Characterization and Sustainable Valorization of Brewers’ Spent Grain for Metal Ion and Organic Substance Removal
Abstract
1. Introduction
2. Materials and Methods
- Protocol
- 2.
- Search (literature review)
- 3.
- Appraisal
- 4.
- Synthesis
- 5.
- Analysis
- 6.
- Report
- (1)
- Image preprocessing
- Conversion to grayscale and gentle CLAHE contrast normalization (Contrast Limited Adaptive Histogram Equalization, clip limit = 0.02) to equalize the lighting without excessively darkening the edges.
- Further steps were performed on an 8-bit image (0–255).
- (2)
- Scale calibration (µm/px)
- Automatic detection of the scale bar length in the bottom left corner:
- -
- cutting out the ROI (bottom ~25% of the height, left ~60% of the width),
- -
- brightness thresholding in the ROI (≥90th percentile), bright pixel mask,
- -
- morphological opening (disk = 1) to remove small disturbances,
- -
- in each line of the mask, the longest horizontal segment of “True” is searched for; its length in pixels is taken as the strip length.
- Conversion factor µm/px = 2 µm / strip length px.
- The following parameters are determined from the converter: FOV (Field of View) width µm, FOV height µm, FOV area µm2.
- (3)
- Pore segmentation (dark according to SEM)
- A slight Gaussian blur (σ ≈ 1.0) for high-frequency noise reduction.
- Global Otsu thresholding on a blurred image.
- Pores operationally defined as pixels below the threshold (darker areas/cavities).
- Morphological opening (disk = 1) with the removal of very small objects (min size = 20 px) to filter out noise.
- Component labeling and object area determination (in px2).
- For each object, the equivalent diameter (the diameter of a circle with the same area) was calculated according to Equation (1):
- Pore density scaled to 1000 µm2 was calculated according to Equation (2):
- (4)
- Texture and edges
- Edge density—the fraction of pixels classified as edges by the Canny detector (σ = 1.5; other parameters are the library defaults). Interpretively: a synthetic image roughness/edginess indicator.
- (5)
- Visual quality control
- For each sample, an overlay with pore boundaries (outlined by mask dilation and XOR) and a page with the detected scale bar (highlighted in green in the SEM images) were generated to verify the accuracy of the calibration and segmentation.
- (6)
- Assumptions and limitations of the analysis method
- Pores are an operational definition (dark areas on SEM); depending on the contrast/SEM settings, they may correspond to depressions, cavities, or topographic shadows.
- Equivalent diameter assumes a circular equivalent; for elongated objects, it provides a conventional measure (good for comparisons, not for shape metrology).
- Edge density also depends on imaging parameters (sharpness/saturation); it is worth comparing images with similar settings.
- Calibration assumes the scale bar is a uniform, horizontal bar in the bottom left corner; unusual positioning/graphics may require manual adjustment.
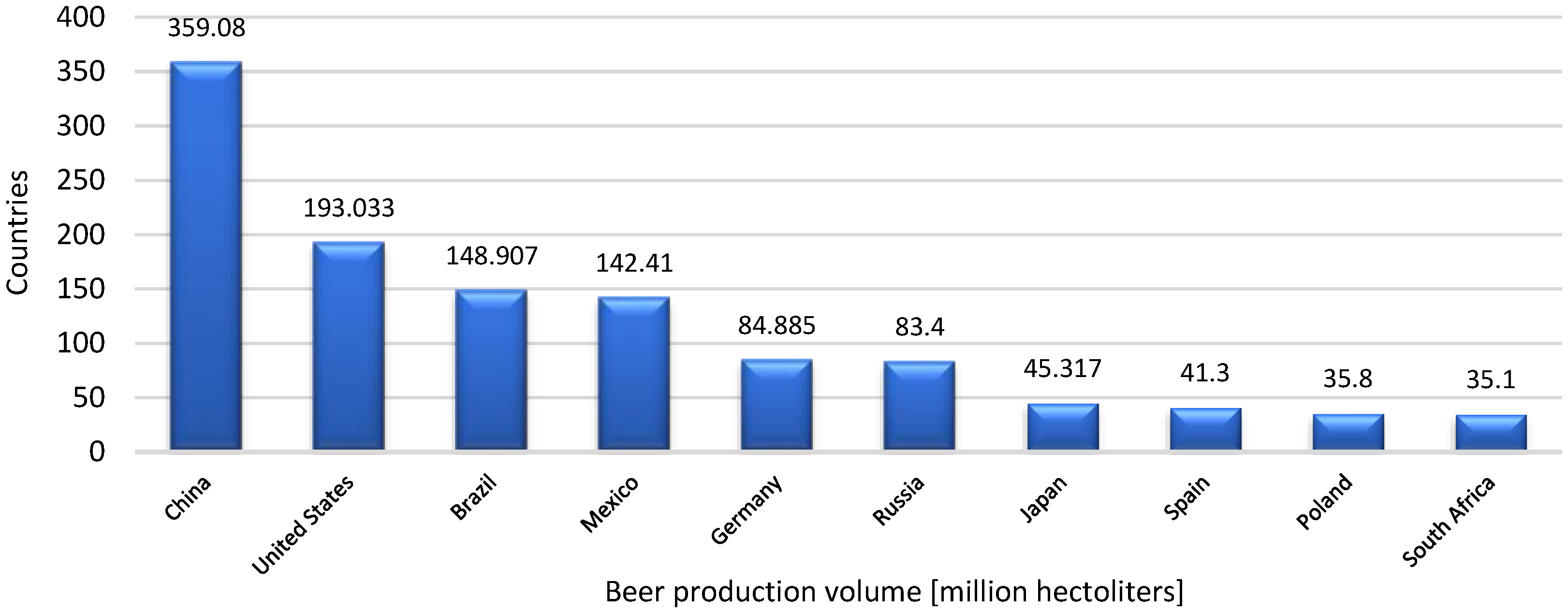
3. The Development of the Brewing Industry: From Fermentation to Global Production
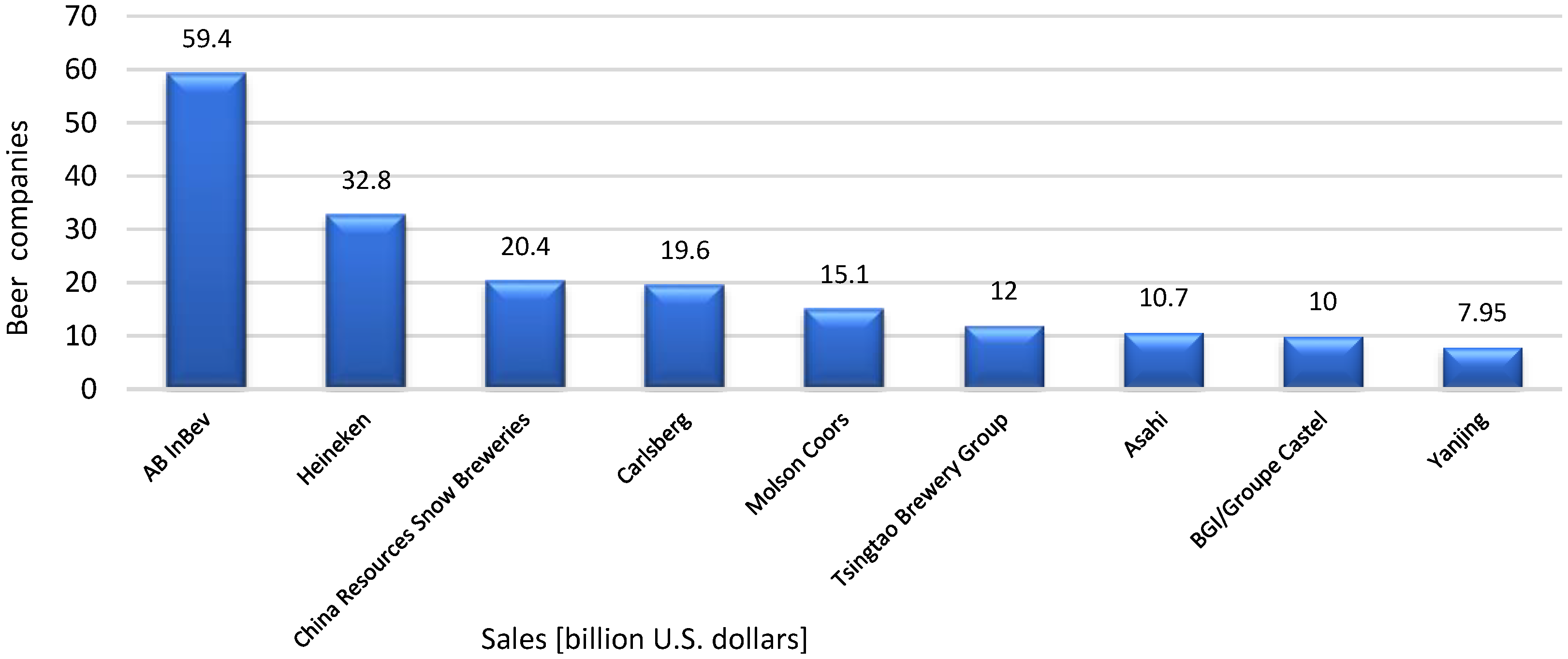
4. Economic Approach
5. The Brewing Process
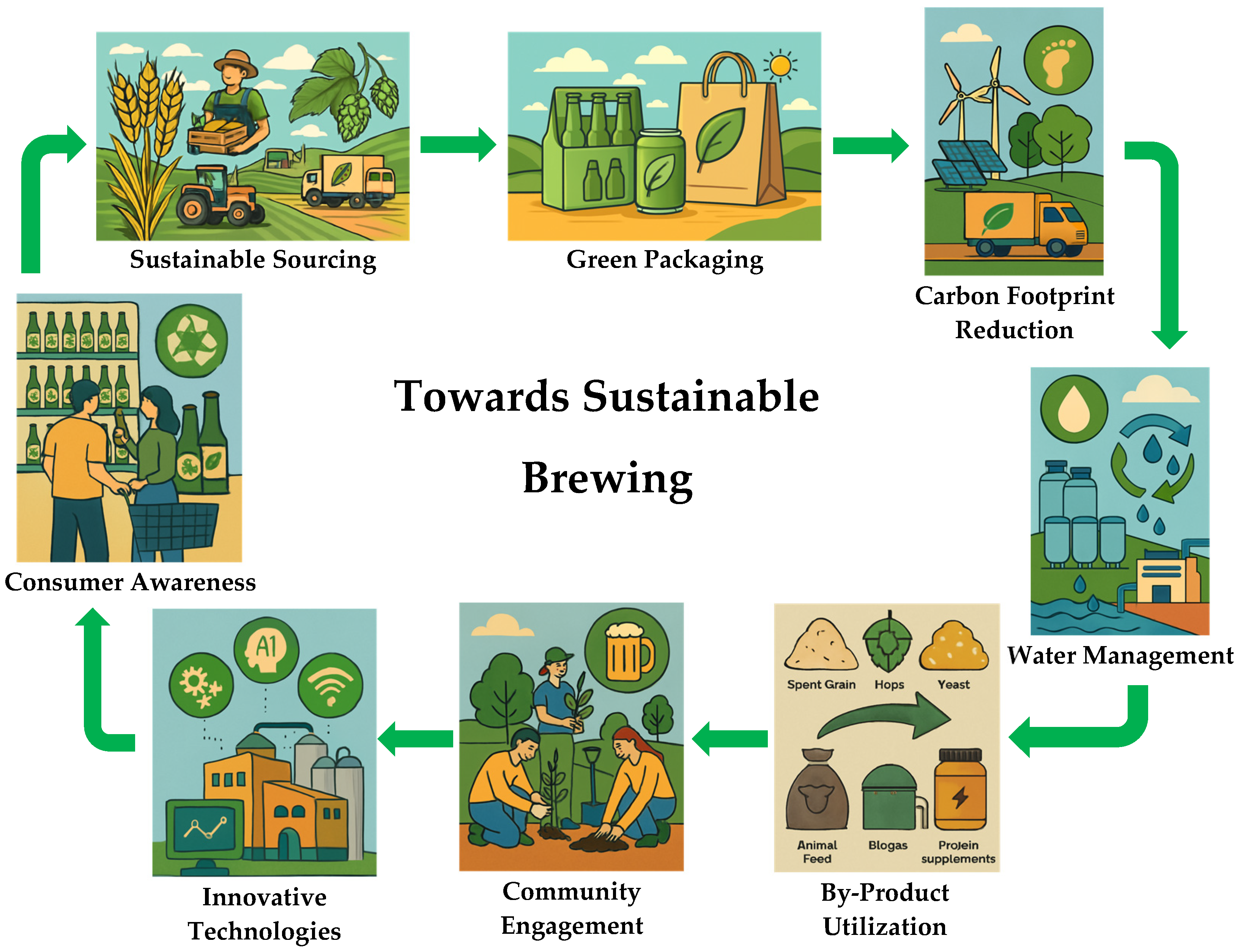
6. Towards Sustainable Brewing
7. Utilization of Brewers’ Spent Grain (BSG): Current Applications and Future Potential
7.1. Biorefining Utilization of BSG
7.2. Application of BSG in the Food Industry
7.3. Using BSG for Energy Production
8. Methods for BSG Preservation and Storage
8.1. Chemical Methods
8.2. Physical Methods
8.3. Ensiling Methods
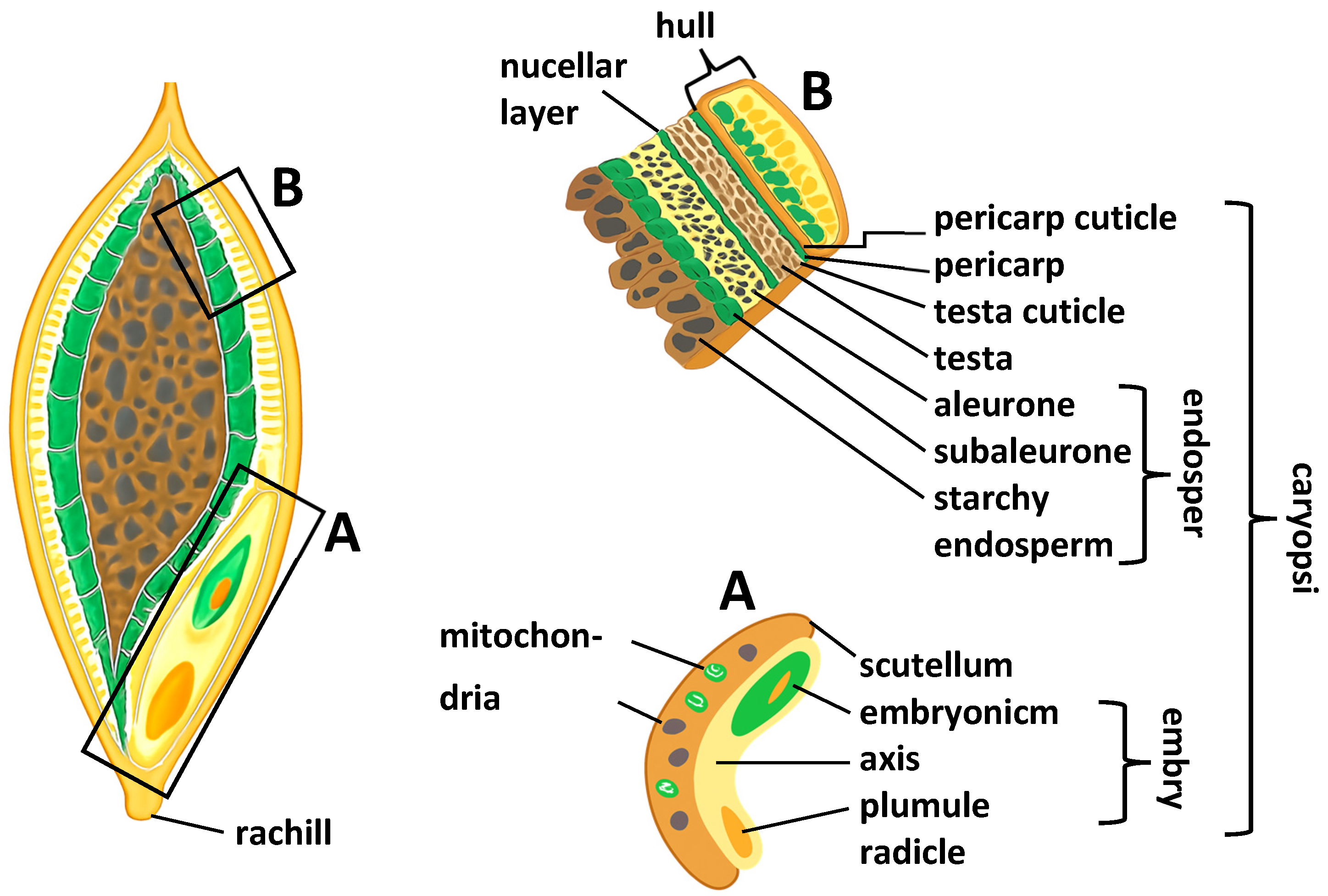
9. Structure of the Barley Grain
10. Chemical Composition and Surface Microstructure Properties of BSG
- Pore count/pore density/pore area share—how many pores are in the field of view, how many per 1000 µm2, and what percentage of the field is occupied by pores (dimensionless).
- Equivalent diameter (p50/p90/max)—circular diameter of an object with the same area; p50 = median, p90 = 90th percentile (upper tail), max = largest detected (the maximum of the distribution of deq in µm).
- Average pore area—average area of a single pore (µm2).
- AR (major/minor)—elongation (1 = circle; >1 = ellipse/slit).
- Circularity (4πA/P2)—roundness (1 = perfect circle; lower values = more jagged).
- Roundness (4A/(π·major2))—another measure of roundness (sensitive to the major axis).
- Eccentricity—elliptical eccentricity (0 = circle, close to 1 = elongated shape).
- Solidity—compactness (A/k. surrounding); lower = more indentations/irregularities.
- GLCM: contrast/homogeneity/energy/correlation/entropy—dimensionless textural descriptors calculated from the co-occurrence matrix; they indicate tone differentiation, homogeneity, orderliness, linear dependence of tones, and randomness of the texture, respectively.
- Edge density (Canny)—the proportion of edge pixels in the image (dimensionless).
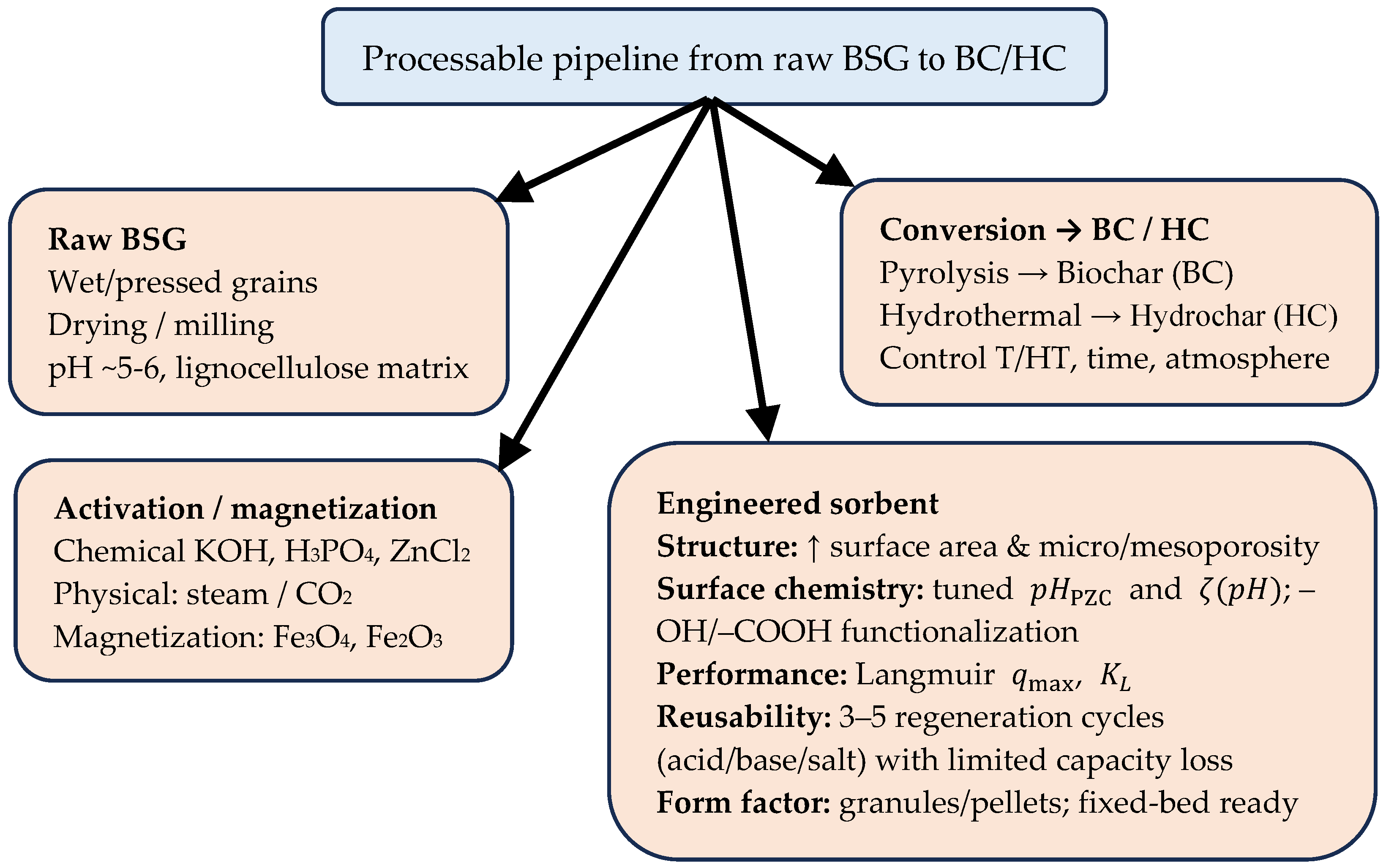
11. Sorption Properties of BSG
11.1. Sorption Processes of Metal Ions
11.2. Sorption Processes of Organic Substances
12. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Simate, G.S.; Cluett, J.; Iyuke, S.E.; Musapatika, E.T.; Ndlovu, S.; Walubita, L.F.; Alvarez, A.E. The Treatment of Brewery Wastewater for Reuse: State of the Art. J. Clean. Prod. 2011, 29–30, 1–10. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef]
- Wierzba, S.; Kłos, A. Heavy metal sorption in biosorbents—Using spent grain from the brewing industry. J. Clean. Prod. 2019, 225, 112–120. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Dancker, P.; Glas, K.; Gastl, M. Potential utilisation methods for brewer’s spent grain: A review. Int. J. Food Sci. Technol. 2025, 60, vvae022. [Google Scholar] [CrossRef]
- Lu, S.; Gibb, S.W. Copper removal from wastewater using spent-grain as biosorbent. Biores. Technol. 2008, 99, 1509–1517. [Google Scholar] [CrossRef]
- Li, Q.; Chai, L.; Wang, Q.; Yang, Z.; Yan, H.; Wang, Y. Fast esterification of spent grain for enhanced heavy metal ions adsorption. Bioresour. Technol. 2010, 101, 3796–3799. [Google Scholar] [CrossRef]
- Su, Y.; Böhm, W.; Wenzel, M.; Paasch, S.; Acker, M.; Doert, T.; Brunner, E.; Henle, T.; Weigand, J.J. Mild hydrothermally treated brewer’s spent grain for efficient removal of uranyl and rare earth metal ions. RSC Adv. 2020, 10, 45116–45129. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wenzel, M.; Paasch, S.; Seifert, M.; Böhm, W.; Doert, T.; Weigand, J.J. Recycling of Brewer’s Spent Grain as a Biosorbent by Nitro-Oxidation for Uranyl Ion Removal from Wastewater. ACS Omega 2021, 6, 19364–19377. [Google Scholar] [CrossRef]
- Vanderheyden, S.R.H.; Vanreppelen, K.; Yperman, J.; Carleer, R.; Schreurs, S. Chromium(VI) removal using in-situ nitrogenized activated carbon prepared from Brewers’ spent grain. Adsorption 2018, 24, 147–156. [Google Scholar] [CrossRef]
- Vanreppelen, K.; Vanderheyden, S.; Kuppens, T.; Schreurs, S.; Yperman, J.; Carleer, R. Activated carbon from pyrolysis of brewer’s spent grain: Production and adsorption properties. Waste Manage. Res. 2014, 32, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Dancker, P.; Glas, K.; Gastl, M. Fixed-bed adsorption study with brewer’s spent grain for the removal of heavy metals in model waters. J. Am. Soc. Brew. Chem. 2025, 1–10. [Google Scholar] [CrossRef]
- He, Y.; Dietrich, A.M.; Jin, Q.; Lin, T.; Yu, D.; Huang, H. Cellulose adsorbent produced from the processing waste of brewer’s spent grain for efficient removal of Mn and Pb from contaminated water. Food Bioprod. Process. 2022, 135, 227–237. [Google Scholar] [CrossRef]
- Kezerle, A.; Velić, N.; Hasenay, D.; Kovačević, D. Lignocellulosic Materials as Dye Adsorbents: Adsorption of Methylene Blue and Congo Red on Brewers’ Spent Grain. Croat. Chem. Acta 2018, 91, 53–64. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Bamforth, C.W. The Microbiology of Malting and Brewing. Microbiol. Mol. Biol. Rev. 2013, 77, 157–172. [Google Scholar] [CrossRef]
- Dancker, P.; Brunner, D.; Glas, K.; Dawid, C.; Gastl, M. Heavy Metal Adsorption of brewer’s Spent Grain in Aqueous Solution: Impact of Mechanochemical Esterification. Brew. Sci. 2025, 78, 47–54. [Google Scholar]
- Mengist, W.; Soromessa, T.; Legese, G. Method for conducting systematic literature review and meta-analysis for environmental science research. MethodsX 2020, 7, 100777. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. Br. Med. J. 2020, 368, l6890. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef]
- Damerow, P. Sumerian Beer: The Origins of Brewing Technology in Ancient Mesopotamia. Cuneiform Digit. Lib. J. 2012, 2, 1–20. [Google Scholar]
- Nelson, M. The Barbarian’s Beverage: A History of Beer in Ancient Europe; Routledge: London, UK, 2005; pp. 1–224. [Google Scholar]
- Unger, R.W. Beer in the Middle Ages and the Renaissance; University of Pennsylvania Press: Philadelphia, PA, USA, 2004; pp. 1–344. [Google Scholar]
- Hornsey, I.S. A History of Beer and Brewing; Royal Society of Chemistry: Cambridge, UK, 2003; pp. 1–742. [Google Scholar]
- Cabras, I.; Higgins, D.M. Beer, Brewing, and Business History. Bus. Hist. 2016, 58, 609–624. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer: Tap into the Art and Science of Brewing, 2nd ed.; Oxford University Press: New York, NY, USA, 2003; pp. 1–233. [Google Scholar]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing: Science and Practice; Woodhead Publishing: Cambridge, UK, 2004; pp. 1–862. [Google Scholar]
- Poelmans, E.; Swinnen, J. A Brief Economic History of Beer. In The Economics of Beer; Swinnen, J., Ed.; Oxford University Press: Oxford, UK, 2011; pp. 3–28. [Google Scholar]
- Elzinga, K.G.; Tremblay, C.H.; Tremblay, V.J. Craft Beer in the United States: History, Numbers, and Geography. J. Wine Econ. 2015, 10, 242–274. [Google Scholar] [CrossRef]
- Garavaglia, C.; Swinnen, J. Economic Perspectives on Craft Beer: A Revolution in the Global Beer Industry; Palgrave Macmillan: London, UK, 2017; pp. 1–380. [Google Scholar]
- Tremblay, V.J.; Tremblay, C.H. The U.S. Brewing Industry: Data and Economic Analysis; MIT Press: Cambridge, MA, USA, 2005; pp. 1–379. [Google Scholar]
- Johnson-Greenough, E.; The World’s Top 40 Brewing Companies. New School Beer 2025. Available online: https://newschoolbeer.com/home/2025/6/worlds-top-40-brewing-companies (accessed on 18 August 2025).
- McGuire, L. The World’s Best Beer for 2025 Has Been Named and It’s Beaten Budweiser, Heineken and Guinness. Available online: https://www.thesun.co.uk/money/35285304/worlds-best-beer-named/ (accessed on 18 August 2025).
- International Brand Equity. 16 Best Beer Brands in the World 2025; International Brand Equity: Bangalore, India, 2025; Available online: https://www.internationalbrandequity.com/best-beer-brands/ (accessed on 18 August 2025).
- VinePair. The 40 Biggest Beer Producers in the World 2025. VinePair Reports 2025. Available online: https://vinepair.com/booze-news/40-biggest-beer-producers-2025 (accessed on 18 August 2025).
- Carlsberg Group. Annual Report 2024; Carlsberg A/S: Copenhagen, Denmark, 2024; pp. 1–204. [Google Scholar]
- Molson Coors Beverage Company. Annual Financial Report 2024; Molson Coors: Chicago, IL, USA, 2024; pp. 1–170. [Google Scholar]
- Constellation Brands Inc. Annual Report 2024; Constellation Brands Inc.: New York, NY, USA, 2024; pp. 1–151. [Google Scholar]
- Forbes. Sales of the Leading Beer Companies Worldwide in 2024. Statista. Available online: https://www-1statista-1com-1s8fui2dd0042.han3.ue.poznan.pl/statistics/257670/sales-of-the-leading-beer-companies-worldwide/ (accessed on 18 August 2025).
- Barth-Haas Group. Leading 10 Countries in Worldwide Beer Production in 2023. Statista. Available online: https://www-1statista-1com-1s8fui2dd0048.han3.ue.poznan.pl/statistics/270269/leading-10-countries-in-worldwide-beer-production/ (accessed on 18 August 2025).
- Barth-Haas Group. Beer Production Worldwide from 1998 to 2022. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 7 August 2024).
- Barth-Haas Group. China Is the World’s Biggest Producer of Beer. Available online: https://www.statista.com/chart/30478/countries-with-the-most-beer-output/ (accessed on 7 August 2024).
- Muster-Slawitsch, B.; Hubmann, M.; Murkovic, M.; Brunner, C. Process modelling and technology evaluation in brewing. Chem. Eng. Proc. Process Intensif. 2014, 84, 98–108. [Google Scholar] [CrossRef]
- Serviss, M.T.; Van Hout, D.; Britton, S.J.; MacIntosh, A.J. Brewing for the Future: Balancing Tradition and Sustainability. J. Am. Soc. Brew. Chem. 2025, 2509059, 1–19. [Google Scholar] [CrossRef]
- Marrucci, L.; Daddi, T.; Iraldo, F. Identifying the most sustainable beer packaging through a Life Cycle Assessment. Sci. Total Environ. 2024, 948, 174941. [Google Scholar] [CrossRef]
- Muster-Slawitsch, B.; Brunner, C.; Ribeiro de Lima, D.; Schnitzer, H. The Green Brewery Concept—Energy Efficiency and the Use of Renewable Energy Sources in Breweries. Chem. Eng. Trans. 2010, 21, 649–654. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Kim, J.; Foley, A.; Rooney, D. Decarbonizing the food and beverages industry: A critical and systematic review of developments, sociotechnical systems and policy options. Renew. Sustain. Energy Rev. 2021, 143, 110856. [Google Scholar] [CrossRef]
- Violino, S.; Figorilli, S.; Costa, C.; Pallottino, F. Internet of Beer: A Review on Smart Technologies from Mash to Pint. Foods 2020, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.A.; Patra, F.; Ghosh, T.; Kumar Mahnot, N.; Dutta, H.; Kumar Duary, R. Advancing food systems with industry 5.0: A systematic review of smart technologies, sustainability, and resource optimization. Sustain. Futures 2025, 9, 100694. [Google Scholar] [CrossRef]
- Talmage, C.A.; Bletscher, C.; Newton, J.D.; Mars, M.M. Community development on tap: How local breweries provide creative community-centered spaces and initiatives for advancing economic and social capital. Community Dev. 2024, 1–22. [Google Scholar] [CrossRef]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Olajire, A.A. The Brewing Industry and Environmental Challenges. J. Clean. Prod. 2012, 31, 102817. [Google Scholar] [CrossRef]
- Sturm, B.; Hugenschmidt, S.; Joyce, S.; Hofacker, W.; Roskilly, A.P. Opportunities and barriers for efficient energy use in a medium-sized brewery. Appl. Therm. Eng. 2013, 53, 397–404. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, M.; Lee, J.; Park, J.H.; Kwon, E.E. Reducing CO2 emissions in brewing industry through sustainable valorisation of brewer’s spent grain using CO2-assisted pyrolysis. J. Anal. Appl. Pyrolysis 2025, 190, 107165. [Google Scholar] [CrossRef]
- Grand, T.; Jenkins, D.; Maskell, D.; Zhuang, S. Valorisation of Carbon Dioxide from Fermentation in Craft Brewing: Potential Technologies, Brewer Interviews, and Implication for a ‘Three-Level Valorisation System. J. Am. Soc. Brew. Chem. 2024, 83, 248–259. [Google Scholar] [CrossRef]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, wastewater and waste management in brewing industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Brito, A.G.; Melo, L.F. Posttreatment of a brewery wastewater using a sequencing batch reactor. Water Environ. Res. 2001, 73, 45–51. [Google Scholar] [CrossRef]
- Shao, X.; Peng, D.; Teng, Z.; Ju, X. Treatment of brewery wastewater using anaerobic sequencing batch reactor (ASBR). Bioresour. Technol. 2008, 99, 3182–3186. [Google Scholar] [CrossRef]
- Čater, M.; Fanedl, L.; Malovrh, Š.; Marinšek Logar, R. Biogas production from brewery spent grain enhanced by bioaugmentation with hydrolytic anaerobic bacteria. Bioresour. Technol. 2015, 186, 261–269. [Google Scholar] [CrossRef]
- Świechowski, K.; Rasaq, W.A.; Syguła, E. Anaerobic digestion of brewer’s spent grain with biochars—Biomethane production and digestate quality effects. Front. Energy Res. 2023, 11, 1141684. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries—Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Romero, I.; López-Linares, J.C.; Castro, E. Brewer’s spent grain as a source of renewable fuel through optimized dilute acid pretreatment. Renew. Energy 2020, 148, 81–90. [Google Scholar] [CrossRef]
- Chen, H.; Chang, S.; Guo, Q.; Hong, Y.; Wu, P. Brewery wastewater treatment using an anaerobic membrane bioreactor. Biochem. Eng. J. 2016, 105, 321–331. [Google Scholar] [CrossRef]
- Pabbathi, N.P.P.; Velidandi, A.; Pogula, S.; Gandam, P.K.; Baadhe, R.R.; Sharma, M.; Sirohi, R.; Thakur, V.K.; Gupta, V.K. Brewer’s spent grains-based biorefineries: A critical review. Fuel 2022, 317, 123435. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Recent advances in biotechnological valorization of brewers’ spent grain. Food Sci. Biotechnol. 2021, 30, 341–353. [Google Scholar] [CrossRef]
- Moirangthem, K.; Knaapila, A.; Lee, Y.; Sandell, M.; Skibinska, I.; Kilcawley, K.N.; O’Connor, P.M.; Maina, H.N.; Niklander, K.; Verhulst, E.P.; et al. Tailored bioprocessing of brewers’ spent grain for the development of upcycled plant-based spoonable snacks. Future Foods 2025, 11, 100621. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Virdi, A.S.; Mahajan, A.; Devraj, M.; Sanghi, R. Brewers’ spent grains: Techno-functional challenges and opportunity in the valorization for food products. LWT 2025, 227, 117785. [Google Scholar] [CrossRef]
- Stojceska, V. Dietary Fiber from Brewer’s Spent Grain as a Functional Ingredient in Bread Making Technology. In Flour and Breads and their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 171–181. [Google Scholar]
- Chattaraj, S.; Mitra, D.; Ganguly, A.; Thatoi, H.; Das Mohapatra, P.K. A critical review on the biotechnological potential of Brewers’ waste: Challenges and future alternatives. Curr. Res. Microb. Sci. 2024, 6, 100228. [Google Scholar] [CrossRef]
- Di Mario, J.; Gambelli, A.M.; Gigliotti, G. Biomethane Production from Untreated and Treated Brewery’s Spent Grain: Feasibility of Anaerobic Digestion After Pretreatments According to Biogas Yield and Energy Efficiency. Agronomy 2024, 14, 2980. [Google Scholar] [CrossRef]
- Zabaleta, R.; Torres, E.; Sánchez, E.; Torres-Sciancalepore, R.; Fabani, P.; Mazza, G.; Rodriguez, R. Brewer’s spent grain-based biochar as a renewable energy source and agriculture substrate. J. Mater. Cycles Waste Manag. 2024, 26, 3787–3801. [Google Scholar] [CrossRef]
- Kang, Y.-G.; Kim, J.-H.; Lee, J.-Y.; Kim, J.-H.; Choi, J.; Oh, T.-K. Potential role of pyrolysis temperature for brewer’s spent grain biochar on mitigating ammonia emissions from urea-fertilized soils. Korean J. Soil Sci. Fert. 2025, 58, 190–201. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P.; Gondek, K. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Manolikaki, I.; Diamadopoulos, E. Agronomic potential of biochar prepared from brewery byproducts. J. Environ. Manag. 2020, 255, 109856. [Google Scholar] [CrossRef]
- Vasileiadou, A. Energy recovery from brewers’ spent grain combustion/co-combustion with lignite. Int. J. Environ. Sci. Technol. 2024, 21, 5335–5350. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Sganzerla, W.G.; Matheus, L.R.; Mançano, R.R.; Ferreira, V.C.; Barroso, T.L.C.T.; da Rosa, R.G.; Colpini, L.M.S. Application of brewers’ spent grains as an alternative biomass for renewable energy generation in a boiler combustion process. Sustain. Chem. Environ. 2023, 4, 100039. [Google Scholar] [CrossRef]
- BDI BioEnergy International AG. Sustainable Brewing Solutions: Obtaining Biogas from Spent Grains. 2024. Available online: https://bdi-bioenergy.com/news/sustainable-brewing-solutionsobtaining-biogas-from-spent-grains/ (accessed on 18 August 2025).
- BrewDog. Green Gas Plant to Power Beer Production and Delivery Vehicles; Institution of Mechanical Engineers: London, UK, 2022; Available online: https://www.imeche.org/news/news-article/cheers%21-biogas-and-waste-heat-help-brewers-boost-sustainability?utm_source=chatgpt.com/ (accessed on 18 August 2025).
- Cenergy. World-Famous Brewery to Benefit from Biogas CHP Cogeneration System, Powering the Plant with Clean Renewable Energy. 2014. Available online: https://img.pr.com/release-file/1409/580516/BreweryBiogasCHP.pdf?utm_source=chatgpt.com/ (accessed on 18 August 2025).
- Scottish & Newcastle. Converting Spent Grain into Renewable Energy; FAO Sustainability Pathways: Rome, Italy, 2009; Available online: https://www.fao.org/nr/sustainability/food-loss-and-waste/database/projects-detail/en/c/135063/ (accessed on 18 August 2025).
- Lv, J.; Fang, X.; Feng, G.; Zhang, G.; Zhao, C.; Zhang, Y.; Li, Y. Effects of Sodium Formate and Calcium Propionate Additives on the Fermentation Quality and Microbial Community of Wet Brewers Grains after Short-Term Storage. Animals 2020, 10, 1608. [Google Scholar] [CrossRef]
- Terefe, G. Preservation techniques and their effect on nutritional values and microbial population of brewer’s spent grain: A review. CABI Agric. Biosci. 2022, 3, 51. [Google Scholar] [CrossRef]
- Moriel, P.; Piccolo, M.B.; Artioli, L.F.A.; Santos, G.S.; Poore, M.H.; Ferraretto, L.F. Method of propionic acid–based preservative addition and its effects on nutritive value and fermentation characteristics of wet brewers grains ensiled in the summertime. Prof. Anim. Sci. 2016, 32, 591–597. [Google Scholar] [CrossRef]
- Moriel, P.; Artioli, L.F.A.; Poore, M.H.; Ferraretto, L.F. Dry matter loss and nutritional composition of wet brewers grains ensiled with or without covering and with or without soybean hulls and propionic acid. Prof. Anim. Sci. 2015, 31, 559–567. [Google Scholar] [CrossRef]
- Ferraretto, L.F.; Fernandes, T.; Silva Filho, W.I.; Sultana, H.; Moriel, P. Dry matter loss, fermentation profile, and aerobic stability of wet brewers grains ensiled with various amounts of dry ground corn. Prof. Anim. Sci. 2018, 34, 642–648. [Google Scholar] [CrossRef]
- Shetty, R.; Petersen, F.R.; Podduturi, R.; Molina, G.-E.S.; Wätjen, A.P.; Madsen, S.K.; Zioga, E.; Ozmerih, S.; Hobley, T.J.; Bang-Berthelsen, C.H. Fermentation of brewer’s spent grain liquids to increase shelf life and give an organic acid enhanced ingredient. LWT 2023, 182, 114911. [Google Scholar] [CrossRef]
- Arranz, J.I.; Miranda, M.T.; Sepúlveda, F.J.; Montero, I.; Rojas, C.V. Analysis of Drying of Brewers’ Spent Grain. Proceedings 2018, 2, 1467. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Anderson, J.L.; Kalscheur, K.F.; Garcia, A.D.; Schingoethe, D.J.; Casper, D.P.; Kleinschmit, D.H. Ensiling characteristics of distillers wet grains with corn stalks and determination of the feeding potential for dairy heifers. Prof. Anim. Sci. 2015, 31, 359–367. [Google Scholar] [CrossRef]
- Robertson, J.A.; I’Anson, K.J.A.; Brocklehurst, T.F.; Faulds, C.B.; Waldron, K.W. Effect of storage conditions on the microbial ecology and biochemical stability of cell wall components in brewers’ spent grain. J. Agric. Food Chem. 2010, 58, 7266–7272. [Google Scholar] [CrossRef]
- Hermansen, C.; Chong, Q.K.; Ho, S.; Natali, F.; Weingarten, M.; Peterson, E.C. Microbiome Evolution of Brewer’s Spent Grain and Spent Coffee Ground Solid Sidestreams Under Industrial Storage Conditions. Appl. Sci. 2024, 14, 9759. [Google Scholar] [CrossRef]
- Heinzen, C.; Agarussi, M.C.N.; Diepersloot, E.C.; Ferraretto, L.F. Effects of microbial inoculation on dry matter losses, fermentation profile, and aerobic stability of wet brewers grain stored with increasing concentrations of dry ground corn. Anim. Feed. Sci. Technol. 2022, 286, 115257. [Google Scholar] [CrossRef]
- Schneider, R.M.; Harrison, J.H.; Loney, K.A. The Effects of Bacterial Inoculants, Beet Pulp, and Propionic Acid on Ensiled Wet Brewers Grains. J. Dairy Sci. 1995, 78, 1096–1105. [Google Scholar] [CrossRef]
- Li, L.; Xie, X.; Zhao, G.; He, J.; Zhang, Y. The effects of applying cellulase and laccase on fermentation quality and microbial community in mixed silage containing corn stover and wet brewer’s grains. Front. Plant Sci. 2024, 15, 1441873. [Google Scholar] [CrossRef]
- de Morais, J.P.G.; Hartung, L.; Nunes, M.A.; Sobires, P.D.; Pereira, F.C.; Vargas, M.E.; Del Valle, T.A.; Campana, M. Calcium oxide reduces fermentation losses and improves the nutritional value of brewery-spent grain silage. Anim. Feed. Sci. Technol. 2025, 319, 116187. [Google Scholar] [CrossRef]
- Killerby, M.A.; Almeida, S.T.R.; Hollandsworth, R.; Guimaraes, B.C.; Leon-Tinoco, A.; Perkins, L.B.; Henry, D.; Schwartz, T.J.; Romero, J.J. Effect of chemical and biological preservatives and ensiling stage on the dry matter loss, nutritional value, microbial counts, and ruminal in vitro gas production kinetics of wet brewer’s grain silage. J. Anim. Sci. 2022, 100, skac095. [Google Scholar] [CrossRef] [PubMed]
- Evers, T.; Millar, S. Cereal Grain Structure and Development: Some Implications for Quality. J. Cereal Sci. 2002, 36, 261–284. [Google Scholar] [CrossRef]
- Brennan, M.; Shepherd, T.; Mitchell, S.; Topp, C.F.E.; Hoad, S.P. Husk to caryopsis adhesion in barley is influenced by pre- and post-anthesis temperatures through changes in a cuticular cementing layer on the caryopsis. BMC Plant Biol. 2017, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.R.; Brennan, M.; Hoad, S.P. The Structure of the Barley Husk Influences Its Resistance to Mechanical Stress. Front. Plant Sci. 2021, 11, 614334. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Wang, Z. Protein accumulation in aleurone cells, sub-aleurone cells and the center starch endosperm of cereals. Plant Cell Rep. 2014, 33, 1607–1615. [Google Scholar] [CrossRef]
- Geng, L.; Li, M.; Zhang, G.; Ye, L. Barley: A potential cereal for producing healthy and functional foods. Food Qual. Saf. 2022, 6, fyac012. [Google Scholar] [CrossRef]
- Li, D.Q.; Wu, X.B.; Wang, H.F.; Feng, X.; Yan, S.J.; Wu, S.Y.; Liu, J.X.; Yao, X.F.; Bai, A.N.; Zhao, H.; et al. Defective mitochondrial function by mutation in THICK ALEURONE 1 encoding a mitochondrion-targeted single-stranded DNA-binding protein leads to increased aleurone cell layers and improved nutrition in rice. Mol. Plant 2021, 14, 1343–1361. [Google Scholar] [CrossRef]
- Betts, N.S.; Wilkinson, L.G.; Khor, S.F.; Shirley, N.J.; Lok, F.; Skadhauge, B.; Burton, R.A.; Fincher, G.B.; Collins, H.M. Morphology, Carbohydrate Distribution, Gene Expression, and Enzymatic Activities Related to Cell Wall Hydrolysis in Four Barley Varieties during Simulated Malting. Front. Plant Sci. 2017, 8, 1872. [Google Scholar] [CrossRef] [PubMed]
- Kovacik, M.; Nowicka, A.; Zwyrtková, J.; Strejčková, B.; Vardanega, I.; Esteban, E.; Pasha, A.; Kaduchová, K.; Krautsova, M.; Červenková, M.; et al. The transcriptome landscape of developing barley seeds. Plant Cell 2024, 36, 2512–2530. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains-Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical composition of lipids in brewer’s spent grain: A promising source of valuable phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y. Development of a Functional Germinated Barley Foodstuff From Brewers’ Spent Grain for the Treatment of Ulcerative Colitis. J. Am. Soc. Brew. Chem. 2001, 59, 59–62. [Google Scholar] [CrossRef]
- Russ, W.; Mörtel, H.; Meyer-Pittroff, R. Application of Spent Grains to Increase Porosity in Bricks. Constr. Build. Mater. 2005, 19, 117–126. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Chemical Characterization and Liberation of Pentose Sugars from Brewer’s Spent Grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Rocha, G.J.M.; Roberto, I.C. Hydrogen peroxide bleaching of cellulose pulps obtained from brewer’s spent grain. Cellul. 2008, 15, 641–649. [Google Scholar] [CrossRef]
- Khidzir, K.M.; Noorlidah, A.; Agamuthu, P. Brewery Spent Grain: Chemical Characteristics and Utilization as an Enzyme Substrate. Malaysian J. Sci. 2010, 29, 41–51. [Google Scholar]
- Adeniran, H.A.; Abiose, S.H.; Ogunsua, A.O. Production of Fungal β-amylase and Amyloglucosidase on Some Nigerian Agricultural Residues. Food Bioprocess. Technol. 2008, 3, 693–698. [Google Scholar] [CrossRef]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT-Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Faulds, C.B.; Robertson, J.A.; Waldron, K.W. Effect of pH on the Solubilization of Brewers’ Spent Grain by Microbial Carbohydrases and Proteases. J. Agric. Food Chem. 2008, 56, 7038–7043. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; del Nozal, M.J. Variability of brewer’s spent grain within a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Esteves, M.P.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Sousa, S.; Rodrigues, J.; Antunes, H.; Porter, J.J.; Gonçalves, I.; Ferreira-Dias, S. Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains. Sep. Purif. Technol. 2004, 40, 309–315. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. The effects of malting and mashing on barley protein extractability. J. Cereal Sci. 2006, 44, 203–211. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour. Technol. 2008, 99, 5427–5435. [Google Scholar] [CrossRef]
- Jay, A.J.; Parker, M.L.; Faulks, R.; Husband, F.; Wilde, P.; Smith, A.C.; Faulds, C.B.; Waldron, K.W. A systematic microdissection of brewers’ spent grain. J. Cereal Sci. 2008, 47, 357–364. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-around characterization of brewers’ spent grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. [Google Scholar] [CrossRef]
- Amirvaresi, A.; Nikounezhad, N.; Amirahmadi, M.; Daraei, B.; Parastar, H. Comparison of Near-Infrared (NIR) and Mid-infrared (MIR) Spectroscopy Based on Chemometrics for Saffron Authentication and Adulteration Detection. Food Chem. 2021, 344, 128647. [Google Scholar] [CrossRef]
- Fărcaş, A.; Tofană, M.; Socaci, S.; Mudura, E.; Scrob, S.; Salanţă, L.; Mureşan, V. Brewers’ spent grain—A new potential ingredient for functional foods. J. Agroaliment. Process Technol. 2014, 20, 137–141. [Google Scholar]
- Belardi, I.; Marrocchi, A.; Alfeo, V.; Sileoni, V.; De Francesco, G.; Paolantoni, M.; Marconi, O. Sequential Extraction and Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy Monitoring in the Biorefining of Brewer’s Spent Grain. Molecules 2023, 28, 7992. [Google Scholar] [CrossRef] [PubMed]
- Mainali, K.; Yadav, M.P.; Sharma, B.K.; Sarker, M.I.; Ngo, H.; Hotchkiss, A.; Simon, S. Isolation and Characterization of the Physiochemical Properties of Brewer’s Spent Grain. Agriculture 2025, 15, 47. [Google Scholar] [CrossRef]
- Fox, G. The Brewing Industry and the Opportunities for Real-Time Quality Analysis Using Infrared Spectroscopy. Appl. Sci. 2020, 10, 616. [Google Scholar] [CrossRef]
- Rego, F.; Soares Dias, A.P.; Casquilho, M.; Rosa, F.C.; Rodrigues, A. Fast Determination of Lignocellulosic Composition of Poplar Biomass by Thermogravimetry. Biomass Bioenergy 2019, 122, 375–380. [Google Scholar] [CrossRef]
- Mallen, E.; Najdanovic-Visak, V. Brewers’ spent grains: Drying kinetics and biodiesel production. Bioresour. Technol. Rep. 2018, 1, 16–23. [Google Scholar] [CrossRef]
- Borel, L.D.M.S.; Lira, T.S.; Ribeiro, J.A.; Ataíde, C.H.; Barrozo, M.A.S. Pyrolysis of brewer’s spent grain: Kinetic study and products identification. Ind. Crops Prod. 2018, 121, 388–395. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Olanrewaju, O.A.; Usman, M.A.; Adeosun, S.O. Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations. Polymers 2023, 15, 2346. [Google Scholar] [CrossRef]
- Balogun, A.O.; Sotoudehniakarani, F.; McDonald, A.G. Thermo-kinetic, spectroscopic study of brewer’s spent grains and characterisation of their pyrolysis products. J. Anal. Appl. Pyrol. 2017, 127, 8–16. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Snoussi, Y.; Garah, M.E.; Ammar, S.; Chehimi, M.M. Brewer’s Spent Grain Biochar: Grinding Method Matters. C 2022, 8, 46. [Google Scholar] [CrossRef]
- Lisci, S.; Mais, L.; Corda, A.; Troncia, S.; Erricob, M.; Grosso, M. Chemometric Models Applied to Raman Spectroscopy for Bioprocess Monitoring. Chem. Eng. Trans. 2023, 99, 571–576. [Google Scholar]
- Krasznai, D.J.; Champagne Hartley, R.; Roy, H.M.; Champagne, P.; Cunningham, M.F. Compositional Analysis of Lignocellulosic Biomass: Conventional Methodologies and Future Outlook. Crit. Rev. Biotechnol. 2017, 38, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.A.; Montero, G.; Montes, D.G.; Valdez-Salas, B.; Ayala, J.R.; García, C.; Carrillo, M.; León, J.A.; Moreno, A. Physicochemical Characterization and SEM-EDX Analysis of Brewer’s Spent Grain from the Craft Brewery Industry. Sustainability 2020, 12, 7744. [Google Scholar] [CrossRef]
- Mishra, P.K.; Gregor, T.; Wimmer, R. Utilising brewer’s spent grain as a source of cellulose nanofibres following separation of protein-based biomass. BioResources 2017, 12, 107–116. [Google Scholar] [CrossRef]
- Kemppainen, K.; Rommi, K.; Holopainen, U.; Kruus, K. Steam explosion of Brewer’s spent grain improves enzymatic digestibility of carbohydrates and affects solubility and stability of proteins. Appl. Biochem. Biotechnol. 2016, 180, 94–108. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Aguilar-Uscanga, M.G.; Pinheiro de Souza Oliveira, R.; Domínguez, J.M. Enhancing the biorefinery of brewery spent grain by deep eutectic solvent pretreatment: Optimisation of polysaccharide enrichment through a response surface methodology. J. Ind. Eng. Chem. 2025, 145, 693–704. [Google Scholar] [CrossRef]
- Nunes, L.F.; Ugalde, G.A.; Anschau, K.F.; Müller, E.I.; Tres, M.V.; Zabot, G.L.; Kuhn, R.C. Clean Production of Sugars from Brewer’s Spent Grains Using Subcritical Water Hydrolysis and Steam Explosion. Sustain. Chem. 2024, 5, 308–323. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Saeb, M.R.; Formela, K. Preparation and characterization of natural rubber composites highly filled with brewers’ spent grain/ground tire rubber hybrid reinforcement. Compos. B Eng. 2018, 145, 182–188. [Google Scholar] [CrossRef]
- Chin, Y.L.; Dinani, S.T.; Chen, W.N.; Boom, R. The emulsifying performance of brewers’ spent grains treated by colloid milling. Innov. Food Sci. Emerg. Technol. 2024, 91, 103541. [Google Scholar] [CrossRef]
- Hejna, A.; Cieśliński, H.; Skórczewska, K.; Kosmela, P.; Aniśko-Michalak, J.; Piasecki, A.; Barczewski, M. The impact of brewers’ spent grain type on the structure and performance of poly(ε-caprolactone)-based composites. Cellulose 2025, 32, 8283–8307. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Chaurin, V.; Reis, S.F.; Gallagher, E. Brewer’s spent grain as a functional ingredient for breadsticks. Int. J. Food Sci. Technol. 2012, 47, 1765–1771. [Google Scholar] [CrossRef]
- da Silva Araújo, F.P.; de Souza Cupertino, G.; de Cássia Superbi de Sousa, R.; de Castro Santana, R.; Pereira, A.F. Use of biochar produced from brewer’s spent grains as an adsorbent. Biomass Conv. Bioref. 2025, 15, 16367–16382. [Google Scholar] [CrossRef]
- Sibono, L.; Tronci, S.; Hajrizaj, R.; Christensen, K.V.; Errico, M.; Grosso, M. Optimization and kinetic analysis of untreated brewers’ spent grain saccharification process via enzymatic hydrolysis. Biochem. Eng. J. 2023, 198, 109044. [Google Scholar] [CrossRef]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential applications of brewery spent grain: Critical an overview. J. Environ. Chem. Eng. 2022, 10, 106951. [Google Scholar] [CrossRef]
- Kalak, T. The use of post-production waste generated in the brewing industry for the effective bioremoval of Cu(II) ions. Desalin. Water Treat. 2022, 271, 124–142. [Google Scholar] [CrossRef]
- Kalak, T.; Walczak, J.; Ulewicz, M. Adsorptive Recovery of Cd(II) Ions with the Use of Post-Production Waste Generated in the Brewing Industry. Energies 2021, 14, 5543. [Google Scholar] [CrossRef]
- Dragana, K.; Marina, Š.; Jelena, P.; Vesna, V.; Jelena, P. Brewer’s spent grain as a potential adsorbent of heavy metal ions from water. Zast. Mater. 2016, 57, 397–403. [Google Scholar]
- Carrasco, K.H.; Höfgen, E.G.; Brunner, D.; Borchert, K.B.L.; Reis, B.; Steinbach, C.; Mayer, M.; Schwarz, S.; Glas, K.; Schwarz, D. Removal of Iron, Manganese, Cadmium, and Nickel Ions Using Brewers’ Spent Grain. Polysaccharides 2022, 3, 356–379. [Google Scholar] [CrossRef]
- Kalak, T.; Cierpiszewski, R. Comparative studies on the adsorption of Pb(II) ions by fly ash and slag obtained from CFBC technology. Pol. J. Chem. Technol. 2019, 21, 72–81. [Google Scholar] [CrossRef]
- Kalak, T.; Kaczmarek, M.; Nowicki, P.; Pietrzak, R.; Tachibana, Y.; Cierpiszewski, R. Preparation of nitrogen-enriched pine sawdust-based activated carbons and their application for copper removal from the aquatic environment. Wood Sci. Technol. 2022, 56, 1721–1742. [Google Scholar] [CrossRef]
- Patel, H. Fixed-Bed Column Adsorption Study: A Comprehensive Review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Šillerová, H.; Chrastný, V.; Čadková, E.; Komárek, M. Isotope fractionation and spectroscopic analysis as an evidence of Cr(VI) reduction during biosorption. Chemosphere 2014, 95, 402–407. [Google Scholar] [CrossRef]
- Zewde, Z.; Asere, T.G.; Yitbarek, M. Porous biochars derived from brewery waste for the treatment of Cr(VI)-contaminated water. PLoS ONE 2024, 19, e0314522. [Google Scholar] [CrossRef]
- Geremias, R.; Pelissari, C.; Libardi, N.; Carpiné, D.; Ribani, R.F. Chromium adsorption studies using brewer’s spent grain biochar: Kinetics, isotherm and thermodynamics. Ciênc. Rural 2023, 53, e20210914. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, H.; Kim, K.W. Removal of cobalt and strontium by adsorption using Brewer’s spent grain formed by pyrolysis. Environ. Geochem. Health 2023, 45, 7131–7144. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wenzel, M.; Seifert, M.; Weigand, J.J. Surface ion-imprinted brewer’s spent grain with low template loading for selective uranyl ions adsorption from simulated wastewater. J. Hazard. Mater. 2022, 440, 129682. [Google Scholar] [CrossRef]
- Ferraz, A.I.; Maria, T.; Tavares, M.T.; Teixeira, J.A. Sorption of Cr (III) from aqueous solutions by spent brewery grain. In Proceedings of the 9th International Chemical Engineering Conference, CHEMPOR 2005, Coimbra, Portugal, 21–23 September 2005; Departamento de Engenharia Química da Universidade de Coimbra: Coimbra, Portugal, 2005. ISBN 972-8055-13-7. [Google Scholar]
- Kukić, D.V.; Vasić, V.M.; Panić, S.N.; Radosavljević, M.S.; Šćiban, M.B.; Prodanović, J.M.; Blagojev, N.T.; Pejin, J.D. Adsorption kinetics of Cr (VI) ions onto biochar from brewer’s spent grain. Acta Period. Technol. 2019, 50, 134–142. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z. Lead sorption from aqueous solutions on chitosan nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 183–190. [Google Scholar] [CrossRef]
- Majiya, H.; Clegg, F.; Sammon, C. Bentonite-Chitosan composites or beads for lead (Pb) adsorption: Design, preparation, and characterization. Appl. Clay Sci. 2023, 246, 107180. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Ofudje, E.A.; Adeogun, A.I.; Aina, P.; Joseph, I.M. Orange peel as low-cost adsorbent in the elimination of Cd(II) ion: Kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour. Bioprocess. 2020, 7, 34. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, L.; Zhang, B.; Wu, G.; Du, D. Adsorptive removal of Cu(II) from aqueous solution using modified rice husk. Int. J. Eng. Res. 2012, 2, 855–863. [Google Scholar]
- Parlayıcı, Ş.; Baran, Y. Removal of hexavalent chromium from aqueous solutions using nano-Fe3O4/waste banana peel/alginate hydrogel biobeads as adsorbent. Biomass Conv. Bioref. 2025, 15, 18695–18721. [Google Scholar] [CrossRef]
- Chanzu, H.A.; Onyari, J.M.; Shiundu, P.M. Brewers’ spent grain in adsorption of aqueous Congo Red and malachite Green dyes: Batch and continuous flow systems. J. Hazard. Mater. 2019, 380, 120897. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Xu, J.; Lu, X.; Wang, C.; Xu, H.; Yuan, H.; Zhang, J. Brewer’s grains with different pretreatments used as bio-adsorbents for the removal of Congo red dye from aqueous solution. BioResources 2020, 15, 6928–6940. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Lončarić, A.; Gašo-Sokač, D.; Bušić, V.; Habuda-Stanić, M. Adsorptive removal of nitrate from wastewater using modified lignocellulosic waste material. J. Mol. Liq. 2019, 285, 535–544. [Google Scholar] [CrossRef]
- Liu, P.; Sun, S.; Huang, S.; Wu, Y.; Li, X.; Wei, X.; Wu, S. KOH Activation Mechanism in the Preparation of Brewer’s Spent Grain-Based Activated Carbons. Catalysts 2024, 14, 814. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Quesada, H.B.; Bergamasco, R.; Vareschini, D.T.; de Barros, M.A.S.D. Activated hydrochar produced from brewer’s spent grain and its application in the removal of acetaminophen. Bioresour. Technol. 2020, 310, 123399. [Google Scholar] [CrossRef]
- Xi, X.; Yan, J.; Cui, L.; Quan, G. Removal of the Pesticide Pymetrozine from Aqueous Solution by Biochar Produced from Brewer’s Spent Grain at Different Pyrolytic Temperatures. BioResources 2014, 9, 7696–7709. [Google Scholar] [CrossRef]
- Deolikar, R.; Patil, R. Chapter 11—Recent Advances in Pesticides Removal Using Agroindustry Based Biochar. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Biswas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 11; pp. 265–290. [Google Scholar]
- Kopp, M.; Anabalón, P.; Rocha, S.; González, M.E.; Romero-García, J.M.; Castro, E.; Cea, M. Synthesis of iron oxide/activated hydrochar composite from residual brewery biomass for remediation of water contaminated with chlorophenol. Sci. Rep. 2025, 15, 10705. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q. Sustainable mechanisms of biochar derived from brewers’ spent grain and sewage sludge for ammonia–nitrogen capture. J. Clean. Prod. 2016, 112, 3927–3934. [Google Scholar] [CrossRef]
- Feng, L.; Qiu, T.; Yan, H.; Liu, C.; Chen, Y.; Zhou, X.; Qiu, S. Removal of Ammonia Nitrogen from Aqueous Media with Low-cost Adsorbents: A Review. Air Soil Pollut. 2023, 234, 280. [Google Scholar] [CrossRef]
- Adam, G.A.; Getye, B.; Gebreslassie, G.; Sisay, T. Magnetically recyclable Activated Carbon Prepared from Brewer’s Spent Grain and Its Chromium (VI) Adsorption study. J. Mater. Proc. Technol. 2024, 1, 100053. [Google Scholar]
- Gomez-Delgado, E.; Morales-Urrea, D.; Jader Alean, J.; López-Córdoba, A. Activated carbons from brewers spent grain improve Orange II removal through combined adsorption and enzymatic oxidation. Sci. Rep. 2025, 15, 22487. [Google Scholar] [CrossRef] [PubMed]


| Components [% Dry Weight] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Cellulose | Hemicellulose | Lignin | Proteins | Ashes | Extractives | Others | Carbohydrates | Crude Fiber | Moisture | Lipids | Acid Detergent Fiber |
| [107] | 49.4 | 8.8 | 14.5 | 4.9 | – | - | – | – | 8.3 | 9.2 | – | |
| [108] | 25.4 | 21.8 | 11.9 | 24 | 2.4 | – | 21.8 | – | – | – | 10.6 | – |
| [109] | 23–25 | 30–35 | 7–8 | 19–23 | 4–4.5 | – | – | – | – | – | – | – |
| [110] | 16.8 | 28.4 | 27.8 | 15.3 | 4.6 | 5.8 | – | – | – | – | – | – |
| [111] | 16.8 | 28.4 | 27.8 | – | 4.6 | – | 22.4 | – | – | – | – | – |
| [112] | – | – | – | 6.4 | 2.3 | – | – | – | – | – | 2.5 | 23.3 |
| [113] | – | – | – | 2.2 | 7.9 | – | – | 79.9 | 3.3 | 6.4 | – | – |
| [114] | – | 22–29 | 13–17 | 20–24 | – | – | 2.7–8.9 | – | – | – | – | – |
| [115] | 51 | 20.1 | 17.6 | – | – | – | – | – | – | 5.4 | – | |
| [116] | – | – | 16 | 31 | 4.0 | – | 1.7–2.0 | – | – | – | 3–6 | – |
| [117] | 21.9 | 29.6 | 21.7 | 24.6 | 1.2 | – | – | – | – | – | – | – |
| [118] | 25.3 | 41.9 | 16.9 | – | 4.6 | – | – | – | – | – | – | – |
| [119] | 0.3 | 22.5 | – | 26.7 | 3.3 | – | 1 | – | – | – | – | – |
| [120] | 12 | 40 | 11.5 | 14.2 | 3.3 | - | 2.0 | - | - | - | 13 | - |
| [121] | 31–33 | 20–22 | 15–17 | 11–13.5 | 6–8 | |||||||
| [122] | 26.0 | 22.2 | – | 22.1 | 1.1 | – | – | – | – | – | – | – |
| [123] | 21.7 | 19.2 | 19.4 | 24.7 | 4.2 | – | – | – | – | – | – | – |
| Parameters | BSG SEM Image A | BSG SEM Image B |
|---|---|---|
| Pores (µm/µm2): | ||
| Pore count | 194 | 220 |
| Pore density [pore count/1000 µm2] | 154.18 | 227.22 |
| Pore area share [14,146] (fraction of FOV) | 0.500 | 0.608 |
| Diameters (equivalent) [µm] | p50 = 0.171; p90 = 0.546; max = 26.251 | p50 = 0.187; p90 = 0.708; max = 15.334 |
| Pore average surface area [µm2] | 3.240 | 2.674 |
| Pore Shape (dimensionless): | ||
| Extension (AR = major/minor) | p50 = 1.88; p90 = 3.17 | p50 = 1.98; p90 = 3.27 |
| Circularity = 4πA/P2 | p50 = 0.799; p10 = 0.353 | p50 = 0.723; p10 = 0.282 |
| Roundness = 4A/(π·major2) | p50 = 0.471 | p50 = 0.441 |
| Eccentricity | p50 = 0.846 | p50 = 0.863 |
| Solidity | p50 = 0.892 | p50 = 0.880 |
| Texture (GLCM, Dimensionless): | ||
| Contrast | 424.570 | 442.706 |
| Homogeneity | 0.156 | 0.142 |
| Energy | 0.014 | 0.013 |
| Correlation | 0.957 | 0.944 |
| Entropy | 17.235 | 17.052 |
| Edge Density (Canny) | 0.077 | 0.093 |
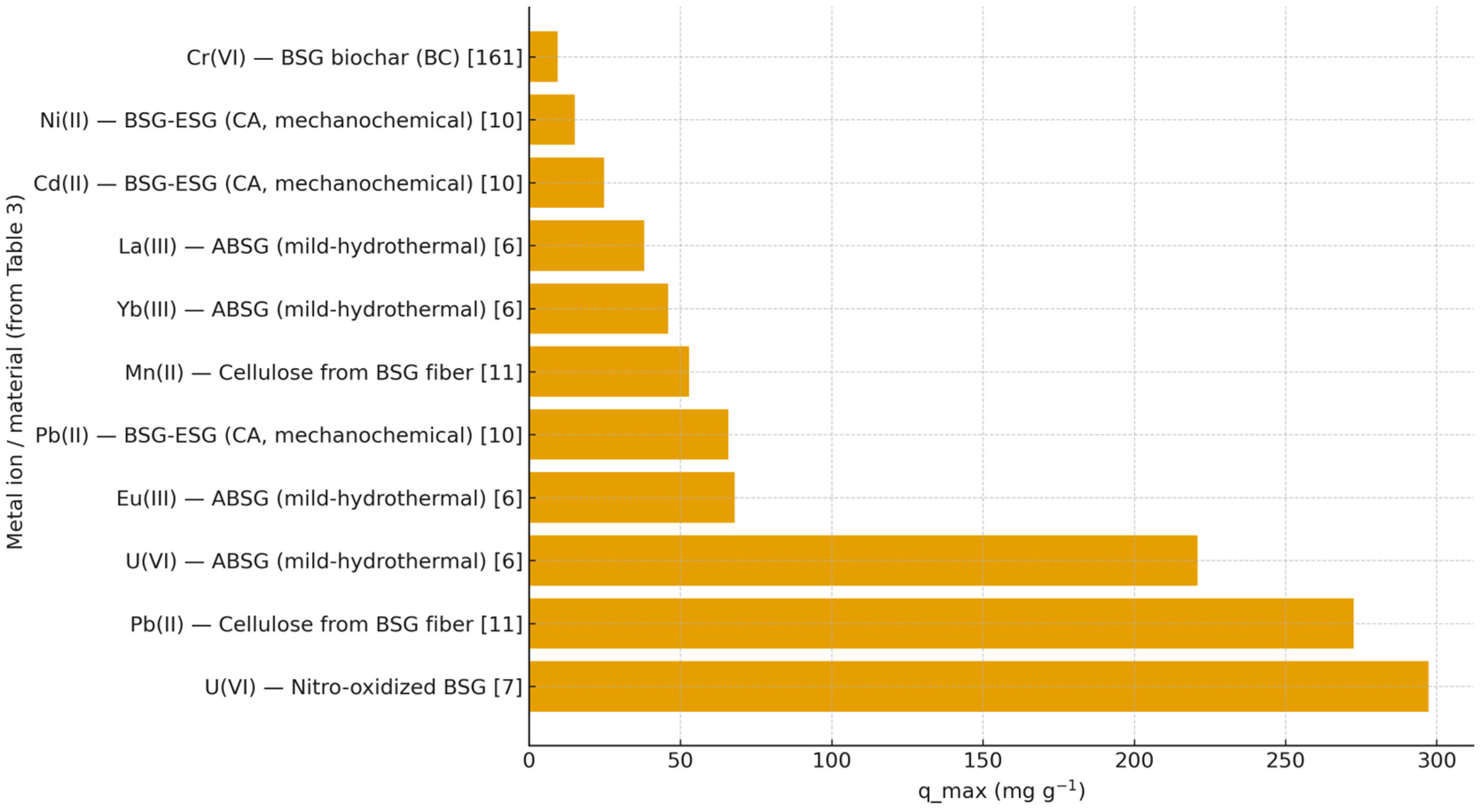
| Ref. | Sorbent BSG | Metals | pH/Matrix | qmax [mg/g] | Best-Fit Isotherm | Kinetics Model | Regeneration | Notes |
|---|---|---|---|---|---|---|---|---|
| [12] | Citric-acid mechanochemically esterified BSG | Pb(II), Cd(II), Ni(II) | pH 4.5 (acetate buffer) | Pb 65.83; Cd 24.72; Ni 15.11 (Langmuir) | Langmuir | ― | ― | Bottle-point isotherm; KL reported |
| [9] | Nitro-oxidized BSG | U(VI) (uranyl) | pH 4.7; batch; C0 = 900 mg/L | 297.3; fast uptake ~1 h | ― | Rapid uptake (∼1 h) | ≈60% capacity retained after 5 cycles | High –COOH (~1.3 mmol/g); works in simulated seawater |
| [8] | Mild-hydrothermally treated BSG (ABSG) | U(VI), La(III), Eu(III), Yb(III) | U: pH 4.7; REE: pH 5.7 | U 221; La 38; Eu 68; Yb 46 | Langmuir | ― | ― | Greener prep; Maillard-derived activation |
| [160] | BSG biochar | Co(II), Sr(II) | ― | C0 3.30–5.52; Sr 1.46–3.04 (298–318 K) | ― | ― | Reusability: C0 75.3→36.2%; Sr 93.6→32.7% over 4 cycles | Competitive ions reduce capacity |
| [162] | Unmodified BSG | Cr(III) | batch + expanded bed column | 16.7 (Langmuir) | Langmuir | Pseudo-second-order (initial) + intraparticle diffusion | Column: breakthrough 58 h; saturation 199 h | Alkali pretreatment not beneficial |
| [6] | Unmodified BSG | Cu(II) | pH 4.2; batch | 10.47 (Langmuir) | Langmuir | Pseudo-second-order | ― | Early demonstration of BSG as biosorbent |
| [159] | BSG biochar (ZnCl2-activated, 700 °C/30 min., 12.5%) | Cr(VI) | batch | 78.13 (Redlich–Peterson) | Redlich–Peterson | Pseudo-first-order; equilibrium <100 min. | ― | Optimized via factorial |
| [13] | BSG fiber-derived cellulose (TEMPO-oxidized) | Pb(II), Mn(II) | Contaminated tap water | Pb 272.5; Mn 52.9 (Langmuir) | Langmuir | ― | ― | Fabricated from fiber-rich product |
| [153] | Unmodified BSG | Fe(III), Mn(II), Cd(II), Ni(II) | Real waters (surface/groundwater) | Fe 11.2; Mn/Cd/Ni 5.5–11.2 | ― | ― | ― | Benchmarked vs guidelines |
| [161] | Surface ion-imprinted BSG (IIP-BSG) | U(VI) (uranyl) | pH 4.6; batch; high ionic strength tolerant | 165.7 (Sips) | Sips | Internal mass transfer controlled | ≈90% capacity retained after 5 cycles | High selectivity vs. Eu(III) (SU > 80%) |
| [7] | Citric acid esterified spent grain (ESG); DMF + NaH2PO2·H2O; 140 °C, 2 h | Cu(II), Pb(II), Zn(II), Cd(II), Ag(I) | pH 6; single-ion nitrates; batch; C0 = 10 mM; dose 2 g/L; 25 °C | Cu 104.13; Pb 293.30; Zn 232.10; Cd 296.61; Ag 205.80 (C0 = 10 mM) | ― | Equilibrium in ~30 min. (fast) | ― | pHPZC: ESG 3.0 vs. RSG 5.8; FTIR ester bands at 1726 and 1167 cm−1; capacities +43–94% vs. RSG |
| [163] | BSG biochar (BC) | Cr(VI) | pH 2 | 9.36 | Freundlich | Pseudo-second-order | ― | Chemisorption; film/external binding dominates |
| [163] | BSG biochar, KOH-activated (ABCK) | Cr(VI) | pH 2 | 8.94 | Freundlich | Pseudo-second-order | ― | Chemisorption |
| [163] | BSG biochar, H3PO4-activated (ABCP) | Cr(VI) | pH 2 | 7.1 | Freundlich | Pseudo-second-order | ― | Chemisorption; intraparticle (pore) diffusion contributes |
| [163] | Raw BSG | Cr(VI) | pH 2 | 7.02 | Freundlich | ― | ― | Not modeled in the paper; capacity reference for comparison |
| Ref. | Biosorbent & Modification | Metal | qmax [mg/g] | Key Conditions | Best-Fit Isotherm/Kinetics |
|---|---|---|---|---|---|
| [164] | Chitosan nanoparticles (cross-linked; nano-size) | Pb(II) | ≈398 | batch; aqueous; pH typically acidic–near-neutral | (reported in study; high monolayer capacity) |
| [165] | Chitosan–bentonite composites / beads | Pb(II) | 42.5–94.6 | batch; Pb(II) aq.; varied pH; composite ratios 90/10–50/50 | Langmuir & Freundlich both fit well |
| [166] | Orange peel (powder, uncarbonized; H2O2-treated / raw) | Cd(II) | up to 128.23 | pH ≈ 4.5; 318 K (batch) | Langmuir (best); kinetics: PFO in this study |
| [167] | Modified rice husk (chemical modification) | Cu(II) | ≈43.5 | 25 °C; pH ≈ 7; = 400 mg/L (batch) | Langmuir/Freundlich modeling performed |
| [168] | Banana-peel/alginate magnetic biobeads (nano-Fe3O4) | Cr(VI) | 370.4 | pH = 2; 25 °C; Langmuir analysis (batch) | Langmuir & Freundlich; kinetics: PSO |
| Ref. | Pollutant | Sorbent | Key Conditions | Isotherm Model, qmax [mg/g] | Kinetic Model | Key Findings |
|---|---|---|---|---|---|---|
| [170] | Congo Red (CR, azo dye) | Chemically pretreated BSG (BGPOH—NaOH; BGPH—H2SO4; BGPB—white-rot fungus) | Batch tests; CR 300 mg/L in some tests; ambient T; dosage varied (0.1–2.0 g) | Langmuir; qmax = 149 (BGPOH), 147 (BGPH), 117 (BGPB) | Pseudo-second order (better fit than PFO) | All pretreatments remove CR; BGPOH highest capacity; adsorption spontaneous; lower T favored |
| [14] | Methylene Blue (cationic dye) | Raw (unmodified) BSG | Batch; pH 7; 20–50 °C; 0.150 g BSG / 50 mL; C0 = 50–500 mg/L | Langmuir; qmax = 80.31 | Pseudo-second order | High removal in synthetic wastewater; favorable RL; good reusability (≤5 cycles) |
| [174] | Pymetrozine (pesticide) | BSG-derived biochar (slow pyrolysis 300–700 °C; best ~400 °C) | Batch; optimal pH ≈ 4; 25–45 °C; 70–80% removed in first 60 min. | Langmuir; qmax = 22.020 (25 °C), 26.032 (35 °C), 31.606 (45 °C) | ― | Adsorption endothermic; Langmuir/Freundlich fits with R ≈ 0.995–0.999 |
| [176] | 2-Chlorophenol (2-CP) | Fe3O4/activated hydrochar from BSG (FeOHC; FeOHC-C) | Batch; pH 3–6; 25 °C; also acts as a heterogeneous Fenton catalyst | Sips; equilibrium capacity ≈ 24.63 (FeOHC), 18.70 (FeOHC-C) | Elovich | Bifunctional: adsorption + Fenton oxidation; spontaneous/exothermic; good reusability |
| [14] | Methylene Blue (MB) | Raw BSG (sieved 53–500 µm) | Batch; 298.15 K; pH ≈ 6.8–7; C0 = 15–150 mg/L; dose = 10 g/L; t = 240 min. | Langmuir (qmax,cal = 37.45; KL = 0.025; R2 = 0.929); Freundlich (n = 1.16; R2 = 0.993) | Pseudo-second order (R2 ≈ 0.999) | 85–96% removal across C0; faster uptake for smaller particles; uptake with pH to ~8–10 |
| [14] | Congo Red (CR) | Raw BSG (sieved 53–500 µm) | Batch; 298.15 K; pH ≈ 6.8–7; C0 = 15–150 mg/L; dose = 10 g/L; t = 240 min. | Langmuir (qmax,cal = 19.65; KL = 0.114; R2 = 0.953); Freundlich (n = 1.46; R2 = 0.944) | Pseudo-second order (R2≈ 0.998–1.000) | 85–96% removal across C0; pH optimum ~7; higher dose removal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalak, T. Characterization and Sustainable Valorization of Brewers’ Spent Grain for Metal Ion and Organic Substance Removal. Sustainability 2025, 17, 9288. https://doi.org/10.3390/su17209288
Kalak T. Characterization and Sustainable Valorization of Brewers’ Spent Grain for Metal Ion and Organic Substance Removal. Sustainability. 2025; 17(20):9288. https://doi.org/10.3390/su17209288
Chicago/Turabian StyleKalak, Tomasz. 2025. "Characterization and Sustainable Valorization of Brewers’ Spent Grain for Metal Ion and Organic Substance Removal" Sustainability 17, no. 20: 9288. https://doi.org/10.3390/su17209288
APA StyleKalak, T. (2025). Characterization and Sustainable Valorization of Brewers’ Spent Grain for Metal Ion and Organic Substance Removal. Sustainability, 17(20), 9288. https://doi.org/10.3390/su17209288









