Life Cycle Assessment of Greenhouse Gas Emissions in Hydrogen Production via High-Calorific Mixed Waste Gasification
Abstract
1. Introduction
2. Methods
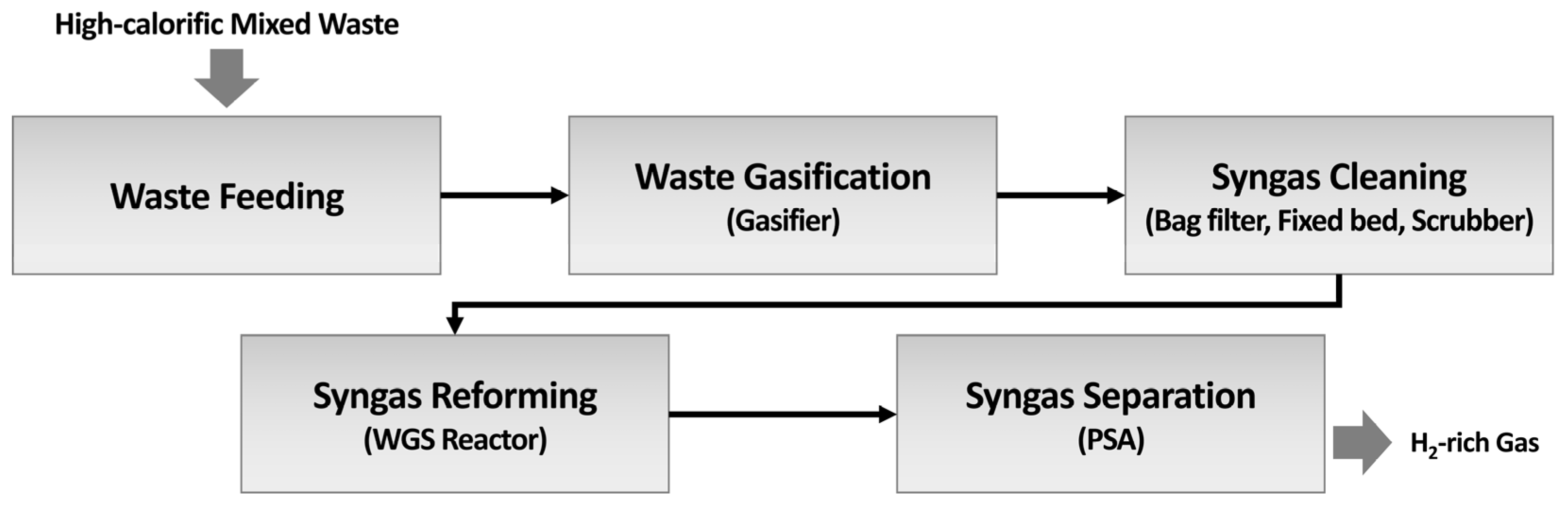
2.1. System Description: Gasification Pilot Plant and Process
2.1.1. Waste Feeding
2.1.2. Gasification
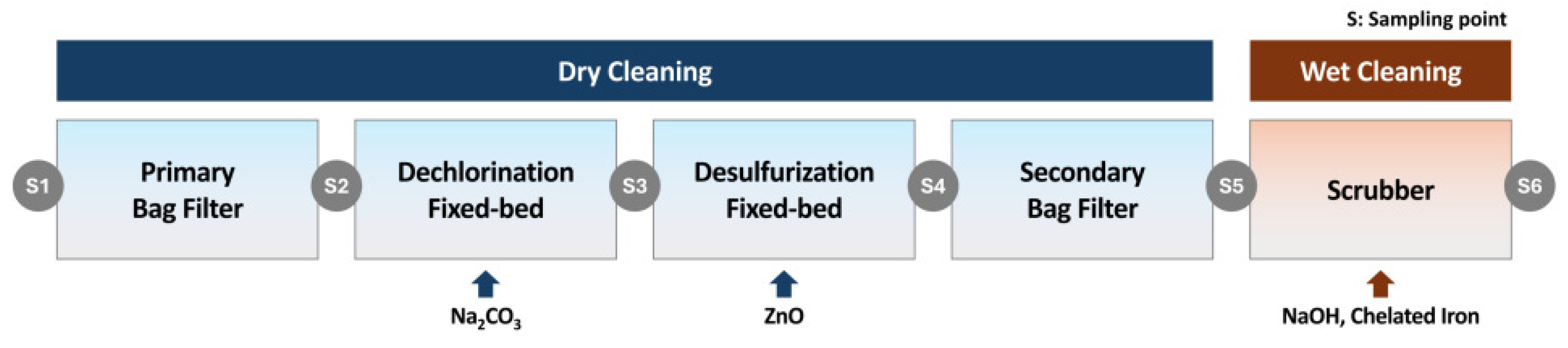
2.1.3. Syngas Cleaning
2.1.4. Syngas Reforming
2.1.5. Hydrogen Separation
2.2. Life Cycle Assessment
2.2.1. Goal and Scope Definition
2.2.2. Inventory Data Acquisition and Impact Assessment
3. Results
3.1. Performance of the Gasification Plant
3.2. Gasification Plant LCA
3.2.1. Life Cycle Inventory
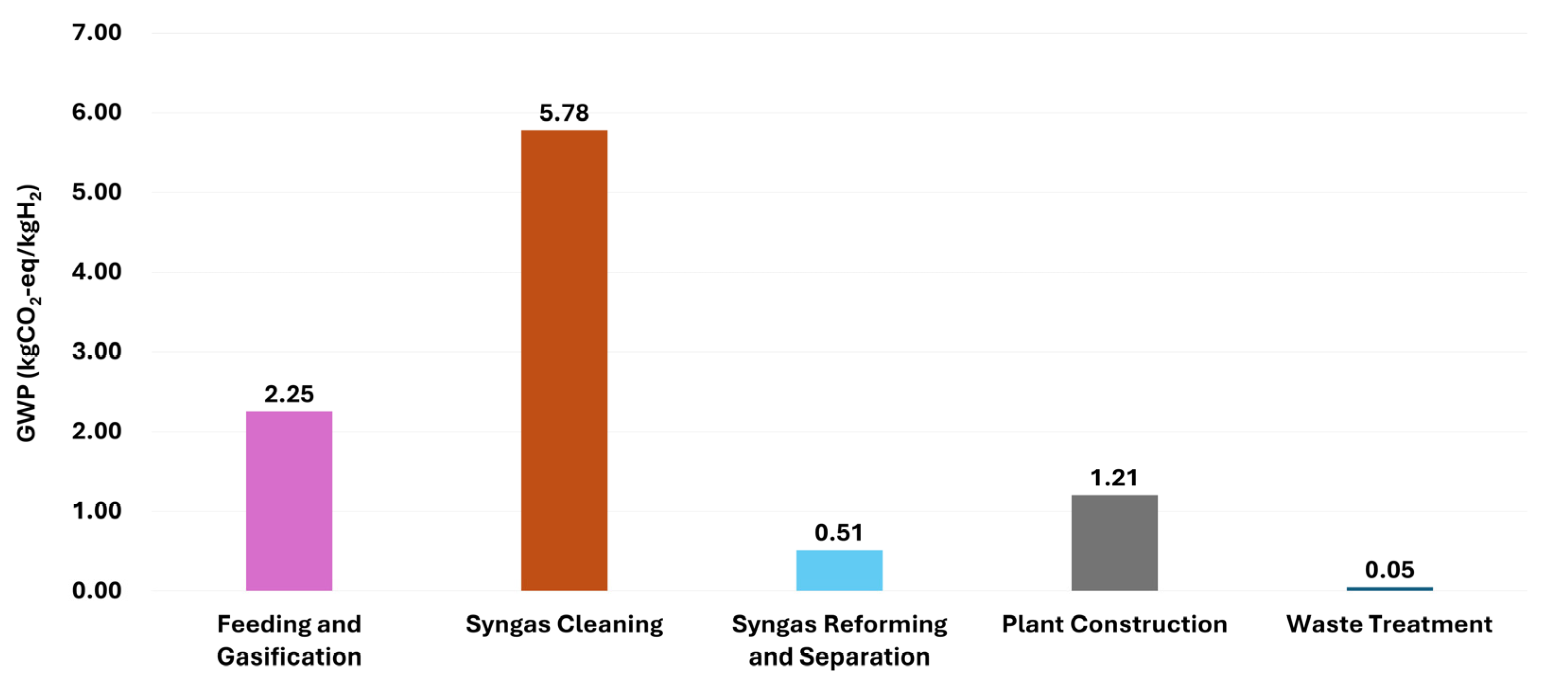
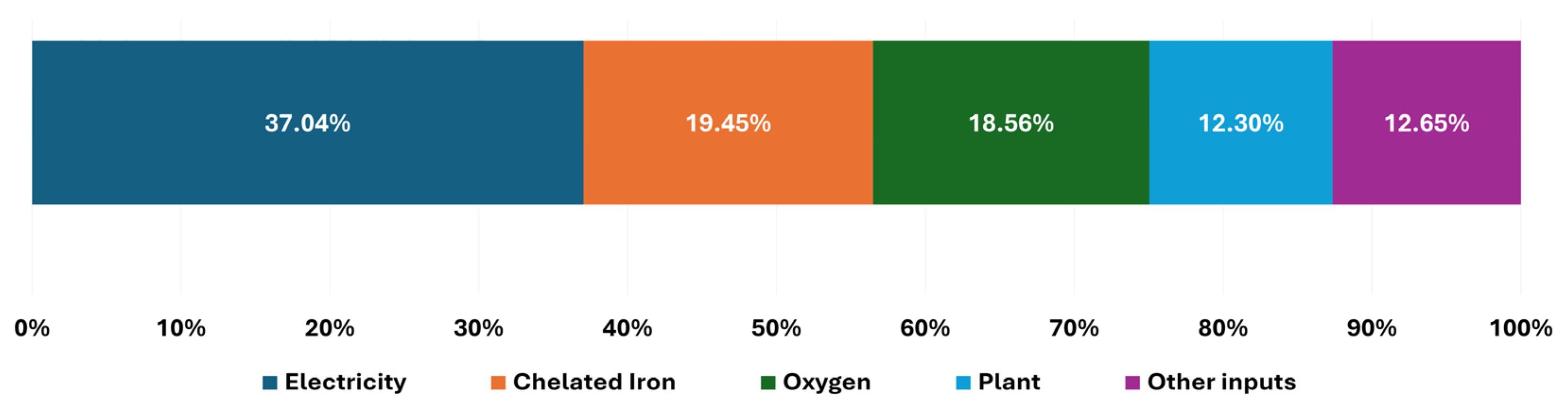
3.2.2. Life Cycle GHG Emissions
3.3. Scenario and Sensitivity Analysis
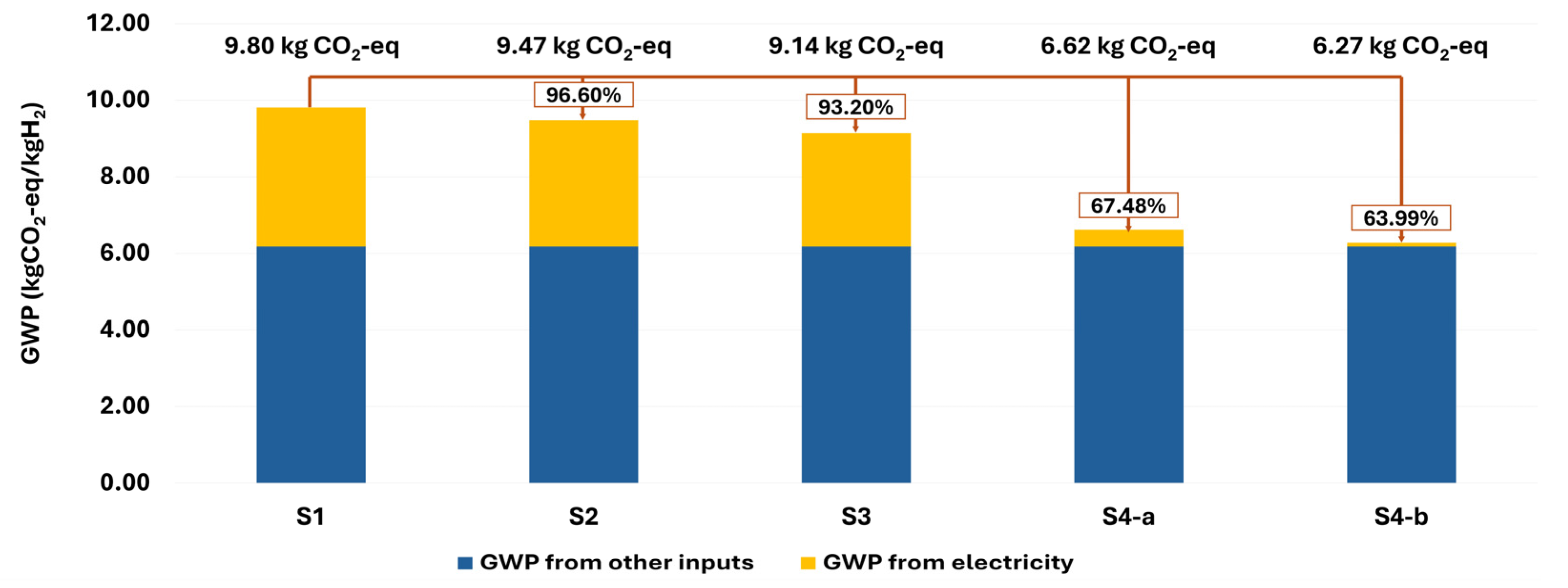
3.3.1. Improvement Scenarios: Impact of Alternative Electricity Supplies
3.3.2. Sensitivity Analysis: Effect of Operational Lifetime
4. Discussion
4.1. Interpretation of Environmental Hotspots
4.2. Comparative Analysis and Regional Context
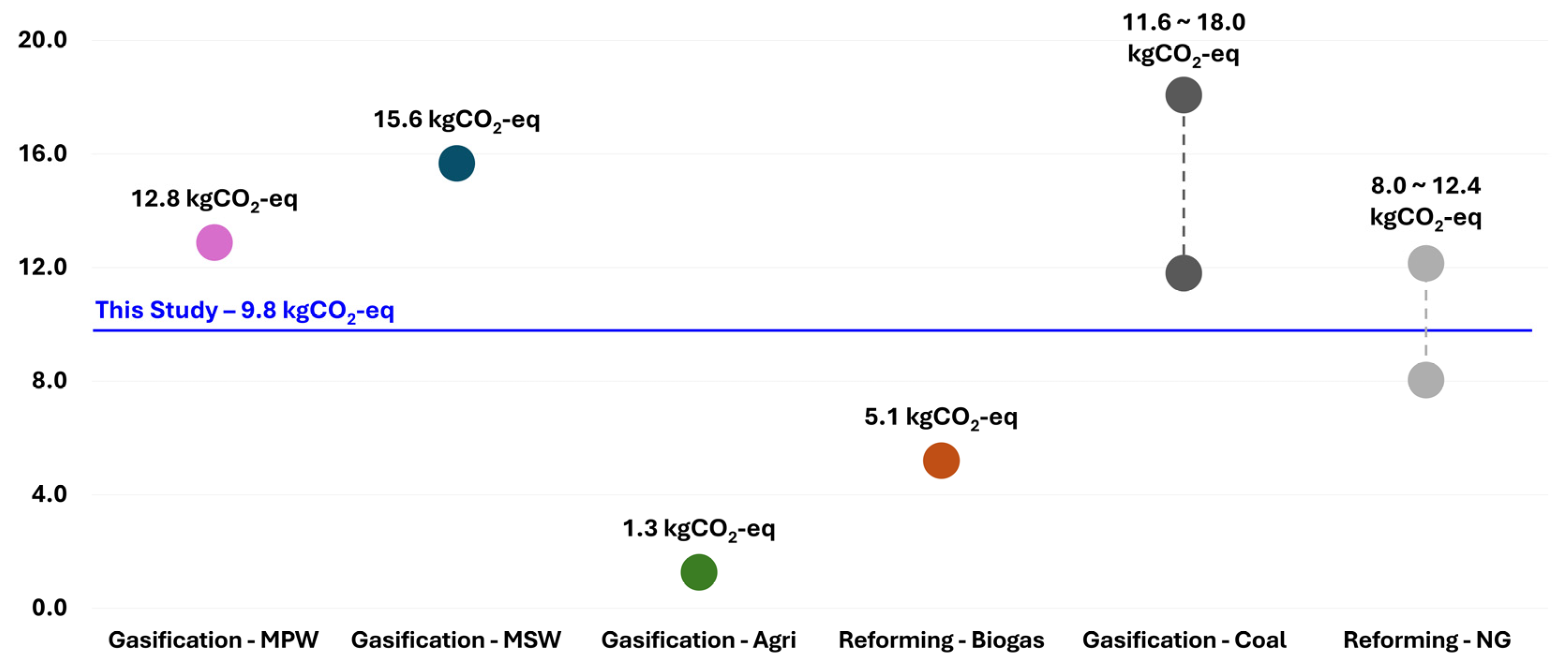
| Category | Main Process | Feedstock | GWP | Ref |
|---|---|---|---|---|
| Waste | Gasification | High-calorific mixed waste | 9.8 | Our Study |
| Gasification | Mixed plastic waste | 12.8 | [50] | |
| Gasification | Municipal solid waste | 15.6 | [50] | |
| Gasification | Agricultural Residue | 1.3 | [51] | |
| Reforming | Biogas from Organic waste | 5.1 | [51] | |
| Fossil Fuel | Gasification | Coal | 11.6–18.0 | [47,48] |
| Reforming | Natural Gas | 8.0–12.4 | [47,48] |
4.3. Methodological Limitations and Uncertainties
4.4. Scientific Contribution and International Transferability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, R. Transition to a hydrogen-based economy: Possibilities and challenges. Sustainability 2014, 14, 15975. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Global Hydrogen Review 2024; IEA: Paris, France, 2024. [Google Scholar]
- Soltani, R.; Rosen, M.A.; Dincer, I. Assessment of CO2 capture options from various points in steam methane reforming for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 20266–20275. [Google Scholar] [CrossRef]
- Soni, A.; Gupta, S.K.; Rajamohan, N.; Yusuf, M. Waste-to-energy technologies: A sustainable pathway for resource recovery and materials management. Mater. Adv. 2025, 6, 4598–4622. [Google Scholar] [CrossRef]
- Javed, M.H.; Ahmad, A.; Rehan, M.; Musharavati, F.; Nizami, A.S.; Khan, M.I. Advancing Sustainable Energy: Environmental and Economic Assessment of Plastic Waste Gasification for Syngas and Electricity Generation Using Life Cycle Modeling. Sustainability 2025, 17, 1277. [Google Scholar] [CrossRef]
- Maitlo, G.; Ali, I.; Mangi, K.H.; Ali, S.; Maitlo, H.A.; Unar, I.N.; Pirzada, A.M. Thermochemical conversion of biomass for syngas production: Current status and future trends. Sustainability 2022, 14, 2596. [Google Scholar] [CrossRef]
- Kun, U.H.; Ksepko, E. Advancing Municipal Solid Waste Management Through Gasification Technology. Processes 2025, 13, 2000. [Google Scholar] [CrossRef]
- Pini, M.; Nocentini, A.; Chiaramonti, D. A Circular Economy Approach to the Gasification of Municipal Solid Waste and Other Biogenic Residues: State of the Art, Challenges, and Perspectives in Europe. Sustainability 2025, 17, 6704. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Life cycle assessment of hydrogen production from biomass gasification systems. Int. J. Hydrogen Energy 2012, 37, 14026–14039. [Google Scholar] [CrossRef]
- Muresan, M.; Cormos, C.C.; Agachi, P.S. Comparative life cycle analysis for gasification-based hydrogen production systems. J. Renew. Sustain. Energy 2014, 6, 013131. [Google Scholar] [CrossRef]
- Hajjaji, N.; Martinez, S.; Trably, E.; Steyer, J.P.; Helias, A. Life cycle assessment of hydrogen production from biogas reforming. Int. J. Hydrogen Energy 2016, 41, 6064–6075. [Google Scholar] [CrossRef]
- Chari, S.; Sebastiani, A.; Paulillo, A.; Materazzi, M. The environmental performance of mixed plastic waste gasification with carbon capture and storage to produce hydrogen in the UK. ACS Sustain. Chem. Eng. 2023, 11, 3248–3259. [Google Scholar] [CrossRef]
- Tavares Borges, P.; Silva Lora, E.E.; Venturini, O.J.; Errera, M.R.; Yepes Maya, D.M.; Makarfi Isa, Y.; Alexander, K.; Zhang, S. A comprehensive technical, environmental, economic, and bibliometric assessment of hydrogen production through biomass gasification, including global and brazilian potentials. Sustainability 2024, 16, 9213. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Longo, S.; Cellura, M.; Miccichè, G.; Ferraro, M. Critical review of life cycle assessment of hydrogen production pathways. Environments 2024, 11, 108. [Google Scholar] [CrossRef]
- Al-Moftah, A.M.S.; Marsh, R.; Steer, J. Life cycle assessment of solid recovered fuel gasification in the state of Qatar. Chem. Eng. 2021, 5, 81. [Google Scholar] [CrossRef]
- Dennis, G.; Brian, L.C. Scaleup Issues from Bench to Pilot. In Proceedings of the 2009 AIChE Annual Meeting, Nashville, TN, USA, 12 November 2009. [Google Scholar]
- Rosyadi, A.A.; Lim, O.A. Comparative Environmental Life Cycle Assessment (LCA) Study of Hydrogen Fuel Production from Different Waste Feedstock in South Korea. Int. J. Automot. Technol. 2025, 26, 1839–1856. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, S.K. A review on recent gasification methods for biomethane gas production. Int. J. Energy Eng. 2016, 6, 32–43. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Salah, C.; Cobo, S.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Environmental sustainability assessment of hydrogen from waste polymers. ACS Sustain. Chem. Eng. 2023, 11, 3238–3247. [Google Scholar] [CrossRef]
- Leuter, P.; Fendt, S.; Spliethoff, H. Requirements on synthesis gas from gasification for material and energy utilization: A mini review. Front. Energy Res. 2024, 12, 1382377. [Google Scholar] [CrossRef]
- Jayanarasimhan, A.; Pathak, R.M.; Shivapuji, A.M.; Rao, L. Tar formation in gasification systems: A holistic review of remediation approaches and removal methods. ACS Omega 2024, 9, 2060–2079. [Google Scholar] [CrossRef]
- Baek, J.I.; Eom, T.H.; Lee, J.B.; Jegarl, S.; Ryu, C.K.; Park, Y.C.; Jo, S.H. Cleaning of gaseous hydrogen chloride in a syngas by spray-dried potassium-based solid sorbents. Korean J. Chem. Eng. 2015, 32, 845–851. [Google Scholar] [CrossRef]
- Marcantonio, V.; Müller, M.; Bocci, E. A review of hot gas cleaning techniques for hydrogen chloride removal from biomass-derived syngas. Energies 2012, 14, 6519. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chen, Y.Y.; Wei, W.C.J. Reforming and Desulphurization of Syngas by 3D-printed Catalyst Carriers. Innov. Ener Res. 2017, 6, 1000172. [Google Scholar] [CrossRef]
- Shibamoto, T.; Yasuhara, A.; Katami, T. Dioxin formation from waste incineration. Rev. Environ. Contam. Toxicol. 2007, 190, 1–41. [Google Scholar] [CrossRef]
- Miao, X.; Ma, Y.; Chen, Z.; Gong, H. Oxidative degradation stability and hydrogen sulfide removal performance of dual-ligand iron chelate of Fe-EDTA/CA. Environ. Technol. 2018, 39, 3006–3012. [Google Scholar] [CrossRef]
- Eddi, I.; Chibane, L. Parametric study of high temperature water gas shift reaction for hydrogen production in an adiabatic packed bed membrane reactor. Rev. Roum. Chim. 2020, 65, 149–164. [Google Scholar] [CrossRef]
- Reddy, G.K.; Kim, S.J.; Dong, J.; Smirniotis, P.G.; Jasinski, J.B. Long-term WGS stability of Fe/Ce and Fe/Ce/Cr catalysts at high and low steam to CO ratios—XPS and Mössbauer spectroscopic study. Appl. Catal. A Gen. 2012, 415, 101–110. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kim, K.J.; Jang, W.J.; Shim, J.O.; Jeon, K.W.; Na, H.S.; Kim, M.K.; Bae, J.W.; Nam, S.C.; Jeon, B.H.; et al. Increase in stability of BaCo/CeO2 catalyst by optimizing the loading amount of Ba promoter for high-temperature water-gas shift reaction using waste-derived synthesis gas. Renew. Energy 2020, 145, 2715–2722. [Google Scholar] [CrossRef]
- Krótki, A.; Bigda, J.; Spietz, T.; Ignasiak, K.; Matusiak, P.; Kowol, D. Performance Evaluation of Pressure Swing Adsorption for Hydrogen Separation from Syngas and Water–Gas Shift Syngas. Energies 2025, 18, 1887. [Google Scholar] [CrossRef]
- Ahn, S.; You, Y.W.; Lee, D.G.; Kim, K.H.; Oh, M.; Lee, C.H. Layered two-and four-bed PSA processes for H2 recovery from coal gas. Chem. Eng. Sci. 2012, 68, 413–423. [Google Scholar] [CrossRef]
- Moon, D.K.; Lee, D.G.; Lee, C.H. H2 pressure swing adsorption for high pressure syngas from an integrated gasification combined cycle with a carbon capture process. Appl. Energy 2016, 183, 760–774. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management-Life Cycle Assessment-Principles and Framework 2006. ISO: Geneva, Switzerland, 2006.
- ISO 14044; Environmental management-Life cycle assessment-Requirements and guidelines 2006. ISO: Geneva, Switzerland, 2006.
- Lijó, L.; González-García, S.; Bacenetti, J.; Fiala, M.; Feijoo, G.; Lema, J.M.; Moreira, M.T. Life Cycle Assessment of electricity production in Italy from anaerobic co-digestion of pig slurry and energy crops. Renew. Energy 2014, 68, 625–635. [Google Scholar] [CrossRef]
- School of Chemical, Biological and Materials Engineering. Municipal Solid Waste to Chemical Products: Supplemental Narrative; University of Oklahoma: Norman, OK, USA, 2018. [Google Scholar]
- National Energy Technology Laboratory. Syngas Composition. In Gasifipedia; U.S. Department of Energy: Morgantown, WV, USA, 2019. [Google Scholar]
- Korea Gas Corporation (KOGAS). 5th Basic Plan for New and Renewable Energy Technology Development, Utilization, and Supply; KOGAS: Incheon, Republic of Korea, 2025. [Google Scholar]
- Niu, F.; Wu, Z.; Chen, D.; Huang, Y.; Ordomsky, V.V.; Khodakov, A.Y.; Van Geem, K.M. State-of-the-art and perspectives of hydrogen generation from waste plastics. Chem. Soc. Rev. 2025, 54, 4948–4972. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Simulation of syngas production from municipal solid waste gasification in a bubbling fluidized bed using aspen plus. Ind. Eng. Chem. Res. 2013, 52, 14768–14775. [Google Scholar] [CrossRef]
- Kaszuba, M.; Ziółkowski, P.; Mikielewicz, D. Comparative study of oxygen separation using cryogenic and membrane techniques for nCO2PP. In Proceedings of the 36th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems (ECOS 2023), Las Palmas de Gran Canaria, Spain, 25–30 June 2023. [Google Scholar]
- Tolley, J.; Estupinan, L. Energy Consumption of Pressure Swing Adsorption vs. Vacuum Swing Adsorption—A Thermodynamic Study; Benchmark Oxygen Solutions: Alberta, Canada, 2022. [Google Scholar]
- Sun, M.; Song, W.; Zhai, L.F.; Cui, Y.Z. Effective sulfur and energy recovery from hydrogen sulfide through incorporating an air-cathode fuel cell into chelated-iron process. J. Hazard. Mater. 2013, 263, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Christopher, L.W.; Paulina, J.; Joe, M.; Constantine, S. Life Cycle Assessment and Grid Electricity: What Do We Know and What Can We Know? ACS Environ. Sci. Technol. 2010, 44, 1895–1901. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen production via steam reforming: A critical analysis of MR and RMM technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef]
- Elgowainy, A.; Vyawahare, P.; Ng, C.; Frank, E.D.; Bafana, A.; Burnham, A.; Sun, P.; Cai, H.; Lee, U.; Reddi, K.; et al. Environmental life-cycle analysis of hydrogen technology pathways in the United States. Front. Energy Res. 2024, 12, 1473383. [Google Scholar] [CrossRef]
- Verma, A. Life Cycle Assessment and Greenhouse Gas Abatement Costs of Hydrogen Production from Underground Coal Gasification. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2014. [Google Scholar]
- Korea Gas Corporation (KOGAS). Global LNG Outlook 2025; KOGAS: Incheon, Korea, 2025. [Google Scholar]
- Afzal, S.; Singh, A.; Nicholson, S.R.; Uekert, T.; DesVeaux, J.S.; Tan, E.C.; Dutta, A.; Carpenter, A.C.; Baldwin, R.M.; Beckham, G.T. Techno-economic analysis and life cycle assessment of mixed plastic waste gasification for production of methanol and hydrogen. Green Chem. 2023, 25, 5068–5085. [Google Scholar] [CrossRef]
- Abawalo, M.; Pikoń, K.; Landrat, M. Comparative Life Cycle Assessment of Hydrogen Production via Biogas Reforming and Agricultural Residue Gasification. Appl. Sci. 2025, 15, 5029. [Google Scholar] [CrossRef]


| RPF Manufacturing Residue | ASR | Incineration Feed Waste | |
|---|---|---|---|
| C (wt.%) | 51.8 | 52.8 | 39.6 |
| H (wt.%) | 7.8 | 7.0 | 5.3 |
| N (wt.%) | 1.1 | 15.8 | 1.5 |
| S (wt.%) | 0.4 | 0.7 | 0.2 |
| Cl (wt.%) | 0.4 | 1.4 | 0.5 |
| O (wt.%) | 18.6 | 5.0 | 18.0 |
| Moisture (wt.%) | 8.5 | 1.2 | 7.5 |
| Ash (wt.%) | 11.4 | 16.1 | 27.4 |
| LHV (kcal/kg) | 5735 | 5162 | 4143 |
| Reactions | Equation | ΔH (MJ/kmol) |
|---|---|---|
| Combustion reaction | C + 1/2O2 = CO | −111 |
| CO + 1/2O2 = CO2 | −283 | |
| H2 + 1/2O2 = H2O | −242 | |
| Boudouard reaction | C + CO2 = 2CO | 172 |
| Water-gas reaction | C + H2O ↔ H2 + CO | 131 |
| Water-gas shift reaction | CO + H2O ↔ H2 + CO2 | −47 |
| Methanation reaction | C + 2H2 ↔ CH4 | −75 |
| Category | Item | Value | Unit |
|---|---|---|---|
| Input | |||
| Feeding and gasification | Mixed waste | 28.8 | kg |
| Oxygen | 23.8 | Nm3 | |
| Electricity | 0.630 | kWh | |
| Cleaning | Adsorbent (ZnO) | 0.579 | kg |
| Adsorbent (Na2CO3) | 93.8 | g | |
| Chelated Iron | 2.68 | kg | |
| NaOH | 0.479 | kg | |
| Water | 49.9 | kg | |
| Electricity | 3.91 | kWh | |
| Reforming and separation | Catalyst (Fe-Al-Cu) | 8.83 | g |
| Steam | 20.6 | kg | |
| Electricity | 0.721 | kWh | |
| Plant construction | Aluminum | 487 | mg |
| Concrete | 0.00104 | m3 | |
| Copper | 761 | mg | |
| Reinforcing steel | 2.28 | kg | |
| Steel (low alloyed) | 0.114 | kg | |
| Diesel | 0.00381 | MJ | |
| Electricity | 2.28 | kWh | |
| Heat | 0.127 | MJ | |
| Output | |||
| Product | Hydrogen | 1.00 | kg |
| Waste treatment | Wastewater | 50.4 | kg |
| Solid residue | 3.28 | kg | |
| After Gasifier | After WGS | After PSA | |
|---|---|---|---|
| H2 (vol%) | 24.78 | 38.93 | 99.99 |
| CO (vol%) | 38.78 | 12.66 | <0.001 |
| CO2 (vol%) | 33.40 | 45.93 | <0.001 |
| CH4 (vol%) | 1.55 | 1.26 | <0.001 |
| CXHY (vol%) | 1.50 | 1.22 | <0.001 |
| S1 | S2 | S3 | S4 | S5 | S6 | |
|---|---|---|---|---|---|---|
| Dust (mg/Nm3) | 36,922.66 | 4351.21 | - | - | 7.20 | <0.001 |
| HCl (ppm) | 170.21 | - | 21.99 | ND | ND | ND |
| H2S (ppm) | 1050.27 | - | 822.75 | 0.23 | 0.05 | ND |
| Category | Item | GWP (kgCO2-eq) |
|---|---|---|
| Input | ||
| Feeding and gasification | Oxygen | 1.82 × 100 |
| Electricity | 4.35 × 10−1 | |
| Cleaning | Adsorbent (ZnO) | 4.17 × 10−1 |
| Adsorbent (Na2CO3) | 3.99 × 10−2 | |
| Chelated Iron | 1.91 × 100 | |
| NaOH | 6.96 × 10−1 | |
| Water | 2.06 × 10−2 | |
| Electricity | 2.70 × 100 | |
| Reforming and separation | Catalyst (Fe-Al-Cu) | 1.76 × 10−2 |
| Electricity | 4.97 × 10−1 | |
| Plant construction | Construction | 1.21 × 100 |
| Output | ||
| Product | Hydrogen | 1.00 |
| Waste treatment | Wastewater | 2.35 × 10−2 |
| Solid residue | 2.55 × 10−2 | |
| Scenario | Electricity Mix Description | GWP from Electricity | Total GWP | GWP Reduction |
|---|---|---|---|---|
| S1 (Baseline) | Current South Korean Grid Mix | 3.63 kg CO2-eq | 9.80 kg CO2-eq | - |
| S2 (Current RE) | 90% Grid Mix + 10% Renewable Mix | 3.30 kg CO2-eq | 9.47 kg CO2-eq | 3.40% |
| S3 (Policy RE) | 80% Grid Mix + 20% Renewable Mix | 2.96 kg CO2-eq | 9.14 kg CO2-eq | 6.80% |
| S4a (100% Solar) | 100% Solar PV Power | 0.443 kg CO2-eq | 6.62 kg CO2-eq | 32.52% |
| S4b (100% Wind) | 100% Wind Power | 0.101 kg CO2-eq | 6.27 kg CO2-eq | 36.01% |
| 10 Years | 20 Years | 30 Years | 40 Years | |
|---|---|---|---|---|
| Total | 1.10 × 101 | 9.80 × 100 | 9.40 × 100 | 9.20 × 100 |
| Plant Construction | 2.41 × 100 | 1.21 × 100 | 8.04 × 10−1 | 6.03 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Park, Y.; Gu, J.-H. Life Cycle Assessment of Greenhouse Gas Emissions in Hydrogen Production via High-Calorific Mixed Waste Gasification. Sustainability 2025, 17, 10308. https://doi.org/10.3390/su172210308
Kim G, Park Y, Gu J-H. Life Cycle Assessment of Greenhouse Gas Emissions in Hydrogen Production via High-Calorific Mixed Waste Gasification. Sustainability. 2025; 17(22):10308. https://doi.org/10.3390/su172210308
Chicago/Turabian StyleKim, Geonyong, Yeongsu Park, and Jae-Hoi Gu. 2025. "Life Cycle Assessment of Greenhouse Gas Emissions in Hydrogen Production via High-Calorific Mixed Waste Gasification" Sustainability 17, no. 22: 10308. https://doi.org/10.3390/su172210308
APA StyleKim, G., Park, Y., & Gu, J.-H. (2025). Life Cycle Assessment of Greenhouse Gas Emissions in Hydrogen Production via High-Calorific Mixed Waste Gasification. Sustainability, 17(22), 10308. https://doi.org/10.3390/su172210308







