Abstract
The market for bio-based pigments is growing rapidly, fuelled by the demand for safe, biodegradable colourants in food, cosmetics, pharmaceuticals and textiles. Bacterial pigments offer vibrant colours as well as antimicrobial, antioxidant, anti-inflammatory and anti-cancer properties that increase product safety and shelf life. Despite their benefits, the production of bacterial pigments is associated with challenges such as low yields, high costs and complex processing. Recent eco-innovations such as metabolic engineering, the use of agro-industrial waste as cheap substrates and environmentally friendly extraction methods are helping to solve these problems while promoting the principles of the circular economy. In addition, extremophilic bacteria from harsh environments provide novel pigments with unique industrial potential. This review highlights key advances in eco-innovations for bacterial biopigment production, focusing on genetic engineering, sustainable substrate use, co-production strategies, process optimisation. The role of artificial intelligence and machine learning in improving the biosynthetic efficiency of biopigments will also be analysed. Finally, current challenges and future research opportunities will be discussed to advance microbial biopigments as scalable, cost-effective and environmentally conscious alternatives to synthetic colourants in various industries.
1. Introduction
Synthetic pigments have been criticised for their hyperallergenicity, carcinogenicity and toxicity, which has drawn the attention of the industry and consumers to their natural alternatives [1]. The increasing global demand for sustainable and environmentally friendly products has accelerated the exploration of eco-innovations in various industries, including the production of natural pigments. The global market for bio-based pigments and colourants, including bacterial pigments, is estimated at USD 31.8 billion in 2023 and is expected to reach USD 43.9 billion by 2031 [2]. The shift from synthetic to natural pigments is driven by the increasing demand for natural, sustainable and safe products, especially in the food, cosmetics and pharmaceutical industries. Pigments of natural origin, including bacterial pigments, have gained attention due to their biodegradability, biocompatibility and biological activities [3,4]. In the medical, cosmetic and food industries, bacterial pigments have gained attention as metabolites with antimicrobial and antioxidant activities. The presence of antiviral, antifungal, antibacterial and antioxidant pigments in products can extend the life of a product, benefiting both manufacturers and consumers [5,6]. Bacterial pigments also have other health-promoting properties such as anti-cancer and anti-inflammatory effects, which further increases their attractiveness [7,8]. Bacterial pigments can also be used as colourants in both the food and textile industries, promising safe and environmentally friendly alternatives to synthetic dyes [9,10].
The use of microorganisms as cell factories for pigments offers advantages for the industry. Microbial production can be carried out in all seasons, product extraction is easy and raw materials are widely available [11]. Apart from their environmentally friendly profile, bacterial pigments can be produced under controlled fermentation conditions, allowing for consistent quality and reducing dependence on agricultural land and seasonal fluctuations that affect plant pigments. Nevertheless, synthetic colourants are still popular and widely used because they are cheaper to produce. To be competitive, the cost of bacterial pigment production must be reduced [12]. Co-production of bacterial pigments with other important compounds such as biopolymers and peptides is one of the ways to reduce overall production costs [13]. The utilisation of waste materials from the agricultural industry and food processing provides cheap and highly nutritious raw materials that are both economically and environmentally beneficial [14]. The search for new microbial products, especially in extreme niches, has become an important aspect of technical progress in industrial biotechnology. Extremophiles that thrive in harsh environments have unique metabolic and genetic adaptations that could prove useful in industrial production [15,16]. Pigments are important secondary metabolites for extremophiles and play a crucial role in oxidative stress mitigation and photoprotection required for survival in many niches they inhabit, converting their ecological importance into commercial value and technological potential [17,18]. However, despite its potential, bacterial pigment production is often limited by challenges related to low yields, high production costs and difficulties in downstream processing. Recent advances in biotechnology and process engineering have triggered a wave of eco-innovations aimed at overcoming these obstacles. Targeted modifications of metabolic pathways, regulatory networks and gene expression can be used to optimise higher yields and pave the way to a scalable and efficient bioproduct production process [19]. In addition, to improve pigment biosynthesis, agro-industrial residues are used as low-cost substrates to promote circular economy principles and green extraction techniques are developed to minimise solvent use and energy consumption.
This review article provides a comprehensive overview of eco-innovations in the production of biopigments by bacteria. The focus is on advances in genetic engineering strategies, extremophilic bacteria as pigment producers, sustainable substrate use, co-production approaches, process optimisation and environmentally friendly extraction methods. Special attention is also paid to artificial intelligence, machine learning technologies and the challenges and future research opportunities in the field of using bacteria as hosts for biopigment synthesis.
2. Extremophillic Bacteria as Pigment Producers
The exploration of extremophilic bacteria as pigment producers represents a cutting-edge frontier in eco-innovation by merging biodiversity with industrial applicability. By expanding pigment diversity beyond mesophilic species, extremophilic bacteria contribute to eco-innovations that reduce reliance on synthetic dyes and promote the use of biological resources under conditions compatible with circular bioeconomy principles. Their use supports the development of robust, scalable, and environmentally friendly pigment production platforms that address both ecological and industrial challenges, representing a promising direction for future research and commercialisation in microbial biotechnology. Extremophilic bacteria, which thrive in environments of extreme temperature, salinity, radiation, pH and pressure, have proven to be valuable sources of unique and robust pigments with promising industrial and biotechnological applications. Pigment production by extremophilic bacteria is not only a survival mechanism but also a valuable source of biotechnologically important bioproducts [20].
Psychrophiles, which thrive in low temperature environments, produce pigments that play a crucial role in photoprotection and the mitigation of oxidative stress. Deinococcus sp. UDEC-P1 and Arthrobacter sp. UDEC-A13 (isolated from the cold environments of Patagonia and Antarctica, respectively) have been shown to produce deinoxanthin and decaprenoxanthin. Both biopigments have been reported to significantly reduce the viability of cancer cells, making them a potential additive in cancer therapy [21]. Chryseobacterium greenlandense UMB34 was also able to synthesise a flexirubin-type pigment [22]. Pigments such as bacterioruberin and its glycosylated derivatives have been reported to be produced by Arthrobacter agilis, with maximum biomass accumulation reaching about 1 g/L [23]. Bacterioruberin is an important pigment due to its strong antioxidant activity, playing a key role in the food, pharmaceutical and cosmetic industries [24].
Thermophiles that thrive at high temperatures also produce a number of biotechnologically important pigments. Bacillus haynesii CamB6 has been claimed to produce pyomelanin, a pigment with remarkable antioxidant properties [25]. Similarly, Thermoflavifilum aggregans P373 is able to synthesise pigments of the xanthin-like family, possibly meso-zeaxanthin or alloxanthin or a version of β-carotene [26]. Carotenoids such as thermozeaxanthins and thermobiszeaxanthins could also be produced by Thermus thermophilus [27].
Radiophilic organisms that withstand high levels of radiation produce pigments that serve as protective agents against radiation-induced oxidative stress. Sphingomonas qomolangmaensis S5–59 and Sphingomonas glaciei S8–45 have gene clusters that are responsible for the biosynthesis of zeaxanthin and other carotenoids. They have been shown to have an antioxidant and radioprotective function, opening the way for the development of new therapies against radiation [28]. In addition, Deinococcus radiodurans R1, one of the most radioresistant microorganisms, produces deinoxanthin, further highlighting the protective role of carotenoid pigments [29].
Halophiles adapted to high salinity environments have been described as prolific producers of carotenoids. Bindiya et al. [30] reported the biosynthesis of carotenoids by Cellulosimicrobium funkei KDR2 (86.2 mg/L) and Halobacillus trueperi KDR3 (200.4 mg/L). It was reported that Cytobacillus depressus KDR7 achieved the highest carotenoid yield (516.9 mg/L). The extracted pigments inhibited biofilm formation by foodborne pathogens such as Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli, which opens up their application in the food industry [30].
Extremophilic bacteria, thriving under diverse and challenging conditions, are a rich yet largely untapped reservoir of biopigments with special properties essential for survival in extreme niches. These microorganisms produce a diverse and abundant array of pigments with bioactive properties, including antioxidant, antibacterial, and photoprotective activities, making them valuable resources for next-generation natural pigments with significant commercial potential. Their unique characteristics have drawn increasing research attention to extreme environments as sources of novel producer strains, with promising applications in the pharmaceutical, cosmetics, and food industries [31]. However, several challenges and drawbacks could limit their broader industrial application. The specialised and often harsh conditions required for extremophilic bacteria to grow present technical difficulties for bioprocess design and scale-up. Maintaining these conditions at large scale increases operational complexity and costs, reducing overall economic competitiveness compared to mesophilic bioprocesses. Additionally, the slower growth rates and lower biomass yields typical of some extremophilic strains can limit pigment productivity, thereby constraining industrial feasibility. Genetic tools and metabolic engineering approaches are less developed for extremophilic bacteria, making strain improvement and optimisation more challenging than for well-studied mesophilic bacteria.
Addressing these drawbacks through innovative bioprocess engineering, strain development, and techno-economic analyses will be essential to fully harness the eco-innovative potential of extremophilic bacteria as sustainable pigment producers.
3. Production of Pigments by Bacteria Using Renewable Resources
Under optimised conditions, bacteria can produce pigments from renewable resources through fermentation processes (Table 1). The utilisation of waste substrates for bacterial production is economically attractive and environmentally friendly due to their low cost, biological origin and the rich nutrient profiles of organic waste [32].

Table 1.
Production of bacterial pigments using renewable resources.
Groundnut and oilseed by-products have proven to be valuable substrates for microbial pigment production. Groundnut cake, a nutrient-rich residue from oil extraction, supports the biosynthesis of prodigiosin by Serratia marcescens TUN02 at a concentration of 6.9 mg/L [33]. Similarly, groundnut oil cake has been utilised by Streptomyces sp. ALAA-R20 to produce undecylprodigiosin at a concentration of 180.5 mg/g dry cell weight (DCW) [34]. The proapoptic activity against bacterial and fungal strains, the anticarcinogenic and antioxidant effect make prodigiosin an important pigment from a medical point of view [54,55]. Cottonseed meal has been successfully used to produce pyocyanin in the culture of Pseudomonas aeruginosa. It was reported that both strain R1 and strain U3 were able to synthesise this biopigment at levels of 4.0 µg/mL and 2.2 µg/mL, respectively [35]. Pyocyanin has gained scientific interest due to its antibacterial, antioxidant and anti-cancer properties. Aquaculture, agriculture, biosensors and medicine are industries in which pyocyanin could find application [56].
Soybean and their derivatives provide a wealth of nutrients that facilitate microbial pigment biosynthesis. A mixture of soybean oil waste and wheat bran enabled Serratia marcescens UCP1549 to produce prodigiosin with a yield of 119.8 g/kg, demonstrating the synergistic potential of the combination of agricultural residues [36]. Soybean oil has also served as an effective substrate for the production of biopigments. Rhodopseudomonas faecalis PA2 produced both β-carotene and lycopene in the cultivation supplemented with soybean oil, reaching titres of 7.2 and 5.6 mg/L, respectively [37]. Effective production of β-carotene and lycopene is highly desirable due to their importance in human nutrition as retinoid precursors and their potent antioxidant activity [57]. In addition, lycopene is considered a nutraceutical that improves protection against cancer, cardiovascular and neurodegenerative diseases, hypertension and inflammation [58].
Cassava wastewater, a by-product of cassava processing, was utilised by Serratia marcescens TNU01 to produce prodigiosin with a yield of 6.2 mg/L [39]. The same species was reported to be able to synthesise prodigiosin at a functional concentration of 10 mg/L in the bioprocess using rice bran as a carbon source [41]. Moreover, rice powder appears to be effective for the growth and synthesis of β-carotene by Bacillus clausii. Korumilli et al. [40] proved that this bacterium produces the biopigment at a concentration of 69.4 mg/g DCW. In addition, residues from seafood processing, which are rich in proteins and minerals, have shown significant potential for pigment production. Shrimp waste served as a substrate for the growth of Serratia marcescens CC17 and the production of prodigiosin at a concentration of 6.3 mg/L [42]. The same residue was used as a substrate for the cultivation of Pediococcus acidilactici CFR2182, resulting in the production of carotenoids at a concentration of 4.2 mg/100 g biomass [43]. Another marine residue, squid pen powder, also supported the production of prodigiosin by Serratia marcescens. Strain TNU01 produced the biopigment at a concentration of 3.5 mg/L [33]. A higher concentration of prodigiosin (6.2 mg/L) was reported for Serratia marcescens cultivated on demineralised shrimp shell powder [44].
Cassarini et al. [45] demonstrated that wheat bran can be utilised by Chitinophaga pinensis to produce flexirubin (0.15 mg/L), by Chromobacterium vaccinii to synthesise violacein (1.47 mg/L) and by Gordonia alkanivorans to produce carotenoids (0.07 mg/L). Sugarcane wastes were utilised for melanin production by Streptomyces sp. with a reported yield of 21.13 g/L [46]. Ahmad [47] demonstrated that sugarcane bagasse supplemented with L-tryptophan enabled Chromobacterium violaceum to achieve a violacein yield of 0.82 g/L.
In addition, the processing of fruit generates considerable organic waste that can be utilised and reused for pigment production. Apple pomace, a by-product of juice extraction, has been successfully utilised as a substrate for the production of carotenoids by Sarcina sp. It has been reported that this bacterial genus can produce the biopigment with a yield of 12.9 mg/100 g dry cell [59]. In addition, orange peel wastes were effectively utilised by Escherichia coli DH416 in the production of lycopene (825.3 mg/L) [49]. Palm date waste was utilised by Lactobacillus plantarum for the production of carotenoids (54.89 mg/kg dry cell) [50]. It was demonstrated that supplementation of Chromobacterium violaceum UTM5 cultivation with liquid pineapple waste resulted in the production of violacein at a concentration of 16.3 mg/L [47]. In addition, Aruldass et al. [51] extracted flexirubin at a concentration of 540 mg/L using the same carbon source in the culture of Chryseobacterium artocarpi CECT 8497.
Other by-products such as brown sugar, whey-based media and crude glycerol supported pigment production. Brown sugar was used in the culture of Serratia marcescens UTM1 for the synthesis of prodigiosin [52]. Strain MN5 of the above species has been reported to produce prodigiosin (870 units per cell) when grown on crude glycerol from biodiesel production [54]. Whey-based medium used in the cultivation of Arthrobacter agilis NP20 supported the production of bacterioruberin and its derivatives at a concentration of 5.13 mg/L [53].
4. Genetic Engineering for Bacterial Pigment Production
4.1. Genetic Modifications of Bacteria to Increase Carotenoid Production
Carotenoids are widely studied bacterial pigments due to their antioxidant properties and industrial importance. Genetic engineering has revolutionised bacterial pigment production by optimising biosynthetic pathways, improving yields and expanding the industrial potential of microbial pigments (Figure 1, Table 2). Astaxanthin biosynthesis has been successfully improved by genetic engineering approaches in several bacterial species. Lemuth et al. [60] achieved 1.4 mg/g DCW in Escherichia coli BW-ASTA by integrating the crtEBIY operon and the crtZ gene from Pantoea ananatis and the crtW148 gene from Nostoc punctiforme. Henke et al. [61] produced astaxanthin and L-lysine in a modified Corynebacterium glutamicum. The authors used several metabolic modifications, including the deletion of crtR, a regulatory gene that suppresses carotenoid biosynthesis, which led to an increased supply of precursors. The crtY gene from Pantotea ananatis was also introduced to enable the conversion of lycopene to β-carotene. In addition, the genes crtE, crtB and crtI from Corynebacterium glutamicum were integrated to improve the synthesis of the precursor. To further increase the astaxanthin yield, the genes crtW and crtZ from Fulvimarina pelagi were introduced. These genes are responsible for the ketolation and hydroxylation of β-carotene and complete the astaxanthin biosynthetic pathway. This genetic modification resulted in the production of 10 mg/L astaxanthin and 48 g/L lysine in a fed-batch fermentation. Lu et al. [62] modified E. coli to increase astaxanthin production through a combination of plasmid-free system engineering, enzyme optimisation and metabolic balancing. The authors compared different β-carotene ketolases from Brevundimonas sp. SD212, Sphingomonas sp. DC18, Paracoccus sp. N81106 and Chlamydomonas reinhardtii to identify the most efficient enzyme combination for astaxanthin biosynthesis. The crtW gene from Brevundimonas sp. SD212 and the crtZ gene from Pantoea ananatis were identified as the optimal combination for astaxanthin production. Further modifications, which included the integration of multiple copies of the crtZ genes and the elimination of plasmid dependence, resulted in the production of 7.4 mg/g DCW astaxanthin as the dominant carotenoid (96.6% of total carotenoids). Furthermore, Park et al. [63] optimised E. coli for astaxanthin biosynthesis through a multi-layered metabolic engineering approach. They introduced a heterologous astaxanthin biosynthetic pathway by incorporating the crtE, crtY, crtI, crtB and crtZ genes from Pantoea ananatis and a truncated BKT gene (trCrBKT) from Chlamydomonas reinhardtii. The stability and localisation of the enzyme was improved by adding fusion tags to trCrBKT, which improved its association with the membrane. Further optimisation of the metabolism was achieved by overexpressing the ispD and ispF genes, resulting in a final carotenoid production yield of 432.82 mg/L in a fed-batch fermentation [63]. Nogueira et al. [64] improved astaxanthin biosynthesis in E. coli by fusing a gene encoding β-carotene hydroxylase (encoded by crtZ gene) and β-carotene ketolase (encoded by crtW gene) into a single polypeptide. This approach improved substrate channelling, reduced the accumulation of intermediates and increased astaxanthin yield by 1.4-fold compared to separate enzyme expression. Henke et al. [65] modified Corynebacterium glutamicum for astaxanthin biosynthesis by introducing the crtY, crtW and crtZ genes, resulting in a productivity of 0.4 mg/L·h. Choi et al. [66] analysed the β-carotene hydroxylase (encoded by the crtZ gene) from different bacterial species and identified the crtZ gene from Brevundimonas sp. SD212 as the most efficient for astaxanthin biosynthesis. In addition, the same authors introduced the CYP175A1 gene from Thermus thermophilus, which enabled the conversion of canthaxanthin to adonirubin, highlighting the potential for novel ketocarotenoid production by genetic engineering.
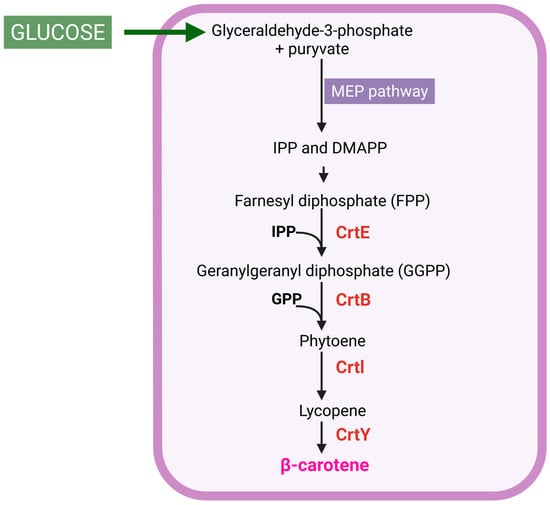
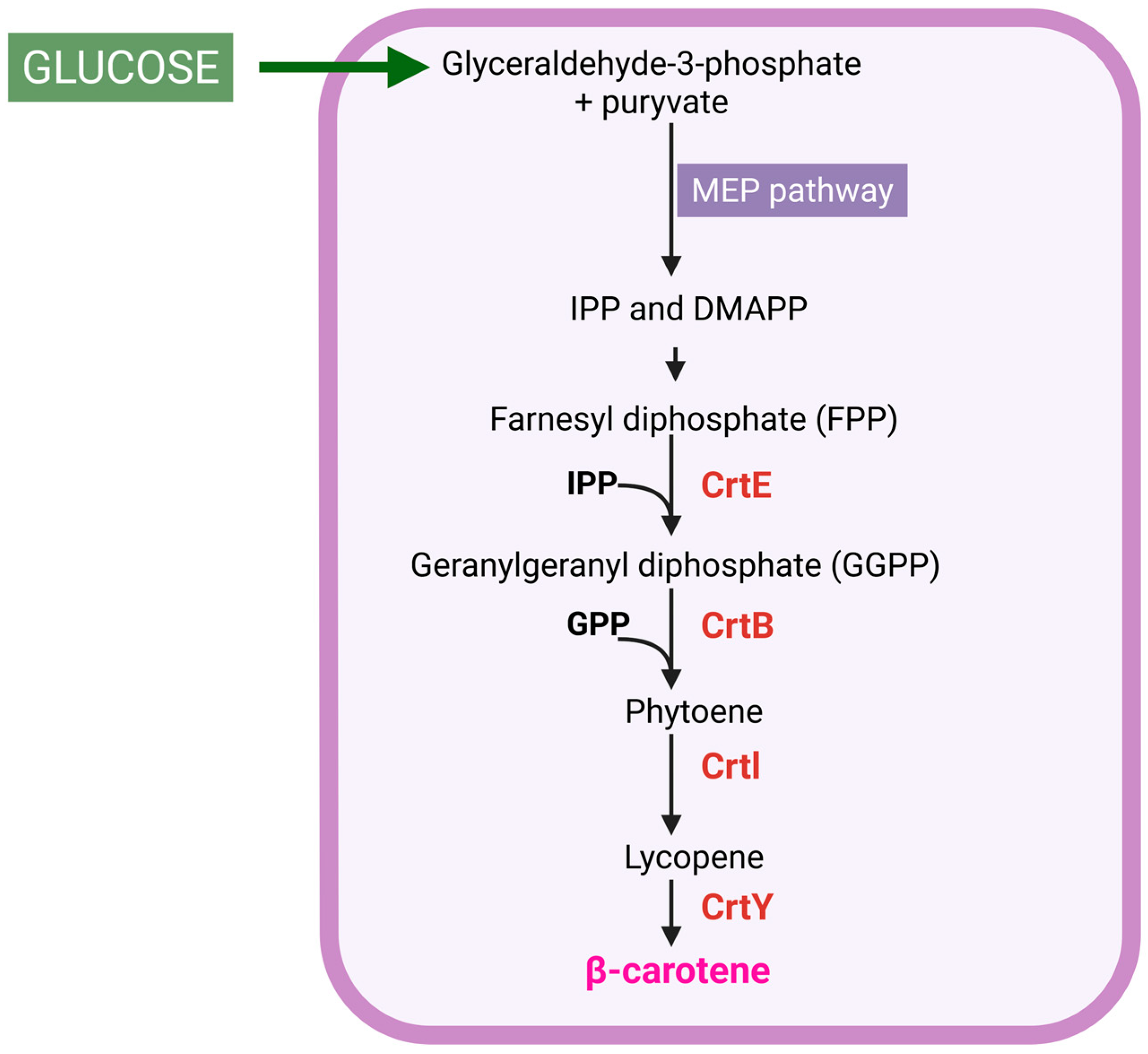
Figure 1.
Diagram of β-carotene production. MEP, 2-C-methyl-D-erythritol 4-phosphate; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl diphosphate; CrtE, GGPP synthase; CrtB, phytoene synthase; CrtI, phytoene dehydrogenase; CrtY, lycopene cyclase. Created in BioRender, https://BioRender.com/63nmkk6 (accessed on 25 October 2025).

Table 2.
Genetic modifications of bacteria for carotenoid production.
Zhao et al. [67] improved β-carotene synthesis to 2.1 g/L by altering key metabolic modules, including ATP synthesis, the pentose phosphate pathway (PPP) and the tricarboxylic acid cycle (TCA), to increase the supply of precursors and cofactors. The combination of overexpression of the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway and modifications to the β-carotene synthesis module resulted in a 64% increase in yield over a high-producing parental strain. Yang and Guo [68] optimised β-carotene biosynthesis by integrating a hybrid mevalonate (MVA) and MEP pathway and co-expressing the dxs, fni, GPPS2, mvaE, mvaS, crtE, crtB, crtI and crtY genes. These modifications resulted in a final production of 3.2 g/L in E. coli BL21(DE3) under aerobic fed-batch fermentation conditions. Yoon et al. [69] increased β-carotene production to 503 mg/L by combining the MEP pathway with an exogenous addition of mevalonate, thus altering the isoprenoid precursor pathway. To achieve this, they overexpressed key genes of the MEP pathway (dxs and ipi) together with heterologous Enterococcus faecalis mvaE and mvaS genes encoding enzymes of the mevalonate-dependent isoprenoid biosynthetic pathway. The implemented strategies in combination with the optimisation of fermentation conditions enabled the authors to produce 465 mg/L of β-carotene.
Li et al. [70] used CRISPR-Cas9 genome editing to systematically optimise β-carotene biosynthesis in E. coli. Through iterative metabolic engineering, they tested 33 genetic modifications and constructed over 100 variants. Their optimised strain, which contained 15 targeted mutations, achieved a production of 2.0 g/L β-carotene in fed-batch fermentation. Wu et al. [71] introduced a membrane engineering strategy to increase the accumulation of β-carotene in E. coli. By overexpressing membrane-bending proteins such as Almgs, MtlA, Tsr, Plsb, Plsc and Dgka, they increased the membrane surface area, enabling higher intracellular β-carotene retention. Their best performing strain reached 44.2 mg/g DCW. Yuan et al. [72] modified an E. coli strain by exchanging the chromosomal promoter. Strong T5 bacteriophage promoters were introduced to upregulate key endogenous genes, resulting in 6 mg/g DCW of β-carotene production. Su et al. [73] optimised lycopene biosynthesis by integrating a 12 kb long synthetic pathway directly into the E. coli genome using CRISPR-Cas9 and λ-Red recombination. Their approach reduced the metabolic burden associated with high copy number plasmids and optimised the efficiency of the pathway, resulting in a 4.4-fold increase in lycopene production. Wei et al. [74] introduced a strategy of chromosomal integration at multiple positions and placed the crtE, crtB and crtI genes at different genomic loci. This strategy distributed the metabolic load across multiple sites and improved both stability and production efficiency. This approach resulted in a lycopene production of 224 mg/L. Zhou et al. [75] focused on redistributing carbon flux by silencing the zwf gene encoding glucose-6-phosphate dehydrogenase to redirect carbon flux to the MEP pathway. This change led to an increased availability of glyceraldehyde-3-phosphate and pyruvate, resulting in a lycopene yield of 7.55 mg/g DCW. Deletion of the crtR gene, a negative regulator of carotenoid biosynthesis, led to a 10- to 30-fold increase in the production of various carotenoids, including β-carotene, lycopene and decaprenoxanthin in Corynebacterium glutamicum [76]. In addition, overexpression of the crtE, crtB, crtI, crtYe, crtYf and crtEb genes increased the accumulation of carotenoids. By overexpressing sigA with simultaneous deletion of sigB, Taniguchi et al. [77] increased the production of lycopene (0.82 mg/g DCW), β-carotene (11.9 mg/g DCW) and bisanhydrobacterioruberin (BABR) (0.52 mg/g DCW) in Corynebacterium glutamicum cells. Yoon et al. [69] compared carotenoid genes from Pantoea agglomerans and Pantoea ananatis to optimise lycopene biosynthesis in E. coli. They reported that the crtE, crtB and crtI genes from P. agglomerans led to a twofold increase in lycopene production (27 mg/L) compared to P. ananatis (12 mg/L). In addition, integration of a mevalonate bottom pathway led to a further increase in lycopene accumulation to 60 mg/L when supplied with exogenous mevalonate. Su et al. [73] modified Rhodobacter sphaeroides by introducing a four-step phytoene dehydrogenase (encoded by crtI4 gene) from Rhodospirillum rubrum while deleting the native three-step phytoene dehydrogenase (encoded by crtI3 gene). They also knocked out crtC gene to block secondary pathways and increase lycopene accumulation. Further optimisations included the deletion of the zwf gene, which redirected the metabolic flux from the pentose phosphate pathway to lycopene biosynthesis, increasing production by 88%. The final manipulated strain yielded 10.32 mg/g DCW lycopene. Shukal et al. [77] developed a CRISPR-Cas9-based genome engineering approach for Escherichia coli BL21 targeting 16 genes associated with metabolic pathways to increase lycopene production. Their strategy involved the use of asymmetric homology arms to facilitate genetic modifications. Iterative gene deletions led to a threefold increase in lycopene yield. CRISPR interference (CRISPRi) was also used to equalise gene expression in the mevalonate pathway by targeting the atoB, mvaS, mvaA, idi and ispA genes. Their approach successfully increased lycopene production in E. coli [78]. Optimisation of ribosome binding sites and redirection of metabolic flux were investigated for lycopene production in E. coli. Modifications included deletion of the crtX and crtY genes in combination with overexpression of the dxs and idi genes, resulting in a final lycopene yield of 3.52 g/L [79].
Corynebacterium glutamicum was also modified to biosynthesise C50 and C40 carotenoids, including decaprenoxanthin, sarcinaxanthin and zeaxanthin. The modification included the introduction of the key genes crtX, crtE, crtB, crtI, crtYe, crtYf and crtEb, which facilitate the expansion and modification of the carotenoid backbones. The introduction of the crtX gene, encoding a glucosyltransferase, enabled the glycosylation of carotenoids, resulting in improved solubility and stability of the pigments [80]. The authors’ strategy led to a production of 3–4 mg/g DCW of C50 and C40 carotenoids. Xie et al. [81] used Cas9-Lag, a CRISPR-based localisation tool, to alter the positioning of genes for carotenoid biosynthetic in the E. coli genome. By replacing the crtZ and crtW genes near membrane-associated regions, the authors improved the efficiency of the enzyme. This approach increased the yield of zeaxanthin and astaxanthin by 29.0% and 26.7%, respectively, demonstrating that the organisation of the genome is a key factor in optimising carotenoid production.
4.2. Genetic Modifications of Bacteria to Increase Non-Carotenoid Production
Genetic modifications play a crucial role in increasing the production of non-carotenoid pigments in bacteria by enabling targeted changes in metabolic pathways. Although non-carotenoid pigments such as melanin, violacein and prodigiosin originate from different biosynthetic pathways, their production can be significantly optimised by metabolic engineering and synthetic biology approaches. Melanin is a dark, polymeric pigment that provides UV protection, resistance to oxidative stress and antimicrobial properties [82]. Wang et al. [83] proposed the expression of the tyr-1 gene from Bacillus megaterium in Vibrio natrigenes. Tyr-1 encodes a tyrosinase enzyme that catalyses the oxidation of L-tyrosine to o-dihydroxyphenylalanine (DOPA) and dopaquinone. DOPA and dopaquinone are cyclised to 5,6-dihydroxyindole (DHI) or 5,6-dihydroxyindole-2-carboxylic acid (DHICA), which then polymerise to melanin. Overexpression of the mel gene from Pseudomonas maltophila in E. coli cells led to a 70.6% increase in melanin synthesis. The introduction of the melA gene from Rhizobium etli into E. coli enabled the conversion of L-tyrosine into melanin [84]. Furthermore, co-expression of the melC gene encoding tyrosinase with the CYP102G4 gene encoding monooxygenase resulted in the production of 3.4 g/L of CYP-melanin [85]. This approach not only improved the efficiency of biopigment production, but also the efficiency of substrate oxidation. The productivity of pyomelanin was improved by genetic modification tools. Pyomelanin is synthesised via the homogentisate (HGA) pathway, in which homogentisic acid spontaneously polymerises to pyomelanin. Park et al. [86] developed an E. coli strain capable of producing 200 mg/L pyomelanin by combining metabolic engineering strategies with the co-production of poly-(3-hydroxybutyrate) [P(3HB)]. This modification strategy involved overexpression of the hppd gene encoding 4-hydroxyphenylpyruvate dioxygenase, which is a key enzyme in HGA synthesis. The homogentisate 1,2-dioxygenase (encoded by the hmgA gene) was switched off to prevent HGA degradation. The bktB, phaB and phaC genes were co-expressed for the simultaneous production of the biopolymer and pyomelanin. The result was that the recombinant E. coli was able to produce 200 mg/L pyomelanin. The efficiency of prodigiosin production was improved by a protoplast fusion strategy between two Streptomyces strains. As a result, the concentration of undecylprodigiosin reached a value of 181.78 mg/g DCW [34].
Industrial chassis microorganisms such as E. coli or C. glutamicum offer rapid growth, well-understood genetics, and scalable fermentation conditions, but may lack the intrinsic ability to produce extremophile-like pigments with comparable stability or diversity. Engineering extremophile pigment biosynthesis genes and stress tolerance traits into these chassis can harness the advantages of robust, easily cultivated hosts while aiming to replicate extremophile pigment qualities. Successful engineering must address challenges in metabolic pathway expression, protein folding under non-extreme conditions, and cofactor availability, while optimising production rates and pigment yields. This integrative approach holds promise for industrial biopigment production by combining extremophile robustness with industrial scalability.
5. Co-Production of Bacterial Pigments with Other Important Bioactive Compounds
Co-production of bacterial pigments with other valuable bioproducts can improve the economic viability and sustainability of microbial biopigment processes. The efficient production of two or more biotechnologically important biomolecules in one fermentation process can be beneficial to create multifunctional product portfolios that fulfil different industrial requirements without the need for several separate cultivation steps. Combining pigment production with other high-value metabolites enables more efficient substrate utilisation and reduces the amount of waste by converting the remaining metabolic intermediates into additional bioproducts.
Some pigment-producing bacteria naturally synthesise biopolymers, such as polyhydroxyalkanoates (PHAs), which have important biotechnological and biomedical applications. These properties increase the industrial importance of bacteria in the production of bioactive compounds. Kumar et al. [87] reported the simultaneous production of violacein and polyhydroxyalkanoates (PHAs) in a cultivation of Iodobacter sp. PCH194 from the Himalayan mountains. In this study, laboratory-scale fermentation in a 22-L bioreactor resulted in the production of P(3HB) (yield of 11.0 g/L, 58% of dry cell mass) and violacein pigment (yield of 1.5 g/L).
The co-production of PHA and bacterial pigments can also combine the beneficial properties of both the biopolymer and the pigment produced. Park et al. [86] successfully produced pyoPHB, a composite of pyomelanin and P(3HB). The composite was co-produced by introducing the HPPD enzyme into P(3HB)-producing Escherichia coli cells. This innovation allowed the use of a single bacterial culture and simplified the extraction process, eliminating the need for two separate biotransformations. As a result, the process saved both time and resources, making it economically and logistically advantageous. The pyoPHB produced had a higher melting temperature and enthalpy of decomposition as well as higher antioxidant activity compared to the control P(3HB) film. The introduction of microbial pigments into the biomaterial can also influence the sensory properties of the produced polymer. Jung et al. [88] produced blue-coloured P(3HB) in a single cultivation and co-extracted indigo pigment and P(3HB). The authors concluded that the introduction of the pigment did not affect the thermal properties of the P(3HB). The co-production led to an improvement in the biotransformation of indole to indigo by introducing PHA synthesis genes into the Escherichia coli host.
In addition, Henke et al. [76] simultaneously produced 48 g/L L-lysine and 10 mg/L astaxanthin in a fed-batch fermentation. The authors modified Corynebacterium glutamicum ASTALYS by introducing genes involved in the heterologous production of β-carotene hydroxylase (encoded by the crtZ gene) and β-carotene ketolase (encoded by the crtW gene). In the same study, the co-production of other valuable carotenoids such as lycopene, β-carotene, zeaxanthin and canthaxanthin with L-lysine was demonstrated.
The simultaneous production of flexirubin-type pigments and antioxidant peptides from feather waste was also demonstrated by Bertolini et al. [13]. It was found that Chryseobacterium sp. kr6 cultured on raw feathers as substrates was able to synthesise 311 mg/g DCW of the above-mentioned biopigment and 342 mg/g DCW of feather proteins. The flexirubin pigments, which are characteristic yellow to orange polyene compounds, exhibited remarkable antioxidant activities as determined by various assays such as hydrogen peroxide scavenging, inhibition of lipid peroxidation and neutralisation of superoxide radicals. The study emphasises the potential of flexirubin to act as a natural antioxidant. Molecular docking analyses indicate that it interacts with antioxidant enzymes such as superoxide dismutase, which may be the basis for its action as a free radical scavenger. The combined production of flexirubin and antioxidant peptides from problematic agro-industrial waste streams illustrates an effective circular bioeconomy strategy by valorising waste into high-value multifunctional biomolecules in an integrated and sustainable bioprocess.
6. Statistical Optimisation for Bacterial Pigment Production
Statistical methodologies have become an important ally in microbial biosynthesis research, optimising yields of pigment and biomass production. analysing of multiple variables allow researchers to identify significant factors, explore their interactions, and determine optimal conditions for microbial processes. Among the most widely used approaches are Response Surface Methodology (RSM), Plackett-Burman Design (PBD), and factorial designs. Application of the statistical methods allow researchers to save both time and resources simultaneously allowing them to achieve higher yields of biopigments (Table S1).
6.1. Response Surface Methodology—Central Composite Design
The Response Surface Methodology (RSM) is a statistical technique used to model and optimise processes in which several variables influence the result. Within RSM, the Central Composite Design (CCD) is an effective method for determining optimal conditions with a relatively small number of trial runs. It integrates a full factorial design with axial and central points, making it an effective tool for finding optimal conditions in microbial pigment production processes. Gharibzahedi et al. [89] optimised the medium and achieved a biomass value of 9.95 g/L, a total carotenoid content of 7.67 mg/L and a canthaxanthin production of 7.10 mg/L in Dietzia natronolimnaea HS-1 using the CCD approach. The authors concluded that the optimal conditions were 25 g/L D-glucose, 15.12 g/L mannose and 36.77 ppm Fe3+. The application of CCD helped to optimise pigment production by halophilic bacteria, including Aquisalibacillus elongatus MB592 (21.4 mg/g fresh biomass), Salinicoccus sesuvii MB597 (27.3 mg/g fresh biomass) and Halomonas aquamarina MB598 (15.7 mg/g fresh biomass) were reported under common conditions of pH 7.5, 37 °C and NaCl concentrations of 2.75 M, 2.5 M and 2.5 M [90].
6.2. Response Surface Methodology—Box–Behnken Design
The Box–Behnken Design (BBD) is another variant of the RSM. Compared to CCD, BBD has a smaller number of test runs, as extreme factor values are excluded. Sudhakar et al. [91] report on the possibility of prodigiosin production by Serratia marcescens CSK using the BBD approach. The authors achieved a biopigment concentration of 2950 mg/L under optimised conditions: pH of 7.0, 4.0 g/L tryptophan, 3.0 g/L sucrose and 60 h incubation. Venil et al. [92] reported a maximum flexirubin pigment yield of 521.6 mg/L in Chryseobacterium artocarpi CECT 8497 under certain concentrations of the culture medium components, e.g., lactose (11.25 g/L), L-tryptophan (6 g/L) and KH2PO4 (650 ppm). Miglani et al. [93] achieved 0.5048 colour value units per mg of prodigiosin isolated from Serratia marcescens CMS2 cultures under optimal conditions with a pH of 6.5, substrate concentration of 1.5%, inoculum size of 1.25% and agitation speed of 150 rpm. The BBD approach was also used to optimise bacterioruberin pigment production in Arthrobacter agilis NP20 [53]. The authors achieved the highest concentration of biopigment (6.01 mg/L) under conditions of 0.86% MgSO4, 0.96% whey and 0.8% inoculum size.
6.3. Plackett-Burman Design and Combined Response Surface Methodology Approaches
The Plackett-Burman Design (PBD) is a screening method that makes it possible to evaluate a large number of variables with a minimum number of experiments. It is often used as a precursor to more advanced optimisation techniques such as RSM. In PBD, each factor is tested at two levels, resulting in a positive or negative effect on the main effect and ignoring the interactions between factors. Guo et al. [94] applied PBD followed by CCD to optimise melanin production in Streptomyces kathirae SC-1 and obtained a yield of 13.7 g/L under conditions of 3.3 g/L amylodextrin, 37 g/L yeast extract and 54.4 μM CuSO4. The production of red pigment in Streptomyces sp. PM4 was also optimised and resulted in a biopigment concentration of 1.874 g/L under conditions of 4.06 g maltose, 7.34 g peptone, 4.34 g yeast extract and 2.89 g tyrosine [95]. PBD and BBD approaches were applied to optimise melanin production in Streptomyces djakartensis NSS-3, achieving 118.73 mg/10 mL under the conditions of 3.71 g/L L-tyrosine, 12.75 days of incubation and 0.57 g/L ferric ammonium citrate [96].
6.4. Factorial Design and Sequential Methodologies
Factorial designs are highly efficient for analysing the effects of multiple variables simultaneously, including their interactions. These designs are used to understand the combined influence of key factors on microbial processes. In a study on Serratia marcescens MN5, a factorial design was used to optimise temperature, pH, peptone concentration, inoculum size and incubation time for the production of prodigiosin. The researchers found that the optimal conditions were 22 °C, pH 9, 1% (w/v) peptone and 6 days incubation and resulted in 870 units/cell of prodigiosin [54]. Sequential methods combine initial screening with advanced optimisation to enable comprehensive analysis of microbial processes. This approach allows researchers to first identify important variables and then optimise them for maximum performance. Khodayian [97] applied a sequential methodology to optimise canthaxanthin production in Dietzia natronolimnaea HS-1. A fractional factorial design was used for initial screening followed by CCD for optimisation. The optimal conditions (pH 7.66, 55.54 g/L whey lactose and 7.36 g/L yeast extract) allow the production of 2871 µg/L canthaxanthin.
7. Challenges and Future Directions
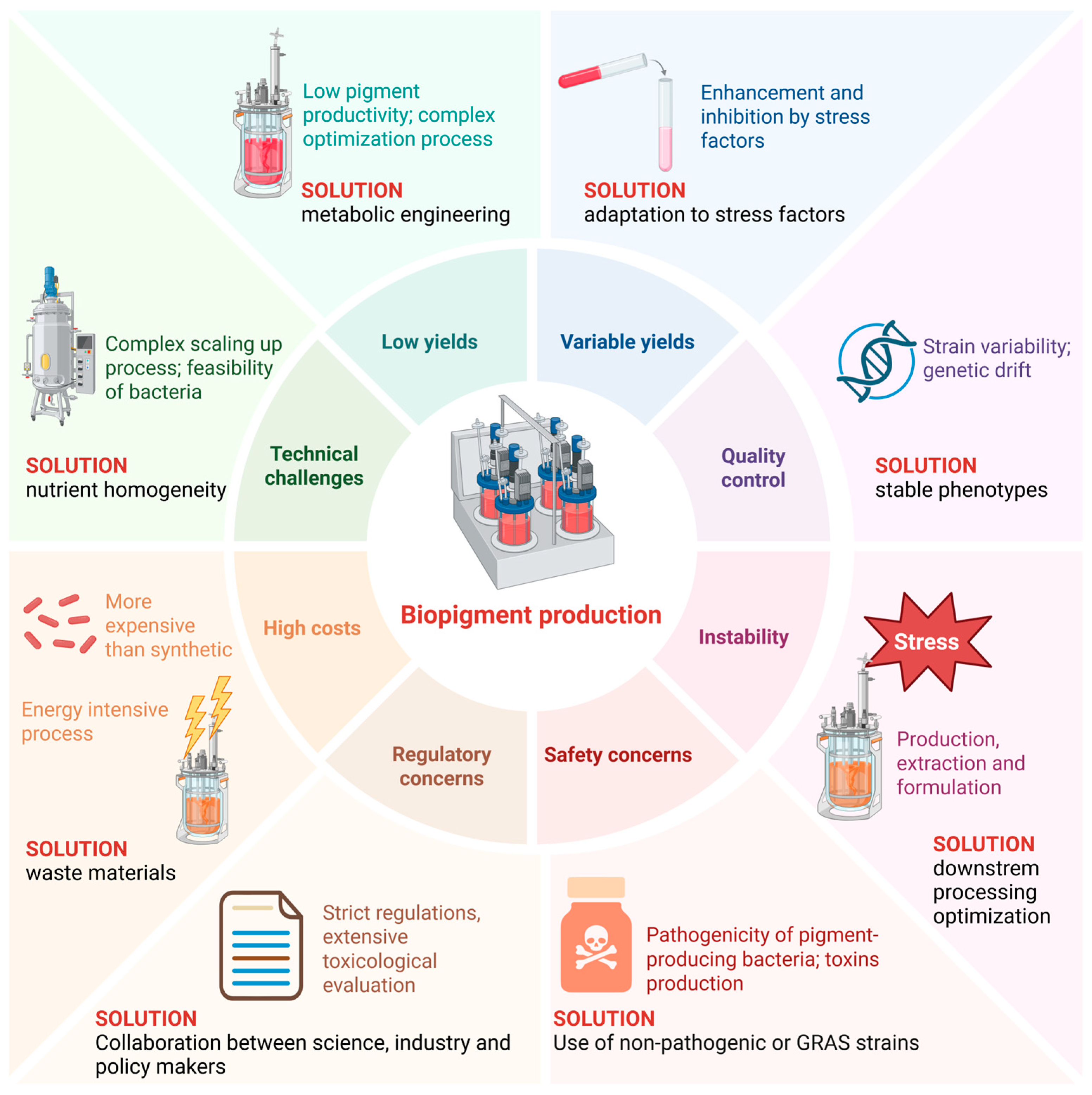
Although bacterial biopigments are promising as natural, environmentally friendly alternatives to synthetic colourants, their production faces several major challenges that stand in the way of widespread industrial use (Figure 2). One major limitation is the often low pigment productivity of indigenous bacterial strains. Many wild-type bacteria produce pigments in concentrations that are insufficient for commercial scale. Pigment biosynthetic pathways are tightly regulated and sensitive to environmental conditions such as pH, temperature, light intensity and nutrient availability, resulting in inconsistent yields [98]. Stress factors such as nutrient deficiency or salinity can both promote and inhibit pigment production, making process optimisation complex and species-specific.
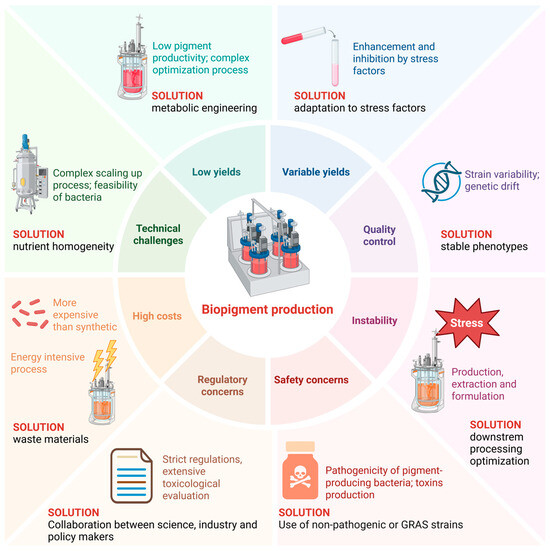
Figure 2.
Challenges in bacterial pigments production. Created in BioRender, https://BioRender.com/muiuah8 (accessed on 25 October 2025).
In addition, natural pigments, especially carotenoids and other polyene-based compounds, are often unstable when exposed to oxygen, light, heat and different pH values during production, extraction or formulation [99]. This instability makes handling difficult and limits shelf life, so formulation innovations such as microencapsulation are required to stabilise them. In addition, strain variability and genetic drift can lead to inconsistent pigment composition and quality, posing a challenge for standardisation and regulatory approval, especially for food and pharmaceutical applications [100]. Despite their environmentally friendly profile, bacterial biopigments are generally more expensive to produce than synthetic colourants due to higher substrate costs, lower yields and energy-intensive processes. Fermentation requires careful optimisation of media composition, aeration, pH and temperature to maximise pigment production, which can be laborious and costly. Organic solvents or multi-stage processes are often used in extraction and purification, which increase operating costs and environmental impact, thus contradicting sustainability goals [101]. Although bacterial pigments are natural, there are safety concerns if pigment-producing bacteria are pathogenic or produce toxins in addition to the pigments [102]. Careful strain selection and genetic engineering to eliminate toxin production are therefore essential. In addition, the regulatory framework for natural dyes is strict and requires extensive toxicological evaluation, which can be costly and time-consuming.
Scaling up pigment production from laboratory to industrial scale is complex due to the interplay of microbial physiology and bioprocess conditions. Maintaining stable phenotypes, avoiding contamination and ensuring oxygen transfer and nutrient homogeneity in large bioreactors can impact pigment yield and quality. Despite significant technical and economic hurdles, ongoing multidisciplinary efforts continue to improve the feasibility of bacterial biopigments as a sustainable, scalable and competitive alternative to synthetic colourants in various industries.
The future of bacterial biopigment production is poised for significant growth and innovation driven by advances in biotechnology, process engineering and sustainability priorities. As the global demand for natural and environmentally friendly colourants increases, extensive research and technological breakthroughs are expected to overcome current production limitations and open up new applications. One of the most promising prospects is the integration of synthetic biology and metabolic engineering to develop genetically optimised bacterial strains with improved pigment biosynthesis capabilities. These genetically engineered microbes will have finely tuned regulatory circuits and metabolic pathways designed to increase pigment yield, stability and synthesis of novel pigments through the introduction or modification of biosynthetic genes. With the help of CRISPR/Cas and other genome editing tools, strain development is becoming faster and more precise [103]. The integration of synthetic biology and metabolic engineering is revolutionising bacterial biopigment production by enabling precise design and optimisation of microbial metabolic pathways for improved pigment biosynthesis. Together, these disciplines enable the development of customised microbial cell factories for efficient, high-yield production of natural pigments with improved quality and novel properties. Metabolic engineering involves the systematic modification of metabolic pathways through the targeted overexpression, deletion or substitution of genes in order to redirect cellular resources towards pigment synthesis. This includes improving the supply of precursors, reducing competing metabolic pathways and optimising the availability of cofactors. For example, the biosynthetic pathway for carotenoids can be altered by overexpressing rate-limiting enzymes, removing feedback inhibitors and balancing enzyme expression to increase the flux to pigment production [104]. Synthetic biology complements metabolic engineering by providing a toolbox of standardised, modular genetic parts such as promoters, ribosome binding sites, terminators and regulatory elements that can be assembled and fine-tuned to build or rewire biosynthetic circuits. This modular approach enables predictable control over gene expression and pathway dynamics. Advanced synthetic biology techniques also enable the construction of novel synthetic pathways or the insertion of heterologous genes for pigment biosynthesis into more robust and faster-growing bacterial hosts, expanding the range of available pigments and improving productivity [105]. Furthermore, coupling synthetic biology with systems biology and multi-omics analyses provides comprehensive insights into cellular metabolism, enabling rational pathway design and iterative optimisation based on genome-scale metabolic models [106].
Artificial intelligence (AI) and machine learning (ML) technologies are increasingly being used to optimise fermentation processes, including predictive models for bacterial growth conditions, substrate utilisation and real-time process control. These tools will significantly reduce experimentation time, improve productivity and create robust, reproducible production systems. AI and ML technologies have been successfully used to predict the next generation of microalgae biomolecules [107]. However, there is still too little data on optimising bacterial biopigment production and overcoming challenges such as low yield, process variability and cost inefficiency using the powerful technologies mentioned above. In the optimisation of fermentation processes, ML algorithms could analyse large data sets from experimental trials to identify key parameters that influence pigment synthesis, such as pH, temperature, nutrient concentration, incubation time and aeration, and predict optimal conditions. Techniques such as the response surface method, which is integrated with AI, can rapidly assess the interactions between variables, significantly reducing experimental labour while improving pigment yield and consistency. For example, ML models have been used to optimise melanin and carotenoid production by predicting culture conditions that simultaneously maximise pigment concentration and biomass [108]. AI-assisted predictive modelling also helps in strain development by guiding genetic modifications. In conjunction with systems biology and multi-omics data, ML can identify metabolic bottlenecks and suggest gene targets for the development of improved pigment biosynthetic pathways. This accelerates the design-build-test cycle in synthetic biology and enables the creation of microbial strains with customised pigment profiles and superior productivity. Recent advances include the use of deep learning for real-time monitoring and adaptive control of bioreactors to ensure stable environmental conditions and timely intervention to avoid yield fluctuations [109]. Hyperspectral imaging in combination with AI algorithms can also enable rapid phenotyping and pigment classification during production [110]. Overall, the integration of AI and ML into bacterial biopigment production holds the potential to facilitate precision fermentation, reduce resource consumption, improve product quality and accelerate the transition of microbial pigments from lab to industry to support sustainable and innovative bio-based pigment production.
In addition, the circular bioeconomy model will continue to play a crucial role, with a focus on valorising waste and by-products from agriculture and the food industry as low-cost substrates for bacterial pigment fermentation. This approach promotes sustainable, cost-efficient bioprocesses with minimal environmental impact. In addition, the development of environmentally friendly extraction and purification methods will ensure that the eco-friendly profile of biopigments extends throughout the production chain [111]. To efficiently separate co-products alongside bacterial biopigments while preserving eco-friendly credentials and economic feasibility, green extraction and purification strategies must be carefully designed and integrated, taking into account the chemical nature of both pigments and co-products, as well as process sustainability. Implementing multi-stage extraction can sequentially recover different co-products based on solubility and polarity differences. Techniques such as ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid extraction with carbon dioxide, subcritical water extraction and high hydrostatic pressure extraction have been shown to be effective in isolating biomolecules [112]. They could increase pigment yield and purity and preserve the structural integrity and bioactivity of sensitive pigment molecules as they operate at milder temperatures and shorter processing times compared to conventional solvent-based methods. In addition, green purification approaches, including chromatography with biodegradable materials and enzyme-assisted purification, reduce the use of chemicals and improve selectivity. By integrating these advanced green technologies, the sustainability profile of bacterial biopigments can be maintained from fermentation to final product formulation. This supports safer, cleaner and more responsible manufacturing that is in line with circular economy principles.
Research into extremophilic and novel bacterial species from unexplored ecosystems is expected to yield new pigments with unique properties such as increased stability, bioactivity and multifunctionality. The combination of pigments with other valuable properties such as antimicrobial or antioxidant activity will expand the range of applications in food preservation, cosmetics, pharmaceuticals and textiles. Finally, increasing regulatory support and consumer awareness of natural and sustainable products will increase market acceptance. Collaboration between science, industry and policy makers will accelerate commercialisation and innovation.
8. Conclusions
Eco-innovations in bacterial biopigment production offer a promising route to sustainable, safe, and high-value natural colourants. While microbial pigments are highly valued for their biodegradability, biocompatibility, and bioactive properties, challenges such as low and inconsistent yields, stability concerns, high production and processing costs, safety issues, and scale-up difficulties persist. Advances in synthetic biology and metabolic engineering are driving breakthroughs by enabling precise optimisation of microbial pathways to enhance pigment yields and customise their features. Additionally, artificial intelligence and machine learning are set to revolutionise strain engineering and process optimisation by utilising large datasets to predict optimal conditions, accelerating development and ensuring consistent productivity. Sustainability is further supported by green extraction methods that reduce energy use and environmental impact, along with valorizing agro-industrial waste as feedstocks, reinforcing circular bioeconomy principles and lowering costs. Co-production strategies that generate multiple valuable products enhance economic feasibility and resource use. Moving forward, sustained multidisciplinary research, regulatory encouragement, and industrial partnerships will be essential to overcome remaining obstacles and fully realise the commercial potential of bacterial biopigments. Together, these innovations promise to establish microbial pigments as competitive, scalable, and eco-friendly alternatives to synthetic colourants across food, cosmetics, pharmaceuticals, textiles, and other sectors, aligning well with sustainability objectives and growing consumer demand for natural products. By integrating diverse approaches and advancing technologies, microbial pigment biotechnology is steadily progressing toward scalable, cost-effective, and environmentally responsible production systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17219897/s1, Table S1: Statistical optimisation for bacterial pigment production.
Funding
This research was funded by University of Warmia and Mazury in Olsztyn (Poland), grant number 12.610.013-110.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Alegbe, E.O.; Uthman, T.O. A review of history, properties, classification, applications and challenges of natural and synthetic dyes. Heliyon 2024, 10, e33646. [Google Scholar] [CrossRef]
- Bio-Based Pigments and Dyes Market 2024 Latest Report with Forecast to 2031. (n.d.). Available online: https://www.insightaceanalytic.com/report/bio-based-pigments-and-dyes-market/2342 (accessed on 9 September 2025).
- Celedón, R.S.; Díaz, L.B. Natural pigments of bacterial origin and their possible biomedical applications. Microorganisms 2021, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Woła̧cewicz, M.; Pyter, W.; Joshi, N.; Drewniak, L.; Pranaw, K. Colorful treasure from Agro-Industrial Wastes: A sustainable chassis for microbial pigment production. Front. Microbiol. 2022, 13, 832918. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Barreto De Oliveira, J.V.; Casanova, L.M.; Neves, A.; Junior Reis-Mansur, M.C.P.P.; Vermelho, A.B. Microbial pigments: Major groups and industrial applications. Microorganisms 2023, 11, 2920. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; Haq, I.U. An overview on industrial and medical applications of Bio-Pigments synthesized by marine bacteria. Microorganisms 2020, 9, 11. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The realm of microbial pigments in the food color market. Front. Sustain. Food Syst. 2021, 5, 603892. [Google Scholar] [CrossRef]
- Kramar, A.; Kostic, M.M. Bacterial secondary metabolites as biopigments for textile dyeing. Textiles 2022, 2, 252–264. [Google Scholar] [CrossRef]
- Devassy, E.; Rebello, S.; Puthur, S.; AN, A.; Aneesh, E.M.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Pandey, A. Bacterial pigments in food production—Associated advantages and disadvantages. Ivanian J. Sci. Res. 2024, 1, 1–5. [Google Scholar]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, D.; Jiménez, M.E.P.; dos Santos, C.; Corrêa, A.P.F.; Brandelli, A. Microbial bioconversion of feathers into antioxidant peptides and pigments and their liposome encapsulation. Biotechnol. Lett. 2021, 43, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Ligabue-Braun, R. Agro-Industrial residues: Eco-Friendly and inexpensive substrates for microbial pigments production. Front. Sustain. Food Syst. 2021, 5, 589414. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Jiang, X.-R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [CrossRef]
- Schultz, J.; Rosado, A.S. Extreme environments: A source of biosurfactants for biotechnological applications. Extremophiles 2019, 24, 189–206. [Google Scholar] [CrossRef]
- Sajjad, W.; Din, G.; Rafiq, M.; Iqbal, A.; Khan, S.; Zada, S.; Ali, B.; Kang, S. Pigment production by cold-adapted bacteria and fungi: Colorful tale of cryosphere with wide range applications. Extremophiles 2020, 24, 447–473. [Google Scholar] [CrossRef]
- Ye, J.-W.; Lin, Y.-N.; Yi, X.-Q.; Yu, Z.-X.; Liu, X.; Chen, G.-Q. Synthetic biology of extremophiles: A new wave of biomanufacturing. Trends Biotechnol. 2022, 41, 342–357. [Google Scholar] [CrossRef]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological advances for improving natural pigment production: A state-of-the-art review. Bioresour. Bioprocess. 2022, 9, 8. [Google Scholar] [CrossRef]
- Somayaji, A.; Dhanjal, C.R.; Lingamsetty, R.; Vinayagam, R.; Selvaraj, R.; Varadavenkatesan, T.; Govarthanan, M. An insight into the mechanisms of homeostasis in extremophiles. Microbiol. Res. 2022, 263, 127115. [Google Scholar] [CrossRef]
- Tapia, C.; López, B.; Astuya, A.; Becerra, J.; Gugliandolo, C.; Parra, B.; Martínez, M. Antiproliferative activity of carotenoid pigments produced by extremophile bacteria. Nat. Prod. Res. 2019, 35, 4638–4642. [Google Scholar] [CrossRef]
- Loveland-Curtze, J.; Miteva, V.; Brenchley, J. Novel ultramicrobacterial isolates from a deep Greenland ice core represent a proposed new species, Chryseobacterium greenlandense sp. nov. Extremophiles 2009, 14, 61–69. [Google Scholar] [CrossRef]
- Fong, N.J.C.; Burgess, M.L.; Barrow, K.D.; Glenn, D.R. Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl. Microbiol. Biotechnol. 2001, 56, 750–756. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Caicedo-Paz, A.V.; Farias, F.O.; Mesquita, L.M.d.S.; Giuffrida, D.; Dufossé, L. Microbial bacterioruberin: The new C50 carotenoid player in food industries. Food Microbiol. 2024, 124, 104623. [Google Scholar] [CrossRef]
- Marín-Sanhueza, C.; Echeverría-Vega, A.; Gómez, A.; Cabrera-Barjas, G.; Romero, R.; Banerjee, A. Stress Dependent Biofilm Formation and Bioactive Melanin Pigment Production by a Thermophilic Bacillus Species from Chilean Hot Spring. Polymers 2022, 14, 680. [Google Scholar] [CrossRef]
- Anders, H.; Dunfield, P.F.; Lagutin, K.; Houghton, K.M.; Power, J.F.; MacKenzie, A.D.; Vyssotski, M.; Ryan, J.L.J.; Hanssen, E.G.; Moreau, J.W.; et al. Thermoflavifilum aggregans gen. nov., sp. nov., a thermophilic and slightly halophilic filamentous bacterium from the phylum Bacteroidetes. Int. J. Syst. Evol. Microbiol. 2014, 64, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A. Thermozeaxanthins, New Carotenoid-glycoside-esters from Thermophilic Eubacterium Thermus thermophilus. Tetrahedron Lett. 1995, 36, 4901–4904. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Yang, R.; Zhang, Y.; Xu, Y.; Liu, G.; Zhang, B.; Wang, J.; Wang, X.; Zhang, W.; et al. Genomic Insights into the Radiation-Resistant Capability of Sphingomonas qomolangmaensis S5-59T and Sphingomonas glaciei S8-45T, Two Novel Bacteria from the North Slope of Mount Everest. Microorganisms 2022, 10, 2037. [Google Scholar] [CrossRef]
- Lemee, L.; Peuchant, E.; Clerc, M.; Brunner, M.; Pfander, H. Deinoxanthin: A new carotenoid isolated from Deinococcus radiodurans. Tetrahedron 1997, 53, 919–926. [Google Scholar] [CrossRef]
- Bindiya, E.; Ramesh, M.D.; Arya, B.; Bhat, S.G. Identification of pigmented extremophilic bacteria from mangrove soil with antibiofilm activity on food pathogens. Microbe 2024, 4, 100136. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Ahangari, H.; Mousazadeh, S.; Hosseini, S.M.; Dufossé, L. Microbial pigments as an alternative to synthetic dyes and food additives: A brief review of recent studies. Bioprocess Biosyst. Eng. 2021, 45, 1–12. [Google Scholar] [CrossRef]
- Ramesh, C.; Prasastha, V.R.; Venkatachalam, M.; Dufossé, L. Natural Substrates and Culture Conditions to Produce Pigments from Potential Microbes in Submerged Fermentation. Fermentation 2022, 8, 460. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, D.N.; Wang, S.-L. Microbial reclamation of chitin and protein-containing marine by-products for the production of prodigiosin and the evaluation of its bioactivities. Polymers 2020, 12, 1328. [Google Scholar] [CrossRef]
- Alzahrani, N.H.; El-Bondkly, A.A.M.; El-Gendy, M.M.A.A.; El-Bondkly, A.M. Enhancement of undecylprodigiosin production from marine endophytic recombinant strain Streptomyces sp. ALAA-R20 through low-cost induction strategy. J. Appl. Genet. 2021, 62, 165–182. [Google Scholar] [CrossRef]
- El-Fouly, M.; Sharaf, A.; Shahin, A.; El-Bialy, H.A.; Omara, A. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J. Radiat. Res. Appl. Sci. 2014, 8, 36–48. [Google Scholar] [CrossRef]
- Dos Santos, R.A.; Rodríguez, D.M.; da Silva, L.A.R.; de Almeida, S.M.; de Campos-Takaki, G.M.; de Lima, M.A.B. Enhanced production of prodigiosin by Serratia marcescens UCP 1549 using agrosubstrates in solid-state fermentation. Arch. Microbiol. 2021, 203, 4091–4100. [Google Scholar] [CrossRef]
- Saejung, C.; Lomthaisong, K.; Kotthale, P. Alternative microbial-based functional ingredient source for lycopene, beta-carotene, and polyunsaturated fatty acids. Heliyon 2023, 9, e13828. [Google Scholar] [CrossRef]
- Schalchli, H.; Hormazábal, E.; Astudillo, Á.; Briceño, G.; Rubilar, O.; Diez, M.C. Bioconversion of potato solid waste into antifungals and biopigments using Streptomyces spp. PLoS ONE 2021, 16, e0252113. [Google Scholar]
- Tran, L.T.; Techato, K.; Nguyen, V.B.; Wang, S.-L.; Nguyen, A.D.; Phan, T.Q.; Doan, M.D.; Phoungthong, K. Utilization of Cassava Wastewater for Low-Cost Production of Prodigiosin via Serratia marcescens TNU01 Fermentation and Its Novel Potent α-Glucosidase Inhibitory Effect. Molecules 2021, 26, 6270. [Google Scholar] [CrossRef] [PubMed]
- Korumilli, T.; Susmita, M. Carotenoid production by Bacillus clausii using rice powder as the sole substrate: Pigment analyses and optimization of key production parameters. J. Biochem. Technol. 2014, 5, 2014. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Refgina Mary, R.; Sekaran, G. Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Wang, S.-L.; Nguyen, D.-N.; Nguyen, A.-D.; Nguyen, T.-H.; Doan, M.-D.; Ngo, V.-A.; Doan, C.-T.; Kuo, Y.-H.; Nguyen, V.-B. Bioprocessing of marine chitinous wastes for the production of bioactive prodigiosin. Molecules 2021, 26, 3138. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, N.; Suresh, P.V.; Sakhare, P.Z.; Sachindra, N.M. Shrimp biowaste fermentation with Pediococcus acidolactici CFR2182: Optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteination/demineralization and carotenoid recovery. Enzym. Microb. Technol. 2007, 40, 1427–1434. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.-L.; Nguyen, A.D.; Phan, T.Q.; Techato, K.; Pradit, S. Bioproduction of Prodigiosin from Fishery Processing Waste Shrimp Heads and Evaluation of Its Potential Bioactivities. Fishes 2021, 6, 30. [Google Scholar] [CrossRef]
- Cassarini, M.; Besaury, L.; Rémond, C. Valorisation of wheat bran to produce natural pigments using selected microorganisms. J. Biotechnol. 2021, 339, 81–92. [Google Scholar] [CrossRef]
- Vasanthabharathi, V.; Lakshminarayanan, R.; Jayalakshmi, S. Melanin production from marine Streptomyces. Afr. J. Biotechnol. 2011, 10, 11224–11234. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Yusof, N.Z.; Nordin, N.; Zakaria, Z.A.; Rezali, M.F. Production and Characterization of Violacein by Locally Isolated Chromobacterium violaceum Grown in Agricultural Wastes. Appl. Biochem. Biotechnol. 2012, 167, 1220–1234. [Google Scholar] [CrossRef]
- Joshi, V.K.; Attri, D.; Rana, N.S. Optimization of apple pomace based medium and fermentation conditions for pigment production by Sarcina sp. Indian J. Nat. Prod. Resour. 2011, 2, 421–427. [Google Scholar]
- Hussain, M.H.; Sajid, S.; Martuscelli, M.; Aldahmash, W.; Mohsin, M.Z.; Ashraf, K.; Guo, M.; Mohsin, A. Sustainable biosynthesis of lycopene by using evolutionary adaptive recombinant Escherichia coli from orange peel waste. Heliyon 2024, 10, e34366. [Google Scholar] [CrossRef] [PubMed]
- Elsanhoty, R.M.; Al-Turki, I.; Ramadan, M.F. Screening of medium components by Plackett–Burman design for carotenoid production using date (Phoenix dactylifera) wastes. Ind. Crop. Prod. 2012, 36, 313–320. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Aziz, A.; Venil, C.K.; Khasim, A.R.; Ahmad, W.A. Utilization of agro-industrial waste for the production of yellowish-orange pigment from Chryseobacterium artocarpi CECT 8497. Int. Biodeterior. Biodegrad. 2016, 113, 342–349. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Brown sugar as a low-cost medium for the production of prodigiosin by locally isolated Serratia marcescens UTM1. Int. Biodeterior. Biodegrad. 2014, 95, 19–24. [Google Scholar] [CrossRef]
- Noby, N.; Khattab, S.N.; Soliman, N.A. Sustainable production of bacterioruberin carotenoid and its derivatives from Arthrobacter agilis NP20 on whey-based medium: Optimization and product characterization. Bioresour. Bioprocess. 2023, 10, 46. [Google Scholar] [CrossRef]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol. Rep. 2017, 14, 47–53. [Google Scholar] [CrossRef]
- Araújo, R.G.; Zavala, N.R.; Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Parra-Arroyo, L.; Rodríguez-Hernández, J.A.; Martínez-Prado, M.A.; Sosa-Hernández, J.E.; Martínez-Ruiz, M.; et al. Recent advances in prodigiosin as a bioactive compound in nanocomposite applications. Molecules 2022, 27, 4982. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.A.; Kamer, A.M.A.; Al-Monofy, K.B.; Al-Madboly, L.A. Pseudomonas aeruginosa’s greenish-blue pigment pyocyanin: Its production and biological activities. Microb. Cell Factories 2023, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.V.; Sambyal, K. An overview of β-carotene production: Current status and future prospects. Food Biosci. 2022, 47, 101717. [Google Scholar] [CrossRef]
- Kapała, A.; Szlendak, M.; Motacka, E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients 2022, 14, 5152. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Pathak, A.P.; Rathod, M.G.; Kamble, G.T.; Murkute, S.D.; Patil, N.P. Industrially significant biomolecules from recently discovered haloalkaliphiles, inhabitants of the coastal mangrove vegetation in Bordi, India. Microbe 2023, 1, 100005. [Google Scholar] [CrossRef]
- Lemuth, K.; Steuer, K.; Albermann, C. Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin. Microb. Cell Factories 2011, 10, 29. [Google Scholar] [CrossRef]
- Henke, N.A.; Heider, S.A.E.; Hannibal, S.; Wendisch, V.F.; Peters-Wendisch, P. Isoprenoid Pyrophosphate-Dependent Transcriptional Regulation of Carotenogenesis in Corynebacterium glutamicum. Front. Microbiol. 2017, 8, 633. [Google Scholar] [CrossRef]
- Lu, Q.; Bu, Y.-F.; Liu, J.-Z. Metabolic Engineering of Escherichia coli for Producing Astaxanthin as the Predominant Carotenoid. Mar. Drugs 2017, 15, 296. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.; Enfissi, E.M.; Welsch, R.; Beyer, P.; Zurbriggen, M.D.; Fraser, P.D. Construction of a fusion enzyme for astaxanthin formation and its characterisation in microbial and plant hosts: A new tool for engineering ketocarotenoids. Metab. Eng. 2019, 52, 243–252. [Google Scholar] [CrossRef]
- Henke, N.A.; Heider, S.A.E.; Peters-Wendisch, P.; Wendisch, V.F. Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124. [Google Scholar] [CrossRef]
- Choi, S.-K.; Matsuda, S.; Hoshino, T.; Peng, X.; Misawa, N. Characterization of bacterial β-carotene 3,3′-hydroxylases, CrtZ, and P450 in astaxanthin biosynthetic pathway and adonirubin production by gene combination in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 1238–1246. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Sun, T.; Zhu, X.; Xu, H.; Tang, J.; Zhang, X.; Ma, Y. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab. Eng. 2013, 17, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, L. Biosynthesis of β-carotene in engineered E. coli using the MEP and MVA pathways. Microb. Cell Factories 2014, 13, 160. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Park, H.-M.; Kim, J.-E.; Lee, S.-H.; Choi, M.-S.; Kim, J.-Y.; Oh, D.-K.; Keasling, J.D.; Kim, S.-W. Increased β-Carotene Production in Recombinant Escherichia coli Harboring an Engineered Isoprenoid Precursor Pathway with Mevalonate Addition. Biotechnol. Prog. 2008, 23, 599–605. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli using CRISPR–Cas9 meditated genome editing. Metab. Eng. 2015, 31, 13–21. [Google Scholar] [CrossRef]
- Wu, T.; Ye, L.; Zhao, D.; Li, S.; Li, Q.; Zhang, B.; Bi, C.; Zhang, X. Membrane engineering—A novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli. Metab. Eng. 2017, 43, 85–91. [Google Scholar] [CrossRef]
- Yuan, L.Z.; Rouvière, P.E.; LaRossa, R.A.; Suh, W. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab. Eng. 2006, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Song, D.; Zhu, H. Homology-dependent recombination of large synthetic pathways into E. coli genome via λ-Red and CRISPR/Cas9 dependent selection methodology. Microb. Cell Factories 2020, 19, 108. [Google Scholar] [CrossRef]
- Wei, Y.; Mohsin, A.; Hong, Q.; Guo, M.; Fang, H. Enhanced production of biosynthesized lycopene via heterogenous MVA pathway based on chromosomal multiple position integration strategy plus plasmid systems in Escherichia coli. Bioresour. Technol. 2018, 250, 382–389. [Google Scholar] [CrossRef]
- Zhou, Y.; Nambou, K.; Wei, L.; Cao, J.; Imanaka, T.; Hua, Q. Lycopene production in recombinant strains of Escherichia coli is improved by knockout of the central carbon metabolism gene coding for glucose-6-phosphate dehydrogenase. Biotechnol. Lett. 2013, 35, 2137–2145. [Google Scholar] [CrossRef]
- Henke, N.A.; Wiebe, D.; Pérez-García, F.; Peters-Wendisch, P.; Wendisch, V.F. Coproduction of cell-bound and secreted value-added compounds: Simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum. Bioresour. Technol. 2018, 247, 744–752. [Google Scholar] [CrossRef]
- Taniguchi, H.; Henke, N.A.; Heider, S.A.; Wendisch, V.F. Overexpression of the primary sigma factor gene sigA improved carotenoid production by Corynebacterium glutamicum: Application to production of β-carotene and the non-native linear C50 carotenoid bisanhydrobacterioruberin. Metab. Eng. Commun. 2017, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Han, G.H.; Seong, W.; Kim, H.; Kim, S.-W.; Lee, D.-H.; Lee, S.-G. CRISPR interference-guided balancing of a biosynthetic mevalonate pathway increases terpenoid production. Metab. Eng. 2016, 38, 228–240. [Google Scholar] [CrossRef]
- Sun, T.; Miao, L.; Li, Q.; Dai, G.; Lu, F.; Liu, T.; Zhang, X.; Ma, Y. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol. Lett. 2014, 36, 1515–1522. [Google Scholar] [CrossRef]
- Heider, S.A.E.; Peters-Wendisch, P.; Netzer, R.; Stafnes, M.; Brautaset, T.; Wendisch, V.F. Production and glucosylation of C50 and C40 carotenoids by metabolically engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2013, 98, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, S.; Zhao, D.; Ye, L.; Li, Q.; Zhang, X.; Zhu, L.; Bi, C. Manipulating the position of DNA expression cassettes using location tags fused to dCas9 (Cas9-Lag) to improve metabolic pathway efficiency. Microb. Cell Factories 2020, 19, 229. [Google Scholar] [CrossRef]
- Qu, Y.; Su, A.; Li, Y.; Meng, Y.; Chen, Z. Manipulation of the regulatory genes ppsR and prrA in Rhodobacter sphaeroides enhances lycopene production. J. Agric. Food Chem. 2021, 69, 4134–4143. [Google Scholar] [CrossRef]
- Wang, Z.; Tschirhart, T.; Schultzhaus, Z.; Kelly, E.E.; Chen, A.; Oh, E.; Nag, O.; Glaser, E.R.; Kim, E.; Lloyd, P.F.; et al. Melanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens through Heterologous Biosynthesis: Characterization and Application. Appl. Environ. Microbiol. 2020, 86, e02749-19. [Google Scholar] [CrossRef]
- Cabrera-Valladares, N.; Martínez, A.; Piñero, S.; Lagunas-Muñoz, V.H.; Tinoco, R.; de Anda, R.; Vázquez-Duhalt, R.; Bolívar, F.; Gosset, G. Expression of the melA gene from Rhizobium etli CFN42 in Escherichia coli and characterization of the encoded tyrosinase. Enzym. Microb. Technol. 2006, 38, 772–779. [Google Scholar] [CrossRef]
- Park, H.; Yang, I.; Choi, M.; Jang, K.-S.; Jung, J.C.; Choi, K.-Y. Engineering of melanin biopolymer by co-expression of MelC tyrosinase with CYP102G4 monooxygenase: Structural composition understanding by 15 tesla FT-ICR MS analysis. Biochem. Eng. J. 2020, 157, 107530. [Google Scholar] [CrossRef]
- Park, S.; Yang, Y.-H.; Choi, K.-Y. One-pot production of thermostable PHB biodegradable polymer by co-producing bio-melanin pigment in engineered Escherichia coli. Biomass-Convers. Biorefinery 2022, 15, 22137–22145. [Google Scholar] [CrossRef]
- Kumar, V.; Darnal, S.; Kumar, S.; Kumar, S.; Singh, D. Bioprocess for co-production of polyhydroxybutyrate and violacein using Himalayan bacterium Iodobacter sp. PCH194. Bioresour. Technol. 2021, 319, 124235. [Google Scholar] [CrossRef]
- Jung, H.-R.; Choi, T.-R.; Han, Y.H.; Park, Y.-L.; Park, J.Y.; Song, H.-S.; Yang, S.-Y.; Bhatia, S.K.; Gurav, R.; Park, H.; et al. Production of blue-colored polyhydroxybutyrate (PHB) by one-pot production and coextraction of indigo and PHB from recombinant Escherichia coli. Dye. Pigment. 2020, 173, 107889. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M.; Moayedi, V. High efficiency canthaxanthin production by a novel mutant isolated from Dietzia natronolimnaea HS-1 using central composite design analysis. Ind. Crop. Prod. 2012, 40, 345–354. [Google Scholar] [CrossRef]
- Fariq, A.; Yasmin, A.; Jamil, M. Production, characterization and antimicrobial activities of bio-pigments by Aquisalibacillus elongatus MB592, Salinicoccus sesuvii MB597, and Halomonas aquamarina MB598 isolated from Khewra Salt Range, Pakistan. Extremophiles 2019, 23, 435–449. [Google Scholar] [CrossRef]
- Sudhakar, C.; Shobana, C.; Selvankumar, T.; Selvam, K. Prodigiosin production from Serratia marcescens strain CSK and their antioxidant, antibacterial, cytotoxic effect and in silico study of caspase-3 apoptotic protein. Biotechnol. Appl. Biochem. 2021, 69, 1984–1997. [Google Scholar]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Optimization of culture conditions for flexirubin production by Chryseobacterium artocarpi CECT 8497 using response surface methodology. Acta Biochim. Pol. 2015, 62, 185–190. [Google Scholar] [CrossRef]
- Miglani, K.; Singh, S.; Singh, D.P.; Krishania, M. Sustainable production of prodigiosin from rice straw derived xylose by using isolated Serratia marcescens (CMS 2): Statistical optimization, characterization, encapsulation & cost analysis. Sustain. Food Technol. 2023, 1, 837–849. [Google Scholar] [CrossRef]
- Guo, J.; Rao, Z.; Yang, T.; Man, Z.; Xu, M.; Zhang, X. High-level production of melanin by a novel isolate of Streptomyces kathirae. FEMS Microbiol. Lett. 2014, 357, 85–91. [Google Scholar] [CrossRef]
- Karuppiah, V.; Aarthi, C.; Sivakumar, K.; Kannan, L. Statistical optimization and anticancer activity of a red pigment isolated from Streptomyces sp. PM4. Asian Pac. J. Trop. Biomed. 2013, 3, 650–656. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Kenawy, E.-R.; Ahmed, S.; El-Sapagh, S. Bioproduction and optimization of newly characterized melanin pigment from Streptomyces djakartensis NSS-3 with its anticancer, antimicrobial, and radioprotective properties. Microb. Cell Factories 2024, 23, 23. [Google Scholar] [CrossRef]
- Khodaiyan, F.; Razavi, S.H.; Mousavi, S.M. Optimization of canthaxanthin production by Dietzia natronolimnaea HS-1 from cheese whey using statistical experimental methods. Biochem. Eng. J. 2008, 40, 415–422. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial pigments: A colorful palette reservoir for biotechnological applications. Biotechnol. Appl. Biochem. 2022, 69, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Felgueiras, H.P.; Leite, B.R.; Soares, G.M.B. Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications. Antibiotics 2025, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Ramakrishnan, E.; Deka, S.; Parasar, D.P. Bacteria as a source of biopigments and their potential applications. J. Microbiol. Methods 2024, 219, 106907. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Coutinho, J.A.; Weingarten, M. Sustainable recovery of microbial-derived natural pigments using deep eutectic solvents: Advances, potential, and challenges. Sep. Purif. Technol. 2025, 361, 131413. [Google Scholar] [CrossRef]
- Agarwal, H.; Bajpai, S.; Mishra, A.; Kohli, I.; Varma, A.; Fouillaud, M.; Dufossé, L.; Joshi, N.C. Bacterial Pigments and Their Multifaceted Roles in Contemporary Biotechnology and Pharmacological Applications. Microorganisms 2023, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Dudeja, C.; Mishra, A.; Ali, A.; Singh, P.P.; Jaiswal, A.K. Microbial Genome Editing with CRISPR–Cas9: Recent Advances and Emerging Applications Across Sectors. Fermentation 2025, 11, 410. [Google Scholar] [CrossRef]
- Kristjansdottir, T.; Ron, E.Y.; Molins-Delgado, D.; Fridjonsson, O.H.; Turner, C.; Bjornsdottir, S.H.; Gudmundsson, S.; van Niel, E.W.; Karlsson, E.N.; Hreggvidsson, G.O. Engineering the carotenoid biosynthetic pathway in Rhodothermus marinus for lycopene production. Metab. Eng. Commun. 2020, 11, e00140. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, M.; Ikezumi, M.; Takaichi, S.; Maoka, T.; Hemmi, H.; Ogawa, T.; Saito, K.; Tobias, A.V.; Umeno, D. A highly selective biosynthetic pathway to non-natural C50 carotenoids assembled from moderately selective enzymes. Nat. Commun. 2015, 6, 7534. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Deng, M.-R.; Zhu, H. Advances in the Discovery and Engineering of Gene Targets for Carotenoid Biosynthesis in Recombinant Strains. Biomolecules 2023, 13, 1747. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Khoo, K.S.; Chew, K.W.; Ting, H.-Y.; Iwamoto, K.; Show, P.L. Digitalised prediction of blue pigment content from Spirulina platensis: Next-generation microalgae bio-molecule detection. Algal Res. 2024, 83, 103642. [Google Scholar] [CrossRef]
- Manochkumar, J.; Jonnalagadda, A.; Cherukuri, A.K.; Vannier, B.; Janjaroen, D.; Chandrasekaran, R.; Ramamoorthy, S. Machine learning-based prediction models unleash the enhanced production of fucoxanthin in Isochrysis galbana. Front. Plant Sci. 2024, 15, 1461610. [Google Scholar] [CrossRef]
- Sammaknejad, N.; Lee, J.; Austria, J.M.; Duenas, N.; Heiba, L.; Sridharan, G.; Davis, J.; Undey, C. A Scalable Deep Learning Approach for Real-Time Multivariate Monitoring of Biopharmaceutical Processes with No Prior Product-Specific History. Biotechnol. Bioeng. 2025, 122, 2333–2352. [Google Scholar] [CrossRef]
- Pouyet, E.; Miteva, T.; Rohani, N.; de Viguerie, L. Artificial Intelligence for Pigment Classification Task in the Short-Wave Infrared Range. Sensors 2021, 21, 6150. [Google Scholar] [CrossRef]
- Rajendran, P.; Somasundaram, P.; Dufossé, L. Microbial pigments: Eco-friendly extraction techniques and some industrial applications. J. Mol. Struct. 2023, 1290, 135958. [Google Scholar] [CrossRef]
- Kopp, G.; Lauritano, C. Greener Extraction Solutions for Microalgal Compounds. Mar. Drugs 2025, 23, 269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).