Abstract
This study addresses water scarcity in arid regions by developing low-temperature-sintered porous ceramics for agricultural water management, utilizing coal gangue solid waste as the primary resource. Systematic single-factor experiments first identified the optimal sintering temperature (615 °C) and polystyrene content (25%) that critically balance pore formation and structural integrity. Building on this, orthogonal experiment optimization yielded an optimal formulation exhibiting exceptional comprehensive performance (coal gangue 20 g, starch 25 g, glass powder 11 g, polystyrene 27 g): 149.70% water absorption, 57.75 h water retention, 77.28% porosity, and 0.55 MPa compressive strength. The material’s graded pore structure, achieved through composite pore-formers (polystyrene/starch) and diatomaceous earth, underlies its enhanced capillary action. The pot experiment of Chinese cabbage confirmed its effect, shortened the emergence time of seedlings to <24 h, and significantly improved the emergence rate and the growth of seedlings in the early stage (7 days). This work provides a new way for the value of coal gangue in dryland agriculture and ecological restoration.
1. Introduction
Dryland ecosystems are a critical component of the global ecosystem, yet they are highly vulnerable to the impacts of human activities and climate change [1,2,3]. Water scarcity, a pressing global issue affecting over 40% of the population and sustaining about 35% of terrestrial biodiversity, severely restricts vegetation growth in these regions [4,5]. Therefore, developing effective strategies for water conservation and efficient utilization is paramount for dryland agriculture and ecological restoration. While modern water-saving irrigation technologies and soil mulching practices (e.g., plastic films) can significantly reduce water evaporation [6,7], the non-biodegradable nature of conventional plastic mulches poses serious threats to soil health [8,9,10]. Alternative mulching materials, such as natural minerals and ceramic grains, have been explored [11,12]. However, unburned terra cotta often requires lengthy curing periods (14–28 days) [13,14,15], while sintered versions typically demand high firing temperatures [16,17]. More importantly, their relatively low water absorption capacity (30–60%) limits their effectiveness in water retention [18,19].
In this context, porous ceramics emerge as a highly promising alternative due to their architecturally tunable, interconnected pore networks. These microstructures generate strong capillary forces, endowing the materials with exceptional water absorption and prolonged retention capabilities—properties crucial for supporting plant growth in arid environments [19,20]. Although porous materials are widely used as catalytic carriers, filters, and thermal insulators [21,22,23,24,25,26,27,28], their application in agriculture is often hindered by high production costs associated with conventional high-temperature sintering. This underscores the need for developing low-temperature fabrication routes.
Concurrently, the pursuit of sustainable materials motivates the valorization of industrial solid waste. Coal gangue (CG), a primary by-product of coal mining, presents significant environmental challenges, including land occupation, risks of spontaneous combustion, and potential heavy metal leaching. In China alone, annual production reaches hundreds of millions of tons, with historical stockpiles amounting to billions of tons, highlighting an urgent need for large-scale, high-value utilization strategies [20,29,30]. Using CG as the main raw material for functional porous ceramics represents a synergistic solution that addresses both solid waste management and the need for advanced agricultural materials.
The properties of porous ceramics are profoundly influenced by their composition and pore structure. In this study, a multi-component material system was designed to optimize performance. Polystyrene (PS) acts as a primary pore-former; its spherical morphology, low packing density, and high burnout rate facilitate the creation of abundant macropores without leaving carbon residue [31,32]. Starch serves a dual role: as a binder providing green strength during forming due to its hydration gelling properties [33,34] and as a secondary pore-former. Diatomaceous earth, a naturally porous material, contributes a multitude of intrinsic nano- to micro-scale pores, significantly enhancing the specific surface area and water absorption capacity [35,36]. Furthermore, the incorporation of glass powder is critical for achieving low-temperature sintering. As an amorphous material, glass softens at relatively low temperatures, facilitating liquid-phase sintering that bonds the ceramic skeleton without requiring excessive energy [37,38]. This combination of raw materials aims to create a hierarchical pore structure for superior water management.
Therefore, this study bridges the gap between waste valorization and agricultural innovation by developing novel, low-temperature-sintered porous ceramics from CG. We systematically investigated the effects of sintering temperature and pore-forming agent content on the water absorption, retention time, and compressive strength. Orthogonal experiments and regression analysis were employed to optimize the proportions of CG, starch, glass powder, and diatomaceous earth. The optimally formulated ceramics (CGPC) were subsequently evaluated in pot experiments with Chinese cabbage to assess their effectiveness in promoting seedling emergence and growth in both soil and standard sand matrices. The primary objective of this study is to expand the range of applications for CG, leverage its potential for high-value utilization, and evaluate its suitability for implementation in agriculture and ecological restoration in arid regions.
2. Experimental Section
2.1. Raw Materials
The CG, sourced from Yangquan, Shanxi Province, appeared gray–black in block form. It was crushed and sieved through a 200-mesh sieve prior to use. The diatomite powder originated from Inner Mongolia, China. Glass powder was obtained from Datong, Shanxi Province. Soluble starch was supplied by Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China) Standard sand meets the standard: GB/T 14684-2022 [39].
The PS used in this experiment is self-made in the laboratory. The preparation process is as follows: using styrene and α—methacrylic acid as raw materials, the volume ratio is 6:1, 100 mL ultra-pure water is added per 6 mL of styrene as the medium, and an oil bath at 75 °C is added in a magnetic heating mixer. After the mixture is stabilized in a three-neck flask for half an hour, 0.14 g potassium persulfate is added as the activator. Then, the mouth of the flask was closed to isolate the air for the complete reaction. After 8 h of reaction, the obtained PS solution was centrifuged, washed, and dried in a blast drying oven at 60 °C until only the PS blocks remained in the centrifuge tube. The prepared PS block was ground into powder with a mortar and pestle.
2.2. CG-Based Porous Ceramic Preparation
Figure 1 illustrates the fabrication process of Coal gangue-based porous ceramics. Initially, predetermined ratio of glass powder (8.24–30%), diatomite (1.47–12.5%), PS (15–30%), CG powder (15.96–46.39%), and starch (6.76–30%) were homogenized through mortar grinding to prepare preforms (unsintered samples). Subsequently, distilled water was added to the mixture, followed by thorough stirring with a glass rod to form a ceramic slurry. The slurry was poured into molds, where its fluidity enabled complete mold filling under gravitational force. The mold-slurry assembly was then dried in an oven at 60 °C for ≥6 h, after which the green body could be easily demolded through gentle vibration. Finally, the green body was sintered in a tubular furnace under programmed thermal conditions: raise the temperature to the target temperature at the rate of 2 °C/min in air atmosphere (600–630 °C), hold for 2 h, and then cool to room temperature at the rate of 5 °C/min. The sintered product constituted the final experimental material.

Figure 1.
Preparation flow of Coal gangue-based porous ceramics.
2.3. Characterization
The crystalline phases of the raw materials and fabricated samples were analysed via X-ray diffraction (XRD; Aeris, Malvern Panalytical, Westborough, MA, USA). X-ray fluorescence spectrometers (XRF, Axios, Malvern Panalytical, Westborough, MA, USA), were employed to analyze the elemental composition and content of the raw materials. Thermogravimetric-differential scanning calorimetry (TG-DSC; STA449F5, NETZSCH, Selb, Germany) was performed on the key raw materials (CG, starch, and PS) from 50 to 700 °C under a flowing air atmosphere at a heating rate of 10 °C/min. The microstructures of the raw materials and the sintered ceramics were examined by scanning electron microscopy (SEM; TESCAN MIRA LMS, Brno, Czech Republic). The compressive strength of the sintered porous ceramic samples was evaluated using a universal testing machine (MTS-e45.105, MTS, Shenzhen, China). All mass measurements are performed using the balance (Zhuojing, BSM-220.4, Shanghai, China) and the data management software attached to the machine.
2.4. Measurement of the Water Absorption and Water Retention Capacity of Coal Gangue-Based Porous Ceramics
The water absorption () and apparent porosity () of the porous ceramics were determined using the Archimedes method (boiling water method) according to a standard protocol [30]. The masses of the porous ceramic sample in three critical states were measured using an analytical balance (Zhuojing, BSM-220.4, Shanghai, China), all reported in grams (g):
Here, m1: The dry mass of the sample after being oven-dried at 105 °C to a constant weight. m2: The apparent mass of the sample when it is fully immersed and suspended in deionized water. This measurement is obtained by suspending the sample in water using a fine thread attached to the balance, ensuring it is completely submerged without touching the container’s walls or bottom. m3: The mass of the water-saturated sample after its pores are filled with water and the surface moisture is carefully removed. The saturation process involves boiling the sample in deionized water for 2 h and then allowing it to cool in the water for an additional 4 h to ensure complete pore filling. After saturation, the sample is taken out, and the surface water is gently blotted away using a damp, lint-free cloth until no visible water film remains on the surface.
The water retention performance was evaluated in a controlled environment maintained at 25 ± 2 °C with 93 ± 2% relative humidity. An analytical balance equipped with integrated data-logging software was employed to monitor mass variations. The testing protocol involved automated mass recording at 15 min intervals until four consecutive stable readings were achieved, indicating the completion of the moisture release process.
2.5. Orthogonal Experiments
The orthogonal experiments were conducted to optimize the comprehensive performance of Coal gangue-based porous ceramics after determining the optimal PS content (25%) and sintering temperature (615 °C) through single-factor experiments.
The primary objective of the orthogonal experimental design was to systematically investigate the effects of four key factors on the performance indicators of porous ceramics, including water absorption rate, water retention time, porosity, and compressive strength. Based on the preliminary single-factor experiments, the PS content and sintering temperature were fixed at optimal values, and the orthogonal array was employed to optimize the proportions of the remaining components.
2.6. Pot Experiments with Coal Gangue-Based Porous Ceramics
Potting experiments were conducted using soil and standard sand collected from the campus. The loess was then sieved through a 20-mesh sieve to remove any extraneous materials, such as small stones and plant roots, from the loess. The sieved loess and standard sand were subjected to a drying process in a blast drying oven for a period of 12 h. Following this, the materials were prepared for use. The prepared gangue-based porous ceramics were mixed with soil and standard sand to form a soil substrate for planting fast-growing Chinese cabbage. The growth conditions of the cabbages were meticulously documented at three distinct time points: 1 d, 3 d, and 7 d postplanting.
Pot experiments were carried out on the soil matrix composed of CG based porous ceramics and standard sand, as well as CG based porous ceramics and the campus soil of Taiyuan University of Technology. The experiment used plastic cans, each with a diameter of 8 cm and a height of 12 cm. The CG-based porous ceramic sample was covered on the seed planting layer, and its mass ratio with soil or standard sand was 1:9. This application method establishes the role of porous ceramics as a surface mulch for the seed planting layer, rather than a soil amendment mixed into the bulk soil. The experiment was conducted at room temperature, and 100 fast-growing Chinese cabbage seeds were sown in each pot. A control group was established for standard sand and soil without adding CG based ceramic samples. Water once every 24 h and add 50 mL water to each basin each time.
Plant growth was evaluated based on two key indicators: seedling emergence rate and above-ground growth. The emergence rate was recorded daily for 7 days. After 7 days, all seedlings were carefully harvested. The following parameters were measured or calculated:
Seedling Emergence Rate (%): This was defined as the percentage of sown seeds that successfully emerged and developed cotyledons above the soil/sand surface. The emergence rate was calculated daily to track the speed of germination and finally determined at Day 7 using the following formula:
Emergence Rate (%) = (Number of Emerged Seedlings / Total Seeds Sown) × 100%.
Above-ground Height (cm): The height of the seedlings was measured from the base of the stem (at the soil/sand surface) to the tip of the longest leaf. The average above-ground height per pot was then calculated and used for statistical analysis.
3. Results and Discussion
3.1. Raw Material Characterization
The chemical compositions of CG and diatomite are summarized in Table 1, while Figure 2a displays the XRD patterns of CG and diatomite. CG primarily consists of SiO2 and Al2O3, with additional transition metal oxides, including Fe2O3, and trace alkali/alkaline earth metal oxides (e.g., K2O and CaO). These components predominantly exist in crystalline phases such as quartz and kaolinite. Diatomite, a naturally porous material, has a SiO2-dominated composition with cristobalite as its primary crystalline phase. As shown in Figure 2b, the raw CG powder consists of irregular, angular particles with a wide size distribution. Starch particles (Figure 2c) exhibit characteristic spherical and oval shapes with smooth surfaces. The synthesized PS (PS) particles (Figure 2d) appear as spherical beads with a relatively uniform size, which is ideal for creating regular pores upon burnout. Diatomaceous earth (Figure 2e) displays its inherent fossilized, porous structure with a high specific surface area, contributing to its water absorption capacity. The spherical morphology of the PS and starch particles is advantageous for generating well-defined macropores, while the intricate porous structure of diatomite contributes to the development of micro- and nano-scale porosity, collectively facilitating the formation of a hierarchical pore network in the final ceramic product.

Table 1.
Chemical compositions of CG and diatomite.
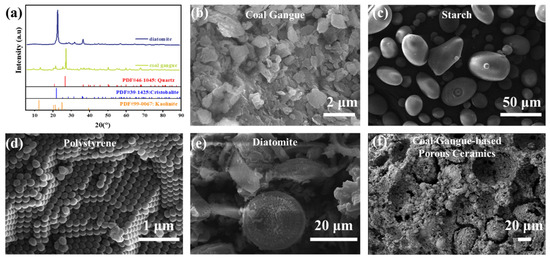
Figure 2.
(a) XRD images of CG and diatomite. SEM images of (b) CG, (c) starch, (d) PS, (e) diatomaceous, and (f) Coal gangue-based porous ceramics.
3.2. Effects of Sintering Temperature and PS Addition on the Physical Phase and Properties of Coal Gangue-Based Porous Ceramics
3.2.1. Determination of the Sintering Temperature
The TG results for CG, starch, and PS are shown in Figure 3. Starting at 400 °C, carbon, organic impurities, and interlayer water in CG begin to decompose, causing significant mass reduction. The mass gradually stabilized after the temperature exceeded 600 °C, with a total mass loss of 30.6% at 625 °C.
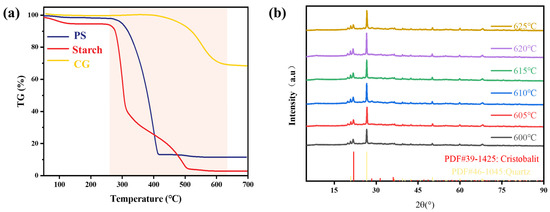
Figure 3.
(a) TG images of CG, starch and PS. (b) XRD patterns of samples sintered at different temperatures.
Starch molecules contain abundant hydroxyl groups that are hydrophilic, leading to surface-adsorbed water. When the temperature increases to 40–120 °C, these physically adsorbed water molecules gradually desorb, resulting in mass loss. As shown in Figure 3a, the weight loss rate reached approximately 5.2% at 120 °C. Chemical bond cleavage and macromolecular decomposition occur between 260 °C and 500 °C. Owing to variations in bond types and stability within starch molecules, less stable bonds decompose first during the initial phase, causing rapid weight loss. More stable bonds break progressively in later stages, slowing the loss rate until the mass stabilizes above 500 °C, with a total weight loss of ~94.1%.
PS exhibits a steep single-stage weight loss between 300 °C and 400 °C, which is attributed to superimposed oxidative decomposition and combustion processes. The weight loss rate reached 87% at 400 °C, leaving residues likely composed of additives and inorganic impurities.
3.2.2. Effects of Temperature on the Physical Phase of Coal Gangue-Based Porous Ceramics
The XRD patterns of samples containing 25% PS (PS) and sintered at various temperatures are presented in Figure 3b. This specific PS content was selected for phase analysis because preliminary single-factor experiments indicated that it yielded porous ceramics with optimal comprehensive performance, exhibiting a superior balance of high water absorption, prolonged water retention time, and acceptable compressive strength, as detailed in Section 3.2.4. All samples exhibit similar phase compositions dominated by quartz and cristobalite. At 600 °C, the quartz diffraction peaks appear relatively weak but gradually intensify with increasing temperature to 625 °C, indicating increased crystallinity. Partial phase transformation from cristobalite (originally present in diatomite) to quartz likely occurred during sintering, as evidenced by reduced cristobalite peak intensities. Kaolinite within CG underwent dehydroxylation at 500–600 °C, forming amorphous metakaolin [37], which eliminated its characteristic diffraction peaks. Starch and PS decomposed completely during sintering, leaving no detectable crystalline phases. Consequently, the overall phase composition remains largely unaffected by variations in the sintering temperature, suggesting a minimal correlation between the phase structure and material performance.
3.2.3. Effects of Temperature on the Properties of Coal Gangue-Based Porous Ceramics
The experimental findings demonstrate that temperature substantially influences the rate of water absorption. A 5 °C temperature fluctuation has been observed to induce a notable variation in the water absorption rate.
The effect of the sintering temperature on water absorption is shown in Figure 4a. The overall water absorption increased and then decreased as the sintering temperature increased from 600 °C to 625 °C, with a distinct maximum observed at 615 °C. This turning point is fundamentally governed by the interplay between the decomposition of pore-forming agents and the sintering behavior of the glass phase, as revealed by our TG analysis and supported by literature.
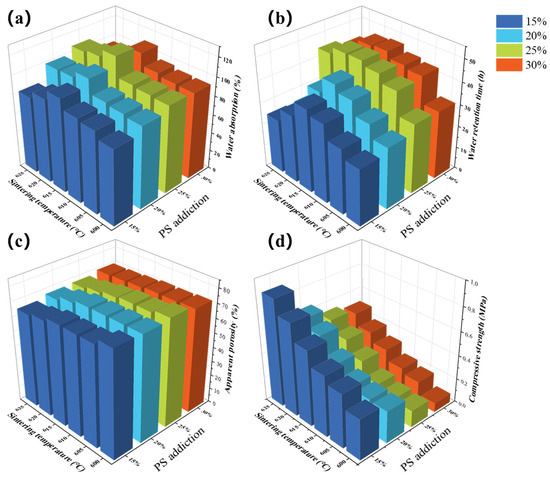
Figure 4.
(a) Water absorption, (b) water retention time, (c) apparent porosity, and (d) compressive strength at different sintering temperatures and PS additions.
The initial increase in water absorption between 600 °C and 615 °C can be attributed to the progressive and complete removal of the pore-forming agents. The TG results in Figure 3a indicate that the major mass loss events for starch (~94.1%) and PS (~87%) are complete by approximately 500 °C and 400 °C, respectively. Furthermore, the mass of the CG, which contains combustible carbon and organic impurities, stabilizes only after 600 °C, with a total mass loss of 30.6% at 625 °C. Therefore, sintering within the 600–615 °C range ensures the full burnout of all organic components, creating a maximal volume of open pores without the risk of carbon residue that could hinder sintering [33]. This process effectively increases the porosity and interconnectivity of the pore network, thereby enhancing the water absorption capacity.
However, when the temperature exceeds the optimum of 615 °C, the water absorption decreases. This is due to the onset of significant viscous flow sintering of the glass powder present in the mixture. At these elevated temperatures, the viscosity of the softened glass phase decreases substantially, increasing its mobility. This leads to the aggregation and flow of the glass phase, which begins to cover, fill, and smooth over the pores created by the decomposed pore-formers. This phenomenon effectively transforms open pores into closed pores and reduces the overall porosity, as visually confirmed by the microstructural evolution seen in the SEM images (Figure 5d–f). The consequent reduction in open porosity and pore connectivity diminishes the material’s capillary force for water absorption. This observation aligns with findings reported by Yang et al. [37] and Chen et al. [20], who also noted that exceeding the optimal sintering temperature in glass-containing porous ceramics leads to pore coarsening and closure, compromising functional properties like water absorption.
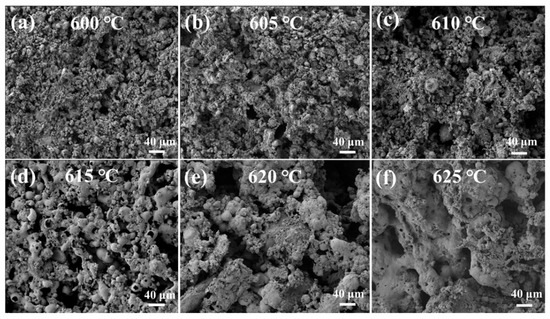
Figure 5.
SEM images of samples sintered at different temperatures: (a) 600 °C, (b) 605 °C, (c) 610 °C, (d) 615 °C, (e) 620 °C, and (f) 625 °C.
Consequently, 615 °C represents the critical balance point where the pore-forming agents have been completely removed, maximizing pore creation, but before the glass phase undergoes excessive flow that degrades the porous structure. This balance is crucial for achieving peak water absorption performance.
The water retention performance of the prepared samples was assessed by subjecting them to a 24 h water absorption period, followed by a natural water loss process at 25 °C and 93% relative humidity. The results of this assessment are presented in Figure 4b. The water retention times of the samples at various temperatures significantly varied. Overall, the water retention time increased but then decreased at elevated temperatures. The longest water retention time was observed for all the samples at 615 °C. The experimental findings indicate a positive correlation between the water retention time and water absorption rate, suggesting that a higher water absorption rate is associated with a longer water retention time. The rate of water release is directly proportional to the amount of water absorbed by the sample; that is, the longer the water is retained, the longer it will take to release. This finding aligns with the observations reported by Zheng et al. [19]. The experimental results indicated a substantial increase in water absorption when the temperature increased from 610 °C to 615 °C. However, the relative increase in water retention time was not significant.
Figure 4c shows the impact of temperature on the porosity of the porous ceramics with various PS contents. The porosity of the porous ceramics gradually decreased with increasing temperature in response to varying PS additions. This phenomenon can be attributed to the pore-forming agent present in the raw material, which ceases to lose weight prior to the glass powder attaining its softening temperature. Upon reaching this temperature, the glass powder produces a liquid glass phase that interconnects the CG and the diatomaceous earth, which have been separated in the raw material. This process results in the formation of a porous ceramic skeleton. As the sintering temperature increased, the degree of softening of the glass powder increased concomitantly. The viscosity of the glass powder mixed with CG and diatomaceous earth decreases as the mixture heats, resulting in increased mobility. This, in turn, leads to slumping and the production of a pore-making agent that fills the pores. Consequently, an increase in the sintering temperature results in a decrease in porosity, and vice versa.
The compressive strength is a critical indicator of the mechanical properties of porous ceramics, and a certain degree of compressive strength is advantageous for the transportation and application of porous ceramics. The effect of varying sintering temperatures on the compressive strength of PS-containing ceramics is illustrated in Figure 4d. Overall, the compressive strength of these materials gradually increases with increasing sintering temperature. For example, when 15% PS was incorporated, the compressive strength was 0.34 MPa at a sintering temperature of 600 °C. As the sintering temperature increased, the compressive strength gradually increased, ultimately reaching a maximum of 0.89 MPa at 625 °C.
SEM images of the samples with various sintering temperatures at 25% PS content are presented in Figure 5. As demonstrated in Figure 5, the melting of the glass powder becomes increasingly intense with increasing temperature. At temperatures below 615 °C, the glass powder exhibited reduced softening and mobility. Consequently, CG and diatomaceous earth, following sintering, are not entirely encased within the melted glass phase. Instead, they are merely interwoven within the glass powder matrix. The process of sintering is known to reach temperatures of 615 °C. This is achieved by the presence of powder particles of varying sizes and shapes within the sintering neck. The sintering neck is a crucial component in this process, as it facilitates the connection of powder particles of different sizes, thereby enabling sintering. Increasing the sintering temperature has been shown to result in the gradual combination of smaller powder particles with larger particles. This process appears to cause the sharp, coarse particles to round and fuse with the surrounding agglomerates of smaller particles. This process results in homogenization of the internal structure, leading to a transition from point contact to surface contact between the particles. This transition serves to mitigate the uneven binding phenomenon, which is often attributed to disparities in the morphology of the powder particles and variations in their dimensions. This approach not only addresses the uneven distribution of pores but also enhances the internal skeleton structure of porous ceramics. When the temperature exceeds 615 °C, the degree of softening of the glass powder increases. Concurrently, the viscosity of the glass powder decreases, the fluidity becomes stronger, and the liquid phase flow plugs the holes left by the pore-making agent.
3.2.4. Effects of PS Addition on the Properties of Porous Ceramics
Considering the PS addition variable, as depicted in Figure 4a, the water absorption rate initially increases but then decreases as the amount of added PS increases. The maximum rate of absorption is attained at a PS addition of 25%, after which it decreases. At temperatures of 600 °C, 605 °C, 610 °C, 615 °C, and 620 °C, the highest water absorption rates of PS with 25% addition were observed, reaching 94.07%, 95.12%, 96.65%, 117.50%, 109.43%, and 106.94%, respectively. The incorporation of PS as a pore-forming agent led to an increase in the pore space of the fired samples, thereby resulting in an increase in the water absorption rate. However, the addition of more than 25% PS led to excessive consumption of the pore-forming agent during the burning process, resulting in the formation of larger holes. These holes, which are too large, hinder the capillary force within the porous material from adsorbing water to the interior of the pore space, consequently leading to a decline in the water absorption rate [19].
As demonstrated in Figure 4b, a consistent trend was observed across all sintering temperatures: the water retention time initially increased with PS (PS) addition, reaching a maximum at 25% PS, and then decreased with further addition. This optimum occurs because a 25% PS content creates an ideal porous network with a high volume of well-sized, interconnected open pores that enhance capillary water uptake and retention. When the PS content exceeds 25%, the pores become excessively large and interconnected, which diminishes the capillary forces necessary for effective water retention, leading to a more rapid release of water and a shorter retention time. The optimal water retention time of 57.75 h was achieved with the sample sintered at 615 °C containing 25% PS, which represents the best combination of sintering temperature and pore-forming agent content. The incorporation of PS as a pore-forming agent has been shown to result in an increase in the internal pores of the material. These pores exhibit an enhanced capacity for water absorption, thereby prolonging the material’s water retention time. However, when the amount of PS exceeds a certain threshold, the pores tend to coalesce, leading to the formation of larger pores. When excess water is introduced, the interconnection of the pores results in the formation of larger pores. This phenomenon is accompanied by a reduction in capillary adsorption, which diminishes the capacity of the pores to absorb water. Consequently, the water retention time decreases.
As demonstrated in Figure 4c, the porosity of the porous ceramics generally increased with increasing polystyrene addition at various temperatures. However, it is important to note that at the lowest sintering temperature of 600 °C, the porosity showed a less pronounced increase with PS addition compared to higher temperatures, likely due to insufficient sintering and incomplete pore formation at this temperature threshold. For example, the porosity of a porous ceramic material was 64.83% when the sintering temperature was 625 °C and the polystyrene addition was 15%. The porosity of the porous ceramic increased gradually with increasing polystyrene addition, and the porosity reached a maximum of 69.58% when the addition reached 30%.
As demonstrated in Figure 4d, the compressive strength of the porous ceramics gradually decreased with increasing addition of PS. The compressive strength decreased from 0.89 MPa to 0.49 MPa in response to increasing addition of PS at a sintering temperature of 625 °C. As the amount of PS incorporated increased, the number of pores formed by the decomposition of PS and the microcracks generated during sintering also increased. These phenomena affect the mechanical properties of the porous ceramics, thereby reducing the compressive strength with increasing PS content within the ceramic embryo.
In summary, the presence of a certain degree of strength renders porous ceramics suitable for practical applications. Higher porosity has been shown to enhance water absorption and retention properties. To determine the optimal application of porous ceramics, specifically for crop root water retention or soil remediation, identifying the ideal combination of parameters is essential. Through rigorous experimentation, it has been ascertained that the porous ceramics obtained by incorporating 25% PS and sintering at a temperature of 615 °C represent the optimal formulation. At this point, the water absorption, water retention time, porosity, and compressive strength of the porous ceramics were 117.50%, 47.75 h, 71.51%, and 0.48 MPa, respectively.
3.3. Determining Other Factors Through Orthogonal Experiments
The preceding experiments demonstrated that the performance of Coal gangue-based porous ceramics is influenced by multiple factors (e.g., raw material ratios and sintering temperature), and their mutual interactions are complex and non-linear. Consequently, relying solely on single-factor experiments is inadequate to reflect the comprehensive experimental outcomes, as such an approach cannot account for inter-factor interactions or identify global optima. To address this, we employed orthogonal experimental design, which efficiently explores the multi-factor space and quantifies the influence of each factor on the target properties. This method provides profound insights into the dominant factors and their optimal combinations for achieving balanced performance.
Based on the single-factor results, which identified 25% PS (PS) as the optimal pore-former and 615 °C as the promising sintering temperature, we designed a four-factor (A: CG, B: starch, C: glass powder, D: diatomaceous earth), five-level orthogonal experiment (L25 array) to optimize the comprehensive performance. The absorption rate and water retention time were set as primary indicators, while porosity and compressive strength served as secondary indicators, ensuring a holistic evaluation of the material’s suitability for dryland agriculture. The factors and levels are detailed in Table 2, and the orthogonal array is presented in Table 3.

Table 2.
Orthogonal experimental factors and levels.

Table 3.
Orthogonal experimental design (L25 (54) array).
The results of the orthogonal experiments are presented in Table 4. In this section, water absorption and water retention time are the primary performance indicators for determining the optimal formulation, whereas porosity and compressive strength are the secondary performance indicators for determining the optimal formulation.

Table 4.
Results of orthogonal experiments.
The variance analysis results of the orthogonal experiment are shown in Tables S1–S4 (see Supplementary Materials). For all four response variables (Water Absorption, Water retention Time, Apparel porosity, compressive strength), analysis of variance showed that at a significance level of α = 0.05, the effects of factors A (CG), B (starch), C (glass dust), and D (diamond) were not significant. This means that within these experimental levels, changes in various factors have no statistically significant impact on the response variables. Possible reasons may include experimental errors or failure to consider the interaction between factors.
The intuitive analysis of orthogonal experiments entails the calculation of the horizontal mean of each factor to derive the extreme deviation of each factor. The magnitude of the extreme deviation is then used to determine the effect of that influencing factor on the performance indicator. A substantial extreme deviation signifies a pronounced impact on the metric by that particular factor.
The results of the visual analysis of the orthogonal experiments are presented in Tables S5–S8 (see Supplementary Materials). When water absorption, water retention time, porosity, and compressive strength are the performance indicators, the priority order of the factors is D > A > B > C, A > D > C > B, D > A > B > C, and A > D > C > B, respectively. The corresponding optimal ceramic formulations are A4B3C2D4, A1B4C1D1, A2B3C3D4, and A1B5C4D1.
The visual analysis of orthogonal experimental results boasts simplicity, intuition, and reduced calculation time. However, it is unable to distinguish between two kinds of fluctuations in the data because of factor variation and experimental errors. Consequently, regression analysis of the experimental results is imperative to achieve enhanced data accuracy and reliability.
The water absorption, water retention time, porosity and compressive strength of CG porous ceramics are used as performance indicators for regression analysis. The regression analysis results are shown in Table S9–S12 (see Supplementary Materials). The following linear dependence regression equations can be derived from the results of the 25 testing cases.
α = 112.431 − 2.588A + 17.732B − 8.590C − 4.229D
Wm = 50.923 − 0.675A − 0.170B + 1.770C − 9.067D
Pa = 76.376 − 0.715A + 1.476B − 0.508C − 1.487D
σ = −0.095 − 0.092A + 0.087B + 0.202C + 0.143D
The regression Equation (3) and the data in Table S5 indicate that when the water absorption rate is used as the performance index, the significance of the four factors in descending order is as follows: BCAD. Factor A is negatively correlated with the water absorption rate, indicating that an increase in CG will result in a decrease in the water absorption rate. Factor B is positively correlated with water absorption, indicating that increasing the amount of starch increases water absorption. The effect of this factor is significant. Factor C is negatively correlated with the rate of water absorption, indicating that increasing the amount of glass powder decreases the rate of water absorption. Factor D exhibited a weak negative correlation with the water absorption rate, suggesting that increasing the dosage of diatomaceous earth would result in a slight reduction in the water absorption rate. In the context of water absorption, factor B emerges as the predominant influencing factor. An increase in the water absorption rate is associated with an increase in the starch content.
As demonstrated in Table S6 and regression Equation (4), when the water retention time was utilized as the performance index, the significance of the four factors in descending order was D > C > A > B. Factor A is negatively correlated with the water retention time, suggesting that an increase in the CG content results in a reduction in the water retention time. Factor B demonstrated a similar correlation, with increasing starch content resulting in a decrease in water retention time. Factors C and D demonstrate a negative correlation with water retention time, although the effect is less pronounced. This finding aligns with the results of the intuitive analysis. Consequently, CG (A) predominantly influences the water retention time.
As demonstrated in Table S7 and regression Equation (5), when porosity is utilized as the performance index, the significance of the four factors in descending order is B > A > D > C. However, the extreme difference in each factor is minimal, indicating that porosity is relatively less affected by each factor. Factor A is negatively correlated with porosity, suggesting that an increase in CG content results in a decrease in porosity. Conversely, factor B is positively correlated with porosity, suggesting that increasing the dosage of starch will lead to an increase in porosity. Factor C is negatively correlated with porosity, suggesting that increasing the dosage of glass powder leads to a decrease in porosity. Factor D exhibited a weak positive correlation with porosity, suggesting that increasing the dosage of diatomaceous earth would result in a modest increase in porosity. Consequently, CG (A) and starch (B) are the primary influencing factors in terms of porosity.
The findings of the regression analysis of compressive strength, as presented in Table S8 and regression Equation (6), reveal the following: In the context of utilizing compressive strength as a performance index, the significance of the four factors is ranked in descending order as follows: C > A > B > D. Factor A is negatively correlated with compressive strength, indicating that an increase in CG dosage results in a reduction in compressive strength. Factors B, C and D have been shown to have positive correlations with compressive strength. These findings suggest that increasing the dosage of starch, glass powder, or diatomaceous earth substantially enhances the compressive strength. With respect to the analysis of compressive strength, CG (A) and diatomite (D) emerge as the more substantial influencing factors, whereas starch (B) and glass powder (C) are found to be inconsequential.
The optimal comprehensive performance scheme for Coal gangue-based porous ceramics, as determined by a combination of intuitive and regression analyses of orthogonal experimental results, was found to be A1B4C4D4. This scheme was derived from a comprehensive consideration of water absorption, water retention time, porosity, and compressive strength. The composition of the CG was 20 g (18.6%), the starch content was 25 g (23.1%), the glass powder content was 25 g (23.1%), the diatomite content was 11 g (10.2%) and the PS content was 27 g (25%). Polystyrene (27%) serves as the primary pore-former, creating well-defined macropores through its spherical morphology and complete burnout. Starch (25%) acts as both a binder and secondary pore-former, contributing to green strength and additional porosity. Diatomaceous earth (11%) provides intrinsic nano- to micro-scale pores that enhance capillary action and specific surface area. Glass powder (23.1%) enables low-temperature sintering through viscous flow, bonding the ceramic skeleton at 615 °C. Coal gangue (18.6%) forms the primary ceramic framework while utilizing solid waste. This combination of components with their specific ratios (PS: 25%, Starch: 23.1%, Diatomite: 10.2%, Glass powder: 23.1%, CG: 18.6%) successfully creates a hierarchical pore structure optimal for water management applications. The Coal gangue-based porous ceramics prepared according to the optimized scheme exhibited the following performance characteristics: water absorption rate of 149.70%, water retention time of 57.75 h, porosity of 77.28%, and compressive strength of 0.55 MPa. These findings demonstrate the efficacy of the measures employed to enhance the performance of porous ceramics, which were optimized via orthogonal experiments. The samples that were subjected to the optimized formulation process were designated CGPC.
Figure 2f shows the microstructure of CGPC. It can be observed that CGPC forms a more uniform and interconnected three-dimensional network pore structure than the single factor optimized sample (Figure 5). The pore size distribution is wider, and there are a large number of micron and submicron pores, which is due to the synergistic effect of composite pore forming agents (PS and starch) and diatomite. This multi-stage pore structure greatly enhanced the capillary force, which explained its high water absorption (149.70%). At the same time, the zigzag pore path effectively delays the evaporation and diffusion of water, which is the key reason for its long water retention time (57.75 h).
Table 5 shows a comparison of the properties of the porous ceramics prepared in different studies with those of the CGPC prepared in this study. It is clear that the porous ceramic samples prepared at lower sintering temperatures in this study could have superior water absorption and retention properties. However, there are also obvious drawbacks. Compared with that of other porous ceramics, the compressive strength of CGPC is lower, which needs to be further improved.

Table 5.
Comparison of the properties of different porous ceramics.
3.4. Pot Experiments with CGPC
As demonstrated in Figure 6a, following the incorporation of gangue-based porous ceramic CGPC into the soil, Chinese cabbage emerged within a 24 h period postplanting, indicating that the introduction of CG-based porous ceramic CGPC led to a reduction in the seedling emergence time of Chinese cabbage in loess soil. Following a period of 7 d during which Chinese cabbage was cultivated, the quantity of seedlings that emerged in the group that had been exposed to the CGPC prepared from the sample was considerably greater than the quantity observed in the control group. As demonstrated in Figure 6b, the emergence of Chinese cabbage seedlings markedly increased from 42% to 93% following 7 d of growth in soil with the incorporation of the CG-based porous ceramic CGPC compared with the control. Compared with that of the control group, the emergence of 7 d of seedling cabbage growth notably increased from 15% to 33% following the incorporation of CG-based porous ceramic CGPC into the standard sand medium. Furthermore, as shown in Figure 6c, the above-ground height of Chinese cabbage after 7 d of planting following the incorporation of the CG-based porous ceramic was notably greater than that of the control group. These results suggest that the incorporation of Coal gangue-based porous ceramics applied as a surface mulch for the seed planting layer may have a beneficial effect on the germination and growth of Chinese cabbage seeds.
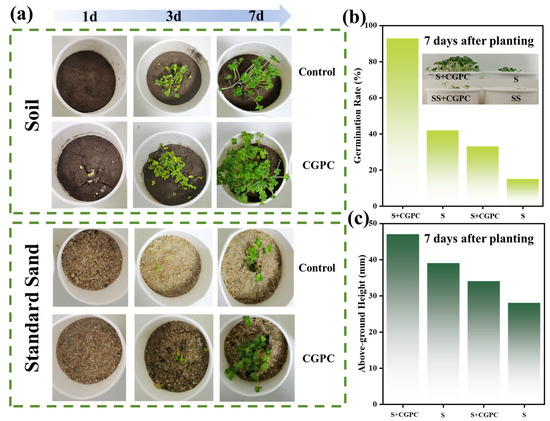
Figure 6.
(a) Growth of fast-growing Chinese cabbage 1 d, 3 d, and 7 d after planting. Growth parameters of fast-growing Chinese cabbage 7 d after planting: (b) Germination rate. (c) Aboveground height. (Note: S: soil; S + CGPC: soil and CGPC; SS: standard sand; SS + CGPC: standard sand and CGPC).
4. Conclusions
In this study, a CG-based low-temperature sintered porous ceramic was successfully prepared. Through material design and process optimization, it provides a new way for the utilization of CG in agriculture in arid areas. The primary conclusions that can be drawn from this analysis are as follows:
- Single-factor experiments identified the sintering temperature and PS (PS) content as the most critical parameters governing the ceramics’ performance. The sintering temperature of 615 °C was determined to be optimal, achieving a delicate balance where the decomposition of pore-forming agents created sufficient pores before the excessive flow of the glass phase could seal them. Furthermore, a PS addition of 25% was found to yield the highest water absorption and retention, beyond which pore coalescence led to a decline in performance. These key findings, illustrated by the systematic trends in water absorption and retention times across different temperatures and PS contents, provided the fundamental parameters for the subsequent compositional optimization.
- Guided by the single-factor results, orthogonal experiment optimization yielded a CG-based porous ceramic (CGPC) with exceptional comprehensive performance. The optimal formula (20 g CG, 25 g starch, 25 g glass powder, 11 g diatomite, 27 g PS) sintered at 615 °C achieved a water absorption rate of 149.70%, a water retention time of 57.75 h, a porosity of 77.28%, and a compressive strength of 0.55 MPa. The synergistic use of composite pore-formers and diatomaceous earth successfully constructed a graded pore structure responsible for these enhanced properties.
- The pot experiment of Chinese cabbage clearly proved the effect of CGPC, which shortened the emergence time of seedlings to less than 24 h, and significantly improved the emergence rate and early growth of seedlings (7 days).
In summary, this work provides a sustainable material strategy for dryland farming by transforming CG into a functional water-management material. As an environmental protection substitute for non-biodegradable plastic film, the developed porous ceramics show great potential. This approach of “turning waste into drought resistance” offers a novel pathway for the resource utilization of industrial solid waste, aligning with the goals of sustainable agriculture and ecological conservation.
5. Future Perspectives
Despite the encouraging results, this study has certain limitations that point to valuable directions for future research.
- Firstly, the compressive strength of the optimized porous ceramic (CGPC, 0.55 MPa), while sufficient for handling and pot experiments, is lower than that of some porous ceramics sintered at higher temperatures [17,18,41]. This relative mechanical weakness could pose a challenge for large-scale transportation and certain mechanical stress scenarios in field applications. Future work will therefore prioritize enhancing the mechanical robustness without compromising the excellent water-absorption properties. Strategies will focus on refining the ceramic skeleton, for instance, by incorporating fibrous reinforcements or exploring alternative low-melting-point cementitious phases to strengthen the inter-particle bonding.
- A primary limitation is the short duration (7 days) and high seeding density (100 seeds/pot) of the pot trials. This experimental design, while effective for a controlled, initial screening of the material’s ability to enhance germination and early establishment, does not capture the long-term effects on plant development. The high seedling density likely induced competition for resources (light, water, nutrients) soon after emergence, which limits the interpretability of the above-ground height data and precludes any assessment of plant health, biomass accumulation, or yield in later growth stages. Consequently, the results should be interpreted as a robust indicator of the material’s positive impact on the very first critical phase of plant life, but not as evidence of sustained growth promotion. Therefore, future work must prioritize long-term pot or field experiments with realistic planting densities and longer observation periods. These studies should track key agronomic indicators over a full growth cycle, including plant biomass, root development, nutrient uptake efficiency, and ultimately, crop yield. This will be essential for validating the practical agricultural value of the CGPC.
- Investigating the material’s long-term stability and its interaction with the soil microbiome in real-field environments will be critical for assessing its durability and ecological impact, paving the way for its practical application in sustainable agriculture.
- Furthermore, the water retention performance was evaluated under stable laboratory conditions (25 °C, 93% RH). While this provides fundamental property comparisons, it does not fully replicate the high-temperature and low-humidity stress typical of real-world dryland environments. Future work should therefore include water retention tests under varied temperature and humidity regimes (e.g., 35–40 °C and 30–50% RH) to better simulate arid field conditions and more accurately predict the material’s efficacy in practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su172210111/s1. Table S1. Variance analysis of water absorption. Table S2. Variance analysis of water retention time. Table S3. Variance analysis of apparent porosity. Table S4. Variance analysis of compressive strength. Table S5. Results of visual analysis of water absorption rate. Table S6. Results of visual analysis of water retention time. Table S7. Results of visual analysis of porosity. Table S8. Compressive strength visual analysis results. Table S9. Results of water absorption regression analysis. Table S10. Results of regression analysis of water retention time. Table S11. Porosity regression analysis results. Table S12. Compressive strength regression analysis results.
Author Contributions
H.W. Writing—original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. H.Z.: Visualization, Software. P.Z.: Writing—review & editing, supervision, project administration, methodology, conceptualization. Y.W.: Supervision, project administration, funding acquisition, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 52371231), the Key R&D Program of Shanxi Province (Grant No. 202302040201008), and the National Science Foundation of Shanxi Province (Grant No. 202303021222013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Albergel, C.; Dutra, E.; Munier, S.; Calvet, J.-C.; Munoz-Sabater, J.; de Rosnay, P.; Balsamo, G. ERA-5 and ERA-Interim driven ISBA land surface model simulations: Which one performs better? Hydrol. Earth Syst. Sci. 2018, 22, 3515–3532. [Google Scholar] [CrossRef]
- Ahlström, A.; Raupach, M.R.; Schurgers, G.; Smith, B.; Arneth, A.; Jung, M.; Reichstein, M.; Canadell, J.G.; Friedlingstein, P.; Jain, A.K.; et al. The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef]
- Tripathi, I.M.; Mahto, S.S.; Kushwaha, A.P.; Kumar, R.; Tiwari, A.D.; Sahu, B.K.; Jain, V.; Mohapatra, P.K. Dominance of soil moisture over aridity in explaining vegetation greenness across global drylands. Sci. Total. Environ. 2024, 917, 170482. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Piao, S.; Chen, A.; Huntingford, C.; Fu, B.; Li, L.Z.X.; Huang, J.; Sheffield, J.; Berg, A.M.; Keenan, T.F.; et al. Multifaceted characteristics of dryland aridity changes in a warming world. Nat. Rev. Earth Environ. 2021, 2, 232–250. [Google Scholar] [CrossRef]
- Papagiannopoulou, C.; Miralles, D.G.; Dorigo, W.A.; Verhoest, N.E.C.; Depoorter, M.; Waegeman, W. Vegetation anomalies caused by antecedent precipitation in most of the world. Environ. Res. Lett. 2017, 12, 074016. [Google Scholar] [CrossRef]
- Wang, J.; Niu, W.; Song, X.; Han, J. Response of tomato root endophytic bacterial communities to water-oxygen coupling under micro/nanoaerated drip irrigation in slightly saline soils. Sci. Hortic. 2023, 321. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, G.; Dou, Y.; Yang, H.; Wang, T.; Wang, Z.; Malhi, S.; Khan, A.A. Plastic mulch increases dryland wheat yield and water-use productivity, while straw mulch increases soil water storage. J. Integr. Agric. 2024, 23, 3174–3185. [Google Scholar] [CrossRef]
- Santini, G.; Probst, M.; Gómez-Brandón, M.; Manfredi, C.; Ceccherini, M.T.; Pietramellara, G.; Santorufo, L.; Maisto, G. Microbiome dynamics of soils covered by plastic and bioplastic mulches. Biol. Fertil. Soils 2023, 60, 183–198. [Google Scholar] [CrossRef]
- Liu, L.; Zou, G.; Zuo, Q.; Li, C.; Gu, J.; Kang, L.; Ma, M.; Liang, K.; Liu, D.; Du, L. Soil bacterial community and metabolism showed a more sensitive response to PBAT biodegradable mulch residues than that of LDPE mulch residues. J. Hazard. Mater. 2022, 438, 129507. [Google Scholar] [CrossRef]
- Macan, G.P.; Anguita-Maeso, M.; Olivares-García, C.; Le, Q.N.P.; Halsall, C.; Landa, B.B. Unravelling the plastisphere-soil and plasticplane microbiome of plastic mulch residues in agricultural soils. Appl. Soil Ecol. 2025, 206. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Haris, M.; Chen, C.; Wang, H.; Guo, J.; Meng, H.; Wu, X.; Liu, X.; Hu, W.; et al. Study of ceramsite-supported iron and manganese oxides for enhancing soil immobilization and reducing rice plants uptake of cadmium. J. Environ. Chem. Eng. 2024, 12. [Google Scholar] [CrossRef]
- Al-Zboon, K.K.; Al-Tabbal, J.A.; Al-Kharabsheh, N.M.; Al-Mefleh, N.K. Natural volcanic tuff as a soil mulching: Effect on plant growth and soil chemistry under water stress. Appl. Water Sci. 2019, 9, 123. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Z.; Yu, Y.; Li, P.; Cosgrove, T. Effect of elevated curing temperature on ceramsite concrete performance. Constr. Build. Mater. 2017, 153, 423–429. [Google Scholar] [CrossRef]
- Tang, K.-J.; An, H.-N.; Liu, C.-B.; Li, Y.-D.; Jia, L.-J.; Tang, Y.; Wang, Q.-Q.; Jiang, Y.; Song, Z.-J. Safety and environmental protection application of high performance solid waste unburned ceramsite and its lightweight high strength concrete. Sustain. Chem. Pharm. 2024, 40. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Zhang, Z.-Y.; Zhou, J.; Yin, X.; Zhu, X.; Wang, X. Preparation of non-sintered building materials with a high content of red mud using magnesium oxychloride cement. Constr. Build. Mater. 2023, 408. [Google Scholar] [CrossRef]
- Liu, B.; Fan, J.; Nian, H.; Li, Y.; Xiang, H.; Zhou, Y. Sintering-resistant porous BaZrO3 ceramics using a particle-stabilized foam method for thermal insulation applications. J. Am. Ceram. Soc. 2025, 108, e20345. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, X.; Li, Y.; Wang, Q.; Pan, L.; Sang, S. The enhanced strength and radiation efficiency of alumina reticulated porous ceramics via coal gangue addition. J. Eur. Ceram. Soc. 2024, 44, 6651–6659. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Li, Y.; Zhang, W.; Huang, Z. Evolution mechanism of pore structure in sintered coal gangue ceramsites. Ceram. Int. 2023, 49, 31385–31395. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Y.; Zhu, Y.; Wang, F.; Zhao, D.; Yang, Y.; Li, H. Preparation of a Novel Structure-Designed Porous Ceramsite for Water Retention. ACS Appl. Mater. Interfaces 2023, 15, 42753–42763. [Google Scholar] [CrossRef]
- Chen, D.; Hu, N.; Wu, Y.; Hou, W.; Guo, Q.; Gui, L.; Tang, R. Preparation and properties of CaO-Al2O3-SiO2 based porous insulation ceramics with addition of phosphogypsum and coal gangue. Ceram. Int. 2025, 51, 23559–23569. [Google Scholar] [CrossRef]
- Liao, M.; Yi, X.; Dai, Z.; Qin, H.; Guo, W.; Xiao, H. Application of metal-BDC-derived catalyst on cordierite honeycomb ceramic support in a microreactor for hydrogen production. Ceram. Int. 2023, 49, 29082–29093. [Google Scholar] [CrossRef]
- Huo, C.; Tian, X.; Nan, Y.; Li, D. Hierarchically porous alumina ceramic catalyst carrier prepared by powder bed fusion. J. Eur. Ceram. Soc. 2020, 40, 4253–4264. [Google Scholar] [CrossRef]
- Miao, L.; Wu, X.; Ji, Z.; Chen, F. Effects of heat-treatment conditions in the preparation of aluminum silicate fiber-based ceramic filter element for hot-gas filtration. Ceram. Int. 2020, 46, 18193–18199. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, R.; Hu, Y.; Liang, S.; Sun, H.; Wang, Z. Fabrication and characterization of mullite fiber-based porous ceramics with mixed fiber lengths for high-temperature gas filtration. Ceram. Int. 2025, 51, 23214–23223. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, T.; Pan, R.; Chun, Y.; Zhou, H.; Zhu, W.; Peng, H.; Zhang, Q. Sintering-free preparation of porous ceramsite using low-temperature decomposing pore former and its sound-absorbing performance. Constr. Build. Mater. 2018, 171, 367–376. [Google Scholar] [CrossRef]
- Guenka, T.d.N.; Machado, M.; Silva, A.; Nunes, M. Freeze-cast porous Al2O3/MgO ceramics as potential acoustic sound absorption. Appl. Acoust. 2024, 220. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, X.; Wu, B.; Liu, B.; Zhang, S. Highly porous ceramics production using slags from smelting of spent automotive catalysts. Resour. Conserv. Recycl. 2021, 166. [Google Scholar] [CrossRef]
- Wang, W.; Lian, W.; Han, L.; Qiao, J.; Liaw, P.K. High-strength and low-thermal-conductivity porous multi-principal cation mullite ceramic. Ceram. Int. 2024, 51, 5821–5831. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Shao, J.; Bai, C.; Li, H.; Lu, S.; Zhang, X.; Yang, K.; Colombo, P. Sodium-based alkali-activated foams from self-ignition coal gangue by facile microwave foaming route. Ceram. Int. 2022, 48, 33914–33925. [Google Scholar] [CrossRef]
- Ren, P.; Zhou, R.; Liu, H.; Huo, Y.; Wang, Y. Preparation of coal gangue based foamed ceramics with SiC as blowing agent and study on its thermal insulation performance. Ceram. Int. 2024, 50, 31680–31690. [Google Scholar] [CrossRef]
- Ramezani, A.; Nemat, S.; Emami, S. Effects of the size of expanded polystyrene as a pore-former on the properties of insulating firebricks. Ceram. Int. 2018, 44, 6641–6644. [Google Scholar] [CrossRef]
- Liu, C.-L.; Du, Q.; Wu, J.-M.; Zhang, G.; Shi, Y.-S. Preparation of porous lead zirconate titanate piezoelectric ceramics via vat photopolymerization combined with burnt polymer spheres technique. Addit. Manuf. 2024, 91. [Google Scholar] [CrossRef]
- Ishii, K.; Shimizu, M.; Sameshima, H.; Samitsu, S.; Ishigaki, T.; Uchikoshi, T. Fabrication of porous (Ba,Sr)(Co,Fe)O3-δ (BSCF) ceramics using gelatinization and retrogradation phenomena of starch as pore-forming agent. Ceram. Int. 2020, 46, 13047–13053. [Google Scholar] [CrossRef]
- Lyckfeldt, O.; Ferreira, J.M.F. Processing of porous ceramics by ‘starch consolidation’. J. Eur. Ceram. Soc. 1998, 18, 131–140. [Google Scholar] [CrossRef]
- Gao, R.; Chen, H.; Li, C.; Liang, X.; Hou, X.; Yang, B. Preparation of diatomite-based porous ceramics and their adsorption properties for Cu2+. Ceram. Int. 2024, 50, 50153–50162. [Google Scholar] [CrossRef]
- Bao, K.; Huang, Y.; Huang, T.; Gu, M.; Wang, L.; Li, Y.; Cheng, X. Preparation of diatomite-based porous ceramics containing interlayer porous MgO by low-temperature sintering for integration of high strength and low thermal conductivity. Mater. Today Commun. 2024, 38. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.-H.; Wu, H.-Q.; Jin, J. Preparation and properties of porous ceramics from spodumene flotation tailings by low-temperature sintering. Trans. Nonferrous Met. Soc. China 2021, 31, 2797–2811. [Google Scholar] [CrossRef]
- Chen, C.-H.; Feng, K.-Q.; Zhou, Y.; Zhou, H.-L. Effect of sintering temperature on the microstructure and properties of foamed glass-ceramics prepared from high-titanium blast furnace slag and waste glass. Int. J. Miner. Met. Mater. 2017, 24, 931–936. [Google Scholar] [CrossRef]
- GB/T 14684-2022; Sand for Construction. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2022.
- Li, Y.; Tang, W.; Sheng, H.; Yang, Y.; Mclean, A. Generation of Pyroxene-Based Porous Ceramics from Steel Refining Slag. ISIJ Int. 2021, 61, 2041–2047. [Google Scholar] [CrossRef]
- Jamaludin, A.R.; Kasim, S.R.; Abdullah, M.Z.; Ahmad, Z.A. Sago starch as binder and pore-forming agent for the fabrication of porcelain foam. Ceram. Int. 2014, 40, 4777–4784. [Google Scholar] [CrossRef]
- Hai, O.; Xiao, X.; Xie, Q.; Ren, Q.; Wu, X.; Pei, M.; Zheng, P. Preparation of three-dimensionally linked pore-like porous atomized ceramics with high oil and water absorption rates. J. Eur. Ceram. Soc. 2023, 43, 4530–4540. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).