Groundwater–Surface Water Interactions and Pollution Assessment Using Hydrochemistry and Environmental Isotopes δ18O, δ2H, and 3H in Puebla Metropolitan Area, Mexico
Abstract
1. Introduction
2. Materials and Methods
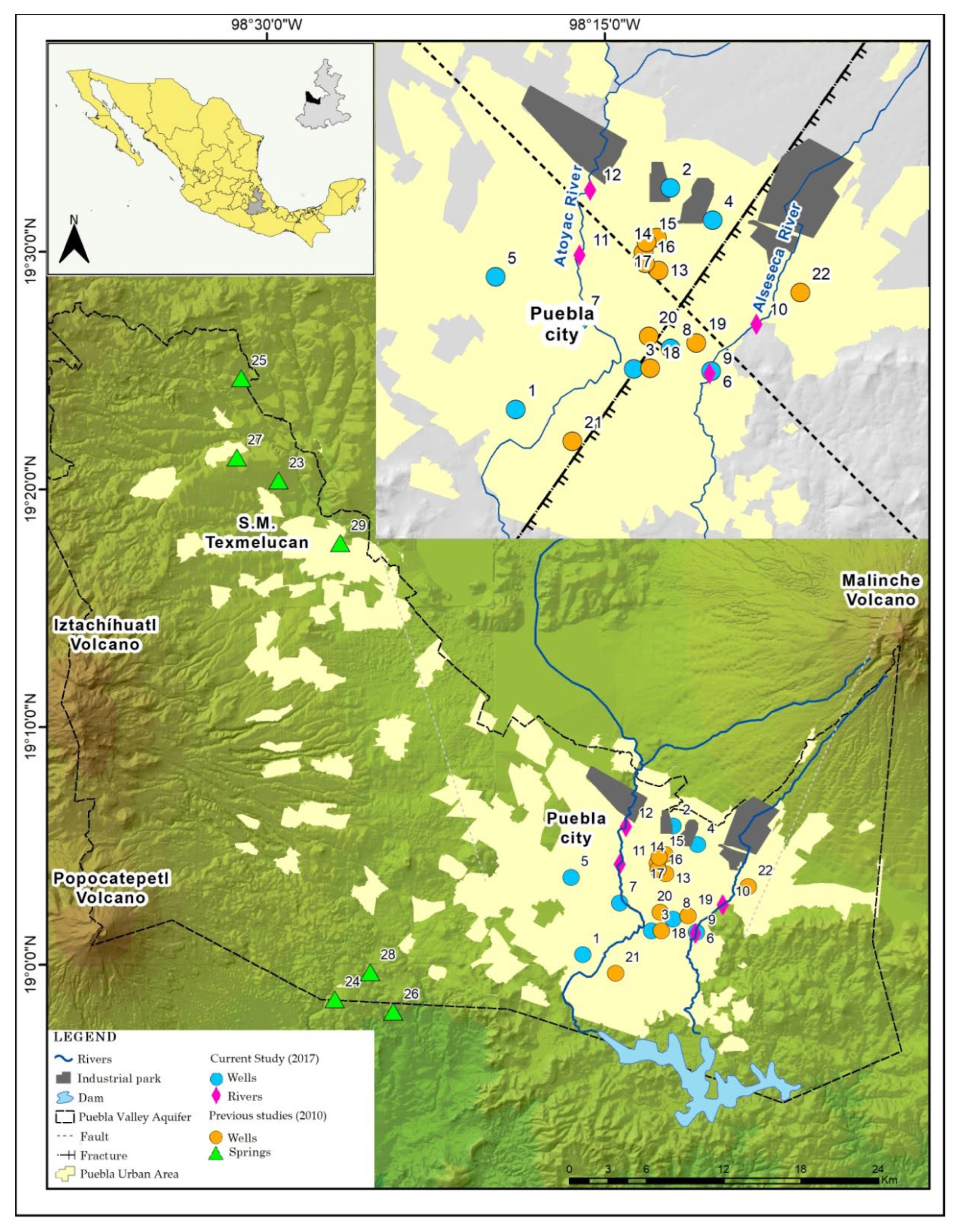
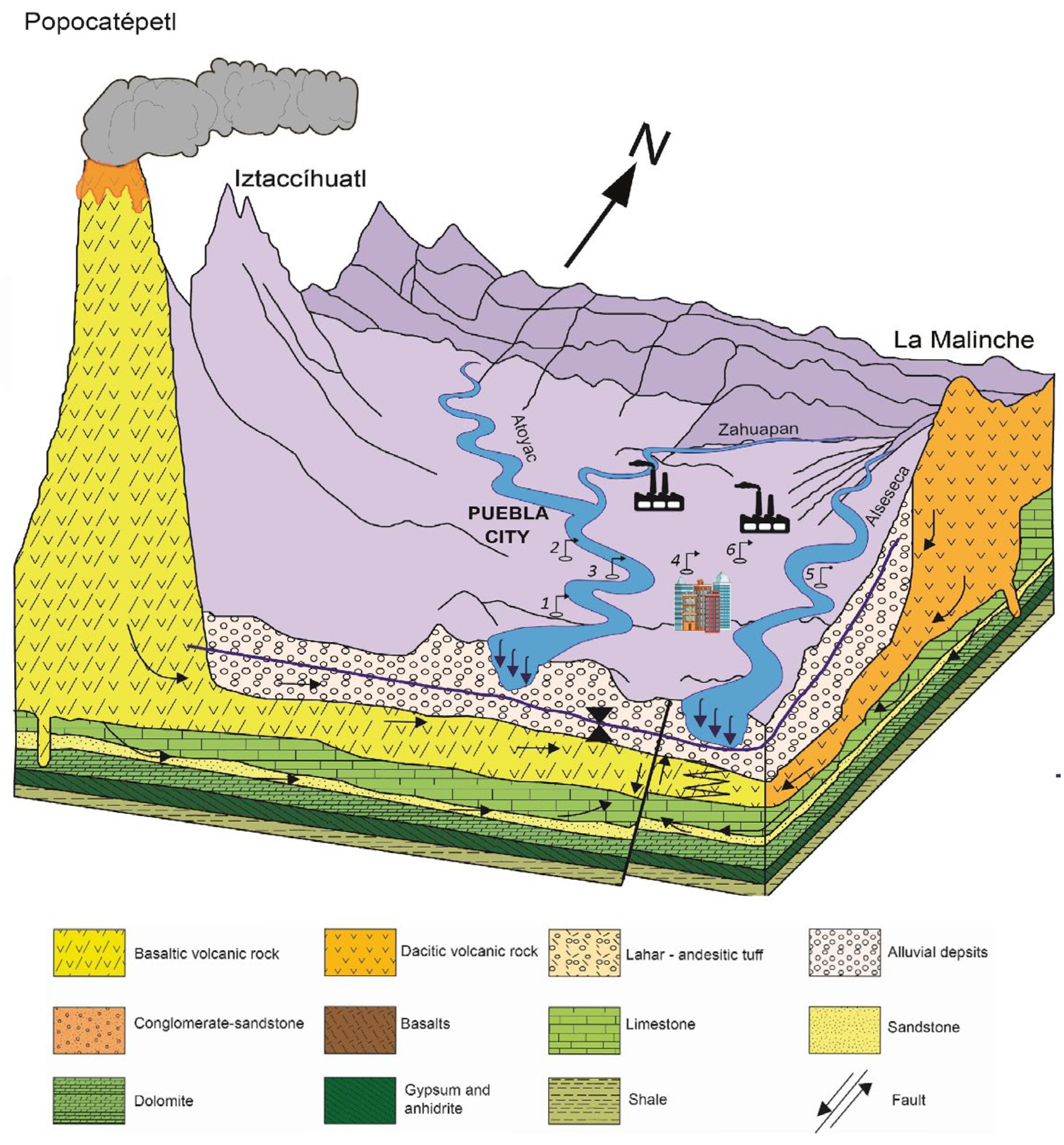
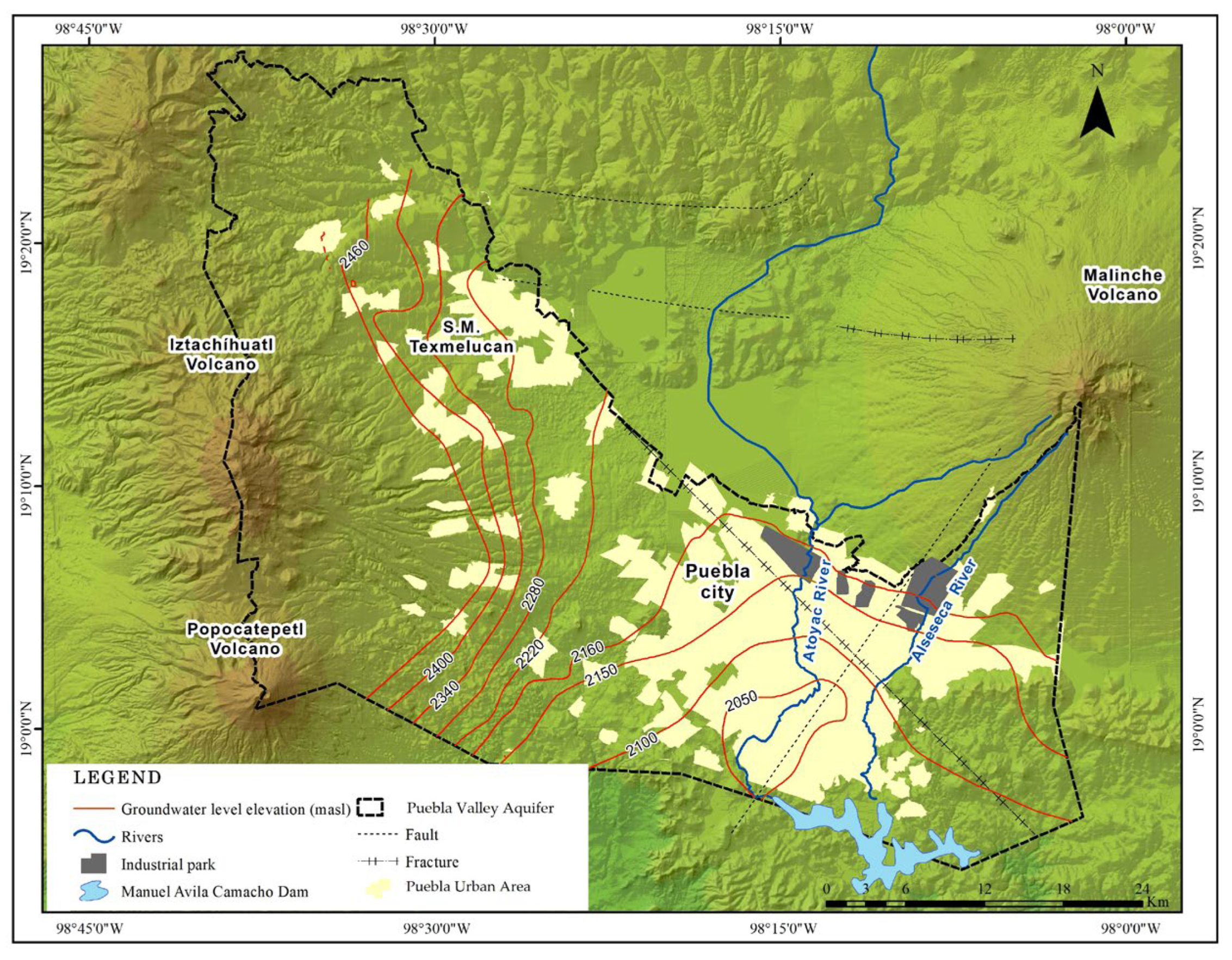
2.1. Study Area Description and Hydrogeological Settings
2.2. Sampling and Analysis
2.3. Isotopic Analysis Methods
2.4. Data Processing, Water Quality Assessment, and Spatial Analysis
3. Results and Discussion
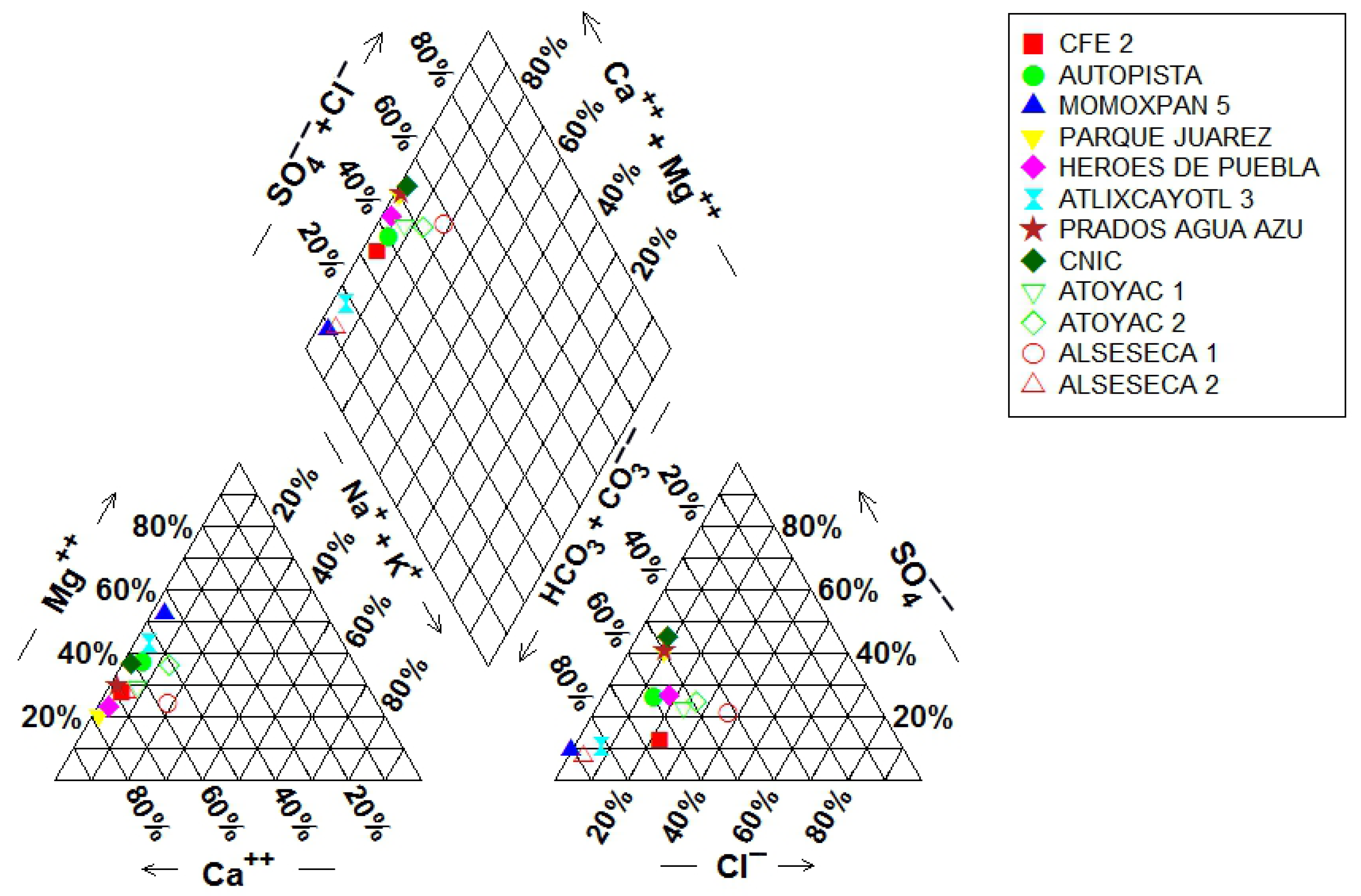
3.1. Water Quality and Hydrochemical Composition
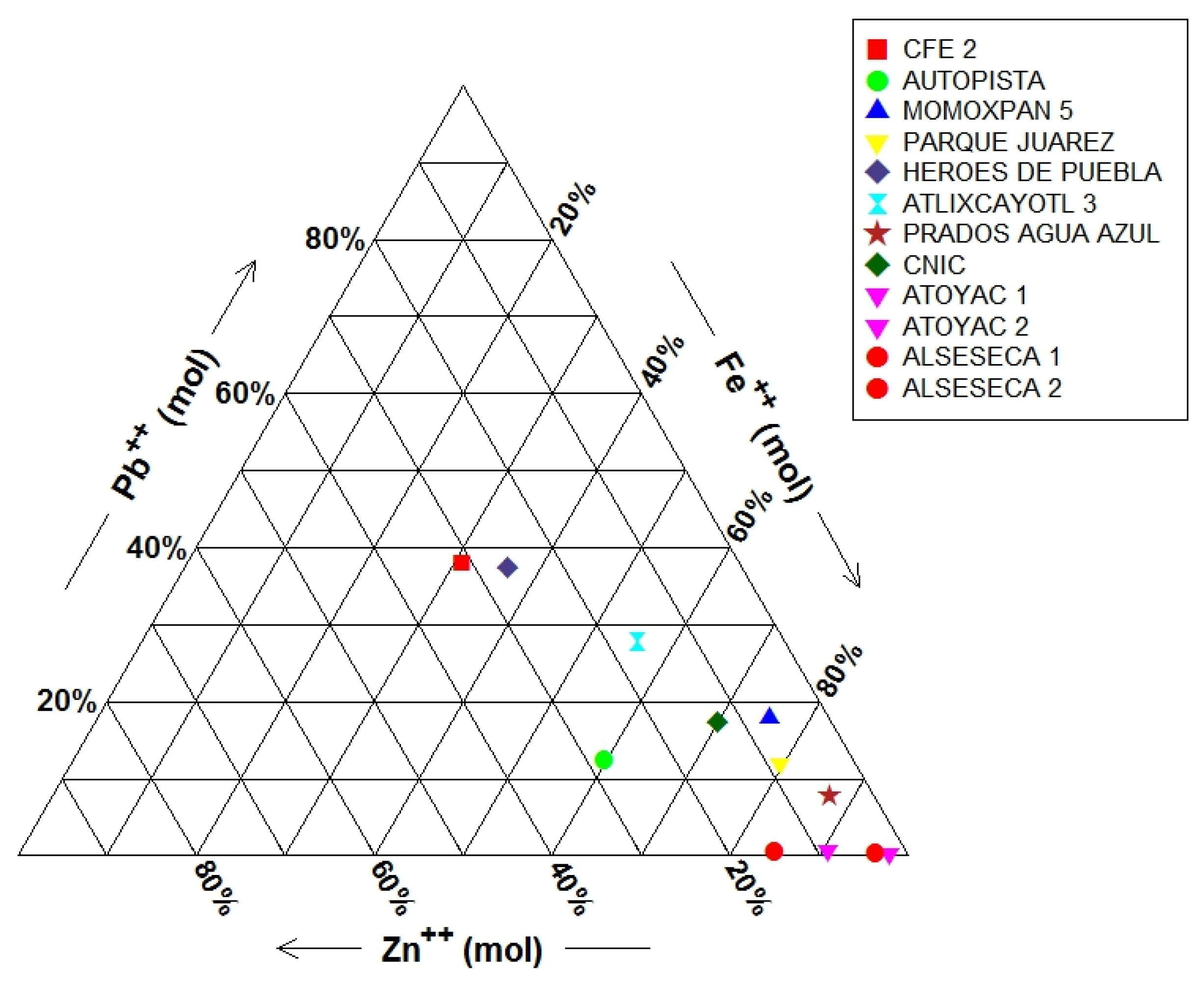
3.2. Potentially Toxic Elements (PTEs) and GW–SW Interactions
3.3. GW–SW Interaction and Residence Time
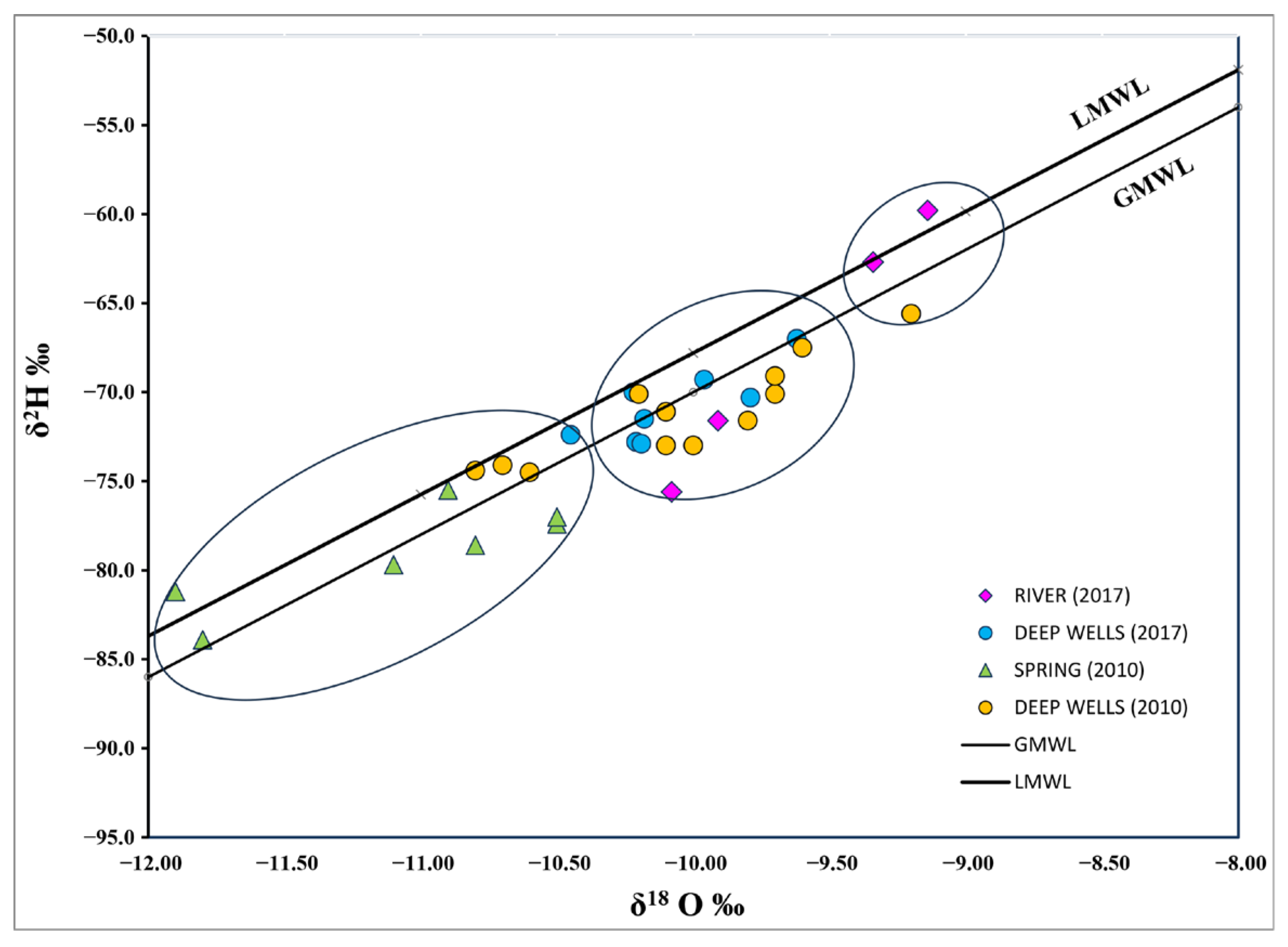
3.3.1. Isotopic Composition
3.3.2. Tritium (3H)
- ▪ Samples with values close to 0 TU indicate pre-bomb water, likely recharged before 1952, representing older groundwater with no tritium left due to radioactive decay.
- ▪ Tritium levels between 0.5 and 1 TU are typically interpreted as a mixture of submodern and modern water, with recharge likely occurring after 1970 but before the complete decay of bomb-derived tritium.
- ▪ Values around 1–2 TU suggest recharge during the late 1980s to early 2000s, while values above 2 TU, such as the 2.18 TU found in surface water (sample ID 10), are consistent with recent recharge events occurring between 1990 and 2017.
3.4. PTE Comparison Studies
| Al | Fe | As | Ba | Cr | Cu | Pb | Zn | Mn | Ni | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (µg L−1) | |||||||||||
| Local studies rivers | |||||||||||
| Pérez et al. [24] | Atoyac River | 0–21,160.0 | 770.0–16,500.0 | 0–7 | 0–89.0 | 0–70.0 | 0–156.0 | ||||
| Hernández et al. [28] | Atoyac River | 4.0–50.0 | 20.0–300.0 | 0.2–13.5 | 15.5–83.1 | 0.5–54.0 | 0.6–5.2 | 0.03–4.24 | 2.0–2.8 | 0.4–417.0 | 0.6–5.2 |
| Present study | Atoyac River | 958.0–2,545.0 | 3461.0–6779.0 | ND–50.0 | 111.0–159.0 | 5.0–10.0 | 67.0–74.0 | 30.0–57.0 | 160.0–393.0 | 355.0–420.0 | 24.0–26.0 |
| Alseseca River | 373.0–4576.0 | 1688.0–3153.0 | 115–530 | 4.0–6.0 | 56.0–76.0 | 28.0–34.0 | 134.0–221.0 | 364.0–838.0 | 12.0–30.0 | ||
| Worldwide studies in rivers | |||||||||||
| Mitra and Bianchi [74] Goolsby et al. [75] Bussan et al. [76] | Mississippi River (USA) | 4.9–27.2 | 3.6–28.9 | 26.0–98.0 | 0.2–27.0 | 0.4–1.2 | 0.04–0.024 | 0.34–1.7 | 1.8–10.0 | ||
| Hou et al. [65] | Yellow River (China) | 0.02–0.1 | 0.03–0.06 | 3.9–6.2 | 20.0 | 2.0–2.5 | 20.0–60.0 | 5.0–47.0 | |||
| Bilgin and Konanç [68] | Coruh River (Turkey) | 0–12,449.0 | ND–6.16 | 2.2–1427.1 | ND–404.5 | ND–914.0 | ND–674.0 | ||||
| Prasad et al. [66]. | Upper Ganga River (India) | 1476.0 | ND–33.0 | 0.0–5.0 | ND–289 | ND–140.0 | |||||
| Avigliano and Schenone [77] | Major River (Argentina) | ND-172 | ND-3.1 | ND-1.2 | ND-220.0 | ND-220.0 | ND-0.16 | ||||
| Canovas et al. [78] | Tinto River (Spain) | ND-151,000.0 | ND-18,900.0 | ND-130.0 | ND-26,000.0 | ND-8000.0 | ND-170.0 | ||||
| Singh and Kumar [67] | Ajay River (India) | ND-1951.0 | ND-720.0 | ND-530.0 | ND-242.0 | ND-160.0 | ND-17.0 | ||||
| Al | Fe | As | Ba | Cr | Cu | Pb | Zn | Mn | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (µg L−1) | ||||||||||
| Mexican studies | ||||||||||
| Fonseca-Montes de Oca et al. [70] | Toluca Valley | 20–268 | 14.0–20.0 | 1.4–1.2 | 60.0–459.0 | 7.0–150.0 | ||||
| Barats et al. [73] | Sierra Huautla | 1.75–844.0 | 1.8–30.4 | 3.4–872.0 | 0.7–14.6 | 0.021–2.4 | 5.5–563.0 | 0.2–504.0 | ||
| Daesslé et al. [79] | Guadalupe Valley | 0.5–100.0 | 0.9–600.0 | 0.23–100.0 | 4.8–140 | 0.05–2.0 | 0.13–15.0 | 0.005–1.0 | 0.5–81.0 | 0.5–740.0 |
| Guédron et al. [80] | Mezquital Valley (deep wells) | 120.0 | 17.0 | 1.0 | 51.0 | 380.0 | 8.0 | |||
| Local studies | ||||||||||
| Salcedo et al. [27,38] | Puebla Valley | 0–90.0 | 30.0–350.0 | 11,232.0 | 0–10.0 | 0–1130.0 | ||||
| Present study | Puebla Valley | - | 6.0–81.0 | 35.0–69.0 | 23.0–292.0 | 2.1–5.0 | 11.0–12.0 | 23.0–32.0 | 4.0–19.0 | 1–1037.0 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTE | Potentially Toxic Elements |
| PMA | Puebla Metropolitan Area |
References
- Fakhari, M.; Raymond, J.; Martel, R. Complementarity of multiple in-situ techniques for spatiotemporal assessment of groundwater/surface-water exchanges. Hydrogeol. J. 2025, 33, 219–235. [Google Scholar] [CrossRef]
- Lin, J.; Ma, R.; Hu, Y.; Sun, Z.; Wang, Y.; McCarter, C. Groundwater sustainability and groundwater/surface-water interaction in arid Dunhuang basin, northwest China. Hydrogeol. J. 2018, 26, 1559–1572. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, G.; Liao, F.; Yang, N.; Jiang, W.; Guo, L.; Shi, Z. Groundwater-surface water interactions derived by hydrochemical and isotopic (222Rn, deuterium, oxygen-18) tracers in the Nomhon area, Qaidam Basin, NW China. J. Hydrol. 2018, 565, 650–661. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; He, X. Interactions between surface water and groundwater in selected tributaries of the Wei River (China) revealed by hydrochemistry and stable isotopes. Hum. Ecol. Risk Assess. 2021, 28, 79–99. [Google Scholar] [CrossRef]
- McArthur, J.M.; Sikdar, P.K.; Hoque, M.A.; Ghosal, U. Wastewater impacts on groundwater: Cl/Br ratios and implications for arsenic pollution of groundwater in the Bengal basin and red river basin, Vietnam. Sci. Total Environ. 2012, 437, 390–402. [Google Scholar] [CrossRef]
- Bayou, W.T.; Mohammed, M.; Ayenew, T. Hydrochemistry and isotope hydrology of groundwater and surface water in the Sor and Gebba watershed, southwestern Ethiopia. Environ. Earth Sci. 2024, 83, 316. [Google Scholar] [CrossRef]
- Li, Z.; Fang, Y.; Meng, B.; Guo, H.; Du, X. Identification of Groundwater–Surface Water Interaction Using Combined Hydraulic and Hydrogeochemical Methods. Water 2024, 16, 2777. [Google Scholar] [CrossRef]
- Irvine, D.J.; Singha, K.; Kurylyk, B.L.; Briggs, M.A.; Sebastian, Y.; Tait, D.R.; Helton, A.M. Groundwater–Surface water interactions research: Past trends and future directions. J. Hydrol. 2024, 644, 132061. [Google Scholar] [CrossRef]
- Nguyen, T.K.H.; Nuottimäki, K.; Jarva, J. Use of a groundwater model to evaluate groundwater-surface water interaction at a riverbank filtration site: A case study in Binh Dinh, Vietnam. Sustain. Water Resour. Manag. 2025, 11, 14. [Google Scholar] [CrossRef]
- Banda, K.; Mulema, M.; Chomba, I.; Chomba, M.; Levy, J.; Nyambe, I. Investigating groundwater and surface water interactions using remote sensing, hydrochemistry, and stable isotopes in the Barotse Floodplain, Zambia. Geol. Ecol. Landsc. 2023, 9, 424–439. [Google Scholar] [CrossRef]
- Zhang, B.; Song, X.; Zhang, Y.; Ma, Y.; Tang, C.; Yang, L.; Wang, Z. The interaction between surface water and groundwater and its effect on water quality in the Second Songhua River basin, Northeast China. J. Earth Syst. Sci. 2016, 25, 1495–1507. [Google Scholar] [CrossRef]
- Oyarzún, R.; Jofré, E.; Morales, P.; Maturana, H.; Oyarzún, J.; Kretschmer, N.; Aravena, R. A hydrogeochemistry and isotopic approach for the assessment of surface water–groundwater dynamics in an arid basin: The Limarí watershed, North-Central Chile. Environ. Earth. Sci. 2015, 73, 39–55. [Google Scholar] [CrossRef]
- Kim, J.; Jeen, S.-W.; Lee, J.; Ko, K.-S.; Koh, D.-C.; Kim, W.; Jo, H. Evaluation of Temporal Contribution of Groundwater to a Small Lake through Analyses of Water Quantity and Quality. Water 2020, 12, 2879. [Google Scholar] [CrossRef]
- Martinez, J.L.; Raiber, M.; Cox, M.E. Assessment of groundwater–surface water interaction using long-term hydrochemical data and isotope hydrology: Headwaters of the Condamine River, southeast Queensland, Australia. Sci. Total Environ. 2015, 536, 499–516. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Sheng, Y.; Han, D.; Shan, K.; Zhu, Z.; Dai, X. Investigating Groundwater–Surface Water Interactions and Transformations in a Typical Dry–Hot Valley Through Environmental Isotopes Analysis. Water 2025, 17, 775. [Google Scholar] [CrossRef]
- Négrel, P.; Petelet-Giraud, E.; Barbier, J.; Gautier, E. Surface water–groundwater interactions in an alluvial plain: Chemical and isotopic systematics. J. Hydrol. 2003, 277, 248–267. [Google Scholar] [CrossRef]
- Rodgers, P.; Soulsby, C.; Petry, J.; Malcolm, I.; Gibbins, C.; Dunn, S. Groundwater–surface-water interactions in a braided river: A tracer-based assessment. Hydrol. Process. 2004, 18, 1315–1332. [Google Scholar] [CrossRef]
- Khan, N.; Khan, H.H.; Siraj, G. Temporal variability in groundwater-surface water interactions using hydraulic and hydrochemical observations in a segment of Kali Nadi, Aligarh, UP, India. Discov. Geosci. 2025, 3, 69. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, W.; Zhao, M.; Ma, Z.; Lu, C.; Li, Y. Interaction between Surface Water and Groundwater in Yinchuan Plain. Water 2020, 12, 2635. [Google Scholar] [CrossRef]
- Clark, I. Groundwater Geochemistry and Isotopes; Taylor and Francis Ltd.: New York, NY, USA, 2015. [Google Scholar]
- Yu, X.; Yuan, X.; Guo, H.; Zhang, Y.; Cao, H.; Luo, T.; Gong, Z.; Huang, H. Coupling Hydrochemistry and Stable Isotopes (δ2H, δ18O and 87Sr/86Sr) to Identify the Major Factors Affecting the Hydrochemical Process of Groundwater and Surface Water in the Lower Reaches of the Yarlung-Zangbo River, Southern Tibet, Southwestern China. Water 2022, 14, 3906. [Google Scholar] [CrossRef]
- Terwey, J.L. Isotopes in Groundwater Hydrology. In Harare Symposium: Challenges in African Hydrology and Water Resources; IAHS: Amsterdam, The Netherlands, 1984; pp. 155–160. [Google Scholar]
- Teng, Y.; Hu, B.; Zheng, J.; Wang, J.; Zhai, Y.; Zhu, C. Water quality responses to the interaction between surface water and groundwater along the Songhua River, NE China. Hydrogeol. J. 2018, 26, 1591–1607. [Google Scholar] [CrossRef]
- Pérez Castresana, G.; Tamariz Flores, V.; López Reyes, L.; Hernández Aldana, F.; Castelán Vega, R.; Morán Perales, J.; Handal Silva, A. Atoyac River pollution in the Metropolitan Area of Puebla, Mexico. Water 2018, 10, 267. [Google Scholar] [CrossRef]
- Rodriguez-Espinosa, P.F.; Jonathan, M.P.; Morales-García, S.S.; Villegas, L.E.C.; Martínez-Tavera, E.; Muñoz-Sevilla, N.P.; Cardona, M.A. Met. Metal enrichment of soils following the April 2012–2013 eruptive activity of the Popocatépetl volcano, Puebla, Mexico. Environ. Monit. Assess. 2015, 187, 717. [Google Scholar] [CrossRef] [PubMed]
- Salcedo Sánchez, E.R.; Garrido Hoyos, S.E.; Esteller, M.V.; Martínez Morales, M.; Ocampo Astudillo, A. Hydrogeochemistry and water-rock interactions in the urban area of Puebla Valley aquifer (Mexico). J. Geochem. Explor. 2017, 181, 219–235. [Google Scholar] [CrossRef]
- Salcedo-Sánchez, E.R.; Ocampo-Astudillo, A.; Garrido-Hoyos, S.E.; Martínez-Morales, M. Effects on groundwater quality of the urban area of Puebla aquifer. In Groundwater and Human Development; Thangarajan, M., Singh, V.P., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 201–214. [Google Scholar] [CrossRef]
- Hernández, A.G.; Martínez, E.; Rodríguez, P.F.; Mendoza, J.A.; Tabla, J.; Escobedo, D.C.; Sujitha, S.B. Detection, provenance and associated environmental risks of water quality pollutants during anomaly events in river Atoyac, Central Mexico: A real-time Monitoring Approach. Sci. Total Environ. 2019, 669, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Espinosa, P.F.; Sabarathinam, C.; Ochoa-Guerrero, K.M.; Martínez-Tavera, E.; Panda, B. Geochemical evolution and boron sources of the groundwater affected by urban and volcanic activities of Puebla Valley, South Central Mexico. J. Hydrol. 2020, 584, 124613. [Google Scholar] [CrossRef]
- Garfias, J.; Arroyo, N.; Aravena, R. Hydrochemistry and origins of mineralized waters in the Puebla aquifer system, Mexico. Environ. Earth Sci. 2010, 59, 1789–1805. [Google Scholar] [CrossRef]
- Dueñas-Moreno, J.; Mora, A.; Narvaez-Montoya, C.; Mahlknecht, J. Trace elements and heavy metal(loid)s triggering ecological risks in a heavily polluted river-reservoir system of central Mexico: Probabilistic approaches. Environ. Res. 2024, 262, 119937. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, P.F.; Shruti, V.C.; Jonathan, M.P.; Martinez-Tavera, E. Metal concentrations and their potential ecological risks in fluvial sediments of Atoyac river basin, Central Mexico: Volcanic and anthropogenic influences. Ecotoxicol. Environ. Saf. 2018, 148, 1020–1033. [Google Scholar] [CrossRef]
- Shruti, V.C.; Rodríguez-Espinosa, P.F.; Martinez-Tavera, E.; Hernández-Gonzalez, D. Metal concentrations in recent ash fall of Popocatépetl volcano 2016, central Mexico: Is human health at risk? Ecotoxicol. Environ. Saf. 2018, 162, 324–333. [Google Scholar] [CrossRef]
- Yan, N.; Liu, W.; Xie, H.; Gao, L.; Han, Y.; Wang, M.; Li, H. Distribution assessment of heavy metals in the surface sediment of the Yellow River China. J. Environ. Sci. 2016, 39, 45–51. [Google Scholar] [CrossRef]
- CONAGUA. Estadísticas del Agua en México; Comisión Nacional del Agua: Coyoacán, México, 2017.
- Weatherbase. Historical Weather for Puebla. Available online: https://www.weatherbase.com (accessed on 27 May 2021).
- Martinez-Tavera, E.; Rodriguez-Espinosa, P.F.; Shruti, V.C.; Sujitha, S.B.; Morales-Garcia, S.; Muñoz-Sevilla, N.P. Monitoring the seasonal dynamics of physicochemical parameters from Atoyac River basin (Puebla), Central Mexico: Multivariate approach. Environ. Earth Sci. 2017, 76, 95. [Google Scholar] [CrossRef]
- Salcedo-Sánchez, E.R.; Esteller, M.V.; Garrido Hoyos, S.E.; Martínez-Morales, M. Groundwater optimization model for sustainable management of the valley of Puebla aquifer, Mexico. Environ. Earth Sci. 2013, 70, 337–351. [Google Scholar] [CrossRef]
- DOF. Norma Oficial Mexicana PROY-NOM-230-SSA1-2002, Salud Ambiental. Diario Oficial de la Federación. 2003. Available online: https://www.gob.mx/cms/uploads/attachment/file/110536/NOM_230_SSA1_2002.pdf (accessed on 18 March 2022).
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA/AWWA/WEF: Washington, DC, USA, 2005. [Google Scholar]
- DOF. Norma Oficial Mexicana NOM-127-SSA1-2021, Agua Para Uso y Consumo Humano. Límites Permisibles de la Calidad del Agua. Diario Oficial de la Federación. 2022. Available online: https://www.dof.gob.mx/nota_detalle_popup.php?codigo=5650705 (accessed on 18 April 2025).
- WHO. Guidelines for Drinking-Water Quality. World Health Organization: Geneva, Switzerland, 2001; Volume 4. [Google Scholar]
- DOF. Acuerdo por el que se Establecen los Criterios Ecológicos de Calidad del Agua, CE-CCA-001/89. Diario Oficial de la Federación, 13 de Diciembre de 1989. 1989. Available online: https://www.dof.gob.mx/index.php?year=1989&month=12&day=13 (accessed on 2 May 2025).
- Craig, H. Standard for reporting concentrations of deuterium and oxygen-18 in natural water. Science 1961, 133, 1833–1834. [Google Scholar] [CrossRef] [PubMed]
- Aqueous Solutions, L.C.C. Geochemist’s Workbench, Version 11; Department of Geology at the University of Illinois Urbana-Champaign: Urbana, IL, USA, 2016. [Google Scholar]
- Masuda, H. Arsenic cycling in the Earth’s crust and hydrosphere: Interaction between naturally occurring arsenic and human activities. Prog. Earth Planet Sci. 2018, 5, 68. [Google Scholar] [CrossRef]
- USEPA. In Situ Treatment of Soil Groundwater Contaminated with Chromium Technical Resource Guide; Office of Research Development, US Environmental Protection Agency: Washington, DC, USA, 2000.
- Katz, S.A.; Salem, H. The Biological and Environmental Chemistry of Chromium. VCH: New York, NY, USA, 1994. [Google Scholar]
- USEPA. Toxicological Review of Hexavalent Chromium; US Environmental Protection Agency: Washington, DC, USA, 1998.
- USEPA. Guidance for Reporting Releases and Other Waste Management Quantities of Toxic Chemicals; US Environmental Protection Agency: Washington, DC, USA, 2001.
- Sankhla, M.S.; Kumar, R.; Prasad, L. Zinc Impurity in Drinking Water and Its Toxic Effect on Human Health. Indian Internet J. Forensic Med. Toxicol. 2019, 17, 84–87. [Google Scholar] [CrossRef]
- Velázquez, M.A.; Pimentel, J.L.; Ortega, M. Estudio de la distribución de boro en fuentes de agua de la cuenca del río Duero, México, utilizando análisis estadístico multivariado. Rev. Int. Contam. Ambient. 2011, 27, 19–30. [Google Scholar]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; US Geological Survey Water-Supply Paper 2254; US Government Printing Office: Washington, DC, USA, 1992.
- Copat, C.; Fiore, M.; Grasso, A.; Arena, G.; Dimartino, A. Boron Levels in Drinking Water Sources from the Volcanic Area of Sicily (South Italy): Risk Evaluation of Developing Chronic Systemic Effects. Ann. Environ. Sci. Toxicol. 2016, 1, 008–012. [Google Scholar] [CrossRef]
- Parent, L.; Campbell, P.G.C. Aluminium bioavailability to the green alga Chlorella Pyrenoidosa in acidified synthetic soft water. Environ. Toxicol. Chem. 1994, 13, 587–598. [Google Scholar] [CrossRef]
- Sacks, L.A.; Herman, J.S.; Kauffman, S.J. Controls on high sulfate concentrations in the Upper Floridan aquifer in southwest Florida. Water Resour. Res. 1995, 31, 2541–2551. [Google Scholar] [CrossRef]
- Cortes, A.; Farvolden, R.N. Isotope studies of precipitation and groundwater in the Sierra de las Cruces, Mexico. J. Hydrol. 1988, 107, 147–153. [Google Scholar] [CrossRef]
- Petelet-Giraud, E.; Négrel, P.; Gourcy, L.; Schmidt, C.; Schirmer, M. Geochemical and isotopic constraints on groundwater-surface water interactions in a highly anthropized site. The Wolfen/Bitterfeld megasite (Mulde subcatchment, Germany). Environ. Pollut. 2007, 148, 707–717. [Google Scholar] [CrossRef]
- Yang, L.; Song, X.; Zhang, Y.; Han, D.; Zhang, B.; Long, D. Characterizing interactions between surface water and groundwater in the Jialu river basin using major ion chemistry and stable isotopes. Hydrol. Earth Syst. Sci. 2012, 16, 4265–4277. [Google Scholar] [CrossRef]
- Ayadi, R.; Trabelsi, R.; Zouari, K.; Saibi, H.; Itoi, R.; Khanfir, H. Hydrogeological and hydrochemical investigation of groundwater using environmental isotopes (18O, 2H, 3H, 14C) and chemical tracers: A case study of the intermediate aquifer, Sfax, Southeastern Tunisia. Hydrogeol. J. 2018, 26, 983–1007. [Google Scholar] [CrossRef]
- Flores Ronces, J.A.; Salcedo Sánchez, E.R.S.; Martínez Morales, M.; Esquivel Martínez, J.M.; Talavera Mendoza, O.; Esteller Alberich, M.V. Trace Metals and Metalloids Present in Springwater of a Mining Area: Assessment Based on Chemical and Isotopic Data (δ2H, δ18O, 3H and 87Sr/86Sr). Water 2023, 15, 1917. [Google Scholar] [CrossRef]
- Horst, A.; Mahlknecht, J.; Merkel, B.J.; Aravena, R.; Ramos-Arroyo, Y.R. Evaluation of the recharge processes and impacts of irrigation on groundwater using CFCs and radiogenic isotopes in the Silao-Romita basin, Mexico. Hydrogeol. J. 2008, 16, 1601–1614. [Google Scholar] [CrossRef]
- Mahlknecht, J.; Daesslé, L.W.; Esteller, M.V.; Torres-Martinez, J.A.; Mora, A. Groundwater flow processes and human impact along the arid US-Mexican border, evidenced by environmental tracers: The case of Tecate, Baja California. Int. J. Environ. Res. Public Health 2018, 15, 887. [Google Scholar] [CrossRef]
- Kurukulasuriya, D.; Howcroft, W.; Moon, E.; Meredith, K.; Timms, W. Selecting Environmental Water Tracers to Understand Groundwater around Mines: Opportunities and Limitations. Mine Water Environ. 2022, 41, 357–369. [Google Scholar] [CrossRef]
- Hou, W.; Sun, S.; Wang, M.; Li, X.; Zhang, N.; Xin, X.; Sun, L.; Li, W.; Jia, R. Assessing water quality of five typical reservoirs in lower reaches of Yellow River, China: Using a water quality index method. Ecol. Indic. 2016, 61, 309–316. [Google Scholar] [CrossRef]
- Prasad, M.B.K.; Ramanathan, A.L.; Shrivastav, S.K.; Anshumali, S.R. Metal fractionation studies in surficial and core sediments in the Achankovil River basin in India. Environ. Monit. Assess. 2006, 121, 77e102. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.K.; Kumar, B. Pathways of heavy metals contamination and associated human health risk in Ajay River Basin, India. Chemosphere 2017, 174, 183–199. [Google Scholar] [CrossRef]
- Bilgin, A.; Konanç, M.U. Evaluation of surface water quality and heavy metal pollution of the Coruh River Basin (Turkey) by multivariate statistical methods. Environ. Earth Sci. 2016, 75, 1029. [Google Scholar] [CrossRef]
- Martínez-Tabche, L.; Cruz-Jiménez, G.; Castañeda-Chávez, M.d.R.; Álvarez-García, D. Evaluación de la toxicidad de los sedimentos del curso alto del río Lerma, México. Rev. Int. Contam. Ambient. 2017, 33, 65–74. [Google Scholar] [CrossRef][Green Version]
- Fonseca-Montes de Oca, R.M.G.; Ramos-Leal, J.A.; Solache-Ríos, M.; Martínez-Miranda, V.; Fuentes-Rivas, R.M. Modification of the relative abundance of constituents dissolved in drinking water caused by organic pollution: A case of the Toluca Valley, Mexico. Water Air Soil Pollut. 2019, 230, 4210. [Google Scholar] [CrossRef]
- Peña-Montenegro, T.D.; Rodríguez-Espinosa, P.F.; Gómez-Salazar, S. Heavy metal contamination of soils and water in the Tula Valley, Mexico. Rev. Int. Contam. Ambient. 2008, 24, 175–182. [Google Scholar]
- Gutiérrez, M.; Cabrera, A.; Arriaga, M.E. Heavy metal pollution in the Nexapa River, Puebla, Mexico. Environ. Geol. 2009, 58, 611–620. [Google Scholar]
- Barats, A.; Renac, C.; Orani, A.M.; Durrieu, G.; Saint Martin, H.; Vicenta Esteller, M.; Garrido Hoyos, S. Tracing source and mobility of arsenic and trace elements in a hydrosystem impacted by past mining activities (Morelos state, Mexico). Sci. Total Environ. 2020, 712, 135565. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Bianchi, T.S. A preliminary assessment of polycyclic aromatic hydrocarbon distributions in the lower Mississippi River and Gulf of Mexico. Mar. Chem. 2003, 82, 273–288. [Google Scholar] [CrossRef]
- Goolsby, D.A.; Battaglin, W.A.; Lawrence, G.B.; Artz, R.S.; Aulenbach, B.T.; Hooper, R.P.; Keeney, D.R.; Stensland, G.J. Flux and Sources of Nutrients in the Mississippi–Atchafalaya River Basin: Topic 3 Report for the Integrated Assessment on Hypoxia in the Gulf of Mexico; NOAA Coastal Ocean Program Decision Analysis Series No. 17; NOAA: Silver Spring, MD, USA, 2000. Available online: https://www.noaa.gov/ (accessed on 2 May 2025).
- Bussan, D.D.; Ochs, C.A.; Jackson, C.R.; Anumol, T.; Snyder, S.A.; Cizdziel, J.V. Concentrations of select dissolved trace elements and anthropogenic organic compounds in the Mississippi River and major tributaries during the summer of 2012 and 2013. Environ. Monit. Assess. 2017, 189, 73. [Google Scholar] [CrossRef] [PubMed]
- Avigliano, E.; Lozano, C.; Plá, R.R.; Volpedo, A.V. Toxic element determination in fish from Paraná River Delta (Argentina) by neutron activation analysis: Tissue distribution and accumulation and health risk assessment by direct consumption. J. Food Compos. Anal. 2016, 54, 27–36. [Google Scholar] [CrossRef]
- Canovas, C.R.; Olías, M.; Nieto, J.M.; Galván, L. Wash-out processes of evaporitic sulfate salts in the Tinto river: Hydrogeochemical evolution and environmental impact. Appl. Geochem. 2010, 25, 288–301. [Google Scholar] [CrossRef]
- Daesslé, L.W.; Andrade-Tafoya, P.D.; Lafarga-Moreno, J.; Mahlknecht, J.; van Geldern, R.; Beramendi-Orosco, L.E.; Barth, J.A.C. Groundwater recharge sites and pollution sources in the wine-producing Guadalupe Valley (Mexico): Restrictions and mixing prior to transfer of reclaimed water from the US-méxico border. Sci. Total Environ. 2020, 713, 136715. [Google Scholar] [CrossRef] [PubMed]
- Guédron, S.; Duwig, C.; Prado, B.L.; Point, D.; Flores, M.G.; Siebe, C. (Methyl) mercury, arsenic, and lead contamination of the World’s largest wastewater irrigation system: The Mezquital Valley (Hidalgo State—Mexico). Water Air Soil Pollut. 2014, 225, 2045. [Google Scholar] [CrossRef]


| ID | Temp | pH | ORP | EC | FC | CBE | DO | TH | TDS | Ca2+ | K+ | Mg 2+ | Na+ | F− | HCO3− | Cl− | NO3− | P | SO42− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | mV | μScm−1 | % | (mg L−1) | ||||||||||||||||
| NS127 | - | 6.5–8.5 | - | - | ND | - | 500 | 1000 | - | - | - | 200 | 1.5 | - | 250 | 44 | - | 400 | ||
| WHO | - | - | - | - | ND | - | 100 | 1000 | 75 | - | 30 | - | - | - | 250 | 50 | - | 200 | ||
| PAL | 6.0–9.0 | 200 | 5 | 31–120 | 1.0 | 1 × 10−4 | 0.005 | |||||||||||||
| Sampling points * | 1 | 20.9 | 8.0 | 520.2 | 591.0 | ND | 3.5 | ND | 146.0 | 296.0 | 39.0 | 5.3 | 9.6 | 11.4 | 0.1 | 123.0 | 25.0 | 1.5 | 0.1 | 18.7 |
| 2 | 19.8 | 7.3 | 757.0 | 883.0 | ND | 0.8 | ND | 212.0 | 441.0 | 49.0 | 8.6 | 19.2 | 16.9 | Bdl | 150.0 | 20.6 | 47.5 | 0.1 | 51.9 | |
| 3 | 26.4 | 6.7 | 391.8 | 3838.0 | 6.0 | 3.8 | ND | 1000.0 | 1542.0 | 151.0 | 23.1 | 110.3 | 119.0 | 0.2 | 1134.0 | 0.1 | 13.6 | 0.1 | 92.9 | |
| 4 | 21.4 | 7.0 | 367.0 | 2293.0 | 91.0 | −1.3 | ND | 645.0 | 1145.0 | 194.0 | 10.4 | 30.3 | 40.3 | ND | 401.0 | 45.7 | 86.3 | 0.1 | 255.3 | |
| 5 | 21.0 | 7.6 | 471.0 | 1233.0 | 70.0 | −2.7 | ND | 299.0 | 616.0 | 86.0 | 7.4 | 16.5 | 25.4 | Bdl | 244.0 | 46.1 | 5.8 | ND | 92.5 | |
| 6 | 24.4 | 7.3 | 429.2 | 3363.0 | ND | 1.6 | ND | 820.0 | 1617.0 | 152.0 | 22.4 | 75.1 | 106.0 | 0.1 | 887.0 | 46.7 | 39.4 | 0.1 | 89.1 | |
| 7 | 23.9 | 6.9 | 356.0 | 4070.0 | ND | −3.2 | ND | 1125.0 | 2037.0 | 274.0 | 17.3 | 72.7 | 93.9 | 0.6 | 766.0 | 88.9 | 19.0 | 0.1 | 498.2 | |
| 8 | 22.0 | 6.8 | 465.0 | 4311.0 | 2.0 | −5.5 | ND | 778.0 | 2156.0 | 256.0 | 21.1 | 93.9 | 101.0 | 0.4 | 810.0 | 87.7 | 11.1 | 0.1 | 622.9 | |
| 9 | 24.9 | 8.1 | 175.0 | 3614.0 | 1.9 × 106 | −4.0 | 2.6 | 227.0 | 1808.0 | 60.0 | 37.5 | 15.4 | 264.2 | 0.3 | 464.0 | 23.4 | 3.2 | 6.0 | 182.8 | |
| 10 | 14.4 | 8.4 | 372.0 | 485.0 | 1.6 × 104 | 4.3 | 7.9 | 773.0 | 242.0 | 87.0 | 12.7 | 21.9 | 74.1 | 0.2 | 471.0 | 14.0 | 7.4 | 0.9 | 31.9 | |
| 11 | 21.2 | 7.9 | 368.0 | 1585.0 | 1.0 × 105 | −5.0 | 2.8 | 279.0 | 785.0 | 69.0 | 16.4 | 19.5 | 64.5 | 0.3 | 308.0 | 79.8 | 10.9 | 3.5 | 102.8 | |
| 12 | 20.6 | 7.7 | 220.0 | 1093.0 | 2.6 × 106 | 5.0 | 2.9 | 284.0 | 596.0 | 46.0 | 23.1 | 19.8 | 117.0 | 0.2 | 215.0 | 68.0 | 20.5 | 3.1 | 86.2 | |
| Max | 26.4 | 8.4 | 757.0 | 4311.0 | 2.61 × 106 | 5.0 | 7.9 | 1125.0 | 2156.0 | 274.0 | 37.5 | 110.3 | 264.2 | 0.6 | 1134.0 | 88.9 | 86.3 | 6.0 | 622.9 | |
| Min | 14.4 | 6.7 | 175.0 | 485.0 | 2.0 | −5.5 | 2.6 | 146.0 | 242.0 | 39.0 | 5.3 | 9.6 | 11.4 | 0.1 | 123.0 | 0.1 | 1.5 | 0.1 | 18.7 | |
| Mean | 21.7 | 7.5 | 407.7 | 2279.9 | 5.88 × 105 | −0.2 | 4.1 | 549.0 | 1106.8 | 121.9 | 17.1 | 42.0 | 86.1 | 0.3 | 497.8 | 45.5 | 22.2 | 1.3 | 177.1 | |
| ID | Al | As | B | Ba | Cr | Cu | Fe | Zn | Pb | Sr | V | Ni | Li | Mn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | |||||||||||||||
| NS127 | 0.200 | 0.025 | - | 0.700 | 0.050 | 2.000 | 0.300 | 5.000 | 0.025 | - | - | - | - | 0.150 | |
| WHO | 0.200 | 0.010 | 0.300 | 0.700 | 0.050 | 1.300 | 0.300 | 3.000 | 0.010 | - | - | - | - | 0.400 | |
| PAL | 0.050 | 0.200 | - | 0.010 | 0.050 | 0.010 | 1.000 | 0.020 | 0.030 | - | - | 0.600 | - | - | |
| Sampling points * | 1 | Bdl. | Bdl. | 0.037 | 0.065 | 0.004 | 0.011 | 0.006 | 0.007 | 0.027 | 0.223 | 0.015 | 0.019 | 0.015 | Bdl |
| 2 | Bdl. | Bdl. | 0.032 | 0.116 | 0.005 | 0.012 | 0.036 | 0.019 | 0.028 | 0.300 | 0.010 | 0.021 | 0.006 | 0.002 | |
| 3 | Bdl. | 0.069 | 1.337 | 0.110 | Bdl. | Bdl. | 0.036 | 0.004 | 0.032 | 1.652 | 0.026 | 0.029 | 0.724 | 1.037 | |
| 4 | Bdl. | Bdl. | 0.622 | 0.057 | Bdl. | Bdl. | 0.044 | 0.005 | 0.024 | 1.386 | 0.011 | 0.018 | 0.135 | 0.001 | |
| 5 | Bdl. | Bdl. | 0.066 | 0.292 | 0.002 | Bdl. | 0.006 | 0.005 | 0.023 | 0.519 | 0.010 | 0.027 | 0.042 | 0.001 | |
| 6 | Bdl. | 0.035 | 1.424 | 0.065 | Bdl. | Bdl. | 0.015 | 0.005 | 0.028 | 1.691 | 0.013 | 0.025 | 0.409 | 0.322 | |
| 7 | Bdl. | 0.042 | 1.996 | 0.023 | Bdl. | Bdl. | 0.081 | 0.005 | 0.026 | 2.585 | 0.013 | 0.023 | 0.467 | 0.023 | |
| 8 | Bdl. | 0.043 | 1.898 | 0.063 | Bdl | Bdl. | 0.027 | 0.006 | 0.025 | 2.513 | 0.008 | 0.027 | 0.486 | 0.553 | |
| 9 | 0.370 | Bdl. | 0.381 | 0.115 | 0.006 | 0.056 | 1.088 | 0.221 | 0.028 | 0.341 | 0.010 | 0.030 | 0.048 | 0.364 | |
| 10 | 4.580 | Bdl. | 0.047 | 0.530 | 0.004 | 0.076 | 3.153 | 0.134 | 0.034 | 0.182 | 0.032 | 0.012 | 0.002 | 0.838 | |
| 11 | 2.550 | Bdl. | 0.139 | 0.159 | 0.010 | 0.067 | 3.461 | 0.393 | 0.057 | 0.261 | 0.032 | 0.026 | 0.025 | 0.420 | |
| 12 | 0.960 | 0.050 | 0.252 | 0.111 | 0.005 | 0.074 | 6.779 | 0.160 | 0.030 | 0.302 | 0.029 | 0.024 | 0.042 | 0.355 | |
| d.l. | 0.020 | 0.001 | 0.002 | - | 0.001 | 0.002 | 0.001 | 0.003 | 0.005 | - | 0.001 | 0.001 | - | 0.001 | |
| q.l. | 0.067 | 0.023 | 0.006 | 0.001 | 0.002 | 0.007 | 0.003 | 0.001 | 0.015 | - | 0.004 | 0.003 | - | 0.001 | |
| YEAR | ID | δ18O (‰) | δ2H (‰) | 3H (TU) | |
|---|---|---|---|---|---|
| 2017 | 1. CFE 2 | Well | −10.21 | −72.80 | ND |
| 2. Autopista | Well | −10.19 | −72.90 | 0.54 | |
| 3. Momoxpan 5 | Well | −9.96 | −69.30 | 0.00 | |
| 4. Parque Juárez | Well | −10.18 | −71.50 | 0.27 | |
| 5. Héroes de Puebla | Well | −9.80 | −70.30 | 1.30 | |
| 6. Atlixcayotl 3 | Well | −9.60 | −67.00 | ND | |
| 7. Prados agua azul | Well | −10.40 | −72.40 | ND | |
| 8. CNIC | Well | −10.20 | −70.00 | 0.20 | |
| 9. Alseseca River 1 | River | −10.10 | −75.60 | 2.00 | |
| 10. Alseseca River 2 | River | −9.10 | −59.80 | 2.20 | |
| 11. Atoyac River 1 | River | −9.30 | −62.70 | 1.30 | |
| 12. Atoyac River 2 | River | −9.90 | −71.60 | 1.10 | |
| 2010 (Garfias et al., 2010) [30] | 1. CFE | Well | −9.70 | −69.10 | |
| 2. Autopista | Well | −10.00 | −73.00 | ||
| 7. Agua Azul | Well | −10.60 | −74.50 | ||
| 13. Baños Paseo Bravo | Well | −10.10 | −71.10 | ||
| 14. Crown Plaza | Well | −10.20 | −70.10 | ||
| 15. Rancho Colorado | Well | −9.70 | −70.10 | ||
| 16. Balneario la paz | Well | −10.80 | −74.40 | ||
| 17. CAPU | Well | −10.70 | −74.10 | ||
| 18. Gabriel pastor | Well | −9.60 | −67.50 | ||
| 19. El An + gel | Well | −9.80 | −71.60 | ||
| 20. Papelera | Well | −10.10 | −73.00 | ||
| 21. Castillotla | Well | −9.20 | −65.60 | ||
| 22. Amalucan | Well | −13.00 | −71.20 | ||
| 23. Zopilocalco | Spring | −10.50 | −77.40 | ||
| 24. Benito Júarez | Spring | −10.90 | −75.50 | ||
| 25. Preciosita | Spring | −11.10 | −79.70 | ||
| 26. Axcopan | Spring | −11.80 | −83.90 | ||
| 27. Piedra Colorada | Spring | −10.80 | −78.60 | ||
| 28. San Baltasar | Spring | −11.90 | −81.20 | ||
| 29. Aan Martín | Spring | −10.50 | −77.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ontiveros-Capurata, R.E.; Martínez Morales, M.; Esteller Alberich, M.V.; Esquivel Martínez, J.M.; Gutiérrez-Macias, T.; Salcedo Sanchez, E.R.; Ocampo Astudillo, A. Groundwater–Surface Water Interactions and Pollution Assessment Using Hydrochemistry and Environmental Isotopes δ18O, δ2H, and 3H in Puebla Metropolitan Area, Mexico. Sustainability 2025, 17, 9258. https://doi.org/10.3390/su17209258
Ontiveros-Capurata RE, Martínez Morales M, Esteller Alberich MV, Esquivel Martínez JM, Gutiérrez-Macias T, Salcedo Sanchez ER, Ocampo Astudillo A. Groundwater–Surface Water Interactions and Pollution Assessment Using Hydrochemistry and Environmental Isotopes δ18O, δ2H, and 3H in Puebla Metropolitan Area, Mexico. Sustainability. 2025; 17(20):9258. https://doi.org/10.3390/su17209258
Chicago/Turabian StyleOntiveros-Capurata, Ronald Ernesto, Manuel Martínez Morales, Maria Vicenta Esteller Alberich, Juan Manuel Esquivel Martínez, Tania Gutiérrez-Macias, Edith Rosalba Salcedo Sanchez, and Ariadna Ocampo Astudillo. 2025. "Groundwater–Surface Water Interactions and Pollution Assessment Using Hydrochemistry and Environmental Isotopes δ18O, δ2H, and 3H in Puebla Metropolitan Area, Mexico" Sustainability 17, no. 20: 9258. https://doi.org/10.3390/su17209258
APA StyleOntiveros-Capurata, R. E., Martínez Morales, M., Esteller Alberich, M. V., Esquivel Martínez, J. M., Gutiérrez-Macias, T., Salcedo Sanchez, E. R., & Ocampo Astudillo, A. (2025). Groundwater–Surface Water Interactions and Pollution Assessment Using Hydrochemistry and Environmental Isotopes δ18O, δ2H, and 3H in Puebla Metropolitan Area, Mexico. Sustainability, 17(20), 9258. https://doi.org/10.3390/su17209258








