifood Production: Advantages and Limitations for Environmental Health. Microorganisms 2022, 10, 2387. [Google Scholar] [CrossRef]
- Xu, X.; Qu, R.; Wu, W.; Jiang, C.; Shao, D.; Shi, J. Applications of Microbial Co-Cultures in Polyketides Production. J. Appl. Microbiol. 2021, 130, 1023–1034. [Google Scholar] [CrossRef]
- Kobayashi, D.Y.; Reedy, R.M.; Bick, J.; Oudemans, P.V. Characterization of a Chitinase Gene from Stenotrophomonas maltophilia Strain 34S1 and Its Involvement in Biological Control. Appl. Environ. Microbiol. 2002, 68, 1047–1054. [Google Scholar] [CrossRef]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and Their Role in Biocontrol by Pseudomonas Bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [PubMed]
- Kafri, M.; Metzl-Raz, E.; Jona, G.; Barkai, N. The Cost of Protein Production. Cell Rep. 2016, 14, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kintaka, R.; Makanae, K.; Namba, S.; Kato, H.; Kito, K.; Ohnuki, S.; Ohya, Y.; Andrews, B.J.; Boone, C.; Moriya, H. Genetic Profiling of Protein Burden and Nuclear Export Overload. eLife 2020, 9, e54080. [Google Scholar] [CrossRef] [PubMed]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved Drought Stress Response in Alfalfa Plants Nodulated by an IAA Over-Producing Rhizobium Strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef]
- Ganesh, J.; Hewitt, K.; Devkota, A.R.; Wilson, T.; Kaundal, A. IAA-Producing Plant Growth Promoting Rhizobacteria from Ceanothus velutinus Enhance Cutting Propagation Efficiency and Arabidopsis Biomass. Front. Plant Sci. 2024, 15, 1374877. [Google Scholar] [CrossRef]
- Lata, D.L.; Abdie, O.; Rezene, Y. IAA-Producing Bacteria from the Rhizosphere of Chickpea (Cicer arietinum L.): Isolation, Characterization, and Their Effects on Plant Growth Performance. Heliyon 2024, 10, e39702. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, E.-G.; Park, J.-R.; Ryu, Y.-H.; Moon, W.; Park, G.-H.; Ubaidillah, M.; Ryu, S.-N.; Kim, K.-M. Effect on Chemical and Physical Properties of Soil Each Peat Moss, Elemental Sulfur, and Sulfur-Oxidizing Bacteria. Plants 2021, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, S.; Mu, W.; Yang, W.; Jönsson, P. Pyrolysis Performance of Peat Moss: A Simultaneous in-Situ Thermal Analysis and Bench-Scale Experimental Study. Fuel 2020, 277, 118173. [Google Scholar] [CrossRef]
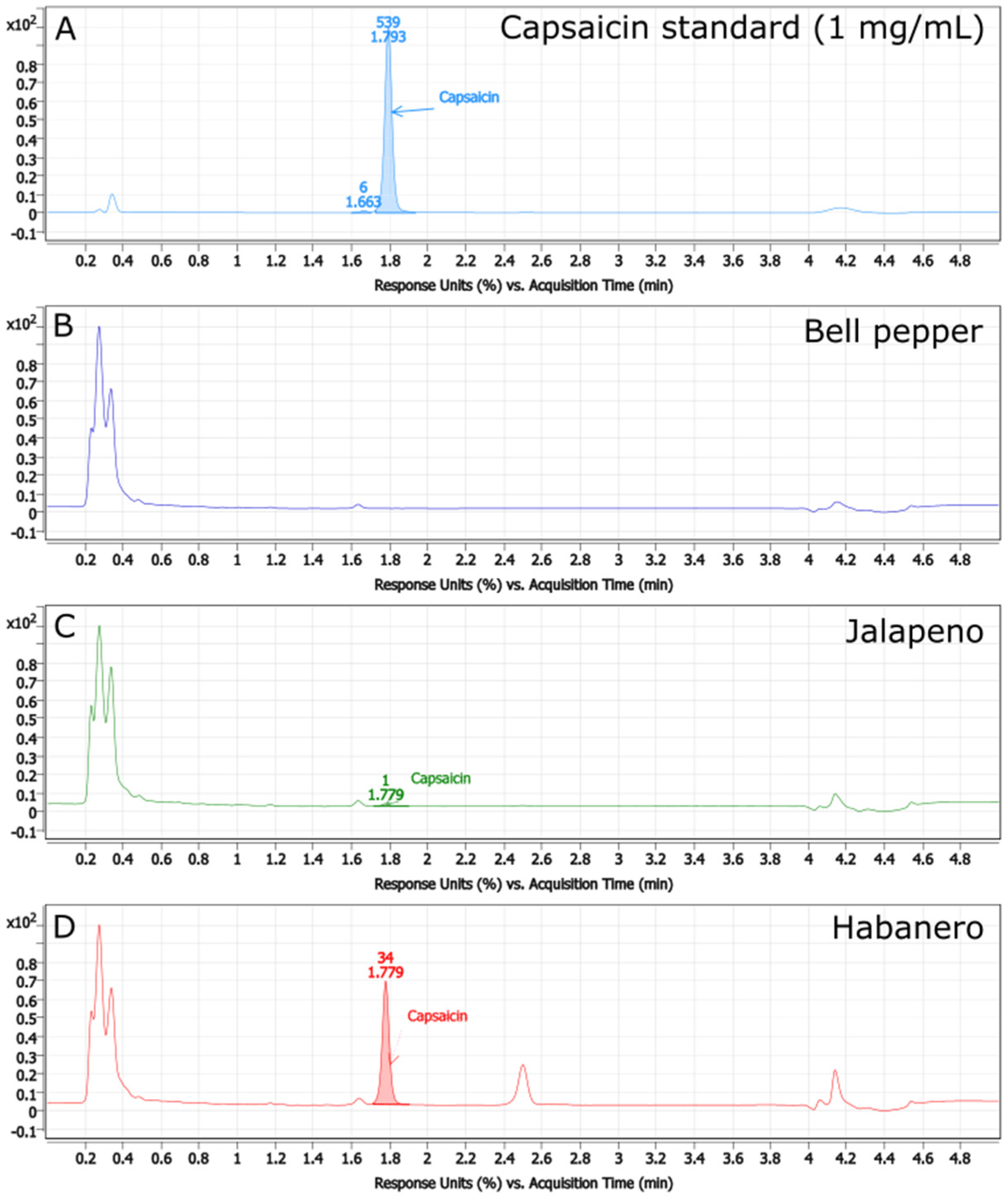
| Sample | Estimated Capsaicin Concentration (μg/g) | Estimated Pungency (Scoville Heat Units) |
|---|---|---|
| Bell Pepper | 0 | 0 |
| Jalapeno | 344.9693513 ± 4.899630199 | 5519.509621 ± 78.39408318 |
| Habanero | 6560.516553 ± 81.99346599 | 104,968.2648 ± 1311.895456 |
| Origin Tissue | ID | Capsaicin Concentration of LB Media in Which Bacteria Was Isolated (μg/mL) | MIC Assay: Capsaicin Concentration at Maximum Inhibition (μg/mL) * |
|---|---|---|---|
| Bell pepper seeds | BPs1 | 150 | 62.5 |
| BPs2 | 150 | ND | |
| BPs3 | 150 | 125 | |
| BPs5 | 0 | 31.25 | |
| Bell pepper fruit flesh | BPf1 | 150 | 250 |
| BPf2 | 150 | 31.25 | |
| BPf6 | 0S | 125 | |
| BPf10 | 150 | 125 | |
| Jalapeno fruit flesh | Jf1 | 150 | 125 |
| Jf2 | 150 | 250 | |
| Jf3 | 0 | 125 | |
| Habanero seeds | Hs1 | 150 | ND |
| Hs3 | 150 | 125 | |
| Hs7 | 150 | 125 | |
| Habanero fruit flesh | Hf1 | 150 | 125 |
| Hf4 | 150 | 500 | |
| Hf5 | 150 | 125 | |
| Hf6 | 150 | 125 | |
| Hf7 | 100 | 250 | |
| Hf11 | 150 | 62.5 |
| Percent Difference in Bacterial Growth at Each Capsaicin Level, Compared to Wells Containing the Growth Control Treatment (0 μg/mL Capsaicin) | ||||||
|---|---|---|---|---|---|---|
| ID | 0 μg/mL | 31.25 μg/mL | 62.50 μg/mL | 125 μg/mL | 250 μg/mL | 500 μg/mL |
| BPs1 | 0 (reference) | −19 | −22 | −6 | +5 | −14 |
| BPs2 | 0 | −1 | +19 | +3 | +11 | +33 |
| BPs3 | 0 | −12 | −15 | −23 | −17 | −21 |
| BPs5 | 0 | −19 | −19 | −18 | +2 | −7 |
| BPf1 | 0 | −33 | −37 | −41 | −50 | −39 |
| BPf2 | 0 | −50 | −39 | −15 | −4 | +5 |
| BPf6 | 0 | −44 | −47 | −50 | −43 | −49 |
| BPf10 | 0 | −6 | −9 | −10 | −4 | +33 |
| Jf1 | 0 | −32 | −66 | −71 | −63 | −59 |
| Jf2 | 0 | +7 | +4 | −25 | −34 | −13 |
| Jf3 | 0 | −4 | −13 | −21 | −15 | −3 |
| Hs1 | 0 | +3 | +8 | +14 | +34 | +90 |
| Hs3 | 0 | −2 | +2 | −5 | −4 | −3 |
| Hs7 | 0 | +7 | −38 | −56 | −50 | −26 |
| Hf1 | 0 | −40 | −48 | −60 | −48 | −32 |
| Hf4 | 0 | −1 | −8 | −16 | 0 | −41 |
| Hf5 | 0 | −29 | −35 | −42 | −41 | −34 |
| Hf6 | 0 | −13 | −17 | −25 | +2 | −24 |
| Hf7 | 0 | +16 | −7 | −12 | −13 | +17 |
| Hf11 | 0 | −38 | −60 | −47 | +10 | −34 |
| Tomato1 | 0 | −45 | −96 | −100 | −77 | −2 |
| Tomato2 | 0 | −77 | −100 | −77 | +15 | −100 |
| Mint1 | 0 | +4 | +9 | −7 | −14 | −43 |
| Mint2 | 0 | +19 | −13 | −16 | −4 | −24 |
| Origin Tissue | ID | Phosphate Solubilization | Potassium Solubilization | Protease Activity | IAA Production (μg/mL) |
|---|---|---|---|---|---|
| Bell pepper seeds | BPs1 | − | − | − | − |
| BPs2 | − | − | + | − | |
| BPs3 | − | − | + | − | |
| BPs5 | − | − | + | − | |
| Bell pepper fruit flesh | BPf1 | − | − | + | − |
| BPf2 | − | − | + | − | |
| BPf6 | − | + | + | − | |
| BPf10 | − | + | + | − | |
| Jalapeno fruit flesh | Jf1 | + | + | − | 220 |
| Jf2 | − | − | − | 263 | |
| Jf3 | + | − | + | 132 | |
| Habanero seeds | Hs1 | − | + | + | − |
| Hs3 | − | − | − | − | |
| Hs7 | + | + | − | 202 | |
| Habanero fruit flesh | Hf1 | − | + | + | − |
| Hf4 | − | − | + | − | |
| Hf5 | − | + | + | − | |
| Hf6 | − | + | + | − | |
| Hf7 | − | + | + | − | |
| Hf11 | − | − | + | − |
| % Radial Inhibition Against Fungal Pathogens | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | 16S rRNA ID—Accession No. | IAA (μg/mL) | Urease Activity | Siderophore Production | F. oxysporum f. sp. lycopersici | C. acutatum | R. solani | C. jacksonii |
| Jf1 | Pseudomonas sp.—PX349277 | 220 | + | − | 28.44 ± 2.75 | 14.12 ± 4.08 | No inhibition | No inhibition |
| Jf2 | Agrobacterium sp.—PX349278 | 263 | + | − | 5.50 ± 3.18 | No inhibition | No inhibition | No inhibition |
| Jf3 | Bacillus sp.—PX349279 | 132 | + | − | No inhibition | 30.59 ± 2.04 | No inhibition | No inhibition |
| Hs7 | Pseudomonas sp.—PX349280 | 202 | + | − | 37.61 ± 9.67 | 11.76 ± 0.00 | No inhibition | No inhibition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaranunt, P.; Wysocki, K.Z.; Kingsley, K.L.; Lindert, S.; Velazquez, F.; White, J.F. Evaluation of Capsaicin as a Selector for Growth Promotional Bacteria Isolated from Capsicum Peppers. Sustainability 2025, 17, 10549. https://doi.org/10.3390/su172310549
Chiaranunt P, Wysocki KZ, Kingsley KL, Lindert S, Velazquez F, White JF. Evaluation of Capsaicin as a Selector for Growth Promotional Bacteria Isolated from Capsicum Peppers. Sustainability. 2025; 17(23):10549. https://doi.org/10.3390/su172310549
Chicago/Turabian StyleChiaranunt, Peerapol, Konrad Z. Wysocki, Kathryn L. Kingsley, Sean Lindert, Fernando Velazquez, and James F. White. 2025. "Evaluation of Capsaicin as a Selector for Growth Promotional Bacteria Isolated from Capsicum Peppers" Sustainability 17, no. 23: 10549. https://doi.org/10.3390/su172310549
APA StyleChiaranunt, P., Wysocki, K. Z., Kingsley, K. L., Lindert, S., Velazquez, F., & White, J. F. (2025). Evaluation of Capsaicin as a Selector for Growth Promotional Bacteria Isolated from Capsicum Peppers. Sustainability, 17(23), 10549. https://doi.org/10.3390/su172310549





