Experimental Study on Adsorption Characteristics of Coal Gangue to Ca2+ in High-Salinity Mine Water
Abstract
1. Introduction
2. Materials and Methods
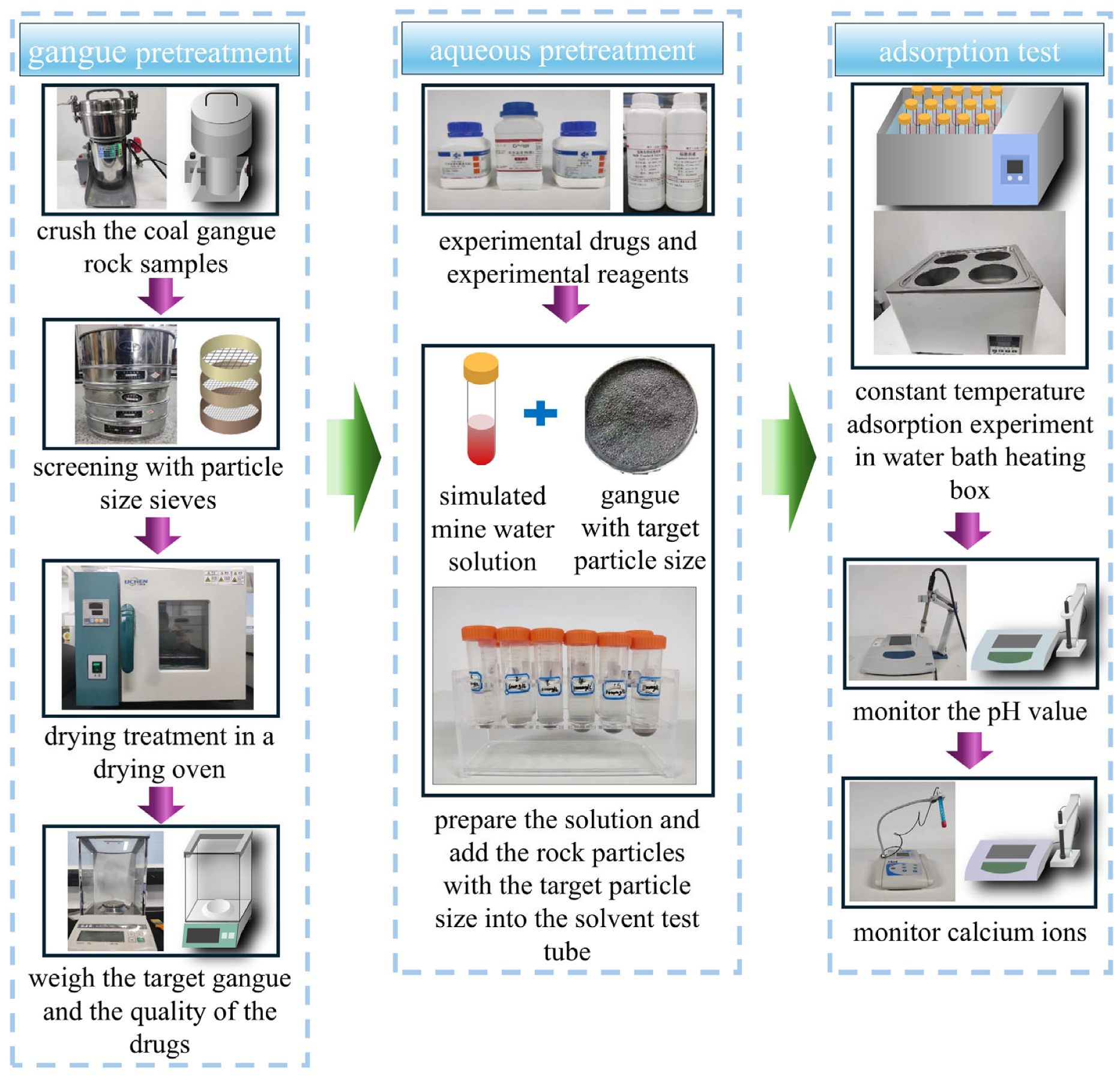
2.1. Adsorbent and Pretreatment
2.2. Adsorbate and Solution Preparation
- ➀
- First, all required equipment including beakers, measuring cylinders, and rubber-headed droppers were thoroughly cleaned with deionized water to ensure they were free of residual impurities. The cleaned glassware was then air-dried naturally until all moisture had completely evaporated before proceeding with solution preparation.
- ➁
- Precisely measured quantities of CaCl2 (0.2775 g, 0.41625 g, 0.555 g, 1.110 g, 1.665 g, 2.220 g, and 2.775 g) were weighed using a high-precision electronic balance (JJ224BC, G&G Measurement Plant, Changshu, China) and added to 1 L of the prepared mine water solution, followed by thorough stirring until complete dissolution was achieved.
- ➂
- Using a rubber-headed dropper, HCl solution was gradually added dropwise while simultaneously activating the stirring apparatus to ensure homogeneous mixing. The pH was monitored in real-time with a pH meter and finely adjusted until the solution reached pH 6. Subsequently, NaOH solution was slowly introduced via dropper while maintaining continuous stirring. Based on real-time pH readings, the solution was precisely adjusted to achieve target pH values of 8 and 9, respectively.
2.3. Characterization of Coal Gangue Materials
2.4. Batch Adsorption Experiment Design
- ➀
- The initially crushed coal gangue was further processed using a mechanical crusher (Ronghao, Rh-600A, Yongkang Ronghao Industry & Trade Co., Ltd., Jinhua, China) and subsequently sieved through mesh screens of different apertures to obtain four particle size fractions: 10–20 mesh, 20–40 mesh, 40–60 mesh, and 200 mesh.
- ➁
- The sieved rock particles were placed in an electric blast drying oven (Model LC-202/101, Lichen Scientific Instruments Co., Ltd., Shaoxing, China) and dried at 105 °C for 5 h.
- ➂
- Mine water solutions with different Ca2+ concentrations and pH values were prepared. Pre-weighed quantities of rock particles were measured using an electronic balance (Model JJ224BC, Shuangjie Testing Instrument Factory, Chuangshu, China) and placed in centrifuge tubes. The tubes were then positioned in a constant-temperature water bath (Model BSH, JoanLab Equipment (Zhejiang) Co., Ltd., Huzhou, China) to conduct batch adsorption experiments under various conditions.
- ➃
- A pH meter and a calcium ion-selective electrode (Model PXS-Ga, Hangzhou Qiwei Instrument Co., Ltd., Hangzhou, China) were employed to monitor changes in solution pH and Ca2+ concentration during the experiments. Intermittent sampling was conducted between 20 and 160 min to obtain concentration-time (Ct-t) and pH-time (pH-t) curves, monitoring both the initial rapid adsorption phase and the subsequent equilibrium phase.
3. Results
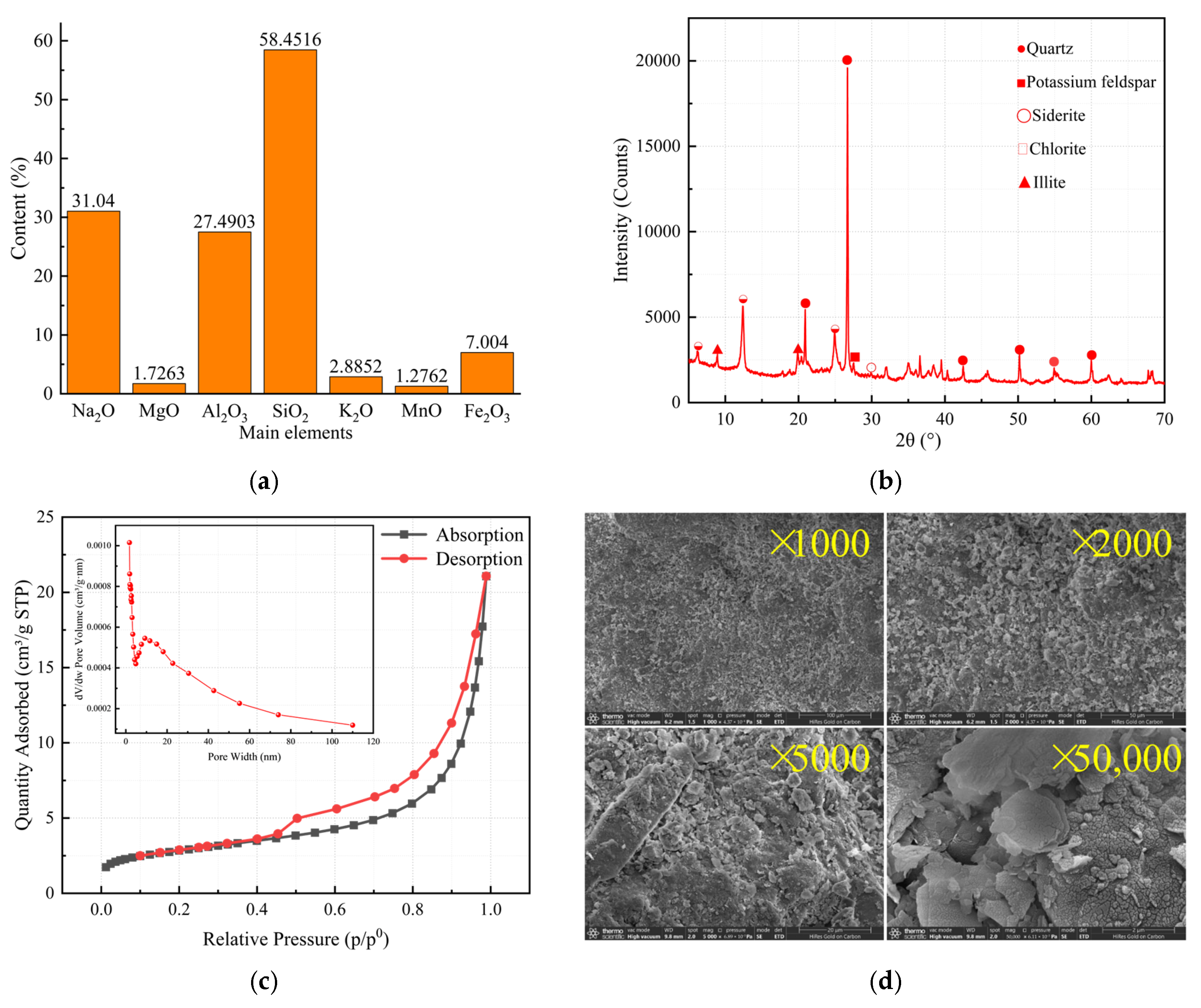
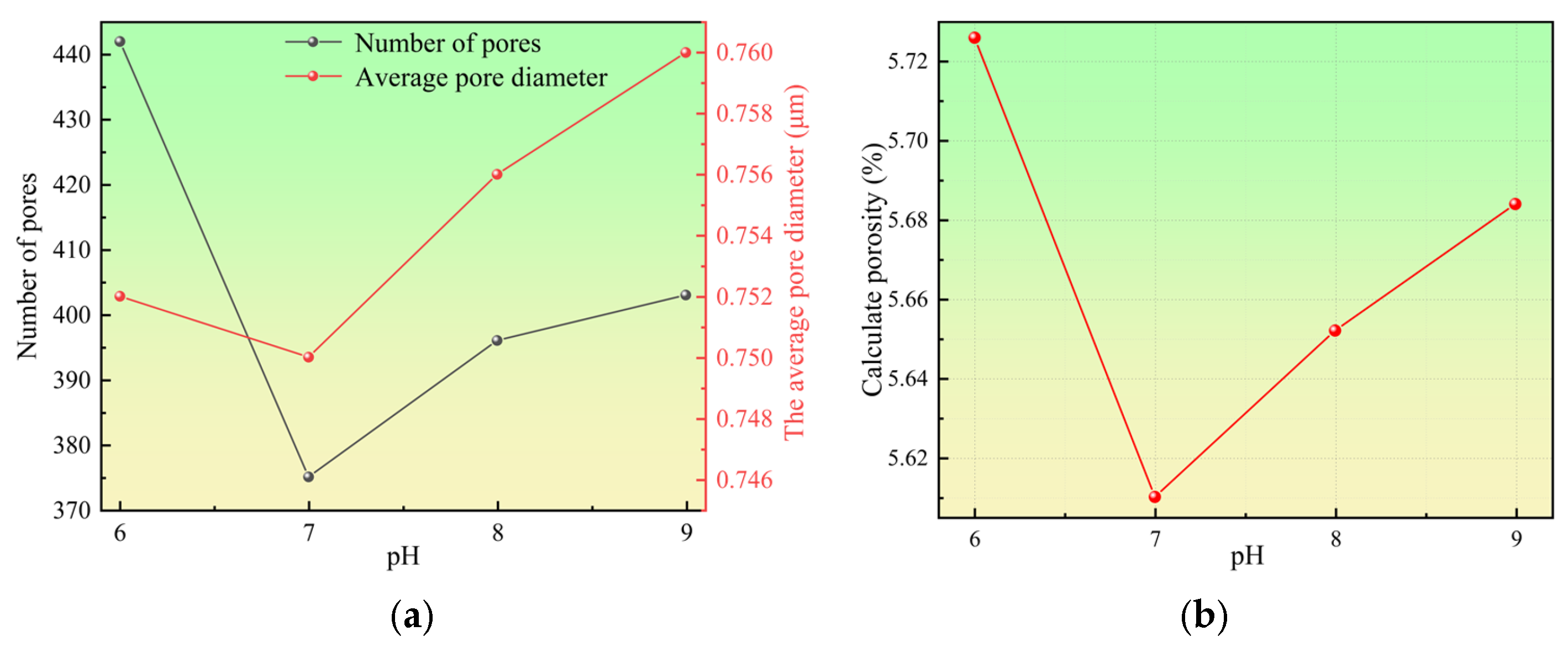
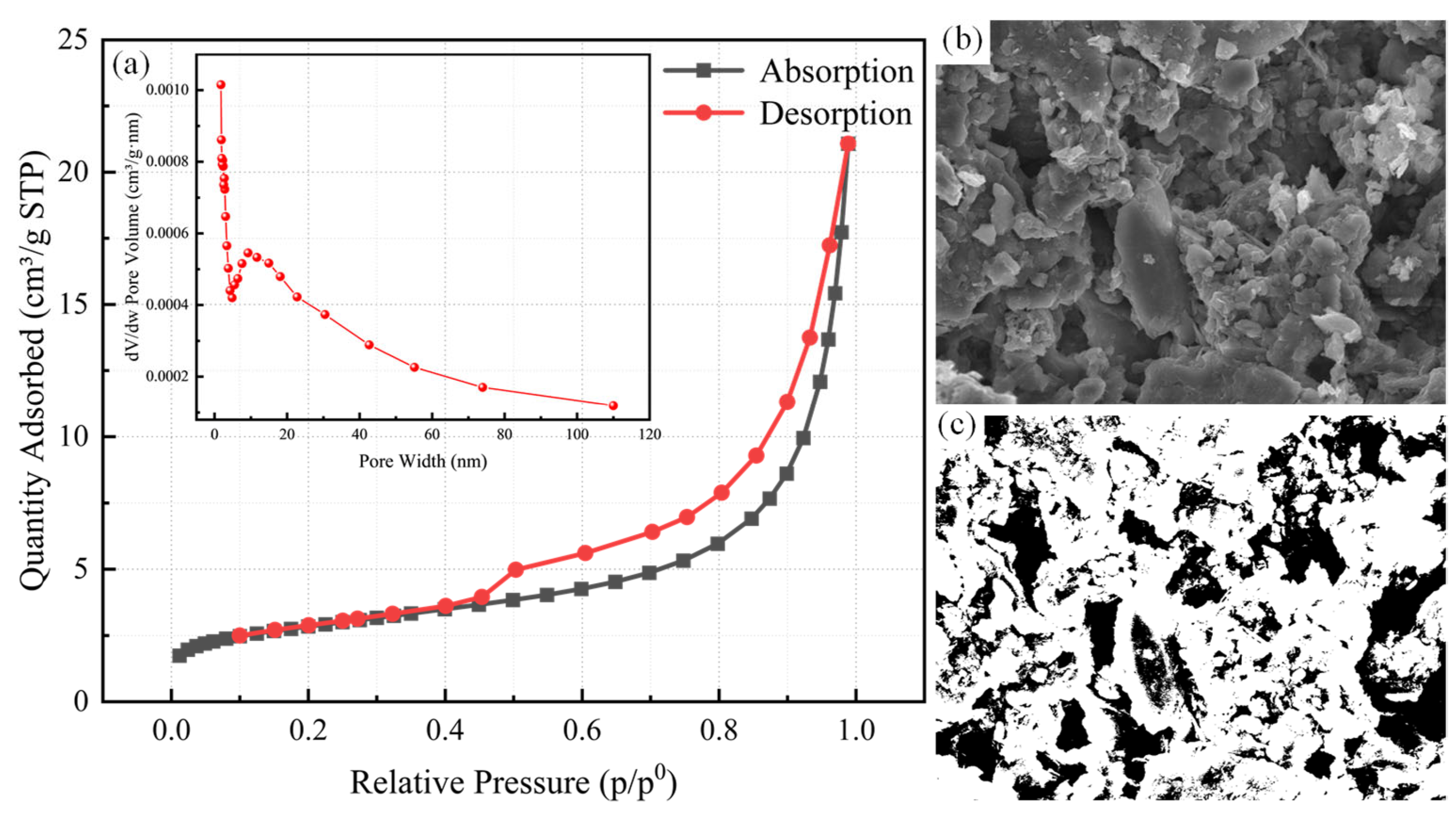
3.1. Key Characterization Results
3.2. Analysis of Key Influencing Factors in the Adsorption Process
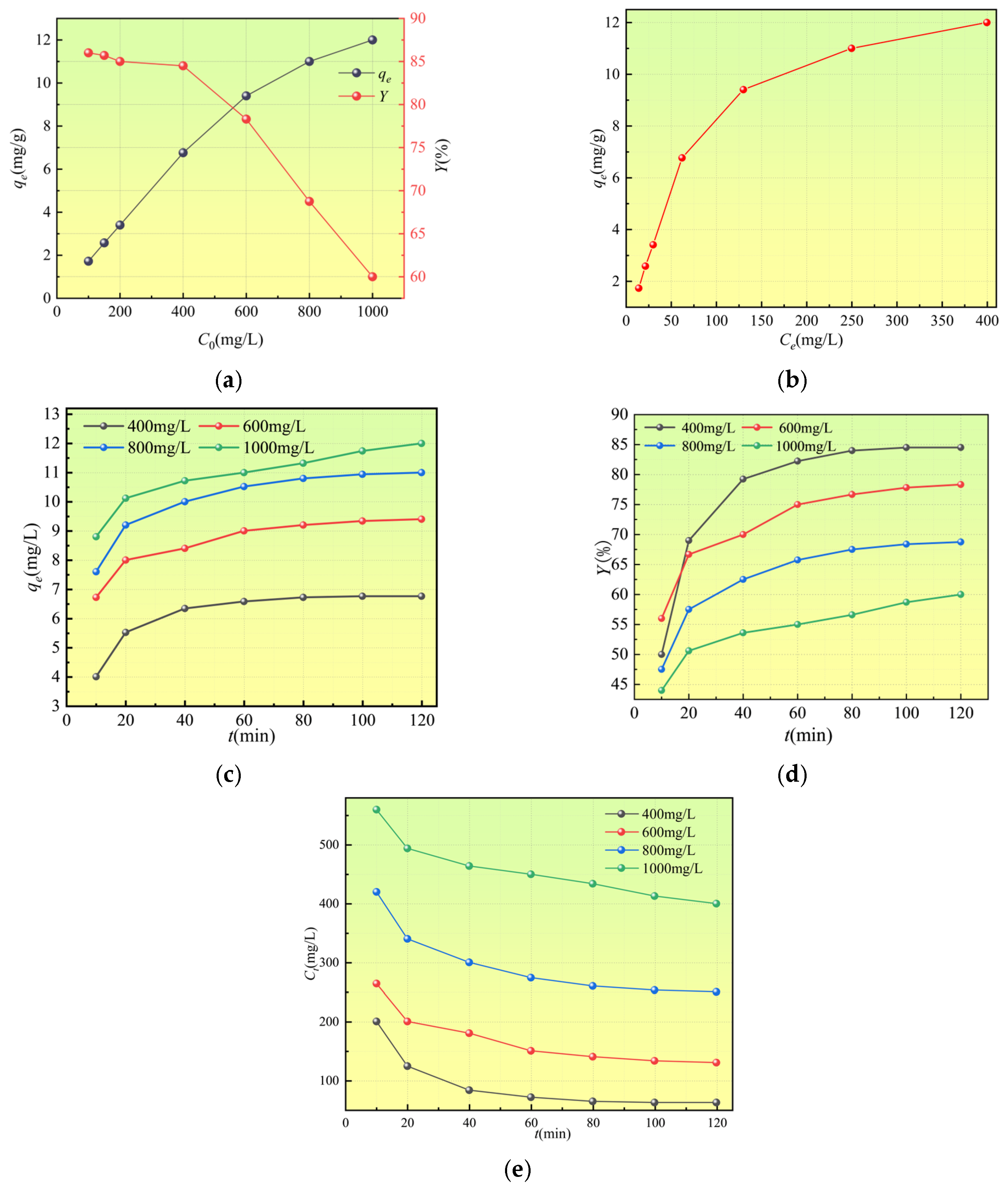
3.2.1. Effect of Initial Concentration and Reaction Time on Adsorption Performance
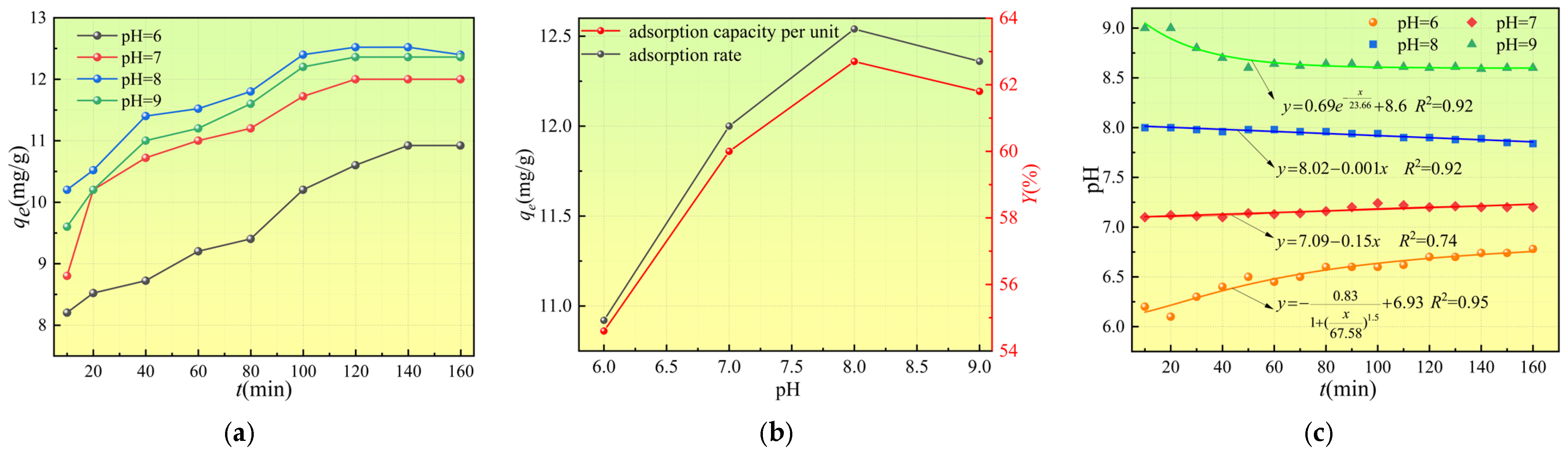
3.2.2. Effect of pH on Adsorption Performance
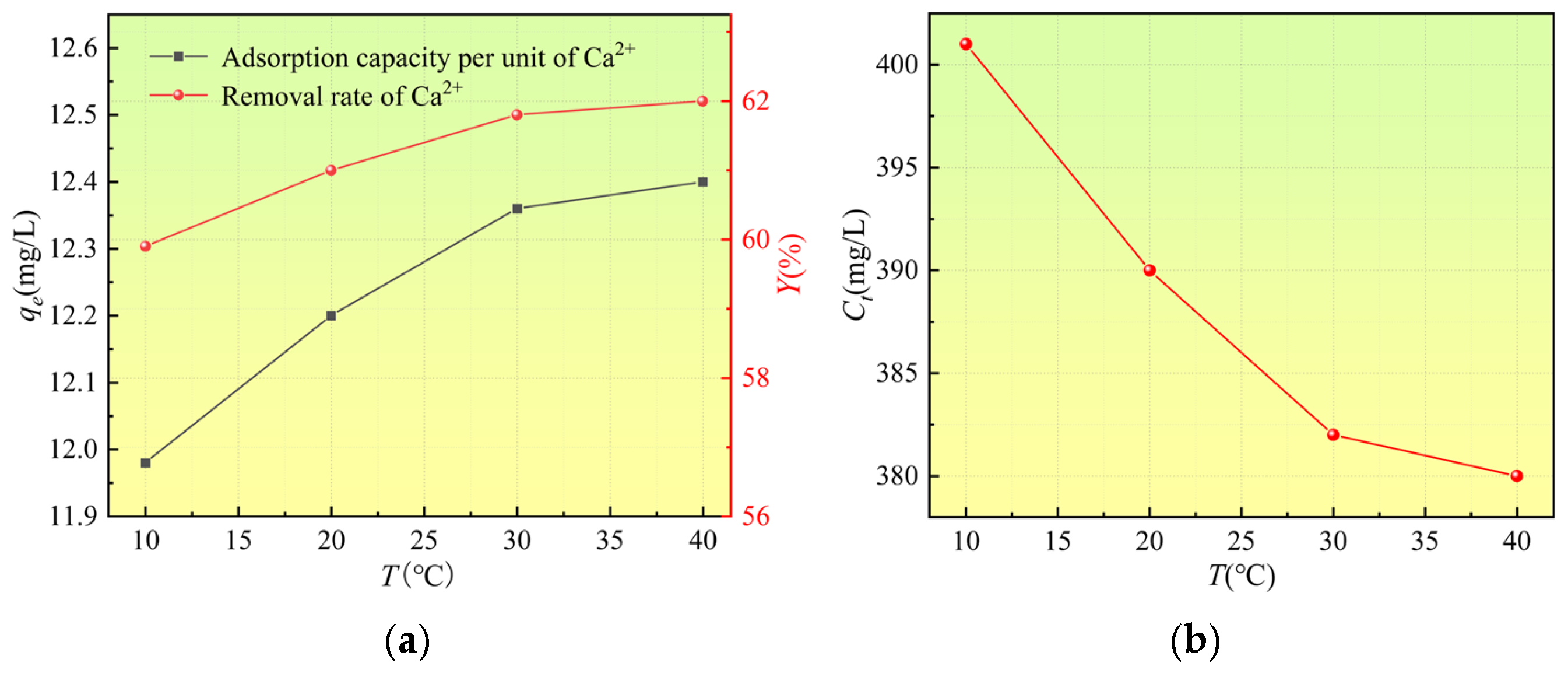
3.2.3. Effect of Temperature on Adsorption Performance
3.2.4. Effect of Particle Size on Adsorption Performance
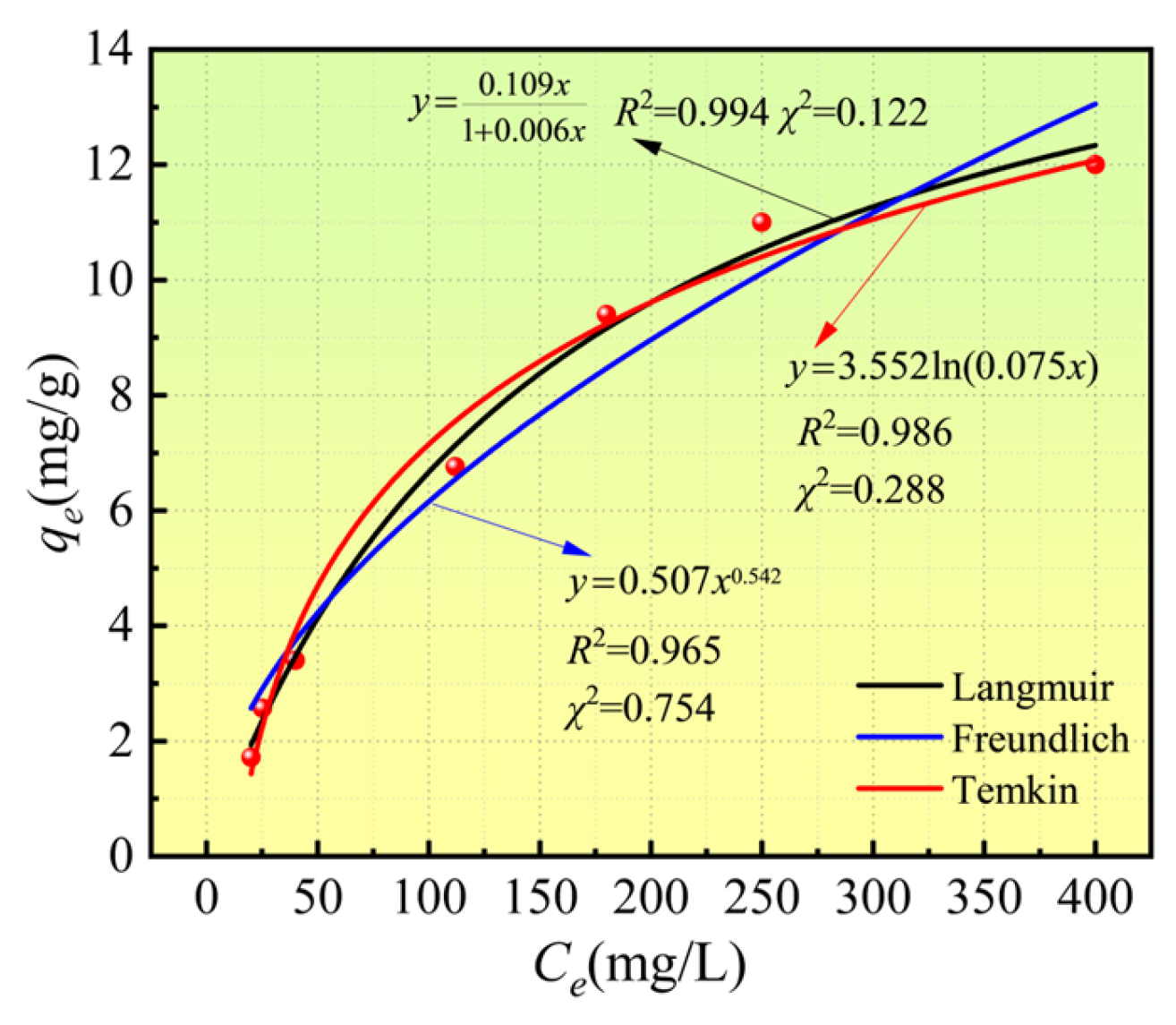
3.3. Isothermal Adsorption Characteristics of Ca2+
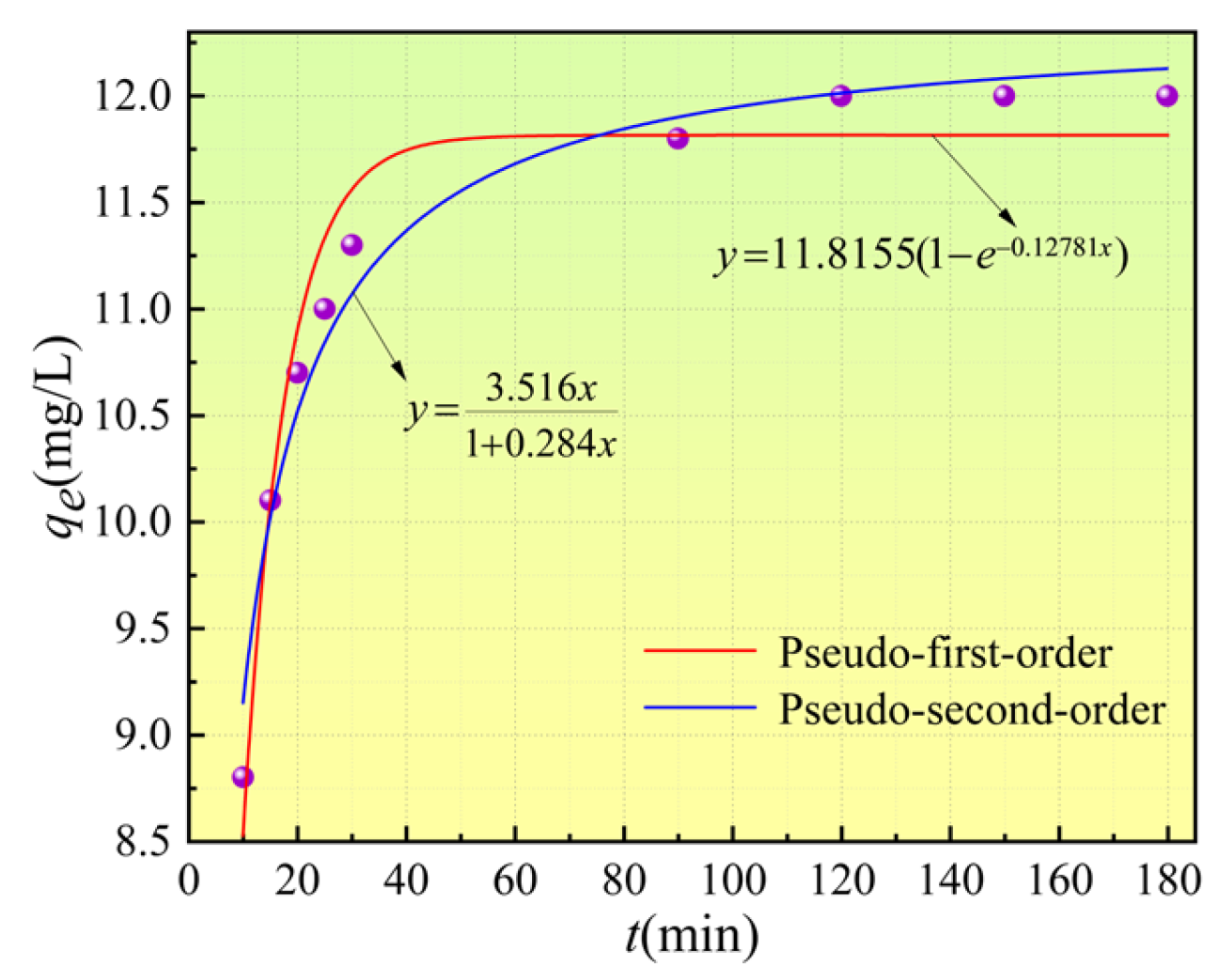
3.4. Adsorption Kinetics of Ca2+
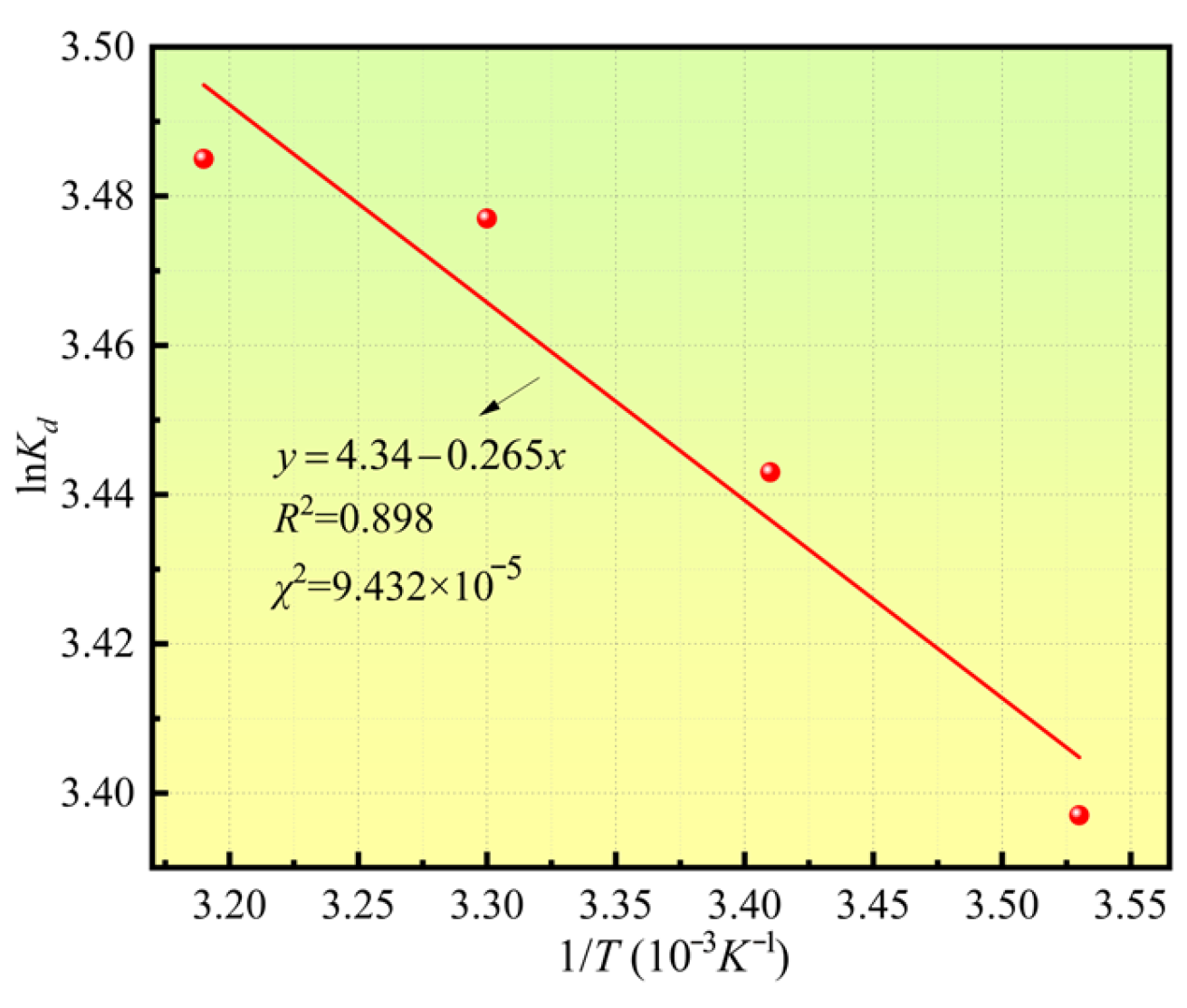
3.5. Adsorption Thermodynamics
4. Discussion
4.1. Synergistic Enhancement of Site Occupation-Dominated Adsorption by Weakly Alkaline Conditions and Moderate-to-High Temperatures
- (1)
- pH-Dependent Modulation of Surface Sites and the Deprotonation Effect
- (2)
- Weak Synergistic Effect from the Solution Side
- (3)
- Mass Transfer Promotion by Temperature and Thermodynamic Validation
4.2. Establishment of the “Structure–Site–Performance” Relationship and the Weakened Particle Size Effect
- (1)
- Abundant Accessible Sites Provided by the Intrinsic Porous Structure
- (2)
- Equilibrium Capacity Constrained by Total Site Density Rather Than Particle Size
- (3)
- Adsorption Site Saturation Dominates Equilibrium Capacity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.H.; Yao, Q.L.; Xia, Z.; Yu, L.; Liu, Z.; Chen, S. Stability Evaluation of the Artificial Dam in Underground Reservoirs Under Storage and Drainage Cycles: A Method Based on Concrete Performance and Case Study. Rock Mech. Rock Eng. 2025, 58, 7725–7744. [Google Scholar] [CrossRef]
- Yao, Q.L.; Yu, L.Q.; Chen, S.Y.; Li, Y.H.; Li, X.H. Mining-affected water resources and ecological effects in ecologically fragile mining areas of western China. J. China Coal Soc. 2025, 50, 748–767. [Google Scholar] [CrossRef]
- Yao, Q.L.; Tang, C.J.; Liu, Z.C. Analysis of coal and water co-mining in ecologically fragile mining areas in Western China. Coal Sci. Technol. 2021, 49, 225–232. [Google Scholar] [CrossRef]
- Wang, W.N.; Yao, Q.L.; Xu, Q.; Chen, X.Y.; Liu, H.Y.; Li, X.H. Experimental Study on the Evolution Law of Coal Mine Underground Reservoir Water Storage Space under the Disturbance and Water-Rock Interaction Effect. Minerals 2022, 12, 1491. [Google Scholar] [CrossRef]
- Xu, Q.; Yao, Q.L.; Wang, F.R.; Xiao, L.; Ma, J.Q.; Kong, F.L.; Shang, X.B. Tracer Test Method to Confirm Hydraulic Connectivity Between Goafs in a Coal Mine. Mine Water Environ. 2024, 43, 104–116. [Google Scholar] [CrossRef]
- Yao, Q.L.; Huang, G.; Xu, Q.; Zhu, L.; Guo, H.T. Experimental study on pre-treatment of high-mineralization mine drainage using gangue of different particle sizes. J. Green Mine 2024, 2, 122–129. [Google Scholar] [CrossRef]
- Gu, D.Z.; Li, J.F.; Cao, Z.G.; Wu, B.Y.; Jiang, B.B.; Yan, Y.; Yan, J.; Chen, Y.P. Technology and engineering development strategy of water protection and utilization of coal mine in China. J. China Coal Soc. 2021, 46, 3079–3089. [Google Scholar] [CrossRef]
- Zhu, L.; Yao, Q.L.; Xu, Q.; Li, Y.H.; Li, X.H. Experimental Study on the Purification Mechanism of Mine Water by Coal Gangue. Water 2023, 15, 697. [Google Scholar] [CrossRef]
- Sun, Y.J.; Chen, G.; Xu, Z.M.; Yuan, H.Q.; Zhang, Y.Z.; Zhou, L.J.; Wang, X.; Zhang, C.H.; Zhang, J.M. Research progress of water environment, treatment and utilization in coal mining areas of China. J. China Coal Soc. 2020, 45, 304–316. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Q. Mine Water Treatment, Resource Utilization and Prospects in Coal Mining Areas of Western China. Mine Water Environ. 2024, 43, 210–230. [Google Scholar] [CrossRef]
- Gu, D.Z.; Cao, Z.G.; Li, J.F.; Wu, B.Y.; Zhang, Y.; Jiang, B.B.; Guo, Q.; Wang, H.P.; Wu, Y.; Shi, X.M.; et al. Original experimental platform system and application of under-ground coal mine reservoirs. J. China Coal Soc. 2024, 49, 100–113. [Google Scholar] [CrossRef]
- Gu, D.Z. Theory framework and technological system of coal mine underground reservoir. J. China Coal Soc. 2015, 40, 239–246. [Google Scholar] [CrossRef]
- Yao, Q.L.; Wang, W.N.; Xia, Z.; Zhu, L.; Wang, A.; Yu, L.Q. Adsorption characteristics of Mn (II) on sandy mudstone and fine sand-stone of coal measures. J. China Univ. Min. Technol. 2020, 49, 411–418. [Google Scholar] [CrossRef]
- Wang, F.T.; Sun, N.; Zhang, C.; Fan, C.H.; Xiong, J.B.; Wei, X.Q.; Hao, W.H. Experimental study on mine water purification mechanism for broken coal and rock masses in the underground reservoir of ecologically vulnerable mining area. Environ. Sci. Pollut. Res. 2024, 31, 21442–21457. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Yadav, V.K.; Yadav, K.K.; Ramar, K.; Alam, J.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, A. Fabrication of different SnO2 nanorods for enhanced photocatalytic degradation and antibacterial activity. Environ. Sci. Pollut. Res. 2023, 30, 71574–71584. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M. Trimesoyl chloride crosslinked chitosan: An efficient adsorbent for Congo red dye removal from water—Kinetic and isotherm analysis. Int. J. Biol. Macromol. 2025, 319, 145538. [Google Scholar] [CrossRef] [PubMed]
- Rajabathar, J.R.; Shukla, A.K.; Ali, A.; Al-Lohedan, H.A. Silver nanoparticle/r-graphene oxide deposited mesoporous-manganese oxide nanocomposite for pollutant removal and supercapacitor applications. Int. J. Hydrogen Energy 2017, 42, 15679–15688. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Mallik, S.; Ruokolainen, J.; Kesari, K.K.; Alhoshan, M. Optimization and prediction of dye adsorption utilising cross-linked chitosan-activated charcoal: Response Surface Methodology and machine learning. J. Mol. Liq. 2024, 411, 125745. [Google Scholar] [CrossRef]
- Mahdi, A.E.; Ali, N.S.; Majdi, H.S.; Albayati, T.M.; Abdulrahman, M.A.; Jasim, D.J.; Kalash, K.R.; Salih, I.K. Effective adsorption of 2-nitroaniline from wastewater applying mesoporous material MCM-48: Equilibrium, isotherm, and mechanism investigation. Desalination Water Treat. 2023, 300, 120–129. [Google Scholar] [CrossRef]
- Al-Hazmi, G.A.A.M.; Alayyafi, A.A.; El-Desouky, M.G.; El-Bindary, A.A. Guava seed activated carbon loaded calcium alginate aerogel for the adsorption of diclofenac sodium: Characterization, isotherm, kinetics, and optimization via Box-Behnken design. Int. J. Biol. Macromol. 2024, 262, 129995. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Alnawmasi, J.S.; Alshammari, N.A.H.; Abomuti, M.A.; Elsayed, N.H.; El-Desouky, M.G. Industrial dye absorption and elimination from aqueous solutions through bio-composite construction of an organic framework encased in food-grade algae and alginate: Adsorption isotherm, kinetics, thermodynamics, and optimization by Box–Behnken design. Int. J. Biol. Macromol. 2024, 274, 133442. [Google Scholar] [CrossRef]
- Yao, Q.L.; Xia, Z.; Tang, C.J.; Zhu, L.; Wang, W.N.; Chen, T.; Tan, Y.M. Characteristics of Heavy Metal Ion Adsorption by Silty Mudstones in Coal Mine Goafs. Geofluids 2020, 2020, 8560151. [Google Scholar] [CrossRef]
- Jiang, B.B.; Li, J.F.; Ma, L.Q.; Wu, M.; Su, C.; Li, J.H. Investigation of the suspended solids removal from mine water by the rock accumulation effect in the underground reservoir of coal mines. Coal Sci. Technol. 2024, 52, 352–361. [Google Scholar] [CrossRef]
- Guo, Y.C.; Huang, Y.L.; Li, J.M.; Ouyang, S.Y.; Fan, B.T.; Liu, Y.H. Study on dynamic adsorption characteristics of gangue to heavy metals in goaf and its water purification mechanism under leaching condition. J. Water Process Eng. 2023, 53, 103741. [Google Scholar] [CrossRef]
- Li, J.M.; Huang, Y.L.; Li, W.; Guo, Y.C.; Ouyang, S.Y.; Cao, G.L. Study on dynamic adsorption characteristics of broken coal gangue to heavy metal ions under leaching condition and its cleaner mechanism to mine water. J. Clean. Prod. 2021, 329, 129756. [Google Scholar] [CrossRef]
- Guo, X.Y.; Dong, Y.R.; Di, J.Z.; Li, Y. Modified Gangue for Coal Mine Wastewater’s Fe2+ and Mn2+ Adsorption. Non-Met. Mines 2017, 40, 83–87. [Google Scholar]
- Liu, B.Y.; Li, S.W.; Song, Y.H.; Yao, Y.X.; Zhou, J.J.; Guo, C.B. Adsorption performance of modified coal gangue for Pb2+ and Zn2+ in wastewater. J. Liaoning Tech. Univ. (Nat. Sci.) 2023, 42, 444–450. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Y.F.; Yang, B.B.; Wang, S.D.; Yang, J.; Sun, C.; Tian, Y.F. Removal efficiencies of dissolved organic matter and ammonium in coalmine water by coal gangue through column experiments. J. China Coal Soc. 2018, 43, 236–241. [Google Scholar] [CrossRef]
- Proust, D. Sorption and distribution of Zn in a sludge-amended soil: Influence of the soil clay mineralogy. J. Soils Sediments 2015, 15, 607–622. [Google Scholar] [CrossRef]
- Wang, X.F.; Gao, Y.B.; Jiang, X.J.; Zhang, Q.; Liu, W.G. Analysis on the Characteristics of Water Pollution Caused by Underground Mining and Research Progress of Treatment Technology. Adv. Civ. Eng. 2021, 2021, 9984147. [Google Scholar] [CrossRef]
- Shah, K.M.; Billinge, I.H.; Chen, X.; Fan, H.; Huang, Y.; Winton, R.K.; Yip, N.Y. Drivers, challenges, and emerging technologies for desalination of high-salinity brines: A critical review. Desalination 2022, 538, 115827. [Google Scholar] [CrossRef]
- Wang, C.S.; Liu, L.; Wang, Y.X.; Hou, P.; Wang, J.B.; Wang, J.W. Treatment of various saline wastewaters by membrane distillation with omniphobic polyvinyl alcohol membrane. Desalination 2024, 591, 118011. [Google Scholar] [CrossRef]
- Ruiz, S.G.; López-Ramírez, J.A.; Zerrouk, M.H.; Lopera, A.E.C.; Alonso, J.M.Q. Study of reverse osmosis membranes fouling by inorganic salts and colloidal particles during seawater desalination. Chin. J. Chem. Eng. 2020, 28, 733–742. [Google Scholar] [CrossRef]
- GB/T 29162-2012; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, & Standardization Administration of the People’s Republic of China. Classification of Gangue. China Standards Press: Beijing, China, 2013.
- Zhao, S.S.; Chen, K.; Xiong, B.L.; Guo, C.L.; Dang, Z. Prediction of adsorption of metal cations by clay minerals using machine learning. Sci. Total Environ. 2024, 924, 171733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.L.; Chen, Q.Z.; Zhou, Q.; Xi, Y.F.; Zhu, J.X.; He, H.P. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.B.; Chen, T.H.; Chen, D.; Frost, R.L. An insight into the removal of Pb(II), Cu(II), Co(II), Cd(II), Zn(II), Ag(I), Hg(I), Cr(VI) by Na(I)-montmorillonite and Ca(II)-montmorillonite. Appl. Clay Sci. 2015, 118, 239–247. [Google Scholar] [CrossRef]
- Yao, S.; Feng, G.R.; Zhao, D.K.; Liu, A.F.; Du, J.; Wang, S.; Yao, X.; Hou, K. Research Progress of Clay Minerals in the Field of Coal Mine Water Treatment. Mater. Rep. 2025, 39, 71–80. Available online: https://link.cnki.net/urlid/50.1078.tb.20241206.0905.002 (accessed on 29 October 2025).
- Gan, W.B.; Liu, Q. Coagulation of bitumen with kaolinite in aqueous solutions containing Ca2+, Mg2+ and Fe3+: Effect of citric acid. J. Colloid Interface Sci. 2008, 324, 85–91. [Google Scholar] [CrossRef]
- Tenea, A.G.; Dinu, C.; Rus, P.A.; Ionescu, I.A.; Gheorghe, S.; Iancu, V.I.; Vasile, G.G.; Pascu, F.L.; Chiriac, F.L. Exploring adsorption dynamics of heavy metals onto varied commercial microplastic substrates: Isothermal models and kinetics analysis. Heliyon 2024, 10, e35364. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Q.; Wu, W.; Khor, S.M. Absorbent cotton-templated manganese oxide nanocomposites for methylene blue promote isothermal adsorption. Int. J. Environ. Sci. Technol. 2024, 21, 417–430. [Google Scholar] [CrossRef]
- Simić, M.; Petrović, J.; Koprivica, M.; Ercegović, M.; Dimitrijević, J.; Vuković, N.S.; Fiol, N. Efficient Adsorption of Lead on Hydro-Pyrochar Synthesized by Two-Step Conversion of Corn Cob in Magnesium Chloride Medium. Toxics 2025, 13, 459. [Google Scholar] [CrossRef]
- Alsuhaibani, A.M.; Alayyafi, A.A.; Albedair, L.A.; El-Desouky, M.G.; El-Bindary, A.A. Efficient fabrication of a composite sponge for Cr(VI) removal via citric acid cross-linking of metal-organic framework and chitosan: Adsorption isotherm, kinetic studies, and optimization using Box-Behnken design. Mater. Today Sustain. 2024, 26, 100732. [Google Scholar] [CrossRef]
- Deng, J.H.; Ma, Z.T.; Qin, Q.Z.; Liu, W.L.; Wang, B. A residue reutilization strategy on fluorine-containing water purification. J. Clean. Prod. 2022, 371, 133613. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1837–1865. [Google Scholar] [CrossRef]
- Alshammari, N.A.H.; Alnawmasi, J.S.; Alotaibi, A.M.; Alshammari, O.A.O.; Abomuti, M.A.; Elsayed, N.H.; El-Bindary, A.A. Efficient adsorption of fluorescein dye from aqueous solutions by Al/Th-MOF bimetal-organic frameworks: Adsorption isotherm, kinetics, DFT computation, and optimization via Box-Behnken design. Process Saf. Environ. Prot. 2024, 190, 353–371. [Google Scholar] [CrossRef]
- Wang, W.J.; Lin, J.X.; Shao, S.B.; Chen, H.J.; Dai, J.W.; Yang, Y. Enhanced adsorption of benzo(a)pyrene in soil by porous biochar: Adsorption kinetics, thermodynamics, and mechanisms. J. Environ. Chem. Eng. 2023, 11, 109002. [Google Scholar] [CrossRef]
- Masuku, M.; Nure, J.F.; Atagana, H.I.; Hlongwa, N.; Nkambule, T.T.I. Pinecone biochar for the Adsorption of chromium (VI) from wastewater: Kinetics, thermodynamics, and adsorbent regeneration. Environ. Res. 2024, 258, 119423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Chen, S.N.; Duan, H.Y.; He, J.; Luo, Y.C. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: Optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef]
- Yang, S.C.; Wang, P.; Wang, W.C.; Cao, Z.; Cao, Y.D. Study on the variability of oxygen adsorption behavior in coal gangue based on pore size structure. Mater. Today Commun. 2024, 41, 110657. [Google Scholar] [CrossRef]
- Al-Hazmi, G.H.; Albedair, L.A.; Alatawi, R.A.S.; Alnawmasi, J.S.; Alsuhaibani, A.M.; El-Desouky, M.G. Enhancing trimethoprim pollutant removal from wastewater using magnetic metal-organic framework encapsulated with poly (itaconic acid)-grafted crosslinked chitosan composite sponge: Optimization through Box-Behnken design and thermodynamics of adsorption parameters. Int. J. Biol. Macromol. 2024, 268, 131947. [Google Scholar] [CrossRef]


| Chemical Reagents | Purity | Manufacturer |
|---|---|---|
| NaOH | 0.01002 mol/L | Yida Technology Co., Ltd. Jurong, China |
| HCl | 0.01002 mol/L | |
| CaCl2 | Analytical Reagent | Fuchen (Tianjin) Chemical Reagents Co., Ltd. Tianjin, China |
| MgCl2·6H2O | Analytical Reagent | |
| KCl | Analytical Reagent | |
| Na2SO4 | Analytical Reagent | |
| NaCl | Analytical Reagent | |
| NaHCO3 | Analytical Reagent | |
| H2O | Analytical Reagent |
| pH | Ion Concentration mg/L | TDS mg/L | Mineralization of Water mg/L | Water Hardness mg/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | K+ | Na+ | Cl− | SO42− | HCO3− | ||||
| 8.17 | 200.2 | 178.9 | 9.4 | 1378 | 1653.76 | 1292 | 488.74 | 4918.72 | 5163.08 | 1092.4 |
| Name | MgCl2·6H2O | NaCl | KCl | NaHCO3 | Na2SO4 |
|---|---|---|---|---|---|
| Mass/g | 1.691 | 1.365 | 0.019 | 0.84 | 1.492 |
| Experiment Name | pH | Temperature (°C) | Ca2+ Concentration (mg/L) | Particle Size (Mesh) | Reaction Time (min) |
|---|---|---|---|---|---|
| Isothermal Adsorption | 7 | 20 | 400, 600, 800, 1000 | 10–20 | 160 |
| Adsorption Kinetics | 7 | 20 | 1000 | 10–20 | 0–180 |
| Adsorption Thermodynamics | 7 | 10, 20, 30, 40 | 1000 | 10–20 | 0–160 |
| Varying Initial Concentration | 7 | 20 | 100, 150, 200, 400, 600, 800, 1000 | 10–20 | 0–210 |
| Varying Reaction Time | 7 | 20 | 400, 600, 800, 1000 | 10–20 | 0–160 |
| Varying pH | 6, 7, 8, 9 | 20 | 1000 | 10–20 | 160 |
| Varying Temperature | 7 | 20 | 1000 | 10–20 | 160 |
| Varying Particle Size | 7 | 20 | 1000 | 10–20, 20–40, 40–60, 200 | 210 |
| Rock Composition | Content | Characteristics |
|---|---|---|
| Sericite | ≈62% | Fine flaky texture, grain size predominantly below 0.1 mm, formed by metamorphic crystallization of early mudstone. Colorless or slightly reddish-brown due to iron staining. Exhibits extremely perfect cleavage. Interference colors, primarily orange-yellow due to fine grain size, rarely reaching vivid secondary hues. |
| Silt-grade minerals | ≈30% | Granular, typically less than 0.05 mm in size, showing weakly developed recrystallization. Mixed among clay and other components, primarily quartz silt grains with trace feldspar. |
| Biotite | <1% | Fine-scale flaky morphology, originating from early mud undergoing metamorphic crystallization. Size less than 0.05 mm, pale green or pale yellow-green in hue, with unusual interference colors. |
| Chlorite | ≈3% | Often exhibits iron staining with a reddish-brown tint; shows weak optical properties under orthoptic polariscope. |
| Other clay minerals | ≈3% | |
| Opaque minerals | ≈1% | Commonly occurs as granular, plate-like, or lamellar textures; predominantly black or dark brown, likely dominated by iron-bearing minerals. |
| Rock structure | Granular-scaly metamorphic texture | |
| Rock fabric | Plate-like foliation structure | |
| Surface Area Results | Parameters |
|---|---|
| Total pore volume | 0.0326 cm3/g |
| Average pore size | 6.4116 nm |
| BET surface area | 10.1629 m2/g |
| Model | Fitting Parameters | |||
|---|---|---|---|---|
| Langmuir | qmax (mg/L) | KL (L/mg) | R2 | χ2 |
| 17.218 | 0.006 | 0.994 | 0.122 | |
| Freundich | Kf | n | R2 | χ2 |
| 0.507 | 1.844 | 0.965 | 0.754 | |
| Temkin | bT (kJ/mol) | AT (L/g) | R2 | χ2 |
| 0.686 | 0.075 | 0.986 | 0.288 | |
| Model | Langmuir | Freundich | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | qe | k1 | R2 | χ2 | qe | k2 | R2 | χ2 |
| 11.812 | 0.128 | 0.952 | 0.057 | 12.365 | 0.023 | 0.967 | 0.039 | |
| Thermodynamic Parameters | T/K | R2 | χ2 | |||
|---|---|---|---|---|---|---|
| 281.5 | 291.5 | 300.5 | 315.5 | |||
| ΔH (kJ·mol−1) | 2.203 | 0.898 | 9.432 × 10−5 | |||
| ΔS (J·mol−1·K−1) | 36.084 | |||||
| ΔG (kJ·mol−1) | −7.95 | −8.31 | −8.64 | −9.18 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Xia, Z.; Mu, H.; Fan, Y.; Zheng, C. Experimental Study on Adsorption Characteristics of Coal Gangue to Ca2+ in High-Salinity Mine Water. Sustainability 2025, 17, 10423. https://doi.org/10.3390/su172210423
Zhao N, Xia Z, Mu H, Fan Y, Zheng C. Experimental Study on Adsorption Characteristics of Coal Gangue to Ca2+ in High-Salinity Mine Water. Sustainability. 2025; 17(22):10423. https://doi.org/10.3390/su172210423
Chicago/Turabian StyleZhao, Nan, Ze Xia, Haokai Mu, Yukuan Fan, and Chuangkai Zheng. 2025. "Experimental Study on Adsorption Characteristics of Coal Gangue to Ca2+ in High-Salinity Mine Water" Sustainability 17, no. 22: 10423. https://doi.org/10.3390/su172210423
APA StyleZhao, N., Xia, Z., Mu, H., Fan, Y., & Zheng, C. (2025). Experimental Study on Adsorption Characteristics of Coal Gangue to Ca2+ in High-Salinity Mine Water. Sustainability, 17(22), 10423. https://doi.org/10.3390/su172210423







