Synergy of Arbuscular Mycorrhizal Fungi and Biochar-Based Fertilizer Reshapes Soybean Nutrient Acquisition and Drives Yield Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Sample Collection and Determination
2.3.1. Plant Sample Collection and Analysis
2.3.2. Soil Sample Collection and Analysis
2.3.3. Determination of Crop Yield and Yield Components
2.4. Statistical Analysis
3. Results
3.1. Effects of AMF and Biochar-Based Fertilizer Interactions on Soybean Plant Growth
3.2. Effects of AMF and Biochar-Based Fertilizer Interactions on Soil Nutrients Content and Enzyme Activities
3.3. Effects of AMF and Biochar-Based Fertilizer Interactions on Soybean Yield
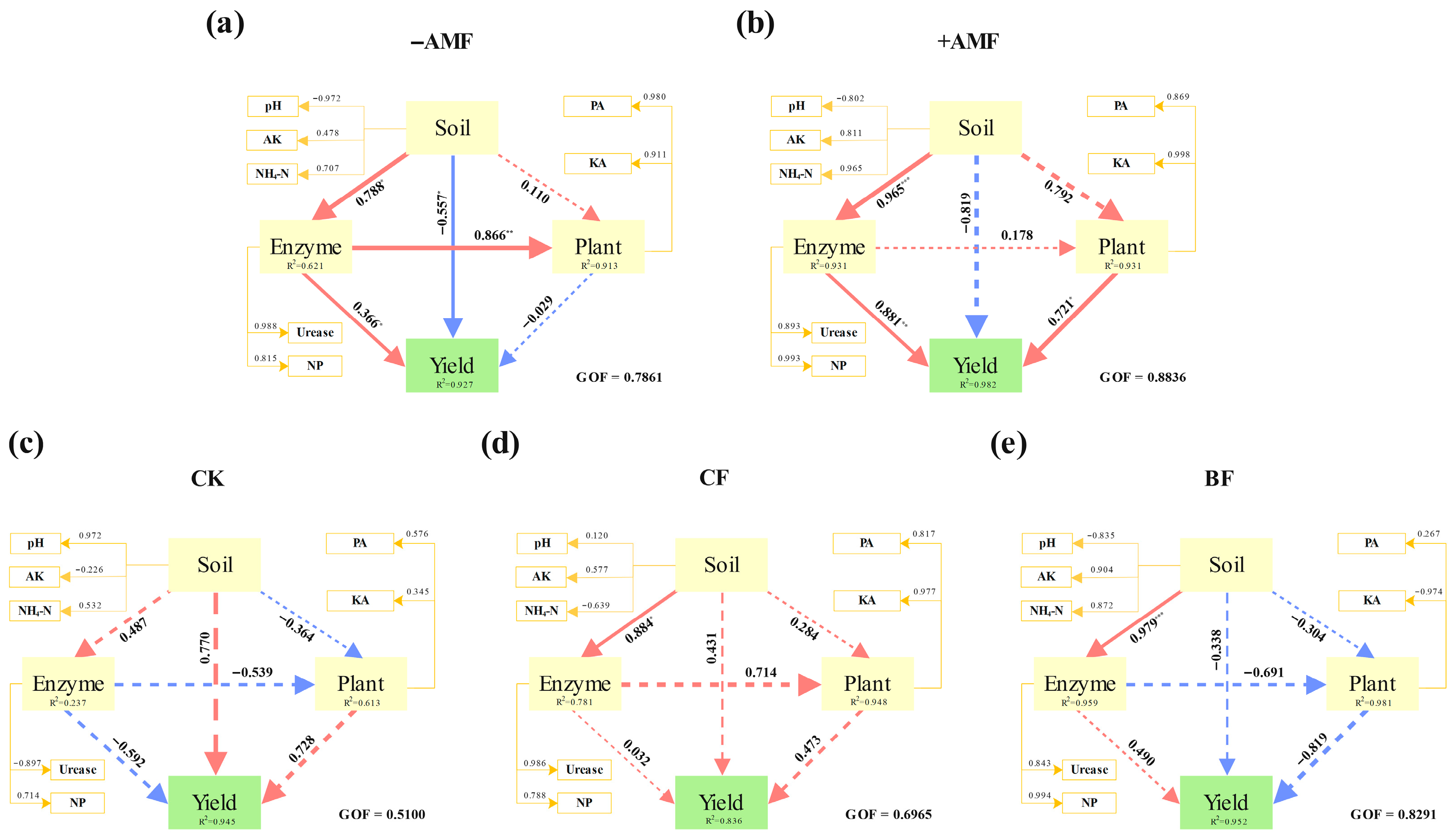
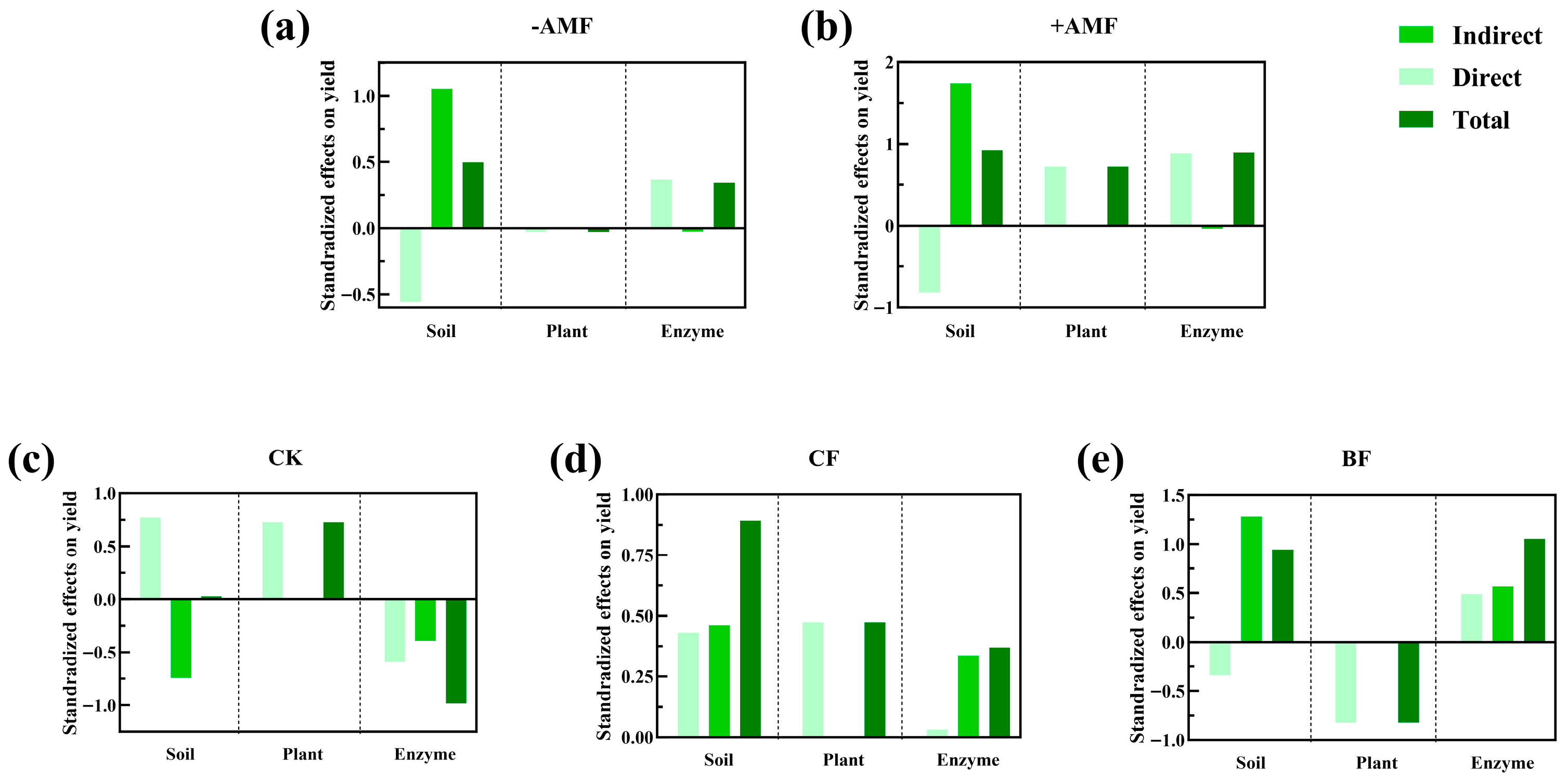
3.4. Comprehensive Analysis of Plant Nutrient Uptake, Soil Nutrient and Yield
4. Discussion
4.1. Effects of AMF and Biochar Basal Fertilizer Interaction on Crop Growth
4.2. Effects of AMF and Biochar Base Fertilizer Interaction on Soil Properties
4.3. Comprehensive Analysis of Plant Nutrient Uptake, Soil Nutrient and Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Zhao, Y.; Xu, Y.; Cui, J.; Li, T.; Hu, Y.; Qian, X.; Li, Z.; Sui, P.; Chen, Y. Yield Performance Response to Field Configuration of Maize and Soybean Intercropping in China: A Meta-Analysis. Field Crop. Res. 2024, 306, 109235. [Google Scholar] [CrossRef]
- Tayade, R.; Imran, M.; Ghimire, A.; Khan, W.; Nabi, R.B.S.; Kim, Y. Molecular, Genetic, and Genomic Basis of Seed Size and Yield Characteristics in Soybean. Front. Plant Sci. 2023, 14, 1195210. [Google Scholar] [CrossRef]
- Taiyawong, A.; Monkham, T.; Sanitchon, J.; Choenkwan, S.; Srisawangwong, S.; Khodphuwiang, J.; Reewarabundit, S.; Chankaew, S. Yield Stability of Soybean Variety Morkhor 60 in Integrated Rotation Systems of Northeastern Thailand. Plants 2025, 14, 2503. [Google Scholar] [CrossRef]
- Marques Pires, M.D.F.; De Souza, H.A.; Medeiros, J.C.; Dalla Rosa, J.; De Souza Martins, R.V.; Sobral, A.H.S.; Pereira Carvalho, S.; De Sousa Vera, G.; De Melo Jorge Vieira, P.F.; Sagrilo, E. Nutrient Uptake by Soybean Plants in Succession of Cover Crops in Northeast of Brazil. Commun. Soil Sci. Plant Anal. 2023, 54, 945–963. [Google Scholar] [CrossRef]
- Mutai, J.; Medvecky, B.; Vanek, S.J.; Ojiem, J.; Bolo, P.; Kihara, J.; Fonte, S.J. Long—Term Cropping System and Manure Effects on Soil Health Parameters and Associated Soil—Borne Pathogens. Soil Sci. Soc. Am. J. 2025, 89, e70076. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, L.; Guo, N.; Cai, B. Transcriptomic Analyses Revealed the Effect of Funneliformis Mosseae on Genes Expression in Fusarium Oxysporum. PLoS ONE 2020, 15, e0234448. [Google Scholar] [CrossRef]
- Huang, L.; Riggins, C.W.; Rodríguez-Zas, S.; Zabaloy, M.C.; Villamil, M.B. Long-Term N Fertilization Imbalances Potential N Acquisition and Transformations by Soil Microbes. Sci. Total Environ. 2019, 691, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Tan, Y.; Lin, H.; Zhang, M.; Kan, L. Effectiveness of Rhizophagus intraradices and Acinetobacter calcoaceticus on Soybean Growth and Thiram Residues in Soybean Grains and Rhizosphere Soil. PeerJ 2025, 13, e19701. [Google Scholar] [CrossRef]
- Nehbandani, A.; Soltani, A.; Rahemi-Karizaki, A.; Dadrasi, A.; Noubakhsh, F. Determination of Soybean Yield Gap and Potential Production in Iran Using Modeling Approach and GIS. J. Integr. Agric. 2021, 20, 395–407. [Google Scholar] [CrossRef]
- Jin, Z.; Jiang, F.; Wang, L.; Declerck, S.; Feng, G.; Zhang, L. Arbuscular Mycorrhizal Fungi and Streptomyces: Brothers in Arms to Shape the Structure and Function of the Hyphosphere Microbiome in the Early Stage of Interaction. Microbiome 2024, 12, 83. [Google Scholar] [CrossRef]
- Duan, S.; Feng, G.; Limpens, E.; Bonfante, P.; Xie, X.; Zhang, L. Cross-Kingdom Nutrient Exchange in the Plant–Arbuscular Mycorrhizal Fungus–Bacterium Continuum. Nat. Rev. Microbiol. 2024, 22, 773–790. [Google Scholar] [CrossRef]
- Zhao, R.; He, G.; Zhou, D.; Li, X.; Kuyper, T.W.; Zhang, F.; Zhang, J. Arbuscular Mycorrhizal Fungi Enhance Nitrate Ammonification in Hyphosphere Soil. New Phytol. 2025, 248, 2516–2527. [Google Scholar] [CrossRef]
- Yin, D.; Yang, X.; Wang, H.; Guo, X.; Wang, S.; Wang, Z.; Ding, G.; Yang, G.; Zhang, J.; Jin, L.; et al. Effects of Chemical-Based Fertilizer Replacement with Biochar-Based Fertilizer on Albic Soil Nutrient Content and Maize Yield. Open Life Sci. 2022, 17, 517–528. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, L.; Yang, Y.; Zhang, X.; Wang, C.; Sun, H.; Chen, H.; Huang, J.; Zhou, S. Production and Subsequent Application of Different Biochar-Based Organic Fertilizers to Enhance Vegetable Quality and Soil Carbon Stability. J. Soil Sci. Plant Nutr. 2025, 25, 147–159. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-Based Slow-Release of Fertilizers for Sustainable Agriculture: A Mini Review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-Based Fertilizer Amendments Improve the Soil Microbial Community Structure in a Karst Mountainous Area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Cheng, Z.; Wang, Y.; Li, S.; Clarke, N. Arbuscular Mycorrhizal Fungal Interacted with Biochar and Enhanced Phosphate-Solubilizing Microorganism Abundance and Phosphorus Uptake in Maize. Agronomy 2024, 14, 1678. [Google Scholar] [CrossRef]
- Meng, L.; Cheng, Z.; Li, S. Response of Soil Nitrogen-Cycling Genes to the Coupling Effects of Arbuscular Mycorrhizal Fungi Inoculation and Biochar Application in Maize Rhizosphere. Sustainability 2024, 16, 3349. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, P.; Chen, Y.; Liu, J.; Wang, X. The Pathway of Arbuscular Mycorrhizal Fungi and Biochar in Regulating Carbon and Nitrogen Metabolism of Ryegrass Under Salt Stress. Period. Ocean. Univ. China 2023, 53, 121–130. [Google Scholar]
- Li, J.; Xu, Z.; Yang, T.; Zhang, J.; Zuo, Y.; Cheng, L. Rhizosphere Ecological Restoration: Interactions between Nutrient Mobilization, Core Microbial Assembly, and Phenylalanine Metabolism Circulation. Biochar 2025, 7, 64. [Google Scholar] [CrossRef]
- Li, Z.; Lin, K.; Wang, Y.; Zhai, Y.; Wang, B.; Ping, M.; Meng, Y.; Luo, W.; Chen, J.; Li, X. Synergistic Superiority of AMF and Biochar in Enhancing Rhizosphere Microbiomes to Support Plant Growth under Cd Stress. Biochar 2025, 7, 105. [Google Scholar] [CrossRef]
- Hiiesalu, I.; Pärtel, M.; Davison, J.; Gerhold, P.; Metsis, M.; Moora, M.; Öpik, M.; Vasar, M.; Zobel, M.; Wilson, S.D. Species Richness of Arbuscular Mycorrhizal Fungi: Associations with Grassland Plant Richness and Biomass. New Phytol. 2014, 203, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Li, L.; Miranda, J.; Tang, Y.; Song, B.; Oosthuizen, M.K.; Wei, W. Experimental Duration Determines the Effect of Arbuscular Mycorrhizal Fungi on Plant Biomass in Pot Experiments: A Meta-Analysis. Front. Plant Sci. 2022, 13, 1024874. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Yang, X.; Wang, B.; Gu, W.; Wang, Y. Effects of Carbon-Based Fertilizer on Maize Root Morphology, Root Bleeding Rate and Components in Northeast China. Agronomy 2023, 13, 814. [Google Scholar] [CrossRef]
- Li, M.; Cai, L. Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus. Sustainability 2021, 13, 3244. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of Arbuscular Mycorrhizal Fungi and Ecosystem Function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct Evidence for Modulation of Photosynthesis by an Arbuscular Mycorrhiza-Induced Carbon Sink Strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef]
- Monreal, M.A.; Grant, C.A.; Irvine, R.B.; Mohr, R.M.; McLaren, D.L.; Khakbazan, M. Crop Management Effect on Arbuscular Mycorrhizae and Root Growth of Flax. Can. J. Plant Sci. 2011, 91, 315–324. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Zhou, J.; Rengel, Z.; George, T.S.; Feng, G. Exploring the Secrets of Hyphosphere of Arbuscular Mycorrhizal Fungi: Processes and Ecological Functions. Plant Soil 2022, 481, 1–22. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Sun, Y.; Yang, S.; Qin, Q.; Xue, Y. Long-Term Biochar-Based Fertilizer Substitution Promotes Carbon, Nitrogen, and Phosphorus Acquisition Enzymes in Dryland Soils by Affecting Soil Properties and Regulating Bacterial Community. Appl. Soil Ecol. 2025, 206, 105801. [Google Scholar] [CrossRef]
- Huang, D.; Liu, N.; Guan, C.; Zhang, J.; Wang, R.; Chen, Q.; Tian, F.; Liu, X.; Wu, Z. Multi Level Synergistic Regulation of Phosphorus Slow-Release Performance through LDH Modified Biochar-Based Slow-Release Fertilizer. Environ. Res. 2025, 285, 122602. [Google Scholar] [CrossRef]
- Grafe, M.; Goers, M.; Von Tucher, S.; Baum, C.; Zimmer, D.; Leinweber, P.; Vestergaard, G.; Kublik, S.; Schloter, M.; Schulz, S. Bacterial Potentials for Uptake, Solubilization and Mineralization of Extracellular Phosphorus in Agricultural Soils Are Highly Stable under Different Fertilization Regimes. Environ. Microbiol. Rep. 2018, 10, 320–327. [Google Scholar] [CrossRef]
- Agnolucci, M.; Battini, F.; Cristani, C.; Giovannetti, M. Diverse Bacterial Communities Are Recruited on Spores of Different Arbuscular Mycorrhizal Fungal Isolates. Biol. Fertil. Soils 2015, 51, 379–389. [Google Scholar] [CrossRef]
- Tesfaye, F.; Liu, X.; Zheng, J.; Cheng, K.; Bian, R.; Zhang, X.; Li, L.; Drosos, M.; Joseph, S.; Pan, G. Could Biochar Amendment Be a Tool to Improve Soil Availability and Plant Uptake of Phosphorus? A Meta-Analysis of Published Experiments. Environ. Sci. Pollut. Res. 2021, 28, 34108–34120. [Google Scholar] [CrossRef] [PubMed]
- Valani, G.P.; Martíni, A.F.; Da Silva, L.F.S.; Bovi, R.C.; Cooper, M. Soil Quality Assessments in Integrated Crop–Livestock–Forest Systems: A Review. Soil Use Manag. 2021, 37, 22–36. [Google Scholar] [CrossRef]
- Jeske, E.S.; Tian, H.; Hanford, K.; Walters, D.T.; Drijber, R.A. Long-Term Nitrogen Fertilization Reduces Extraradical Biomass of Arbuscular Mycorrhizae in a Maize (Zea mays L.) Cropping System. Agric. Ecosyst. Environ. 2018, 255, 111–118. [Google Scholar] [CrossRef]
- Menge, D.N.L.; Hedin, L.O.; Pacala, S.W. Nitrogen and Phosphorus Limitation over Long-Term Ecosystem Development in Terrestrial Ecosystems. PLoS ONE 2012, 7, e42045. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, P.; Yang, L.; Wei, B.; Han, S.; Wu, M.; He, X.; Zeng, W.; He, Z.; Xiao, J.; et al. Effects of Biochar-Based Fertilizers on Fenlong-Ridging Soil Physical Properties, Nutrient Activation, Enzyme Activity, Bacterial Diversity, and Sugarcane Yield. Agronomy 2025, 15, 1594. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The Potential of Biochar as a Microbial Carrier for Agricultural and Environmental Applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of Biochar from Slow Pyrolysis of Papermill Waste on Agronomic Performance and Soil Fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Yang, F.; Sui, L.; Tang, C.; Li, J.; Cheng, K.; Xue, Q. Sustainable Advances on Phosphorus Utilization in Soil via Addition of Biochar and Humic Substances. Sci. Total Environ. 2021, 768, 145106. [Google Scholar] [CrossRef]
- Harun, N.S.N.; Jaafar, N.M.; Sakimin, S.Z. The Effects of Rice Husk Biochar Rate on Arbuscular Mycorrhizal Fungi and Growth of Soursop (Annona muricata L.) Seedlings. Sustainability 2021, 13, 1817. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, Z.; Akhtar, M.A.; Niu, W.; Ren, L.; Zhang, S.; Liu, N.; Cao, H. Synergistic Effects of N-Containing Heterocyclic and Ca Ligand Structures on the Phosphorus Adsorption of N/Ca Co-Doped Biochar. J. Clean. Prod. 2024, 485, 144392. [Google Scholar] [CrossRef]
- Cao, M.-A.; Liu, R.-C.; Xiao, Z.-Y.; Hashem, A.; Abd_Allah, E.F.; Alsayed, M.F.; Harsonowati, W.; Wu, Q.-S. Symbiotic Fungi Alter the Acquisition of Phosphorus in Camellia Oleifera through Regulating Root Architecture, Plant Phosphate Transporter Gene Expressions and Soil Phosphatase Activities. J. Fungi 2022, 8, 800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Li, G.; Qin, P. Interactions between Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Fungus (Mortierella sp.) and Their Effects on Kostelelzkya Virginica Growth and Enzyme Activities of Rhizosphere and Bulk Soils at Different Salinities. Biol. Fertil. Soils 2011, 47, 543. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Wang, J.; Li, Y.; Lou, Y.; Zhuge, Y.; Dong, Y. Soil Available Nitrogen and Yield Effect under Different Combinations of Urease/Nitrate Inhibitor in Wheat/Maize Rotation System. Agronomy 2022, 12, 1888. [Google Scholar] [CrossRef]
- Higo, M.; Tatewaki, Y.; Gunji, K.; Kaseda, A.; Isobe, K. Cover Cropping Can Be a Stronger Determinant than Host Crop Identity for Arbuscular Mycorrhizal Fungal Communities Colonizing Maize and Soybean. PeerJ 2019, 7, e6403. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Azimov, A.; Tyagi, S.; Pengani, K.R.; Sharma, P.; Vikram, K.V.; Poczai, P.; Nasif, O.; Ansari, M.J.; et al. Co-Inoculation of Biochar and Arbuscular Mycorrhizae for Growth Promotion and Nutrient Fortification in Soybean under Drought Conditions. Front. Plant Sci. 2022, 13, 947547. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Meng, L.; Liu, X.; Zhang, Y.; Zhao, S.; Zhao, H. Root Exudation under Maize/Soybean Intercropping System Mediates the Arbuscular Mycorrhizal Fungi Diversity and Improves the Plant Growth. Front. Plant Sci. 2024, 15, 1375194. [Google Scholar] [CrossRef]
- Wang, K.; Ying, S. Better Performance of Organic Fertilizer on Improving Yield and Reducing Nitrogen Losses in a Paddy Field as Compared to Biochar-Based Fertilizer. Water Air Soil Pollut. 2025, 236, 362. [Google Scholar] [CrossRef]



| Treatments | Plant Height (cm) | Bottom Pod Height (cm) | Pod Number of per Plant | Grain Number of per Plant | Seed Weight per Plant (g) | 100-Seed Weight (g) | Yield (kg·ha−1) |
|---|---|---|---|---|---|---|---|
| CK | 116.00 ± 1.9 c | 15.43 ± 1.40 c | 59.20 ± 1.5 c | 121.00 ± 6.24 d | 24.60 ± 1.45 c | 19.54 ± 0.71 b | 2468.58 ± 184.98 d |
| CF | 122.11 ± 2.7 b | 20.55 ± 0.84 a | 64.47 ± 5.7 bc | 138.67 ± 9.45 c | 27.56 ± 1.41 bc | 20.63 ± 0.95 ab | 3164.06 ± 206.03 bc |
| BF | 123.67 ± 3.5 ab | 18.66 ± 1.05 ab | 68.20 ± 4.3 b | 142.67 ± 10.12 bc | 29.04 ± 1.71 b | 20.41 ± 0.75 ab | 3239.35 ± 106.64 b |
| AM + CK | 119.56 ± 2.1 bc | 17.10 ± 0.51 bc | 59.13 ± 3.2 c | 129.00 ± 9.00 cd | 26.42 ± 2.56 bc | 20.21 ± 0.56 ab | 2891.16 ± 146.62 cd |
| AM + CF | 123.78 ± 4.0 ab | 18.77 ± 1.84 ab | 72.53 ± 4.1 b | 156.33 ± 9.02 ab | 32.95 ± 0.95 a | 20.86 ± 0.56 ab | 3622.58 ± 172.81 a |
| AM + BF | 128.22 ± 2.3 a | 18.27 ± 1.13 ab | 82.07 ± 4.4 a | 162.67 ± 11.59 a | 35.14 ± 8.24 a | 21.52 ± 0.63 a | 3869.28 ± 55.76 a |
| ANOVA | |||||||
| A | ** | ns | ** | ** | ** | ns | *** |
| F | ** | ns | ** | ** | ** | * | *** |
| A × F | ns | ns | * | ns | * | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Yang, H.; Fan, Y.; Li, J.; Song, D.; Ma, X.; Li, S. Synergy of Arbuscular Mycorrhizal Fungi and Biochar-Based Fertilizer Reshapes Soybean Nutrient Acquisition and Drives Yield Enhancement. Sustainability 2025, 17, 10355. https://doi.org/10.3390/su172210355
Meng L, Yang H, Fan Y, Li J, Song D, Ma X, Li S. Synergy of Arbuscular Mycorrhizal Fungi and Biochar-Based Fertilizer Reshapes Soybean Nutrient Acquisition and Drives Yield Enhancement. Sustainability. 2025; 17(22):10355. https://doi.org/10.3390/su172210355
Chicago/Turabian StyleMeng, Lingbo, Huawei Yang, Yue Fan, Jiang Li, Diwei Song, Xiaozhe Ma, and Shumin Li. 2025. "Synergy of Arbuscular Mycorrhizal Fungi and Biochar-Based Fertilizer Reshapes Soybean Nutrient Acquisition and Drives Yield Enhancement" Sustainability 17, no. 22: 10355. https://doi.org/10.3390/su172210355
APA StyleMeng, L., Yang, H., Fan, Y., Li, J., Song, D., Ma, X., & Li, S. (2025). Synergy of Arbuscular Mycorrhizal Fungi and Biochar-Based Fertilizer Reshapes Soybean Nutrient Acquisition and Drives Yield Enhancement. Sustainability, 17(22), 10355. https://doi.org/10.3390/su172210355





