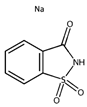
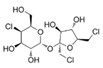
Abstract
The growing consumption of synthetically manufactured sugar substitutes, coupled with the lack of adequate national and international regulations, has led to the presence of various compounds, in different environmental matrices. Within this group, artificial sweeteners, despite their prevalence in mass consumption products, are one of the least studied pollutants. The high consumption of artificial sweeteners, together with the low efficiency of wastewater treatment plants, facilitates their detection in various aquatic ecosystems at concentrations ranging from ng to µg L−1. These concentrations have shown to generate adverse effects on the organisms that inhabit these aquatic ecosystems. The main objective of this review is to provide updated information on the global consumption of sweeteners, reported concentrations in various environmental matrices, and, in particular, the effects of exposure to these compounds on aquatic organisms.
1. Introduction
High-intensity artificial sweeteners, synthetically manufactured sugar substitutes, were introduced to the market in 1983 [1] and their production and consumption have continued to increase. The most widely used sweeteners worldwide are acesulfame-k, aspartame, cyclamate, neotame, saccharin, and sucralose, which are mainly used in beverages and foods, contributing minimal or no calories [2]. Their use is aimed at reducing calorie intake in people with diabetes, athletes, and those seeking to control their weight [3,4,5]. These compounds, which have been introduced into the environment for several years, have acquired the status of emerging contaminants as their detection in the environment has only been possible thanks to advances in highly sensitive analytical techniques [6,7].
These compounds, like other emerging contaminants, are approved for use worldwide. Despite being recognized as safe for human consumption within the recommended average acceptable daily intake (ADI), concerns have grown about their persistence in aquatic systems and their potential chronic effects in non-target organisms and, as an example, cyclamate is restricted in certain countries, such as the United States and Japan [8]. Although there are regulatory agencies around the world, such as the European Food Safety Authority (EFSA), the US Food and Drug Administration (FDA), and the National Institute for Food and Drug Surveillance (INVIMA) in Colombia, the great use of such compounds lies in the excessive consumption of the population, which has increased over time, driven by factors such as disease, trends, and social stereotypes. As an example, global consumption in 2017 was estimated at approximately 159,000 metric tons, with the highest levels of consumption reported in Asia, Oceania, and the United States [9].
The high consumption of artificial sweeteners, coupled with their low absorption rate in the human body, favors their release into wastewater [9]. Due to the limited efficiency of conventional treatment systems, these compounds are not completely eliminated and are consequently discharged into different environmental matrices in concentrations ranging from ng to µg L−1 [10]. While some sweeteners such as aspartame degrade relatively quickly [11] others, such as sucralose and acesulfame-K, resist purification processes and remain in aquatic ecosystems resulting in a high persistence [12,13].
The presence of these compounds in aquatic ecosystems could pose a risk to the organisms that inhabit them. Although there are several studies that demonstrate the effects of exposure to these compounds, most focus on acute effects at high concentrations (mg L−1) [14], which probably do not reflect their real effects on the environment. As a result, sublethal effects originating from molecular alterations derived from chronic exposure to environmentally relevant concentrations are often unnoticed.
The main objective of this review is to provide updated information on the global consumption of sweeteners, reported concentrations in various environmental matrices and, in particular, the effects of exposure to these compounds on aquatic organisms reported to date.
2. Bibliographic Search
The literature search was conducted using online scientific databases such as Google Scholar, PubMed, Scopus, and ScienceDirect, using keywords such as artificial sweeteners, aquatic ecosystems, river, marine ecosystems, and wastewaters. Publications between 1985 and 2025 were considered; however, only 7 articles prior to 2010 were selected, with the aim of contextualizing the historical evolution of the study of these compounds. Most of the works analyzed correspond to the period 2015–2025 and were included according to the following criteria: (I) evaluation of the use and consumption of artificial sweeteners in different countries; (II) analysis of the routes of entry of these compounds into the environment; (III) identification of performance rates in wastewater treatment plants; (IV) reports of concentrations in aquatic ecosystems; and (V) use of aquatic organisms in ecotoxicity tests. In total, 85 articles were selected that met at least one of these criteria.
3. Use and Consumption
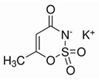
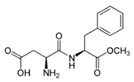
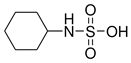
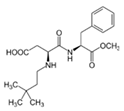
Artificial sweeteners are commonly used in a wide variety of food and beverage products, such as sugar-free foodstuff, nutritional and energy drinks, carbonated beverages, and artificial juices. In addition, these compounds are used in personal care products, such as toothpaste and mouthwashes. Among the most widely consumed sweeteners are acesulfame K (ACE-K), aspartame (ASP), cyclamate (CYC), neotame (NEO), saccharin (SAC), and sucralose (SUC). Table 1 shows the molecular structure and some properties of artificial sweeteners.

Table 1.
Molecular structure and properties of the main artificial sweeteners.
Global consumption of artificial sweeteners has increased significantly over the years, reaching 159,000 metric tons in 2017, with an estimated market value of $2 billion [9]. In 2017, China led global consumption, accounting for 32% of the total, followed by Asia and Oceania, which together represent 23%, the United States with 23%, Europe with 12%, and Africa with 7% [15]. This geographical landscape reflects an uneven distribution of consumption, influenced by factors such as the availability of these products, local regulations, and cultural preferences regarding food and health.
More recently, in 2021, global sweetener market revenue reached approximately $21.3 billion and is estimated to increase to $28.9 billion by 2026 [16], high-lighting the growing interest and expansion of their use in various industries. This is mainly attributed to the demand for low-calorie products created through effective marketing campaigns, the growing acceptance of sweeteners as a health-beneficial option, and technological innovations in the production of next-generation sweeteners.
ASP was the global market leader in 2017, with an estimated consumption of 18.5 thousand metric tons, followed by saccharin with 9.7 thousand metric tons, ACE-K with 6.8 thousand metric tons, and sucralose with 3.3 thousand metric tons [9]. The consumption of these compounds varies considerably between different countries, reflecting both consumer preferences and local regulations in each region. For example, SUC was approved for human consumption in the United States in 1998 and has since then authorized in more than 80 countries with a global growth rate of approximately 5.1% per year from 2008 to 2015 [17]. In 2004, sales of SUC in the United States exceeded $172 million [18], and in 2014, its consumption reached 1500 tons per year in the United States, 400 tons in Europe, and 1090 tons in China [19], reflecting its remarkable acceptance in the global market.
SAC, which has also been approved by various regulatory agencies around the world is consumed in more than 90 countries, establishing itself as one of the most widely used sweeteners globally [20]. On the other hand, Bahndorf and Kienle (2004) reported in 2001 that global consumption of ACE-K was 2500 tons, with America leading consumption at 47%, followed by Europe at 34%, and Asia at only 15%, while Africa and Oceania accounted for just 4% [21]. In the United States, in 2008, 1103 and 974 products containing ACE-K and ASP, respectively, were reported [22]. In contrast, in countries such as Switzerland and Germany, CYC is the most widely consumed artificial sweetener [23]. On the other hand, China is the leading consumer and producer of artificial sweeteners in Asia, with ACE-K and SUC standing out as the market leaders [24]. In Korea, ASP and SAC production reached 838 and 348 tons in 2011, respectively, with per capita consumption of 8.38 mg/day and 17.4 mg/day for ASP and SAC, respectively [25].
Finally, it is estimated that global consumption of these compounds will continue to grow [15], reflecting the continued expansion and growing demand for these products worldwide.
4. Sources of Artificial Sweeteners in Aquatic Ecosystems
The environmental cycle of sweeteners begins with their industrial production and extends to their arrival in aquatic ecosystems, as shown in Figure 1. These compounds are incorporated into a wide variety of food products, beverages and personal care products. Once consumed, sweeteners are not completely metabolized by humans [26,27], leading to their excretion through urine and feces (Figure 1). The resulting waste enters the sewer system and can be discharged directly into surface waters or aquatic ecosystems or after being treated in wastewater treatment plants (WWTPs).
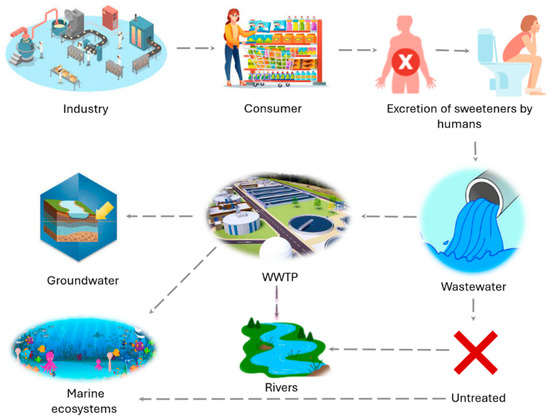
Figure 1.
Sources of artificial sweeteners in aquatic ecosystems.
Removal rates of artificial sweeteners in WWTPs are variable and depends on the compound and the treatment applied. In conventional WWTPs, the highest removal rates have been observed for Saccharin (SAC) [28] and Cyclamate (CYC) [29] with removal efficiencies around 90–97%, with average removal rates of 97.26 ± 3.24% and 96.84 ± 2.47% for SAC and CYC, respectively. On the other side, low or variable removal rates have been reported for Acesulfame (ACE-K) and Sucralose (SUC) are much more persistent, with removal efficiencies ranging from ineffective to occasionally high, depending on the WWTP’s processes. In conventional WWTPs, SUC has shown negative to very low removal (−10% to +10%), indicating potential release from sludge or incomplete degradation [29]. One study showed an overall removal as low as −116% under conventional treatment whereas removal rates up to 99.1% could be achieved under Ultraviolet/Peroxydisulfate Oxidation UV/PDS and Granular Activated Carbon (GAC) adsorption treatments. ACE-K also shows variable removal rates ranging from under 2% [25]. Under conventional treatment up to over 90% [30,31] depending on microbial adaptation and treatment configuration, as well as using conventional activated sludge treatment with denitrification and nitrification.
5. Concentrations of Sweeteners in Environmental Matrices
The reported concentrations of artificial sweeteners in various environmental matrices range from ng to µg L−1 (Table 2) [32]. Analysis reveals interesting patterns that suggest variations depending on the type of matrix and the efficiency of wastewater treatment. Globally, the highest concentrations of artificial sweeteners have been found in wastewater, followed by surface water and, finally, seawater [13], while their detection in marine environments has been limited. Only a few studies have documented their presence in seawater, with concentrations too low, around 5.23 ng L−1 [33].

Table 2.
Concentrations of artificial sweeteners in different countries and aquatic environments.
Among the sweeteners reported here, SUC and ACE-K stand out for having the highest concentrations in the aquatic environment [62] due to their widespread use and high persistence [63]. For example, ACE-K reaches levels of up to 2500 μg L−1 in wastewater effluents in Italy [13]. On the other hand, SUC, has been reported in WWTP effluents at lower concentrations of around 10.8 μg L−1 [63], and in water bodies such as rivers and lakes, with values of up to 9.6 μg L−1 [12]. In contrast, NEO has concentrations in the order of 0.03 μg L−1 in wastewater influents and effluents in China [40].
In the case of CYC, marked differences are observed between matrices. While in WWTP influents values can reach up to 250 μg L−1 [35], in groundwater the levels are considerably lower, with only 0.003 μg L−1 [37] In the case of ASP, the highest concentrations, with 3.1 μg L−1 were found in Vietnam [42], followed by 1.8 μg L−1 in the US [25]. On the other hand, saccharin has been found in remarkably high concentrations, such as 303 μg L−1 in WWTP influents in India [25].
Finally, it is essential to understand that the presence of these compounds in aquatic matrices is influenced by the amount consumed, the type of treatment used in wastewater treatment plants, and the chemical persistence of each sweetener in the receiving environment. While conventional wastewater-treatments may be more effective at removing certain compounds such as CYC, others such as SUC and ACE-K seem to resist degradation processes and remain in the environment. As a result, aquatic ecosystems can act as long-term reservoirs for these emerging pollutants, posing a potential risk to aquatic organisms.
6. Effects
Whilst there is substantial evidence of the adverse effects of artificial sweeteners on freshwater organisms, covering a wide range of biological responses, research in marine environments is still limited and fragmented. Despite detection of these compounds in seawater, especially in coastal areas, little is known about their impact on marine species in the open sea. This gap highlights an urgent need for targeted studies to understand their potential eco-logical risks in saltwater ecosystems.
Several studies have shown that sweeteners can induce measurable biological responses even at extremely low concentrations, similar to those detected in aquatic environments [64]. These alterations range from sublethal responses, such as changes in physiological and behavioral biomarker activity, to lethal effects in acute toxicity tests, indicating a potentially significant ecotoxicological impact (Table 3) [65,66].
A notable observation is that compounds such as SUC and ACE-K had the highest number of studies and a wide range of effects, concentration levels as low as ng L−1 [64]. This highlights their potential to induce long term cumulative sublethal effects if they persist in the environment. In contrast, SAC and CYC showed a smaller range of studied effects, with less diversity in the parameters evaluated, although this does not necessarily imply lower toxicity, but rather indicate a lack of knowledge and further research needs into the potential effects of these compounds in natural environment.
Among the most relevant effects are alterations in physiology (oxidative stress, apoptosis, DNA damage) [67] in the nervous system (alteration of acetylcholinesterase activity, hyperlocomotion, loss of coordination [64,68], as well as in behavior (increased anxiety, decreased learning and memory capacity) [69], and vital functions such as reproduction, feeding, and swimming [64,70]. These effects, although in some cases non-lethal, can compromise the viability of populations and affect the ecological stability of aquatic ecosystems.
It should be noted that chronic tests offer a more complete view of environmental toxicity, revealing cumulative and long-term effects that acute tests do not always detect. Long term monitorization of biomarkers such as lipid peroxidation (LPX), superoxide dismutase (SOD), hydroperoxide content (HPC), protein carbonyl (PCC), sirtuin 1 (SIRT1), and acetylcholinesterase (AChE) allow us to infer the activation of antioxidant defense mechanisms, which could indicate a situation of sustained cellular stress, with the potential to trigger adverse effects on higher organizational levels such as the development, reproduction, and survival of organisms [64,68,71,72]. In Table 3, freshwater fish such as Cyprinus carpio and Carassius auratus showed an increase in SOD activity after Ace-K exposure. This increase has also been observed after exposure to other emerging pollutants such as ciprofloxacin, trimethoprim, and sulfadiazine [73]. The increase in SOD could be related to the increase in superoxide anion, which is catalyzed by SOD, producing oxygen and H2O2 [73].
Freshwater fishes, such as Danio rerio and Cyprinus carpio, have demonstrated their utility for identifying behavioral and molecular alterations [69], while invertebrates, such as Daphnia magna and Nitocra spinipes, are suitable for assessing effects on reproduction, feeding, mobility, and acute or chronic toxicity parameters [66,70]. In combination, this diversity of organisms allows for a more robust assessment of the potential impact of sweeteners at different trophic levels.
Despite the relevance of these findings, there is a significant shortage of studies applying mass molecular analysis techniques, such as proteomic, transcriptomic, and genomic approaches. This gap limits deep understanding of the molecular mechanisms by which sweeteners affect aquatic organisms and restrict the development of predictive models of toxicity at the cellular and physiological levels. Furthermore, most experimental studies have focused on evaluating a single sweetener at a time, without considering the possible synergistic, additive, or antagonistic effects that could arise from combined exposures. This limitation represents a significant gap in knowledge, given that these compounds frequently coexist in complex mixtures in the environment.
Finally, it is important to note that, unlike other emerging pollutants such as antibiotics, many sweeteners do not exhibit obvious acute toxicity in the environment [7]. However, their sublethal and chronic effects, especially on sensitive organisms, highlight the need to reassess their permissible limits in aquatic environments. Furthermore, their widespread presence in WWTP effluents and their persistence suggests that their efficient removal in treatment plants still represents a technical and environmental challenge to be addressed.

Table 3.
Main reported effects of artificial sweeteners on aquatic organisms.
Table 3.
Main reported effects of artificial sweeteners on aquatic organisms.
| Sweeteners | Organisms | Concentration (µg L−1) | Assay | Effects | References |
|---|---|---|---|---|---|
| Ace-K | Fresh water Vertebrate, fish Danio rerio | 50 | Biomarker assay | Increased HPC and LPX activity | [15] |
| 10,000 | Light/dark preference test (LDP) | Increased anxiety | [69] | ||
| 10,000 | Test NTDT | Increased anxiety | [69] | ||
| 10,000 | CPP behavioral testing | Impaired learning and memory capacity | [69] | ||
| >1,000,000 | Acute toxicity test LC50-96 h | Mortality | [48] | ||
| 24 | Toxicity test IC50-24 h | Effects on swimming and feeding | [64] | ||
| Fresh water Vertebrate, fish Cyprinus carpio | 0.05 | Biomarker assay | Increased HPC and SOD activity | [74] | |
| Fresh water Vertebrate, fish Carassius auratus | 100 | Biomarker Assay | Increased SOD activity | [72] | |
| Fresh water crustacean Daphnia magna | 100 | In vivo cardiac toxicity assay | Increased cardiac | [75] | |
| 24 | Toxicity test IC50-24 h | Swimming impairment | [64] | ||
| 28 | Toxicity test IC50-24 h | Affecting feeding activity | [64] | ||
| 1,600,000 | Acute Toxicity Test LC50-48 h | Mortality | [54] | ||
| 0.1 | Biomarker assay | Decreased AChE activity | [64] | ||
| Aspartame | Fresh water crustacean Daphnia magna | 0.1 | Biomarker assay | Increased AChE activity | [64] |
| Fresh water Vertebrate, fish Danio rerio | 0.49 | In situ hybridization assay | Inhibition of neutrophil production | [76] | |
| 20 | Teratogenicity test | Cartilage malformation | [68] | ||
| 20 | In vitro Toxicity Assay | Decreased in locomotor activity | [68] | ||
| 60 | Western blot Technique | Decreased expression of SIRT1 and FOXO3a proteins in neurons | [68] | ||
| Cyclamate | Fresh water Vertebrate, fish Danio rerio | 100 | Biomarker assay | Increased AChE activity | [65] |
| Fresh water crustacean Daphnia magna | 1000 | Chronic toxicity test 21 d | Difficulty in reproduction | [70] | |
| Saccharin | Fresh water Vertebrate, fish Danio rerio | 1000 | Light/dark preference tests (LDP) | Alteration of neurotrans-mitter homeostasis in the brain | [77] |
| 100,000 | Acute Toxicity Test EC50-48 h | Immobilization | [78] | ||
| 1000 | Light/dark preference test (LDP) | Excessive increase during swimming | [79] | ||
| 100 | Biomarker assay | Increased dopamine | [65] | ||
| Sucralose | Fresh water Vertebrate, fish Danio rerio | 0.05 | Biomarker assay | Increased in LPX, HPC, and PCC activity | [71] |
| 0.05 | qRT-PCR molecular technique | Over-expression of Nrf1a and Nrf2a genes | [71] | ||
| 116.5 | Acute Toxicity Test LC50-96 h | Mortality | [71] | ||
| Fresh water Vertebrate, fish Cyprinus carpio | 0.05 | Comet assay | DNA damage | [67] | |
| Tunnel test | Apoptosis | [67] | |||
| 0.05 | Biomarker assay | Increased in HPC, LPX, PCC and SOD activity | [80] | ||
| Salt water crustacean Gammarus zaddachi | 500 | Biomarker assay | Increased AChE and LPX activity | [81] | |
| 5000 | Toxicity test 14 d | Increased respiration | [66] | ||
| Fresh water crustacean Daphnia magna | 20.1 | Biomarker assay | Increased AChE activity | [64] | |
| 5 | Toxicity Test (Toximeter II) | Increased swimming | [66] | ||
| 0.1 | Biomarker assay | Increased AChE activity | [81] | ||
| 175 | Toxicity test IC50-24 h | Abnormal swimming | [64] | ||
| 235 | Toxicity test IC50-24 h | Alteration in feeding activity | [64] | ||
| Saltwater copepod Nitocra spinipes | 0.5 | Acute Toxicity Test LC50-96 h | Mortality | [66] | |
| Saltwater copepod Calanus glacialis | 0.05 | Toxicity test 72 h | Decrease in egg production | [82] | |
| Aquatic plant Lemar minor | 100,000 | Toxicity test 7 days | Growth rate inhibition | [83] | |
| Green algae Pseudokirchneriella subcapitata | 10,000 | Toxicity test 48 h Bioaccumulation | Does not bioaccumulate | [84] | |
| Green algae Scenedesmus vacuolatus | 1,000,000 | Test inhibition reproduction | NOEC | [85] |
Ache: Acetylcholinesterase; CAT: Catalase; SOD: Superoxide dismutase; FOXO3a: Forkhead box protein O3a; HPC: Hydroperoxide content; LPX: Lipid peroxidation; Nrf1a: Nuclear respiratory factor 1a; Nrf2a: Nuclear respiratory factor 2a; PCC: Protein carbonyl; SIRT1: Sirtuin 1; Test CPP: Conditioned Place Preference; Test NTDT: Novel tank diving test; NOEC: No Observed Effect Concentration.
7. Conclusions
The rapidly increasing global consumption of artificial sweeteners, driven by the rising demand for low-calorie products and health concerns, has dangerously escalated their classification as emerging pollutants due to their relentless and persistent presence contaminating multiple environmental matrices. Their extremely low metabolism rate combined with the alarming inefficiency of conventional wastewater treatments has allowed artificial sweeteners to accumulate unchecked in the environment, with sucralose and ACE-K emerging as the most frequently detected and concerning compounds. Even when some sweeteners degrade relatively quickly, their continuous and unregulated release into the environment through domestic and industrial discharges gives them a pseudo-persistent nature that amplifies their potential for severe ecological damage, underscoring the critical need for strict monitoring and control. Although their concentrations are typically low (ng–µg L−1), numerous studies have revealed that they can trigger profound physiological, neurological, reproductive, and behavioral disturbances in aquatic organisms at levels alarmingly close to those currently found in the environment, signaling a significant and growing ecological threat. Yet, the majority of these investigations have been limited to freshwater ecosystems, while studies focusing on marine environments remain scarce and fragmented, leaving a dangerous gap that prevents comprehensive assessment of the environmental risks in these vital habitats. Consequently, there is an urgent and non-negotiable need to promote research focused on understanding the behavior, persistence, and ecotoxicological effects of these compounds in marine and coastal ecosystems before irreversible damage occurs. Like-wise, it is crucial to reinforce environmental regulations, implement advanced water treatment technologies, and encourage mechanistic studies utilizing high-performance molecular approaches to fully comprehend their long-term impacts on population dynamics and ecosystem stability.
Author Contributions
Conceptualization, R.F., V.P., C.G. and S.O.; methodology, R.F., V.P., S.O., H.J.B.-A. and G.A.; writing—original draft preparation, R.F., C.G., V.P., S.O., H.J.B.-A., M.H. and G.A.; writing—review and editing, R.F., G.A. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the reported results can be obtained by contacting the first author directly.
Acknowledgments
This work was supported by Simón Bolívar University, the University of Cádiz and Institute of Marine Sciences of Andalusia (ICMAN-CSIC).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- von Rymon Lipinski, G.W. The New Intense Sweetener Acesulfame K. Food Chem. 1985, 16, 259–269. [Google Scholar] [CrossRef]
- Sang, Z.; Jiang, Y.; Tsoi, Y.K.; Leung, K.S.Y. Evaluating the Environmental Impact of Artificial Sweeteners: A Study of Their Distributions, Photodegradation and Toxicities. Water Res. 2014, 52, 260–274. [Google Scholar] [CrossRef]
- Bowen, A.; Denny, V.; Zahedi, I.; Bidaisee, S. The Whey and Casein Protein Powder Consumption:—ProQuest. Available online: https://www.proquest.com/docview/2136005450?sourcetype=Scholarly%20Journals (accessed on 1 June 2025).
- Higgins, J.P.; Babu, K.; Deuster, P.A.; Shearer, J. Energy Drinks: A Contemporary Issues Paper. Curr. Sports Med. Rep. 2018, 17, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Chan, C.B.; Dworatzek, P.D.; Freeze, C.; Williams, S.L. Nutrition Therapy. Can. J. Diabetes 2018, 42, S64–S79. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Arias Espana, V.A.; Liu, Y.; Jit, J. Emerging Contaminants in the Environment: Risk-Based Analysis for Better Management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef]
- Fernandez, R.; Colás-Ruiz, N.R.; Bolívar-Anillo, H.J.; Anfuso, G.; Hampel, M. Occurrence and Effects of Antimicrobials Drugs in Aquatic Ecosystems. Sustainability 2021, 13, 13428. [Google Scholar] [CrossRef]
- Roberts, A. The Safety and Regulatory Process for Low Calorie Sweeteners in the United States. Physiol. Behav. 2016, 164, 439–444. [Google Scholar] [CrossRef]
- Praveena, S.M.; Cheema, M.S.; Guo, H.R. Non-Nutritive Artificial Sweeteners as an Emerging Contaminant in Environment: A Global Review and Risks Perspectives. Ecotoxicol. Environ. Saf. 2019, 170, 699–707. [Google Scholar] [CrossRef]
- Li, S.; Ren, Y.; Fu, Y.; Gao, X.; Jiang, C.; Wu, G.; Ren, H.; Geng, J. Fate of Artificial Sweeteners through Wastewater Treatment Plants and Water Treatment Processes. PLoS ONE 2018, 13, e0189867. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Stability of Aspartame in the Soft Drinks: Identification of the Novel Phototransformation Products and Their Toxicity Evaluation. Food Res. Int. 2023, 173, 113365. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Z.; Yang, Y.; Zhu, L.; Deng, J.; Lu, S.; Li, X.; Dietrich, A.M. Aqueous Degradation of Artificial Sweeteners Saccharin and Neotame by Metal Organic Framework Material. Sci. Total Environ. 2021, 761, 143181. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-Wide Monitoring Survey on Emerging Polar Organic Contaminants in Wastewater Treatment Plant Effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, K.E.; Nizzetto, L.; Huggett, D.B. Presence, Fate and Effects of the Intense Sweetener Sucralose in the Aquatic Environment. Sci. Total Environ. 2012, 438, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Colín-García, K.; Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M.; García-Medina, S. Influence of Sucralose, Acesulfame-k, and Their Mixture on Brain’s Fish: A Study of Behavior, Oxidative Damage, and Acetylcholinesterase Activity in Danio rerio. Chemosphere 2023, 340, 139928. [Google Scholar] [CrossRef]
- Chen, Z.W.; Shen, Z.W.; Hua, Z.L.; Li, X.Q. Global Development and Future Trends of Artificial Sweetener Research Based on Bibliometrics. Ecotoxicol. Environ. Saf. 2023, 263, 115221. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the Consumption of Low-Calorie Sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Mead, R.N.; Morgan, J.B.; Avery, G.B.; Kieber, R.J.; Kirk, A.M.; Skrabal, S.A.; Willey, J.D. Occurrence of the Artificial Sweetener Sucralose in Coastal and Marine Waters of the United States. Mar. Chem. 2009, 116, 13–17. [Google Scholar] [CrossRef]
- Sharma, V.K.; Oturan, M.; Kim, H. Oxidation of Artificial Sweetener Sucralose by Advanced Oxidation Processes: A Review. Environ. Sci. Pollut. Res. 2014, 21, 8525–8533. [Google Scholar] [CrossRef]
- Cp, K. Determination of the Saccharin Content in Some Ice Creams Available in Market. Int. J. Food Sci. Nutr. 2018, 3, 158–160. [Google Scholar]
- Bahndorf, D.; Kienle, U. World Market of Sugar and Sweeteners; International Association for Stevia Research e.V.: Leinfelden-Echterdingen, Germany, 2004. [Google Scholar]
- Yang, Q. Gain Weight by “Going Diet?” Artificial Sweeteners and the Neurobiology of Sugar Cravings: Neuroscience 2010. Yale J. Biol. Med. 2010, 83, 101–108. [Google Scholar]
- Buerge, I.J.; Buser, H.R.; Kahle, M.; Müller, M.D.; Poiger, T. Ubiquitous Occurrence of the Artificial Sweetener Acesulfame in the Aquatic Environment: An Ideal Chemical Marker of Domestic Wastewater in Groundwater. Environ. Sci. Technol. 2009, 43, 4381–4385. [Google Scholar] [CrossRef]
- Foodchem Consumo de Edulcorantes de Alta Intensidad a Nivel Mundial—FOODCHEM. Available online: https://www.foodchem.cn/News/126.html (accessed on 2 June 2025).
- Subedi, B.; Kannan, K. Fate of Artificial Sweeteners in Wastewater Treatment Plants in New York State, U.S.A. Environ. Sci. Technol. 2014, 48, 13668–13674. [Google Scholar] [CrossRef]
- John, B.A.; Wood, S.G.; Hawkins, D.R. The Pharmacokinetics and Metabolism of Sucralose in the Mouse. Food Chem. Toxicol. 2000, 38, 107–110. [Google Scholar] [CrossRef]
- Roberts, A.; Renwick, A.G.; Sims, J.; Snodin, D.J. Sucralose Metabolism and Pharmacokinetics in Man. Food Chem. Toxicol. 2000, 38, 31–41. [Google Scholar] [CrossRef]
- Li, S.; Geng, J.; Wu, G.; Gao, X.; Fu, Y.; Ren, H. Removal of Artificial Sweeteners and Their Effects on Microbial Communities in Sequencing Batch Reactors. Sci. Rep. 2018, 8, 10298. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Li, Y.; Liu, H.; Wang, Q. Artificial Sweeteners in Wastewater Treatment Plants: A Systematic Review of Global Occurrence, Distribution, Removal, and Degradation Pathways. J. Hazard. Mater. 2025, 494, 138644. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Huang, W.; Kuzma, D.; Kormendi, A. Acesulfame and Other Artificial Sweeteners in a Wastewater Treatment Plant in Alberta, Canada: Occurrence, Degradation, and Emission. Chemosphere 2024, 356, 141893. [Google Scholar] [CrossRef]
- Castronovo, S.; Wick, A.; Scheurer, M.; Nödler, K.; Schulz, M.; Ternes, T.A. Biodegradation of the Artificial Sweetener Acesulfame in Biological Wastewater Treatment and Sandfilters. Water Res. 2017, 110, 342–353. [Google Scholar] [CrossRef]
- Coronado-Apodaca, K.G.; Rodríguez-De Luna, S.E.; Araújo, R.G.; Oyervides-Muñoz, M.A.; González-Meza, G.M.; Parra-Arroyo, L.; Sosa-Hernandez, J.E.; Iqbal, H.M.N.; Parra-Saldivar, R. Occurrence, Transport, and Detection Techniques of Emerging Pollutants in Groundwater. MethodsX 2023, 10, 102160. [Google Scholar] [CrossRef] [PubMed]
- Brumovský, M.; Bečanová, J.; Kohoutek, J.; Borghini, M.; Nizzetto, L. Contaminants of Emerging Concern in the Open Sea Waters of the Western Mediterranean. Environ. Pollut. 2017, 229, 976–983. [Google Scholar] [CrossRef]
- Scheurer, M.; Brauch, H.J.; Lange, F.T. Analysis and Occurrence of Seven Artificial Sweeteners in German Waste Water and Surface Water and in Soil Aquifer Treatment (SAT). Anal. Bioanal. Chem. 2009, 394, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Zirlewagen, J.; Licha, T.; Schiperski, F.; Nödler, K.; Scheytt, T. Use of Two Artificial Sweeteners, Cyclamate and Acesulfame, to Identify and Quantify Wastewater Contributions in a Karst Spring. Sci. Total Environ. 2016, 547, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, K.Y.; Hamm, S.Y.; Kim, M.S.; Kim, H.K.; Oh, J.E. Occurrence and Distribution of Pharmaceutical and Personal Care Products, Artificial Sweeteners, and Pesticides in Groundwater from an Agricultural Area in Korea. Sci. Total Environ. 2019, 659, 168–176. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; Roy, J.W.; Grabuski, J.; Brown, S.J.; Bickerton, G.; Sverko, E. An Artificial Sweetener and Pharmaceutical Compounds as Co-Tracers of Urban Wastewater in Groundwater. Sci. Total Environ. 2013, 461–462, 348–359. [Google Scholar] [CrossRef]
- Baena-Nogueras, R.M.; Traverso-Soto, J.M.; Biel-Maeso, M.; Villar-Navarro, E.; Lara-Martín, P.A. Sources and Trends of Artificial Sweeteners in Coastal Waters in the Bay of Cadiz (NE Atlantic). Mar. Pollut. Bull. 2018, 135, 607–616. [Google Scholar] [CrossRef]
- Arbeláez, P.; Borrull, F.; Pocurull, E.; Marcé, R.M. Determination of High-Intensity Sweeteners in River Water and Wastewater by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2015, 1393, 106–114. [Google Scholar] [CrossRef]
- Guo, W.; Li, J.; Liu, Q.; Shi, J.; Gao, Y. Tracking the Fate of Artificial Sweeteners within the Coastal Waters of Shenzhen City, China: From Wastewater Treatment Plants to Sea. J. Hazard. Mater. 2021, 414, 125498. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.M.; Pang, Z.; Zheng, H.; Ma, X. Mini Review: Will Artificial Sweeteners Discharged to the Aqueous Environment Unintentionally “Sweeten” the Taste of Tap Water? Chem. Eng. J. Adv. 2021, 6, 100100. [Google Scholar] [CrossRef]
- Watanabe, Y.; Bach, L.T.; Van Dinh, P.; Prudente, M.; Aguja, S.; Phay, N.; Nakata, H. Ubiquitous Detection of Artificial Sweeteners and Iodinated X-Ray Contrast Media in Aquatic Environmental and Wastewater Treatment Plant Samples from Vietnam, the Philippines, and Myanmar. Arch. Environ. Contam. Toxicol. 2016, 70, 671–681. [Google Scholar] [CrossRef]
- Li, D.; O’Brien, J.W.; Tscharke, B.J.; Choi, P.M.; Zheng, Q.; Ahmed, F.; Thompson, J.; Li, J.; Mueller, J.F.; Sun, H.; et al. National Wastewater Reconnaissance of Artificial Sweetener Consumption and Emission in Australia. Environ. Int. 2020, 143, 105963. [Google Scholar] [CrossRef]
- Naik, A.Q.; Zafar, T.; Shrivastava, V.K. Environmental Impact of the Presence, Distribution, and Use of Artificial Sweeteners as Emerging Sources of Pollution. J. Environ. Public Health 2021, 2021, 6624569. [Google Scholar] [CrossRef]
- Ordóñez, E.Y.; Quintana, J.B.; Rodil, R.; Cela, R. Determination of Artificial Sweeteners in Water Samples by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2012, 1256, 197–205. [Google Scholar] [CrossRef]
- Mora, A.; Torres-Martínez, J.A.; Capparelli, M.V.; Zabala, A.; Mahlknecht, J. Effects of Wastewater Irrigation on Groundwater Quality: An Overview. Curr. Opin. Environ. Sci. Health 2022, 25, 100322. [Google Scholar] [CrossRef]
- Lange, F.T.; Scheurer, M.; Brauch, H.J. Artificial Sweeteners-A Recently Recognized Class of Emerging Environmental Contaminants: A Review. Anal. Bioanal. Chem. 2012, 403, 2503–2518. [Google Scholar] [CrossRef]
- Belton, K.; Schaefer, E.; Guiney, P.D. A Review of the Environmental Fate and Effects of Acesulfame-Potassium. Integr. Environ. Assess. Manag. 2020, 16, 421–437. [Google Scholar] [CrossRef]
- Alves, P.d.C.C.; Rodrigues-Silva, C.; Ribeiro, A.R.; Rath, S. Removal of Low-Calorie Sweeteners at Five Brazilian Wastewater Treatment Plants and Their Occurrence in Surface Water. J. Environ. Manag. 2021, 289, 112561. [Google Scholar] [CrossRef] [PubMed]
- Sérodes, J.B.; Behmel, S.; Simard, S.; Laflamme, O.; Grondin, A.; Beaulieu, C.; Proulx, F.; Rodriguez, M.J. Tracking Domestic Wastewater and Road De-Icing Salt in a Municipal Drinking Water Reservoir: Acesulfame and Chloride as Co-Tracers. Water Res. 2021, 203, 117493. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Wang, L.; Wei, C.; Li, J.; Zhang, J.; Zhou, Z.; Liang, Y. Sucralose and Acesulfame as an Indicator of Domestic Wastewater Contamination in Wuhan Surface Water. Ecotoxicol. Environ. Saf. 2020, 189, 109980. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Asimakopoulos, A.G.; Thomaidis, N.S. Artificial Sweeteners as Emerging Pollutants in the Environment: Analytical Methodologies and Environmental Impact. Anal. Methods 2012, 4, 3057–3070. [Google Scholar] [CrossRef]
- Hu, L.X.; Olaitan, O.J.; Li, Z.; Yang, Y.Y.; Chimezie, A.; Adepoju-Bello, A.A.; Ying, G.G.; Chen, C.E. What Is in Nigerian Waters? Target and Non-Target Screening Analysis for Organic Chemicals. Chemosphere 2021, 284, 131546. [Google Scholar] [CrossRef]
- Kerberová, V.; Zlámalová Gargošová, H.; Čáslavský, J. Occurrence and Ecotoxicity of Selected Artificial Sweeteners in the Brno City Waste Water. Int. J. Environ. Sci. Technol. 2022, 19, 9055–9066. [Google Scholar] [CrossRef]
- Ens, W.; Senner, F.; Gygax, B.; Schlotterbeck, G. Development, Validation, and Application of a Novel LC-MS/MS Trace Analysismethod for the Simultaneous Quantification of Seven Iodinated X-Ray Contrast Media and Three Artificial Sweeteners in Surface, Ground, and Drinking Water. Anal. Bioanal. Chem. 2014, 406, 2789–2798. [Google Scholar] [CrossRef]
- Tran, N.H.; Hu, J.; Li, J.; Ong, S.L. Suitability of Artificial Sweeteners as Indicators of Raw Wastewater Contamination in Surface Water and Groundwater. Water Res. 2014, 48, 443–456. [Google Scholar] [CrossRef]
- Tran, N.H.; Gan, J.; Nguyen, V.T.; Chen, H.; You, L.; Duarah, A.; Zhang, L.; Gin, K.Y.H. Sorption and Biodegradation of Artificial Sweeteners in Activated Sludge Processes. Bioresour. Technol. 2015, 197, 329–338. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Eaton, A.; Badruzzaman, M.; Haghani, A.W.; Jacangelo, J.G. Occurrence and Suitability of Sucralose as an Indicator Compound of Wastewater Loading to Surface Waters in Urbanized Regions. Water Res. 2011, 45, 4019–4027. [Google Scholar] [CrossRef]
- Loos, R.; Gawlik, B.M.; Boettcher, K.; Locoro, G.; Contini, S.; Bidoglio, G. Sucralose Screening in European Surface Waters Using a Solid-Phase Extraction-Liquid Chromatography–Triple Quadrupole Mass Spectrometry Method. J. Chromatogr. A 2009, 1216, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Thurman, E.M. Analysis of Sucralose and Other Sweeteners in Water and Beverage Samples by Liquid Chromatography/Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2010, 1217, 4127–4134. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Young, R.B.; Vanderford, B.J.; Borch, T.; Snyder, S.A. Artificial Sweetener Sucralose in U.S. Drinking Water Systems. Environ. Sci. Technol. 2011, 45, 8716–8722. [Google Scholar] [CrossRef]
- Cantwell, M.G.; Katz, D.R.; Sullivan, J.C.; Shapley, D.; Lipscomb, J.; Epstein, J.; Juhl, A.R.; Knudson, C.; O’Mullan, G.D. Spatial Patterns of Pharmaceuticals and Wastewater Tracers in the Hudson River Estuary. Water Res. 2018, 137, 335–343. [Google Scholar] [CrossRef]
- Soh, L.; Connors, K.A.; Brooks, B.W.; Zimmerman, J. Fate of Sucralose through Environmental and Water Treatment Processes and Impact on Plant Indicator Species. Environ. Sci. Technol. 2011, 45, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, A.K.E.; Guo, X.; Gorokhova, E. Cardiotoxic and Neurobehavioral Effects of Sucralose and Acesulfame in Daphnia: Toward Understanding Ecological Impacts of Artificial Sweeteners. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 273, 109733. [Google Scholar] [CrossRef]
- Saputra, F.; Lai, Y.H.; Fernandez, R.A.T.; Macabeo, A.P.G.; Lai, H.T.; Huang, J.C.; Hsiao, C. Der Acute and Sub-Chronic Exposure to Artificial Sweeteners at the Highest Environmentally Relevant Concentration Induce Less Cardiovascular Physiology Alterations in Zebrafish Larvae. Biology 2021, 10, 548. [Google Scholar] [CrossRef]
- Wiklund, A.K.E.; Breitholtz, M.; Bengtsson, B.E.; Adolfsson-Erici, M. Sucralose—An Ecotoxicological Challenger? Chemosphere 2012, 86, 50–55. [Google Scholar] [CrossRef]
- Heredia-García, G.; Gómez-Oliván, L.M.; Orozco-Hernández, J.M.; Luja-Mondragón, M.; Islas-Flores, H.; SanJuan-Reyes, N.; Galar-Martínez, M.; García-Medina, S.; Dublán-García, O. Alterations to DNA, Apoptosis and Oxidative Damage Induced by Sucralose in Blood Cells of Cyprinus carpio. Sci. Total Environ. 2019, 692, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Pandaram, A.; Paul, J.; Wankhar, W.; Thakur, A.; Verma, S.; Vasudevan, K.; Wankhar, D.; Kammala, A.K.; Sharma, P.; Jaganathan, R.; et al. Aspartame Causes Developmental Defects and Teratogenicity in Zebra Fish Embryo: Role of Impaired SIRT1/FOXO3a Axis in Neuron Cells. Biomedicines 2024, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Li, X.; Han, G.; Du, L.; Li, M. Zebrafish Neuro-Behavioral Profiles Altered by Acesulfame (ACE) within the Range of “No Observed Effect Concentrations (NOECs)”. Chemosphere 2019, 243, 125431. [Google Scholar] [CrossRef]
- Perkola, N. Fate of Artificial Sweeteners and Perfluoroalkyl Acids in Aquatic Environment. Academic Dissertation, University of Helsinki, Helsinki, Finland, 2014. [Google Scholar]
- Colín-García, K.; Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M.; Islas-Flores, H.; García-Medina, S.; Galar-Martínez, M. Acute Exposure to Environmentally Relevant Concentrations of Sucralose Disrupts Embryonic Development and Leads to an Oxidative Stress Response in Danio rerio. Sci. Total Environ. 2022, 829, 154689. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, J.; Li, F.; Ren, H.; Ding, L.; Xu, K. The Oxidative Stress in the Liver of Carassius auratus Exposed to Acesulfame and Its UV Irradiance Products. Sci. Total Environ. 2016, 571, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Colás-Ruiz, N.R.; Martínez-Rodríguez, G.; Lara-Martín, P.A.; Mancera, J.M.; Trombini, C.; Blasco, J.; Hampel, M. The Antibacterials Ciprofloxacin, Trimethoprim and Sulfadiazine Modulate Gene Expression, Biomarkers and Metabolites Associated with Stress and Growth in Gilthead Sea Bream (Sparus aurata). Aquat. Toxicol. 2022, 250, 106243. [Google Scholar] [CrossRef]
- Cruz-Rojas, C.; SanJuan-Reyes, N.; Fuentes-Benites, M.P.A.G.; Dublan-García, O.; Galar-Martínez, M.; Islas-Flores, H.; Gómez-Oliván, L.M. Acesulfame Potassium: Its Ecotoxicity Measured through Oxidative Stress Biomarkers in Common Carp (Cyprinus carpio). Sci. Total Environ. 2019, 647, 772–784. [Google Scholar] [CrossRef]
- Ferry, S.a.p.u.t.r.a.; Lai, Y.-H.; Fernandez, R.A.; Macabeo, A.P.G.; Lai, H.-T.; Huang, J.-C.; Hsiao, C.-D. In Vivo Modelling of Toxicity of Eight Commercial Artificial Sweeteners in Daphnia Neonates and Zebrafish Embryos through Cardiac Performance Assessments. Preprints 2020, 2020090419. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.; Chen, F.; Zhang, X.; Liu, Y.; Sun, H. Evaluation of Aspartame Effects at Environmental Concentration on Early Development of Zebrafish: Morphology and Transcriptome1. Environ. Pollut. 2024, 361, 124792. [Google Scholar] [CrossRef]
- Li, X.; Dong, G.; Han, G.; Du, L.; Li, M. Zebrafish Behavioral Phenomics Links Artificial Sweetener Aspartame to Behavioral Toxicity and Neurotransmitter Homeostasis. J. Agric. Food Chem. 2021, 69, 15393–15402. [Google Scholar] [CrossRef]
- Kobetičová, K.; Mocová, K.A.; Mrhálková, L.; Fryčová, Z.; Kočí, V. Artificial Sweeteners and the Environment. Czech J. Food Sci. 2016, 34, 149–153. [Google Scholar] [CrossRef]
- Du, L.; Han, G.; Li, X.; Dong, G.; Zhang, L.; Gao, J.; Li, M. Phenotyping Aquatic Neurotoxicity Induced by the Artificial Sweetener Saccharin at Sublethal Concentration Levels. J. Agric. Food Chem. 2021, 69, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Vence, K.; Elizalde-Velázquez, A.; Dublán-García, O.; Galar-Martínez, M.; Islas-Flores, H.; SanJuan-Reyes, N.; García-Medina, S.; Hernández-Navarro, M.D.; Gómez-Oliván, L.M. Toxicological Hazard Induced by Sucralose to Environmentally Relevant Concentrations in Common Carp (Cyprinus carpio). Sci. Total Environ. 2017, 575, 347–357. [Google Scholar] [CrossRef]
- Eriksson Wiklund, A.K.; Adolfsson-Erici, M.; Liewenborg, B.; Gorokhova, E. Sucralose Induces Biochemical Responses in Daphnia magna. PLoS ONE 2014, 9, e92771. [Google Scholar] [CrossRef]
- Hjorth, M.; Hansen, J.H.; Camus, L. Short-Term Effects of Sucralose on Calanus finmarchicus and Calanus glacialis in Disko Bay, Greenland. Chem. Ecol. 2010, 26, 385–393. [Google Scholar] [CrossRef]
- Kobeticová, K.; Mocová, K.A.; Mrhálková, L.; Petrová, Š. Effects of Artificial Sweeteners on Lemna Minor. Czech J. Food Sci. 2018, 36, 386–391. [Google Scholar] [CrossRef]
- Lillicrap, A.; Langford, K.; Tollefsen, K.E. Bioconcentration of the Intense Sweetener Sucralose in a Multitrophic Battery of Aquatic Organisms. Environ. Toxicol. Chem. 2011, 30, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Stolte, S.; Steudte, S.; Schebb, N.H.; Willenberg, I.; Stepnowski, P. Ecotoxicity of Artificial Sweeteners and Stevioside. Environ. Int. 2013, 60, 123–127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).