Abstract
With the rapid increase in solid waste generated worldwide, sustainable approaches for the recovery of resources considering environmental protection are required. As one of the emerging strategies in recent years, biochar has shown great potential due to its high carbon stabilization, adjustable porosity and tunability. This review focuses on the critical assessment of the available technologies for biochar upgrading, with a specific objective of biochar physicochemical functionality improvement and critical materials recovery in line with circular economy targets. We systematically review physicochemical activation methodologies, functionalizations and leaching approaches, accounting for their effects on surface area, porosity and functional group chemistry. Particular attention is paid to the dual functionality of upgraded biochar (i) as a catalyst support for thermochemical processes and (ii) as a medium for the recycling of essential nutrients (e.g., phosphorus, potassium, magnesium, calcium). It is evidenced that customized activation can further improve its adsorption and catalytic efficiency as well as promote near-total nutrition extraction. This review positions advanced biochar as an enabling multipurpose technology across sustainable material production, nutrient cycling and waste valorization in the circular bioeconomy.
1. Introduction
The exponential increase in waste production in all sectors has become a major global burden resulting in serious ecological pollution and threats to environmental and public health as it attracts disease organisms [1,2]. Conventional biowaste management options, including landfilling, are less desirable because of their environmental, social, and economic impacts, such as the taking up of valuable land, production of greenhouse gases (GHGs) like methane, and the potential for soil and groundwater contamination. Worldwide production of solid waste is expected to rise from 2.01 billion tons per year to a staggering 3.40 billion tons by 2050, hence the necessity for creative and sustainable waste management solutions [3,4]. In 2023, the global solid waste sector generated an estimated 173.2 million tonnes of CO2eq with around 70% released from open dumping, a practice that is still in use in many low- and middle-income countries. These projections, illustrated in Figure 1, suggest that emissions under a business-as-usual scenario could reach 203.4 Mt CO2eq in 2030 and 289.5 Mt CO2eq in 2050 if there are no changes to existing practices. This underscores the need to develop more sustainable and systemic solutions. The circular economy (CE) provides an alternative path by stressing the prevention of waste, recovery of materials, and closed-loop resource streams flows [5]. CE deployment in the waste sector could bring us an order of magnitude closer to reaching net-zero emissions by 2050. Switching systems from linear to circular waste is not only key to reaching GHG reduction targets, it is also an important improvement in resource efficiency, preventing environmentally degrading discharges of hazardous and other waste streams, with potential benefits in support of the circular and bioeconomy [6].
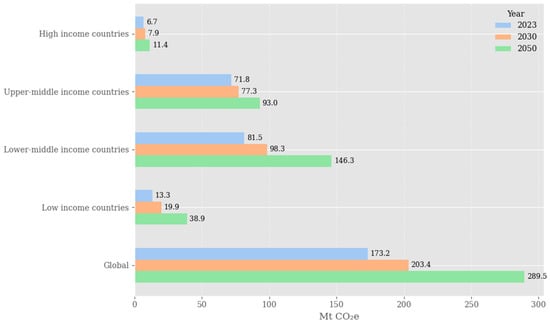
Figure 1.
Estimated global GHG emissions from solid waste management across income groups for the years 2023, 2030, and 2050. Elaborated from: [7].
One promising approach is converting biowaste into biochar through carbonization. This method not only reduces waste, but also recovers raw materials, making it a smart step toward circular- and resource-efficient systems [8]. Resulting biochar can be modified to have high surface area, tunable porosity and rich surface chemistry, which justifies its use in applications including carbon sequestration, CO2 capture, heavy metal immobilization and removal of organic micropollutants from wastewater [9]. Crucially, during biochar upgrading operations it is possible to recover materials, such as phosphorus, potassium, magnesium and calcium, which are considered critical raw materials by the European Union because of their scarcity and increasing demand [10].
Biochar is typically produced through slow pyrolysis of lignocellulosic biomass at temperatures ranging from 400 to 750 °C and in oxygen-limited environments. The derived biochar is a carbon rich solid which contains variable amounts of hydrogen, oxygen, nitrogen, phosphorus, and ash minerals. The processing temperature, residence time, reactor type, and pre-treatment steps can affect the physicochemical characteristics of the produced biochar. These process variables essentially govern the carbonization degree and the development of surface features that characterize biochar’s functioning in diverse applications. Steps like drying, grinding, and activation can further enhance biochar’s performance and functionality [11]. The ash content in biochar is made up of minerals like phosphorus, calcium, aluminum, iron, potassium, and silicon which vary depending on the feedstock sources [12]. Its potential for long-term carbon storage is due to the high proportion (often over 87%) of the stable and resilient carbon it contains [13].
Biochar also exhibits promising performance as a multifaceted catalyst in many reactions, including, among others, methane cracking, biodiesel production, and syngas upgrading [14,15]. Because of its properties, like high porosity and carbon content, and its potentially lower costs, using biochar as an alternative to traditional solid metal-based catalysts shows an interesting potential [16,17]. Two key physicochemical features, including surface functional groups and the dispersion of metal nanoparticles on biochar surfaces, play a crucial role in enhancing its catalytic performance. Moreover, properties like Specific Surface Area (SSA), pores, and its hydrophobic nature also impact the effectiveness in its application as a catalyst [18].
On the basis of the well-established multifunctionality of biochar in the environmental, agricultural, and industrial contexts, the combined thrust of biochar application via upgrading methods with the aim of material recovery as fertilizers and applications of activated biochar as catalyst in methane cracking, biodiesel production, syngas upgrading and even cleaning up environmental contaminants constitutes a derivative approach that simultaneously addresses resource recovery and waste valorization. This represents a win-win strategy which meets the needs of circular bioeconomy approaches. Although a vast amount of literature is available related to the production, properties, and applications of biochar, the original contribution of this work is formed through a deep look at the single stage and integrated chemical–physical activations, the subsequent development of pathways toward upgrading the properties of biochar, catalytic and adsorptive wise (enhanced surface area, porosity and designed functional groups) and also the recovery of valuable nutrients (e.g., phosphorus, potassium, magnesium) from waste derived feedstocks. This dual application serves to contextualize the biochar process in the context of the circular bioeconomy of waste valorization and resource provision. Furthermore, this paper integrates developments, including environmental trade-offs and establishing biochar as a versatile platform to connect sustainable material production with nutrient recovery and circular economy ambitions.
2. Biochar Production Methods
There are several main processes (see Figure 2) for producing char, that differ in feedstock flexibility and operational parameters as well as in the resulting quality and yield of the products. Among the prevalent techniques, slow and intermediate pyrolysis stand out, with slow pyrolysis proving to be the most efficient approach for generating substantial quality of biochar considering the carbon retention and longest residence time [19]. It is worth mentioning two additional pre-treatment methods for transforming carbon from biomass: torrefaction and hydrothermal carbonization (HTC), whose solid products have different structures in comparison to biochar.
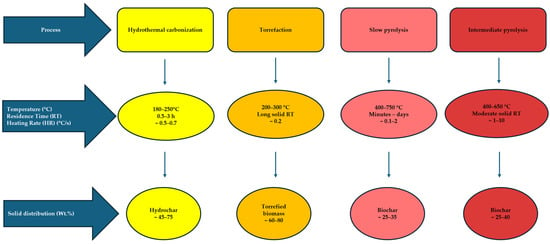
Figure 2.
Operating parameters and typical solid distribution in different processes [20,21,22].
2.1. Hydrothermal Carbonization (HTC)
Hydrothermal carbonization is a thermochemical process that transforms wet biomass (75–90% water content, without requiring a drying pre-treatment step [23]) into a carbon-rich product called hydrochar. In this process, wet biomass is heated to 180–280 °C in water under pressure (10–40 bar). The reaction produces three main outputs: hydrochar (a solid fuel material), process water (rich in dissolved organics), and a small amount of gas (mainly nitrogen and carbon dioxide, comprising approximately 1–2% of the input), the yield of which can vary based on the feed composition [24]. Hydrochar production generally requires less energy consumption than biochar production. It holds potential as a coal substitute due to its comparatively high Higher Heating Value (HHV) [25]. In comparison to biochar, hydrochar has lower carbon, ash content and also a smaller specific surface area and pore volume.
2.2. Torrefaction
Torrefaction is a moderate temperature pre-treatment process in which biomass is heated slowly to about 200–300 °C for a short duration prior to pyrolysis. This process produces a light, porous solid rich in carbon with reduced water content, which in terms of energy density and grindability, is more conducive to handling, storage and transportation of the material [26]. The total carbon yield is influenced by the treatment temperature, residence time, feedstock and furnace atmosphere. For example, lignin from beech starts to decompose at approximately 200 °C, most hemicellulose at about 230 °C and cellulose at temperatures well above 270 °C [27]. Under pilot-scale operations with hardwood and switchgrass pellets, solid yields greater than 77 wt% have been reported [28]. Limited surface area, lower porosity and unstability of torrified biomass make it useful as a fuel, but not for environmental and catalytic processes [29].
2.3. Slow Pyrolysis
Slow pyrolysis is recognized as a suitable method to convert biomass into a stable solid product enriched with carbon and valuable elements (phosphorus, etc.), depending on the feedstock [30]. This thermochemical process is well known, and it offers a sustainable way to produce pyrochar from biomass. In slow pyrolysis, approximately 50% of the feedstock carbon content can be permanently converted into a stable carbon structure [17].
Together with biochar, other condensable (also known as bio-oil) and incondensable fractions (pyrogases) are produced. These fractions can be either valorized and/or used to supply the process energy demand. In the context of the slow pyrolysis process with energy recovery (Figure 3), the biochar yield and quality are highly dependent on the process parameters. Lower temperatures, slower heating rates, longer residence times and larger particle sizes tend to increase char yield because they promote secondary reactions that fix carbon in the solid phase [31]. The composition of feedstock is also important, since biomass with a high lignin content generally generates more biochar due to its higher thermal stability when compared to cellulose and hemicellulose [32]. While the optimal operating conditions depend on feedstock properties, slow pyrolysis generally follows a tendency where more moderate pyrolysis conditions favor carbon recovery in biochar; but higher temperatures and faster heating rates lead to a production distribution shift to bio-oil and pyrogas [33].
In general, since intermediate and fast pyrolysis processes use higher heating rates, the yield of biochar is typically lower compared to the slow pyrolysis [34]. According to the reported studies, at a temperature of 400 °C, by increasing the heating rate from 5 °C/min to 30 °C/min, the biochar yield decreases from 34.2 to 29.7 percent [35]. These considerations allow us to remark that the process conditions and the feedstock quality significantly affect both the final product yield as well as its chemical–physical characteristics.
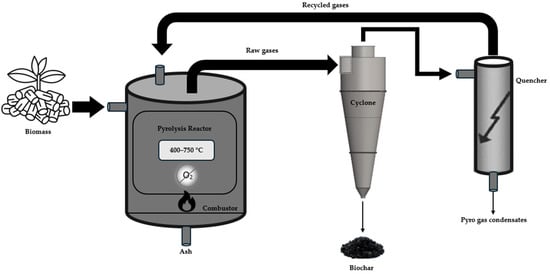

Figure 3.
Basic conceptual scheme of slow pyrolysis for biochar production [36].
The biochar produced by slow pyrolysis (pyrochar) with a low heating rate and high residence time is generally more structurally stable, has a higher carbon content and greater porosity suitable for high temperature conversion processes as a medium, and can even help with the cleanup of pollutants. To put it more simply, pyrochar is more stable in the long term as a conditioner and amendment for soil and environmental use [37]. The production of biochar involves the breakdown of organic materials and the removal of volatile compounds, resulting in a stable matrix with enhanced aromatic carbon structures. These structural features are crucial for biochar’s catalytic functions, as they contribute to its large surface area, increased porosity and abundant active sites, all of which facilitate chemical interactions and make biochar an effective catalyst [38].
Consequently, torrefied biomass obtained at low temperatures has its fuel properties significantly upgraded, but it still does not have the aromaticity and surface area required for more advanced applications [39]. HTC hydrochar also contains high volatiles and higher H/C ratios (>1.4), and it is more suitable as a soil additive and nutrient reuse than catalytic applications [40]. On the contrary, pyrolysis biochar has lower H/C and O/C ratios (<0.7), high aromaticity and structural stability, as well as well-tuned porosity. Moreover, the inherent alkaline nature and rich mineral element content of pyrolysis-derived biochar with the abundant surface functional groups (phenolic and carboxylic acids) also contribute to its catalytic properties [41]. The combined properties described above render slow pyrolysis biochar a potential candidate as multifunctional material for adsorption, environmental applications, and catalyst support [14].
3. Biochar as Catalyst Support
Biochar can stabilize and make nanoparticles uniformly spread on its surface which makes it act as a very effective catalyst as it has active sites for different reactions. The mesoporous structure allows for good dispersion of metals such as manganese, copper, cobalt and iron and immobilization in the pores, preventing leaching into water systems [42]. Its unique features include heterogeneity (easy isolation from reaction mixtures), bifunctionality (promoting both transesterification and esterification reactions), recyclability, porosity, and non-graphitizability, which makes it resistant to the crystallization process at high temperatures [43]. Compared to other catalysts, biochar is low-cost, degradable and recyclable, which as a result is useful in a wide range of applications in energy and environment, such as biodiesel production, methane and tar cracking, syngas and pollutant removal [44]. For example, the native inorganic elements, including Fe and K, in biochar increase tar cracking activity [45] or having biochar support as an eco-friendly and efficient catalyst for methane cracking to produce hydrogen [46]. However, raw biochar often suffers a disadvantage of low porosity, low surface area and poor catalytic performance, since biochar has a plenty of functional surface groups (such as -OH, C=O, etc.) and needs to be modified to improve its functions (including feedstock activation, synthesis control and surface functionalization) [47]. Surface activations and modifications for biochar exhibit marked enhancement in porosity and efficiency, making it widely used in the biorefinery and energy production [48].
3.1. Defined Key Properties of Biochar as Catalyst
In recent years, biochar has been considered as a catalyst for thermochemical processes thanks to a few key qualities: it can handle high heat, resists chemical poisons such as sulfur and contains helpful metals such as iron. These metals serve as active sites, making the reaction more efficient and stable [49]. To boost its performance, the structure of biochar should be optimized. A higher surface area and more pore volume help trap carbon better during the reaction. Thermal treatments can also create intentional flaws and structural defects that act as energetic spots where methane’s strong C-H bonds can break more easily [50]. While temperature and gas composition matter, it is ultimately the catalyst’s design that often determines how much methane actually converts [51].
In the summary of the discussion of biochar upgrading according to the research, the following parameters should be taken into consideration.
3.1.1. Surface Functionality
Biochar inherently consists of oxygenated functional groups including hydroxyl (-OH), carboxyl (-COOH) and carbonyl (-C=O) moieties which markedly improve its catalytic activity [52]. The improved performance is largely due to the presence of active surface sites that promote both the adsorption and redox processes, positioning biochar as a material with considerable potential across numerous applications [53]. Studies have shown the catalytic functions of biochar and focused on the contribution of oxygen-containing groups in pollutant removal and catalytic conversion [54]. Functionalization like sulfonation or metal deposition further contributes to catalytic activity. For example, sulfonated biochar is considered a high-efficiency solid acid catalyst in cellulose hydrolysis [55].
3.1.2. Inorganic Elements
Another important characteristic of biochar is the small presence but significant quantities of inorganic compounds like potassium, sodium, calcium, magnesium and iron. The source feedstock of biochar is an important factor controlling the amount and composition of these minerals. For example, biochar prepared from woody, herbaceous, or aquatic plants generally contains significantly less inorganic substances than that prepared from other sources [56]. To investigate the impact of different types of metallic compounds on the activity of carbon catalysts, two primary upgrading methods can be used [57,58]: the first approach involves removing metals from high-ash carbon materials which contain various metals through acid treatment; the second method introduces different metals onto low-ash carbon-based catalysts to evaluate their effect on catalytic performance.
3.1.3. Particle Size
In catalytic processes, the effect of particle size on mass transfer, as well as reaction rate, is important, because with larger catalyst particles, internal mass transfer resistance is greater, which reduces slower reaction kinetics [59]. It is proved by several studies that smaller catalyst particles give a greater amount of the catalytic surface area, which is good for mass transfer and the process reaction [60,61]. But smaller particles can also result in a greater pressure drop in the reactor, and their application in practice may be restricted [62].
In addition to biochar features, there are also exceptionally stable thermal and mechanical properties of biochar that can be employed well in high-temperature processes. Such inherent stability is believed not only to secure stable performance of biochar under environmentally adverse conditions but also to contribute to the reusability of biochar as a catalyst for multiple cycle [17].
4. Review on Biochar Upgrading Techniques
Biochar quality can be improved using a variety of post-production treatments, such as gas activation, reduction, acid and alkali exposure, oxidations and metal ions treatment [63]. Lately, there has been growing interest in improving biochar’s ability to absorb substances by expanding its surface area, increasing its porosity or introducing functional groups [64]. The selection of a particular method relies upon its intended final field of application [65]. Chemical reagents employed in biochar upgrading can induce alterations in their physicochemical traits, consequently boosting their sorption capabilities. Upgrading processes significantly increases biochar effectiveness and broadens its potential applications, especially in the remediation of pesticide-contaminated environments [66] as can be seen in Table 1.

Table 1.
Biochar upgrading methods [67,68].
4.1. Methods for Biochar Upgrading
Upgrading methods aim to improve biochar properties, widening its range of applications [69]. Biochar upgrading methods are generally divided into two main groups: physical and chemical activation. In physical activation, the material is treated with CO2 gas, steam or a combination of both at temperatures exceeding 700 °C to enhance its porosity and surface area. During this process, highly reactive carbon atoms are eliminated [70,71]. Chemical activation involves using alkali substances such as NaOH, KOH and alkaline earth metal hydroxides (Ca(OH)2) or acids (such as HCl, H3PO4, and HNO3) either during carbonization or after the biochar has been produced to further modify its properties [72].
4.1.1. Physical Methods
Biochar porosity can be enhanced by physical activations like ball milling and also by using mediums like air, CO2, or steam at high temperatures. This process generally occurs in two stages: first, the biomass undergoes a thermochemical transformation, and then it is activated to develop a porous structure [73]. In general, increasing the activation temperature leads to a greater release of volatile organic matter, which in turn generates more pores. Pores larger than 50 nm are classified as macropores, those between 2 and 50 nm are termed mesopores, and pores smaller than 2 nm are referred to as micropores. The formation of pores on biochar’s surface is a key physical feature that helps dissolve toxic pollutants during adsorption and boosts its catalytic performance [74].
- Thermal-oxidation treatment
After the biochar is heated to the desired temperature (800–900 °C) for 1–2 h, it is exposed to hydrogen, air, or argon gases, and new functional groups are formed on its surface [75]. As illustrated in Figure 4, heating biochar in an air environment significantly changes its surface chemistry, which in turn influences its ability to absorb organic and inorganic compounds. Overall, thermal treatment enhances the adsorption of both polar and nonpolar substances. Thermal treatment can significantly increase the porosity and surface acidic groups of biochar and creates new adsorption sites on biochar for organic/inorganic molecules. The optimum thermal air oxidation temperature is in the range of 400–500 °C. Considering the potential variability related to the feedstock, biochar made from lignin-rich feedstocks is preferable for thermal air oxidation treatment [76,77,78].
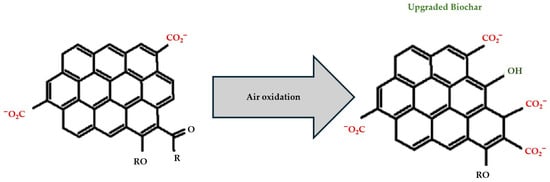
Figure 4.
General thermal treatment mechanism of biochar through air oxidation (Colors on structure: red = oxygenated functional groups, green = new functionalities, black = carbon framework). Adapted from [76], Environmental Functional Materials, 1 (2022), 187–195. © 2022 The Author. Published by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
- Steam activation
In this process, the raw material is first heated to a temperature between 300 and 700 °C for 1 to 2 h without any oxygen present. As the second step, the decomposed sample is subjected to a steam flow at a temperature of 800 to 900 °C for 30 min to 3 h, at a rate of 2.2 to 5 mL/min [79]. Steam improves the properties of biochar by removing elements resulting from incomplete combustion during thermal decomposition and oxidizing the carbon surface. The high-temperature steam reacts with the carbon, removing carbon atoms as CO or CO2, thus developing a more porous structure [80].
- Activation with CO2
CO2 can be used as an oxidizing agent, operating between 200 and 900 °C, resulting in the formation of pores and the increase of the existing ones. The CO2 is usually not very reactive, but when combined with steam at higher temperatures, it becomes active. This enhanced reactivity helps in producing biochar with similar dimensions of pore sizes [70]. The reactivity of CO2 increases with temperature, facilitating its strong interaction with biochar via the reverse Boudouard reaction. The char (C) is activated by the following reaction: C + CO2 ↔ 2CO [81]. During the reaction, carbon dioxide (CO2) is reduced and attaches to the carbon surface to yield a surface oxide (C(O)) and carbon monoxide (CO). The biochar structure post steam activation and CO2 purification is shown in Figure 5. Surface oxide (C(O)) is then desorbed, which further refines the pore structure of the char [82]. In Table 2, the reactions involving biochar in different mediums are presented.

Table 2.
Physical activation of carbon-based material with various mediums.
Table 2.
Physical activation of carbon-based material with various mediums.
| Medium | Reaction | Energy Balance |
|---|---|---|
| Air/O2 [83] | C + O2 → CO2 (1) | Exothermic |
| Steam/H2O [84] | H2O + C → CO + H2 (2) H2 + 2C→ 2C(H) (3) | Endothermic |
| CO2 [85] | CO2 + C→ C(O) + (CO) (4) C(O) → CO (5) CO2 + C→ 2CO (6) | Endothermic |
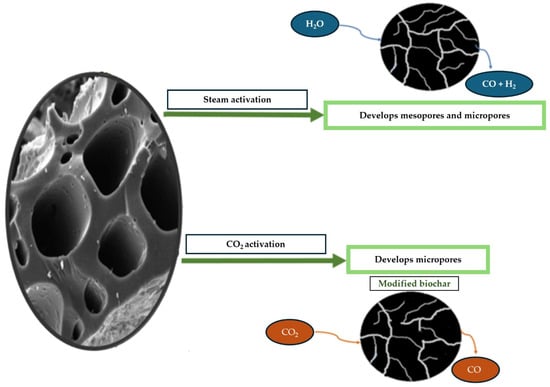
Figure 5.
The microporous structure of biochar forms as a result of steam activation and CO2 treatment. Adapted from [86], Biomass Conversion and Biorefinery, 14 (2020), 5759–5770. © 2020 Springer-Verlag GmbH Germany. Reproduced with permission.
- Ball milling
Another physical modification method is ball milling which represents a solvent free, environmental and convenient method. In this manner, the biochar is subjected to mechanical grinding, either by itself or with pulverized solid additives, in order to enhance its physicochemical properties. Ball milling has the ability to produce a smaller mean diameter, increase specific surface area and add functional groups, etc., leading to a high permeability rate of adsorptive species and catalytic efficiency or mixing dispersion in composites [87].
4.1.2. Chemical Methods
The activation process involves using various agents, including bases like KOH and NaOH, acids such as HNO3 and H3PO4, oxidants like H2O2 and sulfonating agents (SO3H), and ZnCl2. The choice of activation method depends on how the biochar will be used. If it needs to attract negatively charged elements, it is treated with bases to create a positive charge on its surface, improving its ability to bind with the target substance. On the other hand, acid activation can be used when biochar needs to capture positively charged elements [88]. Additionally, activation helps remove moisture and volatile compounds that could lead to tar formation, ultimately increasing its porosity and carbon yield [89]. Activated biochar is carefully washed to remove any leftover chemicals and expose functional groups that enhance its ability to absorb different substances.
- Alkali activation
In this process, biochar is treated with reagents like potassium hydroxide (KOH), sodium hydroxide (NaOH) and ammonium hydroxide (NH4OH) [90].
In a study by Ma [91], Biochar produced from sawdust through pyrolysis in temperatures between 300 and 700 °C was treated with a 2 mol/L potassium hydroxide (KOH) solution for 5 h. During this process, various chemical reactions occurred, as described below:
2KOH → K2O + H2O
BIOCHAR + K2O → BIOCHAR • K2O + H2 + tar
The addition of more functional groups containing nitrogen to the structure of biochar is made possible by using NH4OH, which increases the elemental nitrogen in biochar [67].
At this stage, -NH2 groups were introduced onto the surface of the biochar.
R-COOH + NH4OH → R-CONH2 + H2O
After being treated with NH4OH, due to the significant removal of ash from the pores, the biochar surface exhibited a smoother post-treatment texture. The application of alkalis led to the thinning of micropores, increasing the biochar’s SSA [68].
In another study by Haque et al. [92], activated carbon (AC) was obtained through traditional carbonization and KOH activation, while amorphous silica was derived from rice husk using alkali extraction followed by acid precipitation. The ideal temperature for AC production was found to be 700 °C. AC produced via alkali extraction showed greater activity than that obtained through KOH activation.
- Acid activation
Acid treatment can also modify the surface characteristics of biochar, such as its texture, porosity and surface area, and its effects are different with the types and the concentrations of the acids [93]. The application of acidified biochar has been acknowledged for its notable efficacy as catalyst support for thermochemical processes, for enhancing soil quality and for promoting agricultural productivity. Acidification of biochar is known to influence various physicochemical properties, fostering favorable conditions for nutrient retention, positive ion exchange capacity, and soil fertility [94]. Hydrochloric (HCl), sulfuric (H2SO4), phosphoric (H3PO4) and nitric (HNO3) acids are typically used in biochar upgrading.
The experimental results [95,96,97] show that the modified biochar with the highest specific surface area was obtained through acid treatment followed by KOH activation. Similar to basic activation, acid upgrading increases the number of functional groups containing oxygen in biochar [98]. As a result, the surface charge and functional groups are altered, leading to a lower point of zero charge and improved surface purity. Below are several important reactions reported for the acidic upgrading of biochar using different acids.
Sulfuric acid reacts with the carbon atoms in biochar through the following chemical process [99]:
2H2SO4 + C → 2SO2 + CO2 + 2H2O
Phosphoric acid interacts with the hydroxyl (-OH) groups on the biochar surface, forming ester bonds. This process helps integrate phosphorus atoms into the carbon structure, improving the material’s chemical characteristics:
2ROH + H3PO4 → -O-PO(OH) -OR + 2H2O
Hydrochloric acid undergoes nucleophilic substitution to the biochar [100]:
ROH + HCl → RCl + H2O
RCH2COOH + Cl2 → RCHClCOOH + HCl
RCH = CH2 + HCl→RCHClCH3
RCH = CH2 + Cl2→RCHClCH2Cl
During the upgrading process with nitric acid (HNO3), the aromatic structures within the biochar undergo chemical reactions with the acid. This results in the formation of organic structures containing nitro groups [72]:
BIOCHAR + HNO3 → MODIFIED-BIOCHAR + H2O + CO2
As pointed out by Kasozi et al. [101], although the textural qualities of a biochar may not be improved through organic upgrading, its surface chemistry can be impacted quite considerably.
Organic acids, such as acetic acids, also impact the characteristics of biochar. They decrease the porosity of biochar while increasing the formation of new functional groups on the surface, likely due to esterification reactions. As stated by Xu et al. [102], acid treatment does not change the specific surface area (SSA) and the total pore volume (TPV) of biochar because of pore blockage by adsorbed organic acid molecules. This indicates the biochar upgraded with citric acid. Typically, an increase in the atomic O/C ratio signifies an improvement in biochar’s surface chemistry. Carboxylic groups are among the identified surface functionalities on biochar modified with organic acids. Sheng et al. [103] demonstrated the upgrading of water hyacinth-derived biochar with citric acid; in the study, the biomass was pyrolyzed at 300 °C for 2 h in a nitrogen atmosphere with a 5 °C/min heating rate, followed by treatment with 0.7 mol/L citric acid for 2 h. The application of citric acid led to a reduction in the specific surface area of biochar, likely due to the dissolution and removal of lignin during the acid treatment.
Acid treatment generally extracts metals such as iron, aluminum, titanium, calcium, sodium and sulfur from carbon-based catalysts and adds oxygen-containing groups, like carboxyl, to the biochar surface [61,104]. While this treatment increases the surface area and pore volume of biochar, enhancing its sorption properties, it also alters its composition and reactivity. Bases like NaOH and KOH can react with sulfur and important minerals found in coal to make compounds like silicate and aluminate. These compounds can then be dissolved by acids, chemicals like sulfuric acid and hydrochloric acid, along with other unused minerals [30].
4.1.3. Inorganic Methods
The use of salts, such as chlorides or phosphates, allows for influencing both the physical structure and surface chemistry of biochar. Li et al. [105] noted an alteration in the surface of biochar when it was treated with K3PO4; in their experiment, the particles were soaked in K3PO4 and stirred for 6 h. Afterward, the impregnated biomass was subjected to pyrolysis at 550 °C for 1 h in a nitrogen atmosphere, with a heating rate of 10 °C/min [106]. This pre-treatment procedure resulted in an enrichment of P2O7−4 on the surface of the biochar formed from the breakdown of HPO4−2.
2HPO4−2→ P2O7−4 + H2O
The generated components significantly contribute to the adsorption of heavy metals onto biochar. Treating biochar with K3PO4 enhanced its textural properties and surface chemistry. This modifier expedited the decomposition of biomass polymers, leading to the formation of numerous oxygen-based groups on the surface of biochar [107].
In an experiment, biochar was treated by soaking it with a solution containing 30% ZnCl2 [98]. During this upgrading process, the following chemical transformations took place within the biochar:
BIOCHAR + 2ZnCl2 →BIOCHAR • Zn2OCl2 • 2H2O
Zn2OCl2 • 2H2O → ZnCl2 + ZnO + 2H2O
4.1.4. H2O2 Upgrading
Biochar can also be enhanced using hydrogen peroxide (H2O2), which is often considered a more cost-effective and environmentally friendly alternative to strong acids, bases and salts. In Tan et al. [108], biochar was produced from rape stalk through pyrolysis at 600 °C in a nitrogen atmosphere, with a heating rate of 10 °C/min and a processing time of approximately four hours. To enhance its properties, the biochar was treated with 30% H2O2 for 24 h. During this treatment, H2O2 reacted with the biochar, resulting in gas formation.
2H2O2 → 2H2O + O2
H2O2 + C → H2 + CO2
H2O2 + 2C → H2 + 2CO
The changes in the biochar properties observed across various studies following different upgrading treatments are presented in Table 3.

Table 3.
Physical–chemical methods used in different sources.
Activation techniques result in quite different performances of the biochar. The KOH-activated char had the highest surface area, reached the SSA of 2183.8 m2/g, and developed the sufficient amount of micropores which is suitable for the removal of heavy metal ions and pollutants. Activations by ZnCl2 and H3PO4, which obtained SSA values (>1000 m2/g) and pore volumes as high as 0.6 cm3/g, the resulting hybrid microporous–mesoporous materials are promising for catalysis and tar removal. Physical activation using either CO2 or O2 yielded moderate improvements (SSA 200–850 m2/g) with potentially active surfaces useful for syngas upgrading or methane cracking, while N2 activation was found the least effective (SSA < 122 m2/g) which could be ascribed to both non-reactive atmosphere (inert environment) and few amounts of functional groups being formed. The chemical–physical combined treatments enable synergistic promotion, with a SSA value of up to 1419 m2/g and the PVs of up to 0.83 cm3/g, having potential application in diverse catalysis.
5. Comparative Discussion on Upgrading Methods
Having reviewed the pros and cons of different activation methods (Table 4) and gone through various studies, it was found that activation of biochar through acids like HNO3, HCl, H2SO4 and H3PO4 has been reported to significantly modify biochar surface functional groups toward increased oxygen content, amplified polarity and lessened ash percent. Different acids provide unique surface functionalization: e.g., HNO3 treatment that triggers nitration and oxidation makes it possible for -NO2 and -COOH groups to be attached [72]. However, H3PO4 activation forms phosphorus-containing structures (P=O and P-OOH), which can build stable complexes with pollutants [119]. During HCl treatment, the oxygenated functional groups are enhanced and mainly their carbonyl structures such as phenols, ethers and lactones are reduced. Such functional groups, largely present on the biochar edges and outer surfaces, facilitate hydrophilicity and polarity, whereas demineralization from acid activation tends to reduce ash content and surface charge which favors electrostatic attraction for cationic pollutants [120]. For acids, activation with HNO3 does not significantly affect surface area and porosity in comparison with HCl, H2SO4 and H3PO4. HNO3 treatment at initial stages may cause micropore development and solubilize the inorganic impurities resulting in improved surface area and pore volume. But the long-time activation could cause the coagulation of crystallite and the narrowing of pores, reducing the change in the overall porosity [121]. It is thus claimed that H3PO4 is a milder acid, better preserving the carbon skeleton and more efficient in developing the micropores, and thus particularly useful for porosity engineering. On the other hand, alkaline activation (typically NaOH or KOH) enhances biochar’s alkali groups and nonpolar surface sites, thereby promoting the adsorption of anionic pollutants. These processes mostly enhance the surface area and the pore volume, and KOH-activated biochar has a higher surface area and higher adsorption capacity with respect to steam activation [122]. Nonetheless, an over-alkaline treatment or higher temperature during processing might destruct pore structure because of a vigorous gasification reaction, which in turn results in decreased surface area [123]. From a chemical standpoint, it is well reported that KOH should enhance the surface functional groups (i.e., carboxylic, alcoholic and phenolic), but oxygen groups decompose upon heating, causing reducing overall functionality and raising carbon content [124]. In contrast, NaOH activation has received much less attention, although it is believed to be less expensive, less hazardous and more eco-friendly, and Na+ can enter the biochar structure more effectively than K+ because of its minor ionic radius [125].

Table 4.
Effects, risks and advantages of activation methods.
Physical activation is further developed as a green approach for activating biochar, and steam, CO2 and air/oxygen are the most used agents. The biochar developed by steam activation have excellent microporosity and adsorption capacity; however, the energy demand to produce steam for steam activation is questionable with regard to efficiency and sustainability [126]. On the contrary, CO2 activation prefers the formation of mesoporosity and is efficient in accommodating large molecules, but its slow activation rates and the need for long processing times add up to energy requirements and a carbon footprint [94]. Air or oxygen activation is the simplest and the most economical way to obtain activated carbon, but it has the least control, and often results in overburning, less yield and low stability in biochar [127]. Physical activation, although avoiding toxic residues from chemical reagents such as KOH or H3PO4 and therefore being more environmentally friendly, has high thermal energy needs which remain significant environmental concerns [128]. Generally, in comparison with traditional activated carbon, physically activated biochars consume less in terms of ecosystem service as they are derived from renewable sources with less chemical inputs, especially in the field of wastewater treatment [129]. In addition, the environmental consequences are not limited to carbon costs; for example, soil quality improvements have been recorded for steam-activated biochars, while other activation methods are associated with potential ecotoxic effects [130].
6. Potential Uses of Upgraded Biochar as a Carbon-Based Catalyst in Different Applications
Upgraded biochar holds promise far beyond its traditional uses, finding roles as catalyst support in methane cracking, biodiesel production, syngas upgrading and even cleaning up environmental contaminants. What makes it especially versatile is how its properties can be tailored, especially for catalysis [58,131]. Much of this capability stems from its natural mineral richness; elements like iron and potassium actively support catalytic processes [45].
6.1. Catalytic Methane Cracking
In the concept of methane cracking, research on the pore structure of a catalyst indicates that carbon catalysts with higher meso and macropore content achieve higher hydrogen production compared to those dominated by micropores [132]. This is caused by the diffusion of methane, which is hindered more severely in the presence of micropores, decreasing hydrogen production [133]. Figure 6, adapted from Harun et al. [134], illustrates a study on hydrogen production via catalytic methane decomposition with activated carbon, ruthenium-activated carbon and activated biochar catalysts at 800 °C. It is important to mention that the efficacy of these catalysts is influenced by their surface area, as a larger area enhances the adsorption of methane onto the catalyst surface.
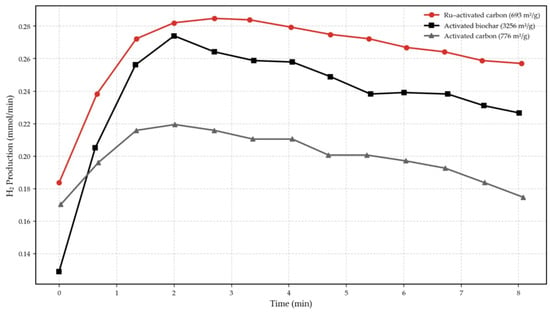
Figure 6.
Hydrogen production from catalytic methane decomposition using Different Activated Carbons. Elaborated data from: [134].
Also, Abbas et al. [135] explored how varying the size of particles affects the methane conversion and decomposition rate using a fixed-bed reactor at a high temperature (850 °C) and a specific methane flow. Their findings suggested that changing the particle size within a fairly broad range (from relatively small to relatively large, specifically 135 to 1095 µm) had little noticeable influence on how much methane was converted, how quickly it decomposed or how long the catalyst remained active. Another study tested both biochar and activated char in methane decomposition, and their results showed that higher temperatures and lower methane concentrations improved conversion. At 900 °C with just 10% methane in a nitrogen stream, they achieved 72.3% conversion with activated char and 66.2% with biochar [49]. During acid treatments of biochar and extraction, some metals like Fe, Ni, Al and Ca, carbon-loaded catalysts showed improved methane conversion efficiency in the methene cracking processes [136].
Biochar’s promise extends into dry methane reforming as well [137]. One study used a tungsten carbide-infused biochar, which maintained performance over 500 h. As CH4/CO2 ratios and temperatures increased, methane conversion decreased, but CO2 transformation and hydrogen production grew steadily [138].
6.2. Support Biodiesel Production
Biochar has been found to be a promising catalyst and support for esterification and transesterification reactions in the processes of biodiesel production [139]. Its low cost, flexibility and mineral-abundant source of biowaste represent an appealing alternative to the typical homogeneous and heterogeneous catalysts [140]. Functionalization is one of the main factors that reinforces biochar catalytic activity. For example, a sulfonated biochar as a solid acid catalyst yielded approximately 97% biodiesel yield from FFAs (free fatty acids) and also produced 88% of productivity via simultaneous esterification and transesterification of vegetable oils [141]. However, biochar with rich content of inorganic minerals (such as calcium and potassium) can play a good role in solid base catalysts. For instance, palm kernel shell biochar, which was rich in CaO after calcination, exhibited 99% FAME (fatty acid methyl ester) content after the metanalysis of sunflower oil [142,143]. In another study, the KOH-activated pomelo peel biochar greatly achieved as high as 98% biodiesel yield because of its high basicity and enhanced surface area/porosity [144].
6.3. Syngas Upgrading (Tar Reforming)
Biochar plays an important role in the prevention of tar generation during biomass gasification and pyrolysis and improving the production of the syngas (a mixture of CO and H2) [145]. The highest H2/CO ratio (1.5) reported was attained by CO2 physically activated biochar (900 °C) with both great SSA and hierarchical porosity, which had an excellent performance with 78.2 wt% of syngas yield and 26 mmol H2/g-biomass of hydrogen production with a gas energy conversion efficiency of 73% [118]. Still, biochar performance can fall short due to its limited porosity and surface area. To overcome this, researchers are refining the material, activating the feedstock, adjusting synthesis settings, adding surface treatments and combining it with other materials. Its structure, high surface area, fine pores and active minerals behave like conventional catalysts (dolomite, olivine, nickel and alkali metals) and as an effective catalyst in tar reforming [146]. For example, Ni/Ca/Fe-loaded biochar-nanocatalysts achieved 87% tar conversion and 42.46 mmol H2/g-biomass which demonstrated the excellent catalytic activity toward metal modifications [147]. In the case of pyrogas, Ren et al. found that using biochar as a catalyst during biomass pyrolysis increased pyrogas yield dramatically from 15% to 46% at 480 °C [148]. Shen et al. noted that the same approach raised hydrogen content in syngas by up to 27% [75].
6.4. Environmental Cleanup
Activated biochar is widely recognized as a cost-effective, environmentally friendly and highly efficient adsorbent of contaminants from water and soil. It does this in two primary ways: by adsorbing pollutants onto its porous surface and by functioning as a catalyst support to accelerate reactions that break down toxic compounds [149]. Biochar can help clean up the environment by immobilizing pollutants, passing on electrons to break them down or enabling oxidants, like peroxides, to generate powerful cleaning agents [150]. In advanced oxidation applications, physically activated biochar (e.g., peanut shell char pyrolyzed at 900 °C) exhibited 98.3% sulfamethotyl removal in 120 min because of its high graphitic content, COOH groups and enhanced specific surface area [151]. Chemically oxidized biochar also performed well, with over 97% removal of the antibiotic sulfamethoxazole in 30 min [152].
7. Waste-Derived Biochar Chemical Leaching and Its Potential for Critical Materials Recovery
The evolving circular economy favors the recovery of critical materials, where biochar can assist. A shift is needed from conventional linear economic systems toward one that focuses on resource reuse and waste reduction. The supply of critical raw materials components of many industries faces potential vulnerabilities. Recovering valuable materials from waste biochar has the advantageous potential for helping to secure the sustainable supply of critical raw materials, which reduces reliance on environmentally harmful extraction processes [153].
Biochar’s characteristics can be influenced by the quality of raw materials and processing conditions, requiring some additional refinement. While biochar has promise, it is often derived from biowaste which is rich in certain inorganic compounds, such as Fe, Si, K and Al, that dilute porosity and may inhibit catalytic processes [154]. Through chemical leaching, valuable materials can be retrieved from waste-derived biochar promoting circularity. Through this form of leaching, helpful nutrients such as phosphorus, potassium, magnesium and calcium can be extracted and used as fertilizers while heavy metals are removed. Leaching enhances biochar’s porosity and adsorption capacity by reducing ash content, thus improving its application in environmental remediation and energy recovery. The process also helps in lowering alkaline earth metals (mostly Ca2+ and Mg2+), preventing carbonate formation that could otherwise diminish biochar’s efficacy [30,155]. For this reason, chemical leaching can be considered as an upgrading process for the biochar.
As has been reviewed, alkali treatments using NaOH, KOH and Ca (OH)2 are particularly effective in extracting silica, alumina and clay minerals from coal, forming hydrated alkali bearing silicate and aluminate. These treatments are also suitable for extracting inorganic and organic sulfur [156]. In a study by Qiu et al. [157], potassium bicarbonate (KHCO3) showed promise as an activating agent in pyrolysis for creating hierarchically porous biochar adsorbents (14.4 mg/g.min) from sewage sludge, supporting material recovery from water leaching which can be used for plant growth (Figure 7).
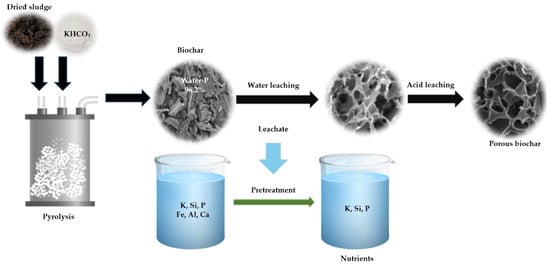
Figure 7.
Biochar after both water and acid leaching treatments. Adapted from [157], Resources, Conservation and Recycling, 176 (2022), 105953. © 2022 Elsevier B.V. Reproduced with permission.
Conversely, acid leaching, which employs acids such as H2SO4, HNO3 and HCl, is more effective in removing carbonates, phosphates, iron (III) oxide and sulfides from low-grade coal. Sequential application of alkali and acid leaching is often beneficial, as alkalis react with sulfur and primary coal minerals to form compounds that acids can later dissolve [158]. Acid leaching is also employed for phosphorus recovery from sewage sludge and incinerated sludge ashes with HCl or H2SO4, as they are particularly effective in dissolving phosphorus bound to aluminum and calcium [159].
In a different investigation carried out by Salimbeni et al., sewage sludge char produced through slow pyrolysis at 450 °C was further treated using HNO3, HCl and KOH through chemical leaching under specific conditions (60–80 °C, 2 h contact time). The main phosphorus and calcium extraction results indicate that leaching could achieve almost complete removal (100% removal) and ash content reduction (61.4% removal). As a result, the biocoal obtained demonstrated substantially higher carbon content (rising from 14.4% to 27.4%) as well as greater surface area (SSA) of 70 m2/g. This greatly improved its potential valorization. While silica remained, and Al/P separation was challenging, single-stage HNO3 leaching was deemed promising for producing P-rich fertilizer precursors and upgraded carbon material [30]. Also, Salimbeni et al. showed how chemical leaching can affect the char derived from industrial sludge and recover the inorganic material for the fertilizers, as can be seen in Figure 8. They started with slow pyrolysis of the dried sludge, continued by acid leaching of the resulting char / yield biocoal and a nutrient rich leachate, and subsequently physically activated the biocoal using CO2 at 700–800 °C. Chemical precipitation was employed for phosphorus recovery from the leachate. The results showed that leaching effectively removed around 88% of ash content in char, with close to 100% of P, Mg, Ca and Fe, and around 90% of Al. The activated carbon derived exhibited a SSA of (417 m2/g), with potential as an adsorbent for emission or wastewater treatment, and suggested other potential applications like metallurgy [160].
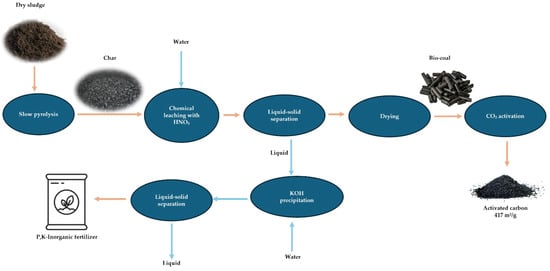
Figure 8.
Elaborated methodology for the thermochemical treatment of derived-sludge char and material recovery [160].
8. From Production to Upgrading: Techno-Economic Concerns and Prospects
The future of biochar upgrading faces opportunities and critical pathways. Many of these are interconnected, and multiple aspects must be approached in order to optimize production processes, improve performance for specific applications and scalably deploy over time.
8.1. Biomass Compatibility
The yield and quality of biochar were determined by the type of biomass. When used as an amendment in soil remediation, carbon sequestration or water treatment technologies, biochar performance is often impeded by contamination and impurities that are present in the biomass. Unsuitable size or shape of feedstock often require expensive pre-treatment operations. It will require long-term commitments to stimulate energy crop production, promote sustainable land management practices and exploit the agro-forestry by-products [161].
8.2. Scale-Up Limitations
Biochar production can suffer from the variability in pyrolysis conditions and operating parameters so that quality is inconsistent and performance is uncertain. Scaling up is difficult due to high costs, inadequate infrastructure and the lack of standard setting in industrial production. Solving these issues will involve advancing reactor designs and energy-efficient processes, as well as common quality standards backed by policy incentives and certification schemes that can promote competition and confidence in the market to foster widespread use [162].
8.3. Balancing Upgrading Efficiency with Environmental Risks
Although the main objective of increasing biochar physicochemical performance is to render it more efficient in different applications, its sustainability may be compromised by the upgrading methods. For example, modifications with metal salt increase the presence of oxygen-containing functional groups, which directly increases inorganic contaminant adsorption. These treatments also alter the leaching potential of metal ions and pose a risk to cationic secondary contamination [163]. Regarding physical activation when it comes to high-energy ball milling, surface area, particle size and functional groups are said to be more important, as they are a solvent free, reproducible and cost-effective green technology. On the other hand, they present issues such as high energy consumption, equipment durability, and dust emission [164]. Also, the polycyclic aromatic hydrocarbons (PAHs) that are formed during pyrolysis need to undergo accurate risk assessments, as PAH-contaminated soil and crops. Therefore, actions must be taken in balancing the efficiency–sustainability trade-off in biochar upgrading using innovation or life-cycle assessments and risk management.
8.4. Economic Obstacles
Economic constraints continue to be a major hurdle for widespread biochar upgrading. Today, the cost of biochar production is still too high and would need to decrease by two-thirds, or carbon subsidies would need to rise between two- and ninefold above current EU emissions trading scheme (ETS) prices (≈88 EUR/t CO2eq) to become economically competitive according to studies [165]. The market itself is still immature and lacks universal quality standards, which constrains investor confidence and adoption. On the agricultural side, where biochar is often treated as a soil amendment, entry capital costs may be high. For instance, when 40 t/ha of biochar was used at a cost of ~€190/t in a Mediterranean study, the break-even time under normal condition was about 5.5 years in an amortization period [166]. Relative to other forms of carbon dioxide removal (CDR), biochar has received small levels of support, while existing subsidies are too weak to ensure widespread uptake. Despite all of the tunable properties of activated biochar, some difficulties such as high fabrication cost, poor long-term stability, industrial scale-up and lack of understanding of their catalysis mechanism still exist. These issues must be addressed in order to realize the full potential of biochar in a sustainability circular bioeconomy with respect to industrial scale. In the future, biochar upgrading will go beyond agriculture and forestry by offering new research opportunities in environmental remediation, horticulture, construction and materials science. These choices necessitate pathways, low in cost, to upgrade them, such as the production of ‘green’ biochar from a variety of residues, which also may contribute to reductions in costly feedstock dependence and to circular resource use. It is also crucial to establish the lowest effective application levels of advanced biochar at which they can yield top performance while minimizing costs and risks of collateral pollution. Ultimately, the translation from bench to application will depend on the success of extended field trials and weathering studies to guarantee novel modification approaches yield highly performant biochar that is both durable and safe at large-scale production. Collectively, they envision the opportunities and difficulties that form the basis for the next phase of biochar upgrading technologies.
9. Conclusions
This review has discussed the comparative properties of different thermochemical conversion processes, highlighting that slow pyrolysis continues to be the most efficient pathway for producing well-tuned pore structure and stable biochar, making it suitable to valorize waste streams and supports through the short-term mitigation of greenhouse gases by sequestering carbon. The following upgrading steps substantially enhance biochar performance with the specific surface area up to 2100 m2/g by KOH activation, and hybrid microporous–mesoporous structure is obtained with a pore volume of 0.83 cm3/g through a combined (KOH-N2) treatment while acid activation provides near-total removal of ash (removal factors of up to 88–100%) enriched with the surface oxygen functionalities that are necessary for suitable adsorption and catalytic activity. At the catalytic level, upgraded biochar significantly outcompetes common catalysts. In the case of methane cracking, activated char gave 72.3% CH4 conversion at 900 °C and 66.2% in the case of raw biochar. As the catalyst support for biodiesel production, the sulfonated biochar was capable of achieving a 97% biodiesel yield from the free fatty acids. For syngas utilization, CO2-activated biochar co-gasified performed a high 78.2 wt% vol syngas yield at 26 mmol H2/g biomass and produced energy conversion efficiencies of up to 73%. Environmental remediation performance was similarly strong, with oxidized biochars removing >97% of antibiotics, and thermally activated chars removing 98.3% of sulfamethoxazole. Regarding materials recovery, sequential acid–alkali leaching of waste-derived biochar enabled a nearly 100% recovery of P, Mg, Ca and Fe, as well as the substantial decrease of ash content by 61–88%. This not only allowed the recovery of EU-listed critical raw materials but also increased carbon content (from 14.4% to 27.4%) and specific surface area (up to 417 m2/g), at the same time improving the biochar’s catalytic and adsorptive capacity.
Despite all of the progress in biochar upgrading, there are several issues which need to be considered, such as production cost, scaling up the upgrading process and disposal of solid residues. Overcoming these barriers will require technological advancements and innovative policy frameworks. Crucially, biochar upgrading provides real-world opportunities in developed economies. It is consistent with circular economy objectives and carbon neutrality commitments; decentralized schemes can displace open burning, enhance soil fertility and decrease dependence on imported fertilizers. Moving forward it will be crucial to interface the emerging technologies with sound economic projections and enabling regulations if biochar is to realize its potential as a sustainable climate change mitigation and resource recovery.
Author Contributions
P.D.: Data Collection, Literature Review, Writing—Original Draft; M.P.: Supervision, Methodology, Conceptualization, Writing—Review and Editing; A.S.: Data Analysis, Visualization—Review and Editing; V.N.: Review and Editing; D.C.: Supervision, Project Administration, Writing—Review and Editing, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the MICS (Made in Italy–Circular and Sustainable) Extended Partnership and received funding from the European Union Next Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)–MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.3–D.D. 1551.11-10-2022, PE00000004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this study.
Acknowledgments
The authors want to thank colleagues at the Energy Department of Politecnico di Torino and RE-CORD (Renewables Energy Consortium for Research and Demonstration) for their valuable technical feedback and support during the preparation of this manuscript. This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SSA | Specific Surface Area |
| TPV | Total Pore Volume |
| PV | Pore Volume |
| HRT | Highest Reactor Temperature |
| HR | Heating Rate |
| HHV | Higher Heating Value |
| RT | Residence Time |
| PAH | Polycyclic Aromatic Hydrocarbons |
| HTC | Hydrothermal Carbonization |
| OCFGs | Oxygen-Containing Functional Groups |
| AC | Activated Carbon |
| CE | Circular Economy |
| GHGs | Greenhouse Gases |
| ETS | Emissions Trading Scheme |
References
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Rangasamy, G. A review on regeneration of biowaste into bio-products and bioenergy: Life cycle assessment and circular economy. Fuel 2023, 338, 127221. [Google Scholar] [CrossRef]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. (Eds.) What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Rahman, I.U.; Mohammed, H.J.; Bamasag, A.; Peshawar, T.; Pukhtunkhwa, K. An exploration of recent waste-to-energy advancements for optimal solid waste management. Discov. Chem. Eng. 2025, 5, 1–26. [Google Scholar] [CrossRef]
- Accelerating Towards a Circular Economy Transition|Report. Available online: https://www.ellenmacarthurfoundation.org/towards-a-circular-economy-business-rationale-for-an-accelerated-transition (accessed on 30 July 2025).
- Noor, T.; Javid, A.; Hussain, A.; Bukhari, S.M.; Ali, W.; Akmal, M.; Hussain, S.M. Types, sources and management of urban wastes. Urban Ecol. Emerg. Patterns Soc.-Ecol. Syst. 2020, 131, 239–263. [Google Scholar] [CrossRef]
- Oo, P.Z.; Prapaspongsa, T.; Strezov, V.; Huda, N.; Oshita, K.; Takaoka, M.; Ren, J.; Halog, A.; Gheewala, S.H. The role of global waste management and circular economy towards carbon neutrality. Sustain. Prod. Consum. 2024, 52, 498–510. [Google Scholar] [CrossRef]
- Khawaja, M.K.; Alkayyali, K.; Almanasreh, M.; Alkhalidi, A. Waste-to-energy barriers and solutions for developing countries with limited water and energy resources. Sci. Total Environ. 2024, 926, 172096. [Google Scholar] [CrossRef] [PubMed]
- Masrura, S.U.; Dissanayake, P.; Sun, Y.; Ok, Y.S.; Tsang, D.C.W.; Khan, E. Sustainable use of biochar for resource recovery and pharmaceutical removal from human urine: A critical review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 3016–3048. [Google Scholar] [CrossRef]
- Zhu, F.; Cakmak, E.K.; Cetecioglu, Z. Phosphorus recovery for circular Economy: Application potential of feasible resources and engineering processes in Europe. Chem. Eng. J. 2023, 454, 140153. [Google Scholar] [CrossRef]
- Ferraro, G.; Pecori, G.; Rosi, L.; Bettucci, L.; Fratini, E.; Casini, D.; Rizzo, A.M.; Chiaramonti, D. Biochar from lab-scale pyrolysis: Influence of feedstock and operational temperature. Biomass Convers. Biorefinery 2024, 14, 5901–5911. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Lehmann, J.; Berruti, F.; Giudicianni, P.; Sanei, H.; Masek, O. Biochar is a long-lived form of carbon removal, making evidence-based CDR projects possible. Biochar 2024, 6, 81. [Google Scholar] [CrossRef]
- Zhou, T.; Deng, J.; Zeng, Y.; Liu, X.; Song, B.; Ye, S.; Li, M.; Yang, Y.; Wang, Z.; Zhou, C. Biochar Meets Single-Atom: A Catalyst for Efficient Utilization in Environmental Protection Applications and Energy Conversion. Small 2024, 20, 2404254. [Google Scholar] [CrossRef]
- Velusamy, K.; Isabel, J.B.; Periyasamy, S.; Thiruvenkadam, A.; Ravikumar, H.; Gupta, S.K.; López-Maldonado, E.A. Role of biochar as a greener catalyst in biofuel production: Production, activation, and potential utilization—A review. J. Taiwan Inst. Chem. Eng. 2024, 177, 105732. [Google Scholar] [CrossRef]
- Yu, J.T.; Dehkhoda, A.M.; Ellis, N. Development of biochar-based catalyst for transesterification of canola oil. Energy Fuels 2011, 25, 337–344. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, Y.K. Applications of Modified Biochar-Based Materials for the Removal of Environment Pollutants: A Mini Review. Sustainability 2020, 12, 6112. [Google Scholar] [CrossRef]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Lotti, G.; Vaccari, F.P.; Sanei, H. Assessment of long-lived Carbon permanence in agricultural soil: Unearthing 15 years-old biochar from long-term field experiment in vineyard. Biomass Bioenergy 2024, 191, 107484. [Google Scholar] [CrossRef]
- Kazawadi, D.; Ntalikwa, J.; Kombe, G. A Review of Intermediate Pyrolysis as a Technology of Biomass Conversion for Coproduction of Biooil and Adsorption Biochar. J. Renew. Energy 2021, 2021, 5533780. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.W.; Kim, H.J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Miliotti, E.; Casini, D.; Rosi, L.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Lab-scale pyrolysis and hydrothermal carbonization of biomass digestate: Characterization of solid products and compliance with biochar standards. Biomass Bioenergy 2020, 139, 105593. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Akaeme, F.C.; Georgin, J.; de Oliveira, J.S.; Franco, D.S.P. Biomass Hydrochar: A Critical Review of Process Chemistry, Synthesis Methodology, and Applications. Sustainability 2025, 17, 1660. [Google Scholar] [CrossRef]
- Kota, K.B.; Shenbagaraj, S.; Sharma, P.K.; Sharma, A.K.; Ghodke, P.K.; Chen, W.H. Biomass torrefaction: An overview of process and technology assessment based on global readiness level. Fuel 2022, 324, 124663. [Google Scholar] [CrossRef]
- Onyenwoke, C.; Tabil, L.G.; Mupondwa, E.; Cree, D.; Adapa, P. Effect of Torrefaction on the Physiochemical Properties of White Spruce Sawdust for Biofuel Production. Fuels 2023, 4, 111–131. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A. Fast pyrolysis of biomass thermally pretreated by torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 95–102. [Google Scholar] [CrossRef]
- Bukhsh, K.; Chen, C.; Su, G.; Dong, S.; Li, L.; Deng, S.; Li, X.; Ma, J.; Wang, X. Comparative advantages of biomass-derived biochars via torrefaction under flue gas and nitrogen atmosphere. J. Anal. Appl. Pyrolysis 2025, 190, 107120. [Google Scholar] [CrossRef]
- Salimbeni, A.; Di Bianca, M.; Lombardi, G.; Rizzo, A.M.; Chiaramonti, D. Opportunities of Integrating Slow Pyrolysis and Chemical Leaching for Extraction of Critical Raw Materials from Sewage Sludge. Water 2023, 15, 1060. [Google Scholar] [CrossRef]
- Mengesha, T.T.; Ancha, V.R.; Nigussie, A.; Afessa, M.M.; Bhandari, R. Effect of Particle Size and Heating Rate on Formation of Polycyclic Aromatic Hydrocarbons During Corn Cob Biomass Pyrolysis. Sustainability 2025, 17, 4962. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Tsekoa, P.; Kammies, L.R.D.; Chelechele, K.; Oluwalana-Sanusi, A.E.; Chaukura, N. Influence of biomass baseline potential on biochar properties and performance for targeted applications. Discov. Water 2025, 5, 77. [Google Scholar] [CrossRef]
- di Laurea, C.; Pinzuti, R.C.A. Biochar: Analysis and Economic Potential with a Focus on European Producers; Politecnico di Torino: Turin, Italy, 2023. [Google Scholar]
- Slezak, R.; Unyay, H.; Szufa, S.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Reactors Torrefaction vs. Biomass Pyrolizers—Part 2. Energies 2023, 16, 2212. [Google Scholar] [CrossRef]
- Gorshkov, A.; Berezikov, N.; Kaltaev, A.; Yankovsky, S.; Slyusarsky, K.; Tabakaev, R.; Larionov, K. Analysis of the Physicochemical Characteristics of Biochar Obtained by Slow Pyrolysis of Nut Shells in a Nitrogen Atmosphere. Energies 2021, 14, 8075. [Google Scholar] [CrossRef]
- Salimbeni, A.; Lombardi, G.; Rizzo, A.M.; Chiaramonti, D. Techno-Economic feasibility of integrating biomass slow pyrolysis in an EAF steelmaking site: A case study. Appl. Energy 2023, 339, 120991. [Google Scholar] [CrossRef]
- Afshar, M.; Mofatteh, S. Biochar for a sustainable future: Environmentally friendly production and diverse applications. Results Eng. 2024, 23, 102433. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Piersa, P.; Unyay, H.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Huang, J.; Feng, Y.; Xie, H.; Wu, P.; Wang, M.; Wang, B.; Zhang, Q.; Zhang, S.; Liu, Z. A bibliographic study reviewing the last decade of hydrochar in environmental application: History, status quo, and trending research paths. Biochar 2023, 5, 1–27. [Google Scholar] [CrossRef]
- Ercan, B.; Alper, K.; Ucar, S.; Karagoz, S. Comparative studies of hydrochars and biochars produced from lignocellulosic biomass via hydrothermal carbonization, torrefaction and pyrolysis. J. Energy Inst. 2023, 109, 101298. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Khan, N.; Gupta, A.; Ahamad, S.; Hussain, M.K.; Khan, M.U.; Siddiqui, Z.N. Functionalized biochar catalysts: Advancing green chemistry in synthesis of O- and N-heterocycles. Environ. Res. 2025, 284, 122136. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Tagliaferro, A. A Comprehensive Overview on Biochar-Based Materials for Catalytic Applications. Catalysts 2023, 13, 1336. [Google Scholar] [CrossRef]
- Hervy, M.; Berhanu, S.; Weiss-Hortala, E.; Chesnaud, A.; Gérente, C.; Villot, A.; Minh, D.P.; Thorel, A.; Le Coq, L.; Nzihou, A. Multi-scale characterisation of chars mineral species for tar cracking. Fuel 2017, 189, 88–97. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; Wang, S.; Sun, S.; Shang, Q.; Zhao, Y. Effect of biochar support on the catalytic performance of Fe-based catalysts for CH4 cracking. Fuel Process. Technol. 2023, 247, 107794. [Google Scholar] [CrossRef]
- Chellappan, S.; Nair, V.; Sajith, V.; Aparna, K. Synthesis, optimization and characterization of biochar based catalyst from sawdust for simultaneous esterification and transesterification. Chin. J. Chem. Eng. 2018, 26, 2654–2663. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kundu, S.; Halder, P.; Marzbali, M.H.; Chiang, K.; Surapaneni, A.; Shah, K. Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int. J. Hydrogen Energy 2020, 45, 29978–29992. [Google Scholar] [CrossRef]
- Prabowo, J.; Lai, L.; Chivers, B.; Burke, D.; Dinh, A.H.; Ye, L.; Wang, Y.; Wang, Y.; Wei, L.; Chen, Y. Solid carbon co-products from hydrogen production by methane pyrolysis: Current understandings and recent progress. Carbon 2024, 216, 118507. [Google Scholar] [CrossRef]
- Mirkarimi, S.M.R.; Bensaid, S.; Negro, V.; Chiaramonti, D. Review of methane cracking over carbon-based catalyst for energy and fuels. Renew. Sustain. Energy Rev. 2023, 187, 113747. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Zheng, W.; Leng, S.; Ai, Z.; Zhang, W.; Yang, Z.; Yang, J.; Xu, Z.; Cao, J.; et al. A complete review on the oxygen-containing functional groups of biochar: Formation mechanisms, detection methods, engineering, and applications. Sci. Total Environ. 2024, 946, 174081. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yu, J.; Li, W.; He, X.; Li, X. The Effects of Chemical Oxidation and High-Temperature Reduction on Surface Functional Groups and the Adsorption Performance of Biochar for Sulfamethoxazole Adsorption. Agronomy 2022, 12, 510. [Google Scholar] [CrossRef]
- Sharmila, V.G.; Tyagi, V.K.; Varjani, S.; Banu, J.R. A review on the lignocellulosic derived biochar-based catalyst in wastewater remediation: Advanced treatment technologies and machine learning tools. Bioresour. Technol. 2023, 387, 129587. [Google Scholar] [CrossRef] [PubMed]
- Azar, F.Z.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. Cellulose hydrolysis catalysed by mesoporous activated carbons functionalized under mild conditions. SN Appl. Sci. 2019, 1, 1739. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Chowdhury, A.A.; Almomani, F.; Khan, N.A.; Badruddin, I.A.; Kamangar, S. Biochar produced from waste-based feedstocks: Mechanisms, affecting factors, economy, utilization, challenges, and prospects. GCB Bioenergy 2024, 16, e13175. [Google Scholar] [CrossRef]
- Wang, J.; Jin, L.; Zhou, Y.; Li, Y.; Hu, H. Effect of Ca(NO3)2 addition in coal on properties of activated carbon for methane decomposition to hydrogen. Fuel Process. Technol. 2018, 176, 85–90. [Google Scholar] [CrossRef]
- Liu, F.; Xuan, G.; Ai, L.; Liu, Q.; Yang, L. Key factors that affect catalytic activity of activated carbon-based catalyst in chemical looping methane decomposition for H2 production. Fuel Process. Technol. 2021, 215, 106745. [Google Scholar] [CrossRef]
- Hamdani, I.R.; Ahmad, A.; Chulliyil, H.M.; Srinivasakannan, C.; Shoaibi, A.A.; Hossain, M.M. Thermocatalytic Decomposition of Methane: A Review on Carbon-Based Catalysts. ACS Omega 2023, 8, 28945–28967. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.U.; Nam, W.; Yoon, K.J.; Han, G.Y. Hydrogen production by catalytic decomposition of methane over carbon catalysts in a fluidized bed. Korean J. Chem. Eng. 2007, 24, 674–678. [Google Scholar] [CrossRef]
- Kim, M.H. Hydrogen production by catalytic decomposition of methane over activated carbons: Kinetic study. Int. J. Hydrogen Energy 2004, 29, 187–193. [Google Scholar] [CrossRef]
- Jin, Z.; Xiao, S.; Dong, H.; Xiao, J.; Tian, R.; Chen, J.; Li, Y.; Li, L. Adsorption and catalytic degradation of organic contaminants by biochar: Overlooked role of biochar’s particle size. J. Hazard. Mater. 2022, 422, 126928. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A critical review on production, modification and utilization of biochar. J. Anal. Appl. Pyrolysis 2021, 161, 105405. [Google Scholar] [CrossRef]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Dai, S.J.; Zhao, Y.C.; Niu, D.J.; Li, Q.; Chen, Y. Preparation and reactivation of magnetic biochar by molten salt method: Relevant performance for chlorine-containing pesticides abatement. J. Air Waste Manag. Assoc. 2019, 69, 58–70. [Google Scholar] [CrossRef]
- Wang, P.; Stenrød, M.; Wang, L.; Yuan, S.; Mao, L.; Zhu, L.; Zhang, L.; Zhang, Y.; Jiang, H.; Zheng, Y.; et al. Characterization of Montmorillonite–Biochar Composite and Its Application in the Removal of Atrazine in Aqueous Solution and Soil. Front. Environ. Sci. 2022, 10, 888252. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; Abdelmonem, N.M.; Ismail, I.M.A.; Ragab, S.; El Nemr, A. Ozone and Ammonium Hydroxide Modification of Biochar Prepared from Pisum sativum Peels Improves the Adsorption of Copper (II) from an Aqueous Medium. Environ. Process. 2020, 7, 973–1007. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Liu, Y.; Lin, X.; Wang, J.; Zhang, Z.; Li, N.; Li, Y.; Zhang, Y. KOH modification effectively enhances the Cd and Pb adsorption performance of N-enriched biochar derived from waste chicken feathers. Waste Manag. 2021, 130, 82–92. [Google Scholar] [CrossRef]
- Al Masud, A.; Shin, W.S.; Sarker, A.; Septian, A.; Das, K.; Deepo, D.M.; Iqbal, M.A.; Islam, A.R.M.T.; Malafaia, G. A critical review of sustainable application of biochar for green remediation: Research uncertainty and future directions. Sci. Total Environ. 2023, 904, 166813. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.; Ahamed, T.S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Peiris, C.; Nayanathara, O.; Navarathna, C.M.; Jayawardhana, Y.; Nawalage, S.; Burk, G.; Karunanayake, A.G.; Madduri, S.B.; Vithanage, M.; Kaumal, M.N.; et al. The influence of three acid modifications on the physicochemical characteristics of tea-waste biochar pyrolyzed at different temperatures: A comparative study. RSC Adv. 2019, 9, 17612–17622. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated carbon as catalyst support: Precursors, preparation, modification and characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Greenough, S.; Dumont, M.J.; Prasher, S. The physicochemical properties of biochar and its applicability as a filler in rubber composites: A review. Mater. Today Commun. 2021, 29, 102912. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface Chemical Functional Groups Modification of Porous Carbon. Recent Pat. Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Xiao, F. A review of biochar functionalized by thermal air oxidation. Environ. Funct. Mater. 2022, 1, 187–195. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lü, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef]
- Sun, Z.; Dai, L.; Lai, P.; Shen, F.; Shen, F.; Zhu, W. Air oxidation in surface engineering of biochar-based materials: A critical review. Carbon. Res. 2022, 1, 32. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.-C.; Cho, J.-S.; Lee, S.-E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Sinha, S.; Ojha, P. Liquid-phase adsorption of phenols using activated carbons derived from agricultural waste material. J. Hazard. Mater. 2008, 150, 626–641. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Veksha, A.; Bhuiyan, T.I.; Hill, J.M. Activation of Aspen Wood with Carbon Dioxide and Phosphoric Acid for Removal of Total Organic Carbon from Oil Sands Produced Water: Increasing the Yield with Bio-Oil Recycling. Materials 2016, 9, 20. [Google Scholar] [CrossRef]
- Bushra, B.; Remya, N. Biochar from pyrolysis of rice husk biomass—Characteristics, modification and environmental application. Biomass Convers. Biorefinery 2020, 14, 5759–5770. [Google Scholar] [CrossRef]
- Lu, X.; Sun, J.; Pan, G.; Qi, W.; Zhang, Z.; Xing, J.; Gao, Y. Ball-Milling-Modified Biochar with Additives Enhances Soil Cd Passivation, Increases Plant Growth and Restrains Cd Uptake by Chinese Cabbage. Horticulturae 2025, 11, 168. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.-B.; Liu, Y.-G.; Gu, Y.-L.; Zeng, G.-M.; Hu, X.-J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Dalai, A.K.; Azargohar, R. Production of activated carbon from biochar using chemical and physical activation: Mechanism and modeling. ACS Symp. Ser. 2007, 954, 463–476. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef]
- Ma, Y. Comparison of Activated Carbons Prepared from Wheat Straw via ZnCl2 and KOH Activation. Waste Biomass Valorization 2017, 8, 549–559. [Google Scholar] [CrossRef]
- Haque, M.; Islam, M.S. Preparation and characterization of activated carbon & amorphous silica from rice husk. Bangladesh J. Sci. Ind. Res. 2015, 50, 263–270. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef] [PubMed]
- Jedynak, K.; Charmas, B. Adsorption properties of biochars obtained by KOH activation. Adsorption 2024, 30, 167–183. [Google Scholar] [CrossRef]
- Jamil, U.; Zeeshan, M.; Khan, S.R.; Saeed, S. Synthesis and two-step KOH based activation of porous biochar of wheat straw and waste tire for adsorptive exclusion of chromium (VI) from aqueous solution; thermodynamic and regeneration study. J. Water Process Eng. 2023, 53, 103892. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wu, R.; Liu, Y.; Lin, X.; Kan, H.; Zheng, Y. Preparation of Acid- And Alkali-Modified Biochar for Removal of Methylene Blue Pigment. ACS Omega 2020, 5, 30906–30922. [Google Scholar] [CrossRef]
- Hafeez, A.; Pan, T.; Tian, J.; Cai, K. Modified Biochars and Their Effects on Soil Quality: A Review. Environments 2022, 9, 60. [Google Scholar] [CrossRef]
- Han, Y.; Zheng, J.; Jiang, C.; Zhang, F.; Wei, L.; Zhu, L. Hydrochloric Acid-Modified Algal Biochar for the Removal of Microcystis Aeruginosa: Coagulation Performance and Mechanism. SSRN Electron. J. 2022, 10, 108903. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, S.; Tan, X.; Zeng, G.; Zeng, W.; Ding, Y.; Cao, W.; Zheng, B. Enhanced adsorption of methylene blue by citric acid modification of biochar derived from water hyacinth (Eichornia crassipes). Environ. Sci. Pollut. Res. 2016, 23, 23606–23618. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, J.; Cui, Q.; Zhang, W.; Zhu, X. A feasible biochar derived from biogas residue and its application in the efficient adsorption of tetracycline from an aqueous solution. Environ. Res. 2022, 207, 112175. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in Preparation and Application of Modified Biochar for Improving Heavy Metal Ion Removal From Wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Li, B.; Wei, D.; Li, Z.; Zhou, Y.; Li, Y.; Huang, C.; Long, J.; Huang, H.; Tie, B.; Lei, M. Mechanistic insights into the enhanced removal of roxsarsone and its metabolites by a sludge-based, biochar supported zerovalent iron nanocomposite: Adsorption and redox transformation. J. Hazard. Mater. 2020, 389, 122091. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, W.; Zhou, X.; Shao, J.; Chen, Y.; Zhang, S.; Chen, H. Coeffect of pyrolysis temperature and potassium phosphate impregnation on characteristics, stability, and adsorption mechanism of phosphorus-enriched biochar. Bioresour. Technol. 2022, 344, 126273. [Google Scholar] [CrossRef]
- Zheng, Z.; Duan, X. Mitigating the Health Effects of Aqueous Cr(VI) with Iron-Modified Biochar. Int. J. Environ. Res. Public Health 2022, 19, 1481. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, X.; Wang, L.; Gao, B.; Luo, J.; Fang, R.; Zou, W.; Meng, N. Sorption of tetracycline on H2O2-modified biochar derived from rape stalk. Environ. Pollut. Bioavailab. 2019, 31, 198–207. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Chen, Y.; Cai, L.; Wang, Y.; Song, W. An abundant porous biochar material derived from wakame (Undaria pinnatifida) with high adsorption performance for three organic dyes. Bioresour. Technol. 2020, 318, 124082. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yakaboylu, G.A.; Yumak, T.; Zondlo, J.W.; Sabolsky, E.M.; Wang, J. Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew. Energy 2020, 155, 38–52. [Google Scholar] [CrossRef]
- Guo, F.; Peng, K.; Liang, S.; Jia, X.; Jiang, X.; Qian, L. Evaluation of the catalytic performance of different activated biochar catalysts for removal of tar from biomass pyrolysis. Fuel 2019, 258, 116204. [Google Scholar] [CrossRef]
- Wang, L.; Sun, F.; Hao, F.; Qu, Z.; Gao, J.; Liu, M.; Wang, K.; Zhao, G.; Qin, Y. A green trace K2CO3 induced catalytic activation strategy for developing coal-converted activated carbon as advanced candidate for CO2 adsorption and supercapacitors. Chem. Eng. J. 2020, 383, 123205. [Google Scholar] [CrossRef]
- Lobos, M.L.N.; Sieben, J.M.; Comignani, V.; Duarte, M.; Volpe, M.A.; Moyano, E.L. Biochar from pyrolysis of cellulose: An alternative catalyst support for the electro-oxidation of methanol. Int. J. Hydrogen Energy 2016, 41, 10695–10706. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.R.; Duimovich, N.; Tiraferri, A.; Laurell, P.; Borghei, M.; Zimmerman, J.B.; Vahala, R. Tailored mesoporous biochar sorbents from pinecone biomass for the adsorption of natural organic matter from lake water. J. Mol. Liq. 2019, 291, 111248. [Google Scholar] [CrossRef]
- Premchand, P.; Demichelis, F.; Galletti, C.; Chiaramonti, D.; Bensaid, S.; Antunes, E.; Fino, D. Enhancing biochar production: A technical analysis of the combined influence of chemical activation (KOH and NaOH) and pyrolysis atmospheres (N2/CO2) on yields and properties of rice husk-derived biochar. J. Environ. Manag. 2024, 370, 123034. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, J.H.; Ahmed, M.A.; Choi, J.W. Evaluation of pyrochar and hydrochar derived activated carbons for biosorbent and supercapacitor materials. J. Environ. Manag. 2021, 298, 113436. [Google Scholar] [CrossRef]
- Yang, H.; Cui, Y.; Jin, Y.; Lu, X.; Han, T.; Sandström, L.; Jönsson, P.G.; Yang, W. Evaluation of Engineered Biochar-Based Catalysts for Syngas Production in a Biomass Pyrolysis and Catalytic Reforming Process. Energy Fuels 2023, 37, 5942–5952. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Wang, Y.; Li, R.X.; Cao, H.L.; Li, Y.F.; Lü, J. Impacts of temperatures and phosphoric-acid modification to the physicochemical properties of biochar for excellent sulfadiazine adsorption. Biochar 2022, 4, 14. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. The effects of chemical modification on adsorbent performance on water and wastewater treatment—A review. Bioresour. Technol. Rep. 2022, 20, 101259. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2018, 35, 777–815. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Álvarez, M.L.; Białowiec, A.; Jerzykiewicz, M. Characterization and Sodium Cations Sorption Capacity of Chemically Modified Biochars Produced from Agricultural and Forestry Wastes. Materials 2021, 14, 4714. [Google Scholar] [CrossRef]
- Chew, T.W.; H’ng, P.S.; Abdullah, B.C.T.G.L.C.; Chin, K.L.; Lee, C.L.; Hafizuddin, B.M.S.M.N.; TaungMai, L. A Review of Bio-Based Activated Carbon Properties Produced from Different Activating Chemicals during Chemicals Activation Process on Biomass and Its Potential for Malaysia. Materials 2023, 16, 7365. [Google Scholar] [CrossRef]
- Pinij, P.; Tippayawong, N.; Chimupala, Y.; Chaiklangmuang, S. Performances of functional groups and KOH-transformation in corn stover waste through catalytic pyrolysis. J. Anal. Appl. Pyrolysis 2021, 157, 105234. [Google Scholar] [CrossRef]
- Hafizuddin, M.S.; Lee, C.L.; Chin, K.L.; H’ng, P.S.; Khoo, P.S.; Rashid, U. Fabrication of Highly Microporous Structure Activated Carbon via Surface Modification with Sodium Hydroxide. Polymers 2021, 13, 3954. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Xu, Z.; He, M.; Xu, X.; Cao, X.; Tsang, D.C.W. Impacts of different activation processes on the carbon stability of biochar for oxidation resistance. Bioresour. Technol. 2021, 338, 125555. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.V.N.; Arun, J.; Gopinath, K.P. Production, characterization, activation and environmental applications of engineered biochar: A review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.U.; Summers, R.S.; Cook, S.M. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef]
- Kołtowski, M.; Charmas, B.; Skubiszewska-Zięba, J.; Oleszczuk, P. Effect of biochar activation by different methods on toxicity of soil contaminated by industrial activity. Ecotoxicol. Environ. Saf. 2017, 136, 119–125. [Google Scholar] [CrossRef]
- Ali, H.; Ali, S.; Baloch, S.; Naheed, F.; Amjad, E.; Saeed, Q.; Naveed, M.; Mustafa, A. Biochar Application for Soil Quality Improvement: An Overview; Intechopen: London, UK, 2024. [Google Scholar] [CrossRef]
- Bai, Z.; Li, W.; Bai, J.; Li, B.; Chen, H. The Effects of Textural Properties and Surface Chemistry of Activated Carbon on Its Catalytic Performance in Methane Decomposition for Hydrogen Production. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 1145–1153. [Google Scholar] [CrossRef]
- Pham, C.Q.; Siang, T.J.; Kumar, P.S.; Ahmad, Z.; Xiao, L.; Bahari, M.B.; Cao, A.N.T.; Rajamohan, N.; Qazaq, A.S.; Kumar, A.; et al. Production of hydrogen and value-added carbon materials by catalytic methane decomposition: A review. Environ. Chem. Lett. 2022, 20, 2339–2359. [Google Scholar] [CrossRef]
- Harun, K.; Adhikari, S.; Jahromi, H. Hydrogen production via thermocatalytic decomposition of methane using carbon-based catalysts. RSC Adv. 2020, 10, 40882–40893. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.F.; Daud, W.M.A.W. Influence of reactor material and activated carbon on the thermocatalytic decomposition of methane for hydrogen production. Appl. Catal. A Gen. 2010, 388, 232–239. [Google Scholar] [CrossRef]
- Bai, Z.; Chen, H.; Li, W.; Li, B. Hydrogen production by methane decomposition over coal char. Int. J. Hydrogen Energy 2006, 31, 899–905. [Google Scholar] [CrossRef]
- Mani, S.; Kastner, J.R.; Juneja, A. Catalytic decomposition of toluene using a biomass derived catalyst. Fuel Process. Technol. 2013, 114, 118–125. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Kumar, S.; Soomro, S.A.; Harijan, K.; Uqaili, M.A.; Kumar, L. Advancements of Biochar-Based Catalyst for Improved Production of Biodiesel: A Comprehensive Review. Energies 2023, 16, 644. [Google Scholar] [CrossRef]
- Park, G.; Lee, D.-J.; Kwon, D.; Kim, J.Y.; Jung, S.; Tsang, Y.F.; Kwon, E.E. Use of biochar as a catalyst for biodiesel production. J. Ind. Eng. Chem. 2025, 142, 408–415. [Google Scholar] [CrossRef]
- di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- Jung, J.M.; Oh, J.I.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Jung, J.M.; Oh, J.I.; Park, Y.K.; Lee, J.; Kwon, E.E. CO2-mediated chicken manure biochar manipulation for biodiesel production. Environ. Res. 2019, 171, 348–355. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Cao, J.-P.; Liu, T.-L.; Ren, J.; Zhao, X.-Y.; Wu, Y.; Wang, J.-X.; Ren, X.-Y.; Wei, X.-Y. Preparation and characterization of nickel loaded on resin char as tar reforming catalyst for biomass gasification. J. Anal. Appl. Pyrolysis 2017, 127, 82–90. [Google Scholar] [CrossRef]
- Asadullah, M.; Ito, S.I.; Kunimori, K.; Yamada, M.; Tomishige, K. Biomass Gasification to Hydrogen and Syngas at Low Temperature: Novel Catalytic System Using Fluidized-Bed Reactor. J. Catal. 2002, 208, 255–259. [Google Scholar] [CrossRef]
- Yang, G.; Hu, Q.; Hu, J.; Yang, H.; Yan, S.; Chen, Y.; Wang, X.; Chen, H. Hydrogen-rich syngas production from biomass gasification using biochar-based nanocatalysts. Bioresour. Technol. 2023, 379, 129005. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J. Hydrocarbon and hydrogen-rich syngas production by biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts. RSC Adv. 2014, 4, 10731–10737. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Chandola, D.; Rana, S.; Chandola, D.; Rana, S. Biochar for Environmental Remediation. In Biochar-Productive Technologies, Properties and Applications; Intechopen: London, UK, 2022. [Google Scholar] [CrossRef]
- Gasim, M.F.; Choong, Z.-Y.; Koo, P.-L.; Low, S.-C.; Abdurahman, M.-H.; Ho, Y.-C.; Mohamad, M.; Suryawan, I.W.K.; Lim, J.-W.; Oh, W.-D. Application of Biochar as Functional Material for Remediation of Organic Pollutants in Water: An Overview. Catalysts 2022, 12, 210. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Li, J.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.; Wang, S.; Song, H.; et al. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon Res. 2022, 1, 4. [Google Scholar] [CrossRef]
- Barragán-Mantilla, S.P.; Gascó, G.; Méndez, A. Perspectives on the use of biochar in the valorization of mining wastes from sulfide minerals flotation: Recovery of metals and effects on toxicity. Waste Manag. 2023, 171, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Z.-F.; Pan, X.-W.; Tan, J.-Y.; Yang, S.-S.; Wu, J.-T.; Chen, C.; Yuan, Y.; Ren, N.-Q. Sewage sludge derived biochar for environmental improvement: Advances, challenges, and solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kumari, U.; Meikap, B.C. A Review of Chemical Leaching of Coal by Acid and Alkali Solution. J. Min. Metall. 2018, 54, 1–24. [Google Scholar] [CrossRef]
- Pochampally, S.V.; Blanco, J.G.; Ayalew, K.; Murph, S.E.H.; Moon, J. Novel alkali intercalated and acid-exfoliated biochars with enhanced surface areas for contaminant adsorption applications. Sep. Purif. Technol. 2024, 350, 127793. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, H.; Liang, S.; Yang, L.; Gan, Q.; Tao, S.; Yu, W.; Lv, R.; Ding, L.; Xiao, K.; et al. Hierarchically porous biochar preparation and simultaneous nutrient recovery from sewage sludge via three steps of alkali-activated pyrolysis, water leaching and acid leaching. Resour. Conserv. Recycl. 2022, 176, 105953. [Google Scholar] [CrossRef]
- Rahman, M.; Pudasainee, D.; Gupta, R. Review on chemical upgrading of coal: Production processes, potential applications and recent developments. Fuel Process. Technol. 2017, 158, 35–56. [Google Scholar] [CrossRef]
- Jama-Rodzeńska, A.; Sowiński, J.; Koziel, J.A.; Białowiec, A. Phosphorus recovery from sewage sludge ash based on cradle-to-cradle approach—Mini-review. Minerals 2021, 11, 985. [Google Scholar] [CrossRef]
- Salimbeni, A.; Di Bianca, M.; Rizzo, A.M.; Chiaramonti, D. Activated Carbon and P-Rich Fertilizer Production from Industrial Sludge by Application of an Integrated Thermo-Chemical Treatment. Sustainability 2023, 15, 14620. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Santos, D.C.B.D.; Evaristo, R.B.W.; Dutra, R.C.; Suarez, P.A.Z.; Silveira, E.A.; Ghesti, G.F. Advancing Biochar Applications: A Review of Production Processes, Analytical Methods, Decision Criteria, and Pathways for Scalability and Certification. Sustainability 2025, 17, 2685. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, C.; Zhang, Y.; Lu, L.; Xu, D.; Peng, X. Biochar modification methods and mechanisms for salt-affected soil and saline-alkali soil improvement: A review. Soil Use Manag. 2024, 40, e12992. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Xiang, L.; Dou, Q.; Liu, Y.; Wang, M.; Wen, X.; Fu, Y.; Ul Islam, M.; Chang, S.X.; et al. Effects of ball milling on biochar adsorption of contaminants in water: A meta-analysis. Sci. Total Environ. 2023, 882, 163643. [Google Scholar] [CrossRef]
- Jokubė, M.; Hyyrynen, M.; Pihlainen, S.; Hyytiäinen, K. Economic feasibility of biochar for carbon stock enhancement in Finnish agricultural soils. Carbon Manag. 2025, 16, 2465328. [Google Scholar] [CrossRef]
- Aguirre, J.L.; Martín, M.T.; González, S.; Peinado, M. Effects and Economic Sustainability of Biochar Application on Corn Production in a Mediterranean Climate. Molecules 2021, 26, 3313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).