Lignin Valorization from Lignocellulosic Biomass: Extraction, Depolymerization, and Applications in the Circular Bioeconomy
Abstract
1. Introduction
- To analyze general research trends. To summarize the developments in lignin extraction and conversion technologies described in the latest literature, emphasizing the evolution of chemical, biological, and thermochemical processes.
- Identify recognized methodologies and types of lignin. Categorize the main methods of lignin isolation (e.g., Kraft, sulfite, alkaline, organosolv) and relate them to structural and chemical properties associated with valorization potential.
- Explore opportunities and limitations. Discuss industrial and environmental aspects of lignin utilization, identify key opportunities in the circular bioeconomy, as well as challenges related to process efficiency, scale-up, and product selectivity.
2. Sources, Composition, and Valorization Potential of Lignocellulosic Biomass
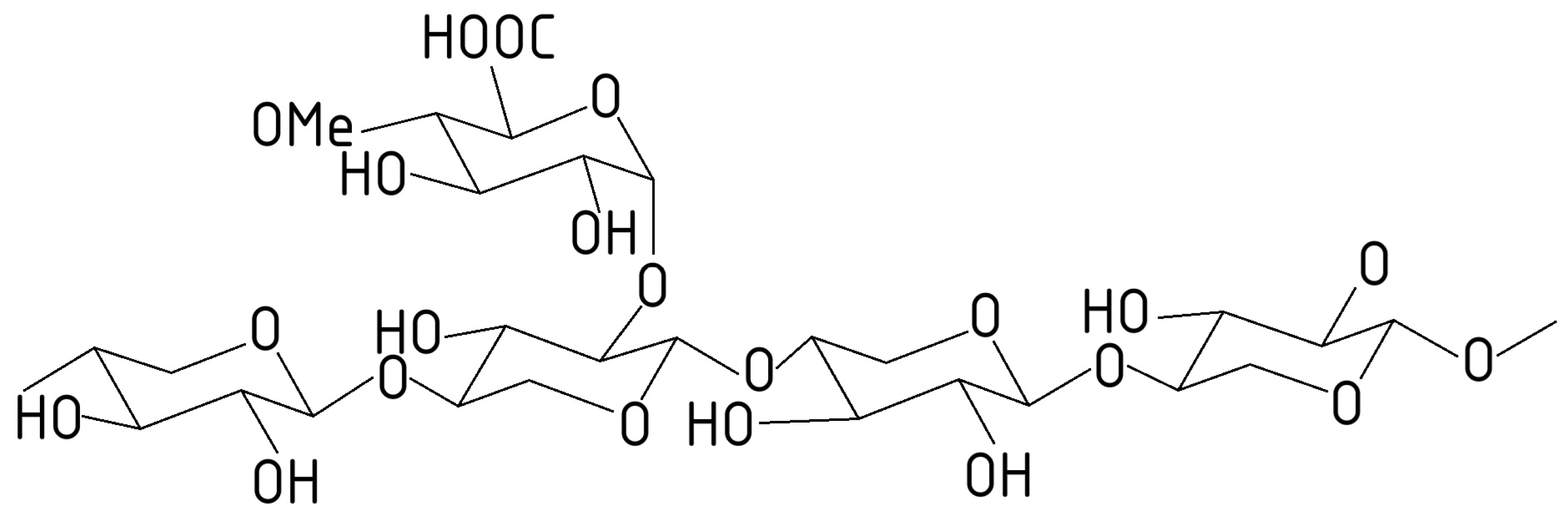
2.1. Composition of Lignocellulosic Biomass
2.2. Representative Sources of Lignocellulosic Biomass
2.2.1. Cereal Residues
2.2.2. Industrial Residues
2.2.3. Nut and Fruit Residues
2.3. Structure of Plant Cell Walls
3. Lignin as a Multifunctional Biopolymer
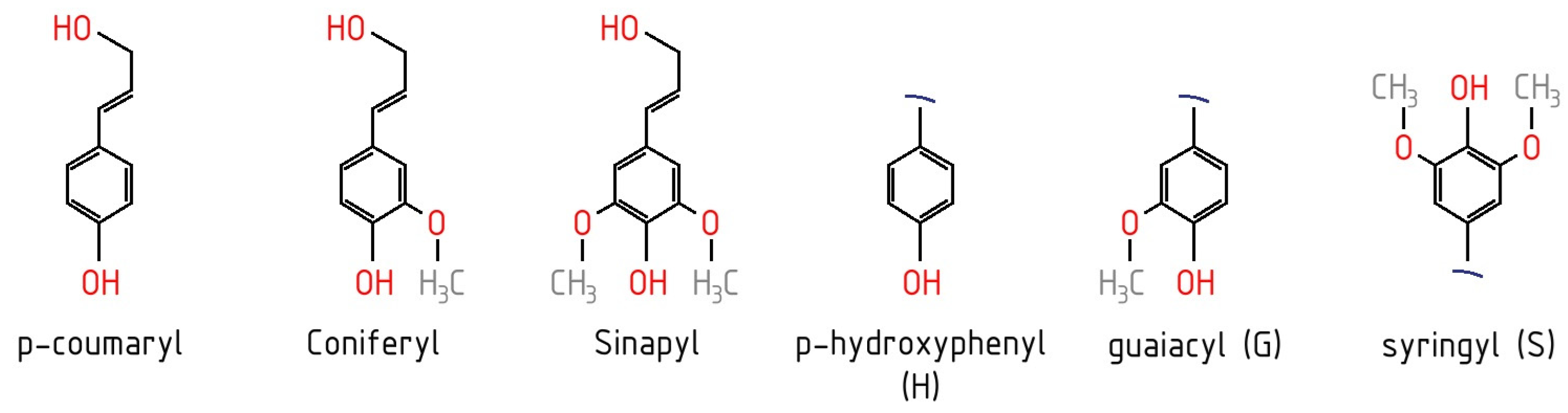
Structure, Composition, and Tissue-Specific Variability
- Guaiacyl (G) lignin: Predominantly coniferyl alcohol, typical of gymnosperms (softwoods).
- Syringyl (S) lignin: Predominantly sinapyl alcohol, commonly found in angiosperms (hardwoods).
- p-Hydroxyphenyl (H) lignin: Contains more p-coumaryl alcohol, often found in grasses and some other plants [27].
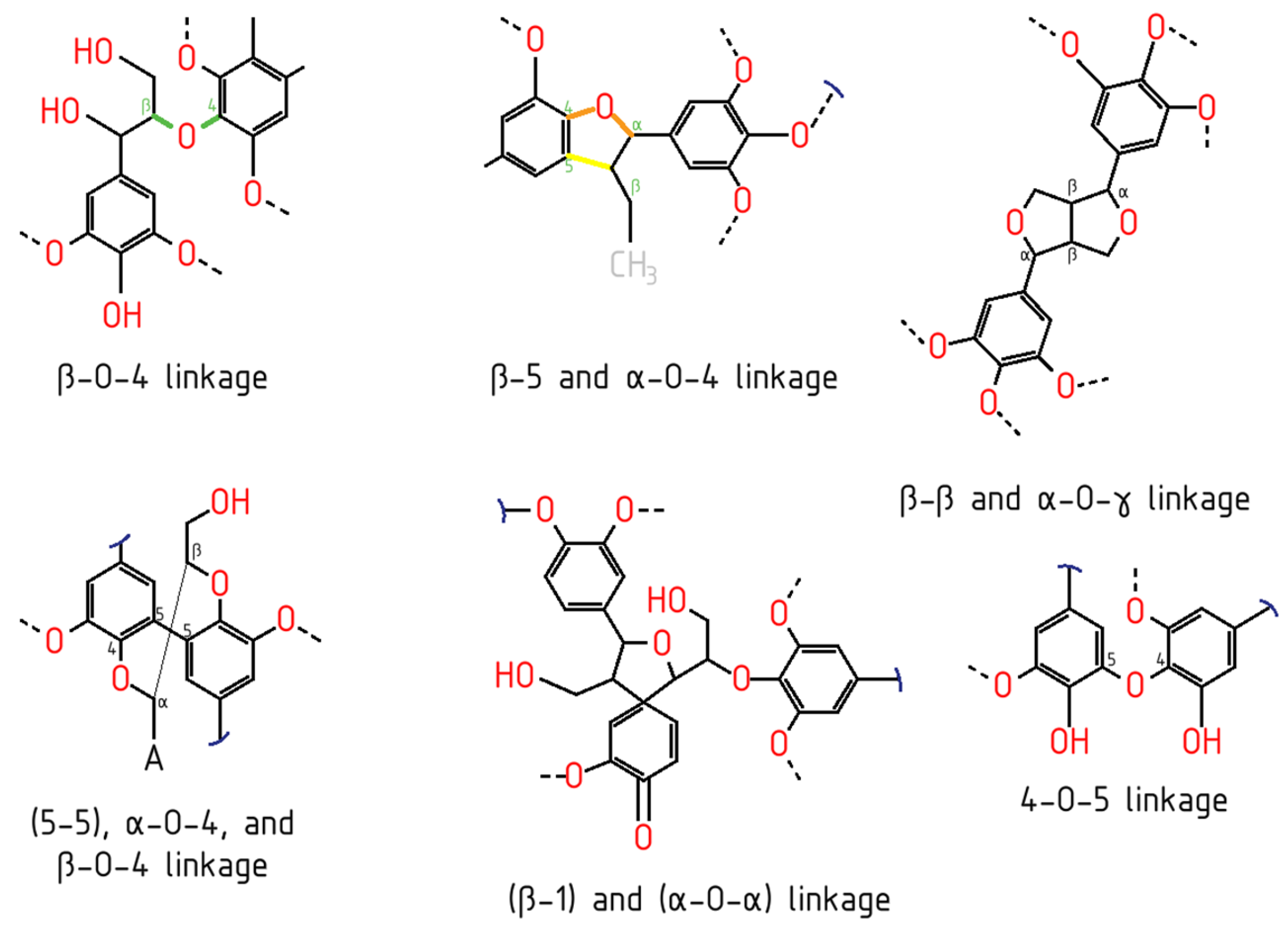
4. Lignin Extraction, Fractionation and Industrial Lignin Types
4.1. Lignin Fractionation
- Technical lignin: derived from industrial paper, pulp, and cellulose production.
- Kraft lignin: obtained via the sulfate-based pulping process.
- Lignosulfonate: produced through sulfite pulping.
- Alkali lignin: extracted from biomass using alkaline solutions.
- Organosolv lignin: isolated with organic solvents.
4.2. Industrially Relevant Lignin Types
5. Lignin Depolymerization and Upgrading
5.1. Chemical and Catalytic Depolymerization
- Pyrolysis is a process in which oxygen is not required for the thermal treatment of lignin. The temperature can range from 300 °C to 600 °C. The absence of oxygen is necessary to prevent the reaction from continuing and CO2 from forming. The extent to which this process transforms the ring structures of lignin depends on the type of raw material, temperature, and heating rate [23].
- Hydrogenolysis. In a typical lignin reduction depolymerization system, lignin or lignocellulose is treated at a temperature of 180–300 °C and a pressure of 0–5 MPa H2 (at room temperature). The solvent is usually a polar alcohol solvent or another hydrogen-donating solvent. Solvolysis breaks down the lignin in the matrix and depolymerizes it into small fragments. Some of these fragments interact with the catalyst and are hydrogenated to form stable phenolic monomers [88].
- Oxidative depolymerization is attractive due to its relatively mild operating conditions and the possibility of producing targeted products with multiple functionalities. During oxidative depolymerization, lignin is converted in the presence of an oxidant, typically O2 or H2O2. Oxidation can cause the breakdown of side chains, forming phenolic aldehydes and acids, but it can also break down aromatic rings in lignin, forming aliphatic carboxylic acids [89].
- Gasification requires higher temperatures (between 700 °C and 1000 °C) compared to the other thermochemical methods, and it focuses primarily on the production of non-condensable gases, such as H2, CO, CO2, and CH4. Once syngas is produced, cleaned and filtered to remove problematic chemical compounds, it can then be used to generate energy [90].
- Combustion takes place in the presence of oxygen and at extremely high temperatures (around 800 °C to 1000 °C) and can be used to produce heat, electricity, gas, and solid carbon residue [23].
5.2. Biological Depolymerization and Hybrid Routes
6. Lignin Properties and Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanoca, A.; de Vries, L.; Boerjan, W. Lignin engineering in forest trees. Front. Plant Sci. 2019, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Kim, K.H. Lignin to materials: A focused review on recent novel lignin applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Madigal, J.P.T.; Terasaki, M.; Takada, M.; Kajita, S. Synergetic effect of fungal pretreatment and lignin modification on delignification and saccharification: A case study of a natural lignin mutant in mulberry. Biotechnol. Biofuels Bioprod. 2025, 18, 13. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Sand, A.; Tuteja, J. Introductory chapter: Introduction to structure properties and application of lignin. In Lignin-Chemistry, Structure, and Application; Sand, A., Tuteja, J., Eds.; IntechOpen: London, UK, 2023; pp. 3–7. [Google Scholar] [CrossRef]
- Shah, T.A.; Li, Z.; Li, Z.; Zhang, A. Composition and role of lignin in biochemicals. In Lignin-Chemistry, Structure, and Application; Sand, A., Tuteja, J., Eds.; IntechOpen: London, UK, 2023; pp. 9–27. [Google Scholar] [CrossRef]
- Maceda, A.; Terrazas, T. Fluorescence Microscopy methods for the analysis and characterization of lignin. Polymers 2022, 14, 961. [Google Scholar] [CrossRef]
- Balk, M.; Sofia, P.; Neffe, A.T.; Tirelli, N. Lignin, the lignification process, and advanced, lignin-based materials. Int. J. Mol. Sci. 2023, 24, 11668. [Google Scholar] [CrossRef]
- Petrik, D.L.; Karlen, S.D.; Cass, C.L.; Padmakshan, D.; Lu, F.; Liu, S.; Le Bris, P.; Antelme, S.; Santoro, N.; Wilkerson, C.G.; et al. P-Coumaroyl-CoA: Monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. Plant J. 2014, 77, 713–726. [Google Scholar] [CrossRef]
- Bandounas, L.; Wierckx, N.J.P.; de Winde, J.H.; Ruijssenaars, H.J. Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol. 2011, 11, 94. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Callone, E.; Ungureanu, E.; Ungureanu, O.C.; Popa, V.I. Structure of cellulose isolated from rapeseed stalks. Polymers 2025, 17, 1032. [Google Scholar] [CrossRef]
- Przypis, M.; Wawoczny, A.; Gillner, D. Biomass and cellulose dissolution—The important issue in renewable materials treatment. Appl. Sci. 2023, 13, 1055. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A.; Lata. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Murugesan, T. A “Fourier Transformed Infrared” compound study of lignin recovered from a formic acid process. Procedia Eng. 2016, 146, 1312–1319. [Google Scholar] [CrossRef]
- Nadányi, R.; Ház, A.; Lisý, A.; Jablonský, M.; Šurina, I.; Majová, V.; Baco, A. Lignin modifications, applications, and possible market prices. Energies 2022, 15, 6520. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibers and lignin in bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A.J. Lignin as a natural antioxidant: Chemistry and applications. Macromol 2025, 5, 5. [Google Scholar] [CrossRef]
- McNeill, D.C.; Pal, A.K.; Nath, D.; Rodriguez-Uribe, A.; Mohanty, A.K.; Pilla, S.; Gregori, S.; Dick, P.; Misra, M. Upcycling of ligno-cellulosic nutshells waste biomass in biodegradable plastic-based biocomposites uses—A ciomprehensive review. Compos. Part C Open Access 2024, 14, 100478. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.A.; Delgado-Blanca, I.; Mañas-Villar, M.; Orriach-Fernández, F.J.; Soriano-Cuadrado, B. Extraction and depolymerization of lignin from different agricultural and forestry wastes to obtain building blocks in a circular economy framework. Polymers 2024, 16, 1981. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva-Komlenić, D.; Bucić-Kojić, A. A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Bischof, S.; Mayr, S.; Gritsch, S.; Bartolome, M.J.; Schwaiger, N.; Guebitz, G.M.; Weiss, R. The biomodified lignin platform: A review. Polymers 2023, 15, 2694. [Google Scholar] [CrossRef] [PubMed]
- Welker, C.M.; Balasubramanian, V.K.; Petti, C.; Rai, K.M.; DeBolt, S.; Mendu, V. Engineering plant biomass lignin content and composition for biofuels and bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar] [CrossRef]
- Gordobil, O.; Diaz, R.H.; Sandak, J.; Sandak, A. One-step lignin refining process: The influence of the solvent nature on the properties and quality of fractions. Polymers 2022, 14, 2363. [Google Scholar] [CrossRef] [PubMed]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of lignin using organic solvents: A combined experimental and theoretical study. Int. J. Biol. Macromol. 2020, 168, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huigen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651. [Google Scholar] [CrossRef]
- Creteanu, A.; Lungu, C.N.; Lungu, M. Lignin: An adaptable biodegradable polymer used in different formulation processes. Pharmaceuticals 2024, 17, 1406. [Google Scholar] [CrossRef]
- Mateo, S.; Fabbrizi, G.; Moya, A.J. Lignin from plant-based agro-industiral biowastes: From extraction to sustainable applications. Polymers 2025, 17, 952. [Google Scholar] [CrossRef]
- Cazier, E.A.; Pham, T.-N.; Cossus, L.; Abla, M.; Ilc, T.; Lawrence, P. Exploring industrial lignocellulosic waste: Source, types, and potential as high-value molecules. Waste Manag. 2024, 188, 11–38. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Ajayi, V.A.; Lateef, A. Microbial valorization of corncob: Novel route for biotechnological products for sustainable bioeconomy. Environ. Technol. Innov. 2021, 24, 102073. [Google Scholar] [CrossRef]
- Isroi; Millati, R.; Syamsiah, S.; Niklasson, C.; Nur Cahyanto, M.; Lundquist, K.; Taherzadeh, M.J. Biological pretreatment of lignocelluloses with white-rot fungi and its applications: A review. BioResources 2011, 6, 5224–5259. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Corcione, C.E. Efficient and environmentally friendly techniques for extracting lignin from lignocellulose biomass and subsequent uses: A review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- Sharma, S.; Moharana, S.; Sharma, A.; Srivastava, A. Development of lignin-based biodegradable polymer from agro-waste. Mater. Chem. 2024, 3. [Google Scholar] [CrossRef]
- Smith, R.A.; Cass, C.L.; Mazaheri, M.; Sekhon, R.S.; Heckwolf, M.; Keappler, H.; de Leon, N.; Mansfield, S.D.; Keappler, S.M.; Sedbrook, J.C.; et al. Suppression of CINNAMOYL-CoA REDUCTASE increases the level of monolignol ferulates incorporated into maize lignins. Biotechnol. Biofuels 2017, 10, 109. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Calderón-Santoyo, M.; de Souza Oliveira, R.P.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Deconstructing sugarcane bagasse lignocellulose by acid-based deep eutectic solvents to enhance enzymatic digestibility. Carbohyd. Polym. 2022, 298, 120097. [Google Scholar] [CrossRef] [PubMed]
- Tardy, B.L.; Lizundia, E.; Guizani, C.; Hakkarainen, M.; Sipponen, M.H. Prospects for the integration of lignin materials into the circular economy. Mater. Today 2023, 65, 122–132. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Costa-Trigo, I.; Ramírez-Pérez, A.M.; de Blas, E.; Calderón-Santoyo, M.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Development of sustainable biorefinery processes applying deep eutectic solvents to agrofood wastes. Energies 2022, 15, 4101. [Google Scholar] [CrossRef]
- Amensisa, Y.E.; Demsash, H.D.; Tefera, M.E. Extraction and chracterization of cellulose from coffee husk and brewery’s spent grain fibers using alkali-hydrogen peroxide treatment method. Adv. Mater. Sci. Eng. 2024, 2024, 5101871. [Google Scholar] [CrossRef]
- Anuchi, S.O.; Sedransk Campbell, K.L.; Hallett, J.P. Effective pretreatment of lignin-rich coconut wastes using a low-cost ionic liquid. Sci. Rep. 2022, 12, 6108. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential uses of spent coffee grounds in the food industry. Foods 2002, 11, 2064. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Duong, C.T.T.; Vu, C.N.M.; Nguyen, H.M.; Pham, T.T.; Tran-Thuy, T.-M.; Nguyen, L.Q. Data on chemical composition of coffee husks and lignin microparticles as their extracted product. Data Brief 2023, 52, 109781. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.N.; Bracco, P.; Cecone, C.L.; Parola, V.L.; Vineis, C.L.; Testa, M.L. Antibacterial properties of functionalized cellulose extracted from deproteinized soybean hulls. Cellulose 2023, 30, 7805–7824. [Google Scholar] [CrossRef]
- Corsi, L.; Mateo, S.; Spaccini, F.; Buratti, C.; Moya, A.J. Optimization of microwave-assisted organosolv pretreatment for enzymatic hydrolysis of cherry tree pruning and pistachio shells: A step to bioethanol production. BioEnergy Res. 2024, 17, 294–308. [Google Scholar] [CrossRef]
- Ollani, S.; Peano, C.; Sottile, F. Recent innovations on the reuse of almond and hazelnut by-products: A review. Sustainability 2024, 16, 2577. [Google Scholar] [CrossRef]
- Quiceno-Suarez, A.; Cadena-Chamorro, E.M.; Ciro-Velásquez, H.J.; Arango-Tobón, J.C. By-products of the cocoa agribusiness: High value-added materials based on their bromatological and chemical characterization. Rev. Fac. Nac. Agron. Medellín 2024, 77, 10585–10599. [Google Scholar] [CrossRef]
- Paës, G. Fluorescent probes for exploring plant cell wall deconstruction: A review. Molecules 2014, 19, 9380–9402. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Jarvis, M.C. Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant Sci. 2012, 3, 204. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Pẽna-Valdivia, C.B.; Trejo, C.; Terrazas, T. Characterization of lignocellulose of Opuntia (Cactaceae) species using FTIR spectroscopy: Possible candidates for renewable raw material. Biomass Convers. Biorefinery 2022, 12, 5165–5175. [Google Scholar] [CrossRef]
- Haughn, G.W.; Western, T.L. Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Front. Plant Sci. 2012, 3, 64. [Google Scholar] [CrossRef]
- Büyüksarı, Ü.; As, N.; Dündar, T. Mechanical properties of earlywood and latewood sections of Scots pine wood. BioResources 2017, 12, 4004–4012. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Peciulyte, A.; Kiskis, J.; Larsson, P.T.; Olsson, L.; Enejder, A. Visualization of structural changes in cellulosic substrates during enzymatic hydrolisis using multimodal nonlinear microscopy. Cellulose 2016, 23, 1521–1536. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Karthäuser, J.; Biziks, V.; Mai, C.; Militz, H. Lignin and lignin-derived compounds for wood applications—A review. Molecules 2021, 26, 2533. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Liebner, F.; van Herwijnen, H.W.G.; Solt, P.; Konnerth, J. Ligneous resole adhesives for exterior-grade plywood. Eur. J. Wood Wood Prod. 2018, 76, 251–258. [Google Scholar] [CrossRef]
- Weng, J.-K.; Mo, H.; Chapple, C. Over-expression of F5H in COMT-deficient Arabidopsis leads to enrichment of an unusual lignin and disruption of pollen wall formation. Plant J. 2010, 64, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tobimatsu, Y.; Jackson, L.; Nakashima, J.; Ralph, J.; Dixon, R.A. Novel seed coat lignins in the Cactaceae: Structure, distribution and implications for the evolution of lignin diversity. Plant J. 2013, 73, 201–211. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Kapsokalyvas, D.; Loos, J.; Boogers, I.A.L.A.; Appeldoorn, M.; Kabel, M.A.; Zandvoort, M.V. Quantification of morphochemical changes during in situ enzymatic hydrolysis of individual biomass particles based on autofluorescence imaging. Biopolymers 2019, 111, e23347. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Chemistry of lignin-based materials. Green Mater. 2013, 1, 137–160. [Google Scholar] [CrossRef]
- Lourenço, A.; Rencoret, J.; Chemetova, C.; Gominho, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Lignin composition and structure differs between xylem, phloem and phellem in Quercus suber L. Front. Plant Sci. 2016, 7, 1612. [Google Scholar] [CrossRef]
- Kamimura, N.; Takahashi, K.; Mori, K.; Araki, T.; Fujita, M.; Higuchi, Y.; Masai, E. Bacterial catabolism of lignin-derived aromatics: New findings in a recent decade. Eniviron. Microbiol. Rep. 2017, 9, 679–705. [Google Scholar] [CrossRef]
- Zeng, J.; Singh, D.; Laskar, D.D.; Chen, S. Degradation of native wheat straw lignin by Streptomyces viridosporus T7A. Int. J. Environ. Sci. Technol. 2013, 10, 165–174. [Google Scholar] [CrossRef]
- Renault, H.; Werck-Reichhart, D.; Weng, J.-K. Harnessing lignin evolution for biotechnological applications. Curr. Opin. Biotechnol. 2019, 56, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hussin, M.H.; Latif, N.H.A.; Hamidon, T.S.; Idris, N.N.; Hashim, R.; Appaturi, J.N.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Fatriasari, W.; et al. Latest advancements in high-performance bio-based wood adhesives: A critical review. J. Mater. Res. Technol. 2022, 21, 3909–3946. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 12. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef]
- Abdelazis, O.Y.; Li, K.; Tunå, P.; Hulteberg, C.P. Continuous catalytic depolymerisation and conversion of industrial kraft lignin into low-molecular-weight aromatics. Biomass Convers. Biorefinery 2018, 8, 455–470. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K. Lignin for bioeconomy: The present and future role of technical lignin. Int. J. Mol. Sci. 2021, 22, 63. [Google Scholar] [CrossRef]
- Stark, N.M.; Yelle, D.J.; Agarwal, U.P. Techniques for characterizing lignin. In Lignin in Polymer Composites; Faruk, O., Sain, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 49–66. [Google Scholar] [CrossRef]
- Reddy, S.M.S.; Chen, F.; Shadle, G.; Jackson, L.; Aljoe, H.; Dixon, R.A. Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proc. Natl. Acad. Sci. USA 2005, 102, 16573–16578. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech. Histochem. 2007, 82, 209–216. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Kim, H.; Ralph, J. Hydroxystilbenes are monomers in palm fruit endocarp lignins. Plant Physiol. 2017, 174, 2072–2082. [Google Scholar] [CrossRef]
- Liu, B.; Tang, L.; Chen, Q.; Zhu, L.; Zou, X.; Li, B.; Zhou, Q.; Fu, Y.; Lu, Y. Lignin distribution on cell wall micro-morphological regions of fibre in development Phyllostachys pubescens Culms. Polymers 2022, 14, 312. [Google Scholar] [CrossRef]
- Marques, A.V.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Ferulates and lignin structural composition in cork. Holzforschung 2016, 70, 275–289. [Google Scholar] [CrossRef]
- Eudes, A.; Dutta, T.; Deng, K.; Jacquet, N.; Sinha, A.; Benites, V.T.; Baidoo, E.E.K.; Richel, A.; Sattler, S.E.; Northen, T.R.; et al. SbCOMT (Bmr12) is involved in the biosynthesis of tricin-lignin in sorghum. PLoS ONE 2017, 12, e0178160. [Google Scholar] [CrossRef]
- Kim, H.; Li, Q.; Karlen, S.D.; Smith, R.A.; Shi, R.; Liu, J.; Yang, C.; Tunlaya-Anukit, S.; Wang, J.P.; Chang, H.-M.; et al. Monolignol benzoates incorporate into the lignin of transgenic Populus trichocarpa depleted in C3H and C4H. ACS Sustain. Chem. Eng. 2020, 8, 3644–3654. [Google Scholar] [CrossRef]
- Feldman, D. Lignin nanocomposites. J. Mol. Sci. Part A 2016, 53, 382–387. [Google Scholar] [CrossRef]
- Abbadessa, A.; Oinonen, P.; Henriksson, G. Characterization of two novel bio-based materials from pulping process side streams: Ecohelix and CleanFlow black lignin. BioResources 2018, 13, 7606–7627. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Elder, T.; Kim, H.; Ralph, J. Lignin monomers from beyond the canonical monolignol biosynthetic pathway: Another brick in the wall. ACS Sustain. Chem. Eng. 2020, 8, 4997–5012. [Google Scholar] [CrossRef]
- Chen, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Dixon, R.A.; Ralph, J. A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. USA 2011, 109, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Dorn, L.; Thirion, A.; Ghorbani, M.; Olaechea, L.M.; Mayer, I. Exploring fully biobased adhesives: Sustainable kraft lignin and 5-HMF adhesives for particleboards. Polymers 2023, 15, 2668. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Brink, D.P.; Prothmann, J.; Ravi, K.; Sun, M.; García-Hidalgo, J.; Sandahl, M.; Hulteberg, C.P.; Turner, C.; Líden, G.; et al. Biological valorization of low molecular weight lignin. Biotechnol. Adv. 2016, 34, 1318–1346. [Google Scholar] [CrossRef] [PubMed]
- Sadan, R.; Alaoui, C.H.; Ihammi, A.; Chigr, M.; Fatimi, A. A brief overview of lignin extraction and isolation processes: From lignocellulosic biomass to added-value biomaterials. Environ. Earth Sci. Proc. 2024, 31, 3. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and application of lignosulfonates and sulfonated lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.G.; Agrawal, K.; Verma, P. Organosolv pretreatment: An in-depth purview of mechanics of the system. Bioresour. Bioprocess. 2023, 10, 50. [Google Scholar] [CrossRef]
- Shen, Z.; Shi, C.; Liu, F.; Wang, W.; Ai, M.; Huang, Z.; Zhang, X.; Pan, L.; Zou, J.-J. Advances in heterogeneous catalysts for lignin hydrogenolysis. Adv. Sci. 2024, 11, 2306693. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the oxidative valorization of lignin to high-value chemicals: A critical review of opportunities and challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef]
- Garcia, A.C.; Cheng, S.; Cross, F.S. Lignin gasification: Current and future viability. Energies 2022, 15, 9062. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Manaenkov, O.V.; Markova, M.E.; Sidorov, A.I.; Bykov, A.V.; Sulman, M.G.; Kiwi-Minsker, L. Lignin Hydrogenolysis over Bimetallic Ni–Ru Nanoparticles Supported on SiO2@HPS. Catalysts 2023, 13, 856. [Google Scholar] [CrossRef]
- Weng, C.; Peng, X.; Han, Y. Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis. Biotechnol. Biofuels 2021, 14, 84. [Google Scholar] [CrossRef]
- Zhong, N.; Chandra, R.; Yamamoto, M.; Leskinen, T.; Granström, T.; Saddler, J. Sulphite addition during steam pretreatment enhanced both enzyme-mediated cellulose hydrolysis and ethanol production. Bioresour. Bioprocess. 2022, 9, 71. [Google Scholar] [CrossRef]
- Gałązka, A.; Jankiewicz, U.; Orzechowski, S. The role of ligninolytic enzymes in sustainable agriculture: Applications and challenges. Agronomy 2025, 15, 451. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Śwideska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: Micoorganisms, enzymes invloved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Olughu, O.O.; Tabil, L.G.; Dumonceaux, T.; Mupondwa, E.; Cree, D. Optimization of solid-state dermentation of switchgrass using white-rot fungi for biofuel production. Fuels 2022, 3, 730–752. [Google Scholar] [CrossRef]
- Martinez, D.; Larrondo, L.F.; Putnam, N.; Gelpke, M.D.S.; Huang, K.; Chapman, J.; Helfenbein, K.G.; Ramaiya, P.; Detter, J.C.; Larimer, F.; et al. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat. Biotechnol. 2004, 22, 695–700. [Google Scholar] [CrossRef]
- Houtman, C.J.; Maligaspe, E.; Hunt, C.G.; Fernández-Fueyo, E.; Martínez, A.T.; Hammel, K.E. Fungal lignin peroxidase does not produce the veratryl alcohol cation radical as a diffusible ligninolytic oxidant. J. Biol. Chem. 2018, 293, 4702–4712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandra, R. Lininolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
- Christpher, L.P.; Yao, B.; Ji, Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods and applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Ando, D.; Lu, F.; Kim, H.; Eugene, A.; Tobimatsu, Y.; Vanholme, R.; Elder, T.J.; Boerjan, W.; Ralph, J. Incorporation of catechyl monomers into lignins: Lignification from the non-phenolic end via Diels-Alder cycloaddition? Green Chem. 2021, 23, 8995. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Zhai, R.; Wen, Z.; Jin, M. Recent advances in lignin valorization with bacterial cultures: Microorganisms, metabolic pathways, and bio-products. Biotechnol. Biofuels 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent Advances in Lignin-Based Biofuel Production. Energies 2023, 16, 3382. [Google Scholar] [CrossRef]
- Jiju, P.S.; Patel, A.K.; Shruthy, N.S.; Shalu, S.; Dong, C.-D.; Singhania, R.R. Sustainability through lignin valorization: Recent innovations and applications driving industrial transformation. Bioresour. Bioprocess. 2025, 12, 88. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A biopolymer from forestry biomass for biocomposites and 3D printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef]
- Shorey, R.; Salaghi, A.; Fatehi, P.; Mekonnen, T.H. Valorization of lignin for advanced material applications: A review. RSC Sustain. 2024, 2, 804. [Google Scholar] [CrossRef]
- Djikanović, D.; Kalauzi, A.; Radotić, K.; Lapierre, C.; Jeremić, M. Deconvolution of lignin fluorescence spectra: A contribution to the comparative structural studies of lignins. Russ. J. Phys. Chem. A 2007, 81, 1425–1428. [Google Scholar] [CrossRef]
- Donaldson, L. Softwood and hardwood lignin fluorescence spectra of wood cell walls in different mounting media. IAWA J. 2013, 34, 3–19. [Google Scholar] [CrossRef]
- Kránitz, K.; Sonderegger, W.; Bues, C.T.; Niemz, P. Effects of aging on wood: A literature review. Wood Sci. Technol. 2016, 50, 7–22. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, M.; Jin, W.; Zhang, J.; Fan, G.; Feng, Y.; Li, Z.; Wang, S.; Lee, J.S.; Luan, G.; et al. A comprehensive review of unlocking the potential of lignin-derived biomaterials: From lignin structure to biomedical application. J. Nanobiotechnol. 2025, 23, 538. [Google Scholar] [CrossRef]
- Menezes, F.; Rocha, G.; Maciel Filho, R. Obtainment and characterization of lignin from enzymatic hydrolysis of sugarcane bagasse of 2G process in pilot scale. Chem. Eng. Trans. 2016, 50, 397–402. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef]
- Gil-Chávec, J.; Gurikov, P.; Hu, X.; Meyer, R.; Reynolds, W.; Smirnova, I. Application of novel and technical lignins in food and pharmaceutical industries: Structure-function relationship and current challenges. Biomass Convers. Biorefinery 2021, 11, 2387–2403. [Google Scholar] [CrossRef]
- Solt, P.; Konnerth, J.; Gindl-Altmutter, W.; Kantner, W.; Moser, J.; Mitter, R.; van Herwijnen, H.W.G. Technological performance of formaldehyde-free adhesive alternatives for particleboard industry. Int. J. Adhes. Adhes. 2019, 94, 99–131. [Google Scholar] [CrossRef]
- Punia, P.; Singh, L. Optimization of alkali pre-treatment of sweet sorghum [Soghum bicolor (L.) Moench] residue to improve enzymatic hydrolysis for fermentable sugars. Waste Manag. Bull. 2024, 2, 131–141. [Google Scholar] [CrossRef]
- Silva, T.A.L.; Zamora, H.D.Z.; Varão, L.H.R.; Prado, N.S.; Baffi, M.A.; Pasquini, D. Effect of steam explosion pretreatment catalysed by organic acid and alkali on chemical and structural properties and enzymatic hydrolysis of sugarcane bagasse. Waste Biomass Valorization 2018, 9, 2191–2201. [Google Scholar] [CrossRef]
- Billings, A.F.; Fortney, J.L.; Hazen, T.C.; Simmons, B.; Davenport, K.W.; Goodwin, L.; Ivanova, N.; Kyrpides, N.C.; Mavromatis, K.; Woyke, T.; et al. Genome sequence and description of the anaerobic lignin-degrading bacterium Tolumonas lignolytica sp. nov. Stand. Genom. Sci. 2015, 10, 106. [Google Scholar] [CrossRef]
- Kylili, A.; Koutinas, M.; Georgali, P.Z.; Fokaides, P.A. Lignin valorisation: Life cycle assessment (LCA) considerations for enabling circular bioeconomy. Int. J. Sustain. Energy 2023, 42, 1008–1027. [Google Scholar] [CrossRef]



| Biomass Source | Origin | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Other Major Components | Notes/Potential Applications |
|---|---|---|---|---|---|---|
| Corncob | Cereal residue | 69.2 | 22.8 | 8.0 | - | High cellulose content; bioethanol feedstock [30] |
| Wheat straw | Cereal residue | 33–45 | 26–32 | 17.5–30 | - | Common agricultural byproduct [33] |
| Rice straw | Cereal residue | 35–40 | 30 | 15.6–25 | - | High lignin limits digestibility [33] |
| Sugarcane bagasse | Industrial residue | 35–45 | 26–35 | 11–25 | 3–14% other extractives | Abundant; used for power and bioproducts [35] |
| Brewery spent grain | Industrial residue | 16–25 | 28–35 | 11–27 | 15–24% protein | Used for ethanol, xylitol, food additives [38] |
| Coffee husk | Industrial residue | 30–35 | 18–21 | 19–22 | 25–28% waxes and inorganic matter | Biochar, composting, polymers [38,41] |
| Coconut shells | Nut residue | 20–30 | 15–30 | ~50 | - | Very high lignin; energy applications [39] |
| Almond shells | Nut residue | ~46 | 23 | 21 | - | Source of fibrous material [44] |
| Hazelnut shells | Nut residue | 15.4 | 22.4 | 25.9 | - | Dense lignocellulosic biomass [44] |
| Extraction Method | Main Reagents/Conditions | Lignin Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Kraft process | NaOH + Na2S, 160–180 °C | Sulfur-rich, condensed | Industrially dominant, robust | Odorous, limited reactivity | [54,60,83,85] |
| Sulfite process | SO2 + CaSO3/MgSO3, 120–180 °C | Lignosulfonates, water-soluble | Produces sulfonated lignin, easy to handle | Low purity, sulfur content | [54,83,85,86] |
| Organosolv process | Organic solvent (ethanol, acetic acid), 150–200 °C | Low-sulfur, high-purity lignin | Easy to depolymerize, suitable for fine chemicals | Higher cost, solvent recovery needed | [7,17,87] |
| Alkali process | H2SO4, HCl or HNO3, 100–150 °C | Phenolic, partially degraded | Simple and inexpensive | Structural modification, lower yield | [7,87] |
| Depolymerization Method | Catalyst/Conditions | Products | References |
|---|---|---|---|
| Pyrolysis | 300–600 °C (no O2) | Bio-oil, phenols, gases, biochar | [23] |
| Hydrogenolysis | 180–300 °C | Phenolics, aromatics | [88] |
| Oxidative depolymerization | O2 or H2O2 | Phenolic acids, aldehydes | [89] |
| Gasification | High temperature (700–1000 °C) | H2, CO, CO2, and CH4 | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makaveckas, T.; Šimonėlienė, A.; Šipailaitė-Ramoškienė, V. Lignin Valorization from Lignocellulosic Biomass: Extraction, Depolymerization, and Applications in the Circular Bioeconomy. Sustainability 2025, 17, 9913. https://doi.org/10.3390/su17219913
Makaveckas T, Šimonėlienė A, Šipailaitė-Ramoškienė V. Lignin Valorization from Lignocellulosic Biomass: Extraction, Depolymerization, and Applications in the Circular Bioeconomy. Sustainability. 2025; 17(21):9913. https://doi.org/10.3390/su17219913
Chicago/Turabian StyleMakaveckas, Tomas, Aušra Šimonėlienė, and Vilma Šipailaitė-Ramoškienė. 2025. "Lignin Valorization from Lignocellulosic Biomass: Extraction, Depolymerization, and Applications in the Circular Bioeconomy" Sustainability 17, no. 21: 9913. https://doi.org/10.3390/su17219913
APA StyleMakaveckas, T., Šimonėlienė, A., & Šipailaitė-Ramoškienė, V. (2025). Lignin Valorization from Lignocellulosic Biomass: Extraction, Depolymerization, and Applications in the Circular Bioeconomy. Sustainability, 17(21), 9913. https://doi.org/10.3390/su17219913





