Influence of Stress on Gas Sorption Behavior and Induced Swelling in Coal: Implications for Sustainable CO2 Geological Storage
Abstract
1. Introduction
1.1. Stress Effects on Gas Adsorption Capacity
1.2. Stress Effects on Adsorption Kinetics
1.3. Stress Effects on Adsorption-Induced Deformation
1.4. Research Gaps and Objectives
2. Experimental Methodology
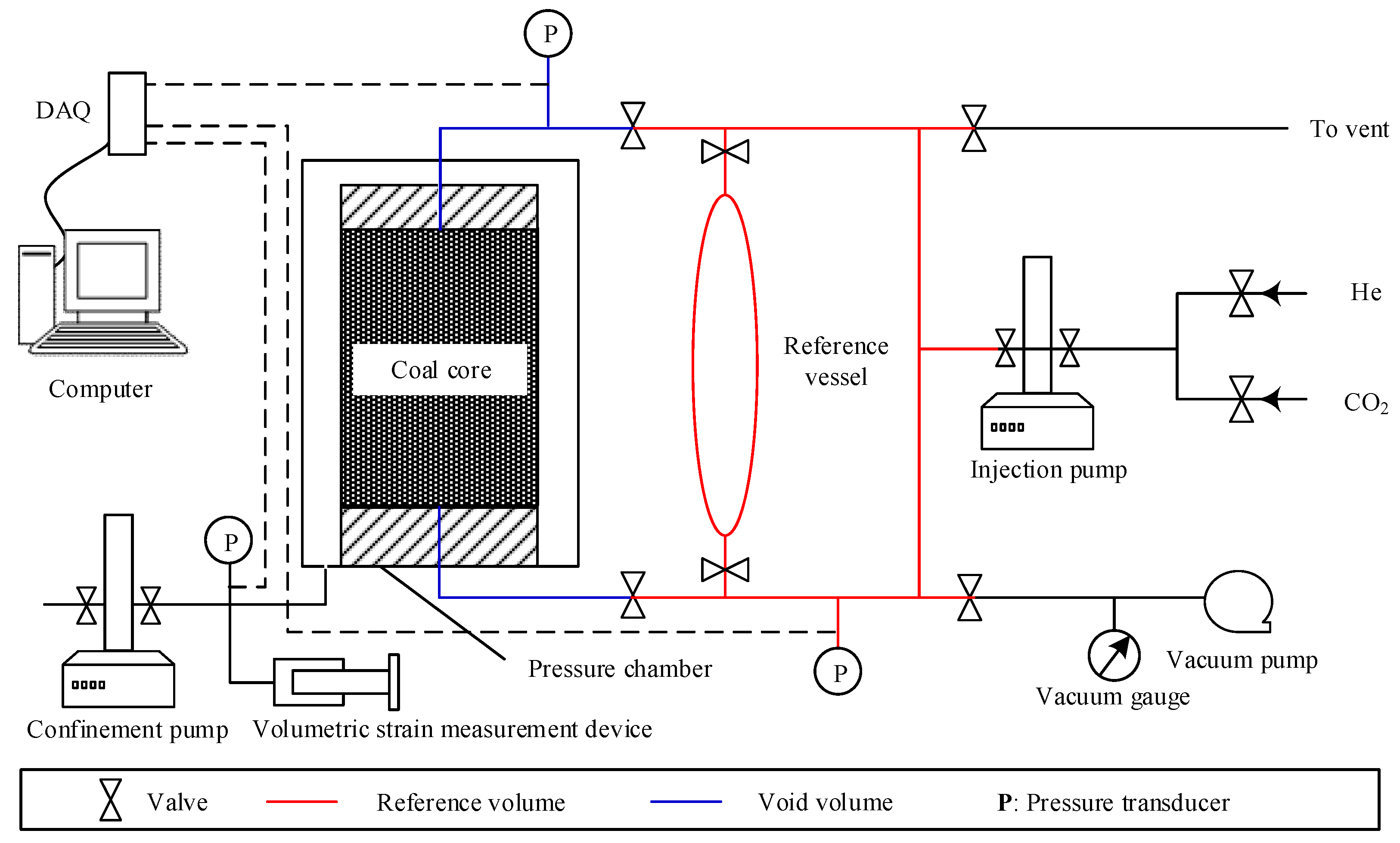
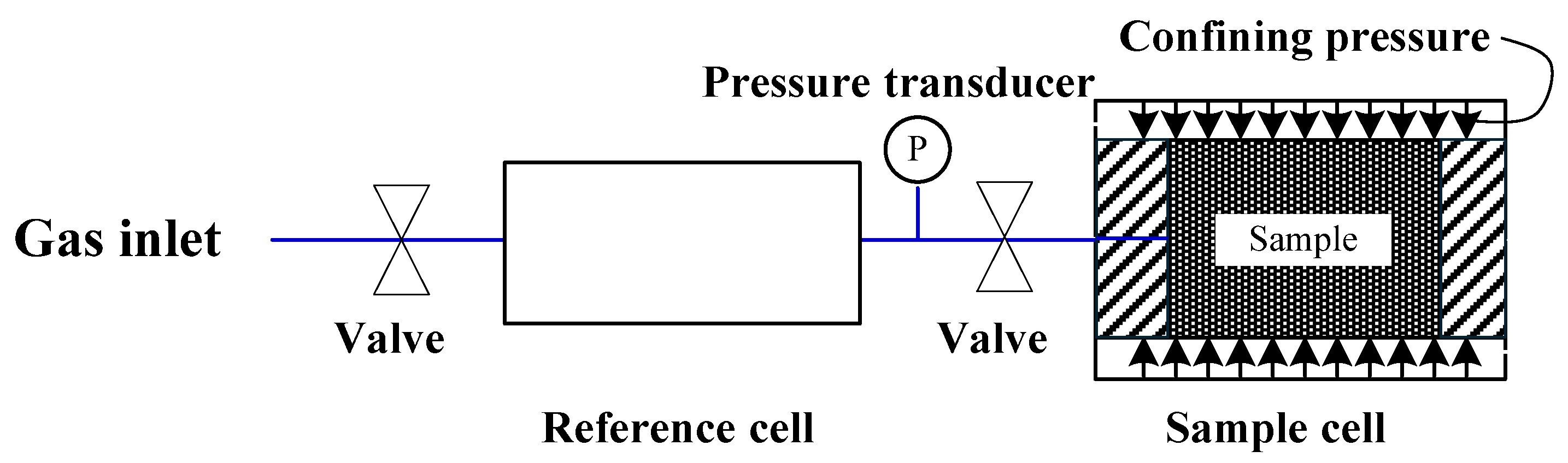
2.1. Experimental Apparatus
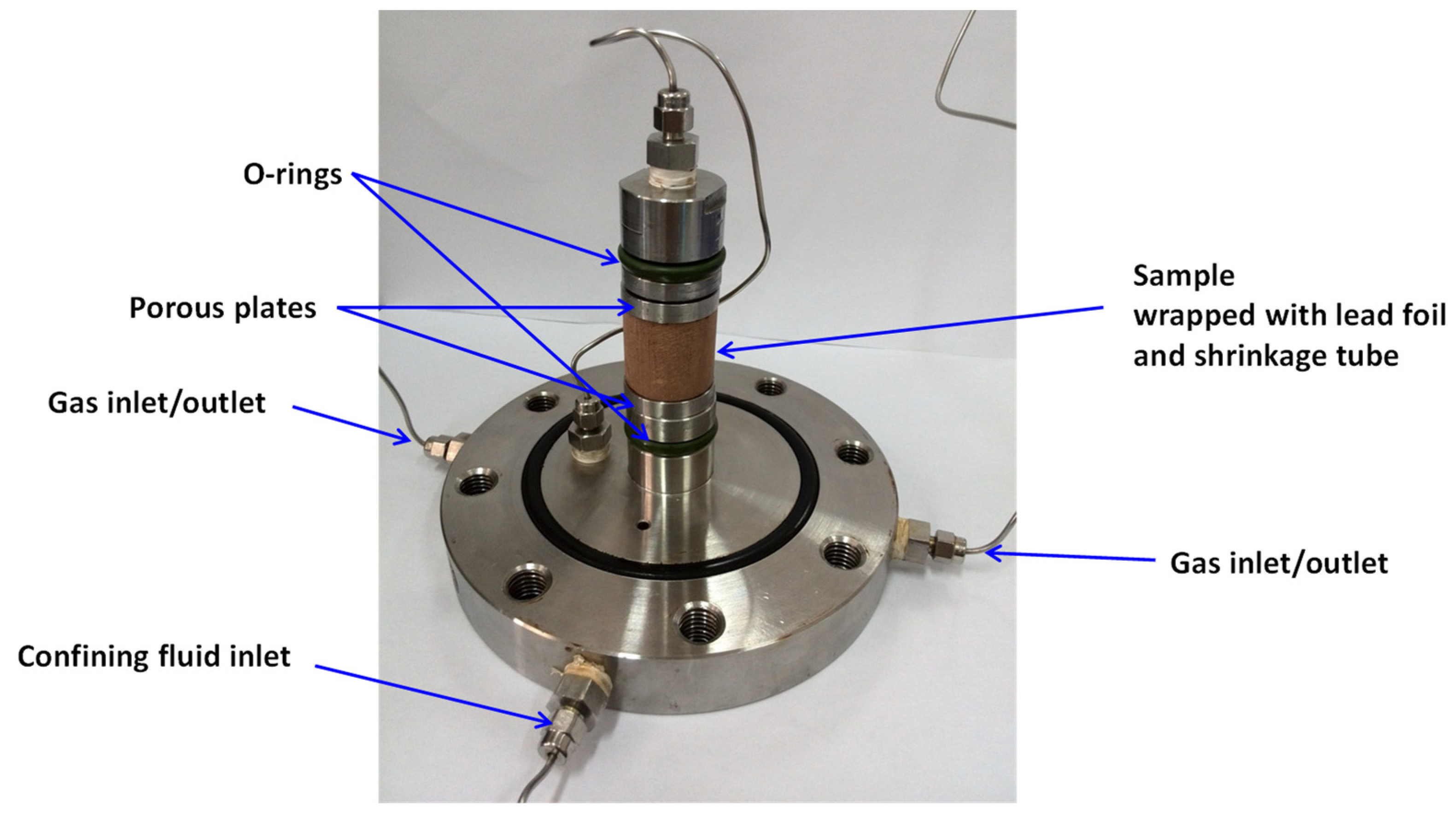
2.2. Coal Sample Preparation
2.3. Experimental Conditions
- (1)
- Constraint 1 (Constant confining pressure): The confining pressure was maintained at a constant value throughout the experiment to simulate a stable external stress environment. This setup allowed the coal sample to undergo sorption-induced deformation without the need for external pressure adjustments. To achieve this condition, the target confining pressure was first applied to the sample using the confinement pump. Subsequently, the valve connecting the confinement pump and the pressure chamber was closed to isolate the system. During the entire adsorption test, the confining pressure was kept constant by fine-tuning the volumetric strain measurement device.
- (2)
- Constraint 2 (Constant volume): The total volume of the confining fluid (used to apply confining pressure) and the specimen was kept constant throughout the experiment. Under this condition, the volume of the confining fluid varies in response to gas sorption-induced deformation of the coal sample, which in turn alters the confining pressure. This setup ensures that variations in the experimental system are primarily driven by internal pressure fluctuations resulting from adsorption, rather than active regulation of the confining fluid volume. To establish this condition, the confining pressure was initially raised to the target value using the confinement pump. The valve between the confinement pump and the pressure chamber was then closed to disconnect the two systems. During the experiment, although adsorption-induced deformation of the coal sample resulted in fluctuations in confining pressure, the total volume of the confining fluid and the specimen was strictly maintained.
2.4. Experimental Procedure
2.4.1. Sample Assembly
- Mount the prepared coal core sample into the pressure chamber. The internal structure of the pressure chamber is illustrated in Figure 2.
2.4.2. Confining Pressure Application
- Use the confinement pump to apply confining pressure to a present value—15 MPa was adopted in this study—to simulate the target external stress environment.
2.4.3. Vacuum Treatment
- Evacuate the entire experimental system (including the tubing network and the coal sample) using a vacuum pump for a period of 24 to 48 h. This step is critical to thoroughly remove residual moisture and air, eliminating their interference with subsequent gas sorption measurements.
2.4.4. Gas Injection
- Inject the high-purity test gas (CO2) into the pre-calibrated reference vessel.
- Allow the gas to equilibrate thermally for a sufficient duration (several minutes). Thermal equilibrium is confirmed by the stabilization of pressure readings in the reference vessel.
2.4.5. Adsorption Initiation
- Open the valve connecting the reference vessel and the pressure chamber to establish gas communication between the two units, thereby initiating the gas adsorption process of the coal sample.
2.4.6. Pressure Monitoring & Equilibrium Determination
- Continuously monitor the pressures in both the reference vessel and the pressure chamber until they stabilize. A steady pressure in these components indicates that the coal sample has reached gas adsorption equilibrium.
- Analyze the gas adsorption capacity and adsorption kinetics of coal sample using the method detailed in Section 2.5 and Section 2.6.
2.4.7. Multi-Pressure Measurement
- Repeat Steps 2.4.4–2.4.6 with different preset pressures in the reference vessel. This iterative process enables the determination of coal adsorption capacities and adsorption kinetics under a series of distinct equilibrium pressures.
2.4.8. Sorption-Induced Swelling Measurement
- Under different stress conditions, the sorption-induced swelling deformation of the coal sample is measured using the method described in Section 2.7.
2.5. Adsorption Measurement Method
2.6. Sorption Kinetics Measurement Method
- Mt: mass of gas sorbed at time t,
- M∞: equilibrium sorbed mass,
- D: diffusion coefficient (units: cm2/s),
- rp: particle radius (units: cm).
2.7. Swelling Measurement Method
3. Results and Discussion
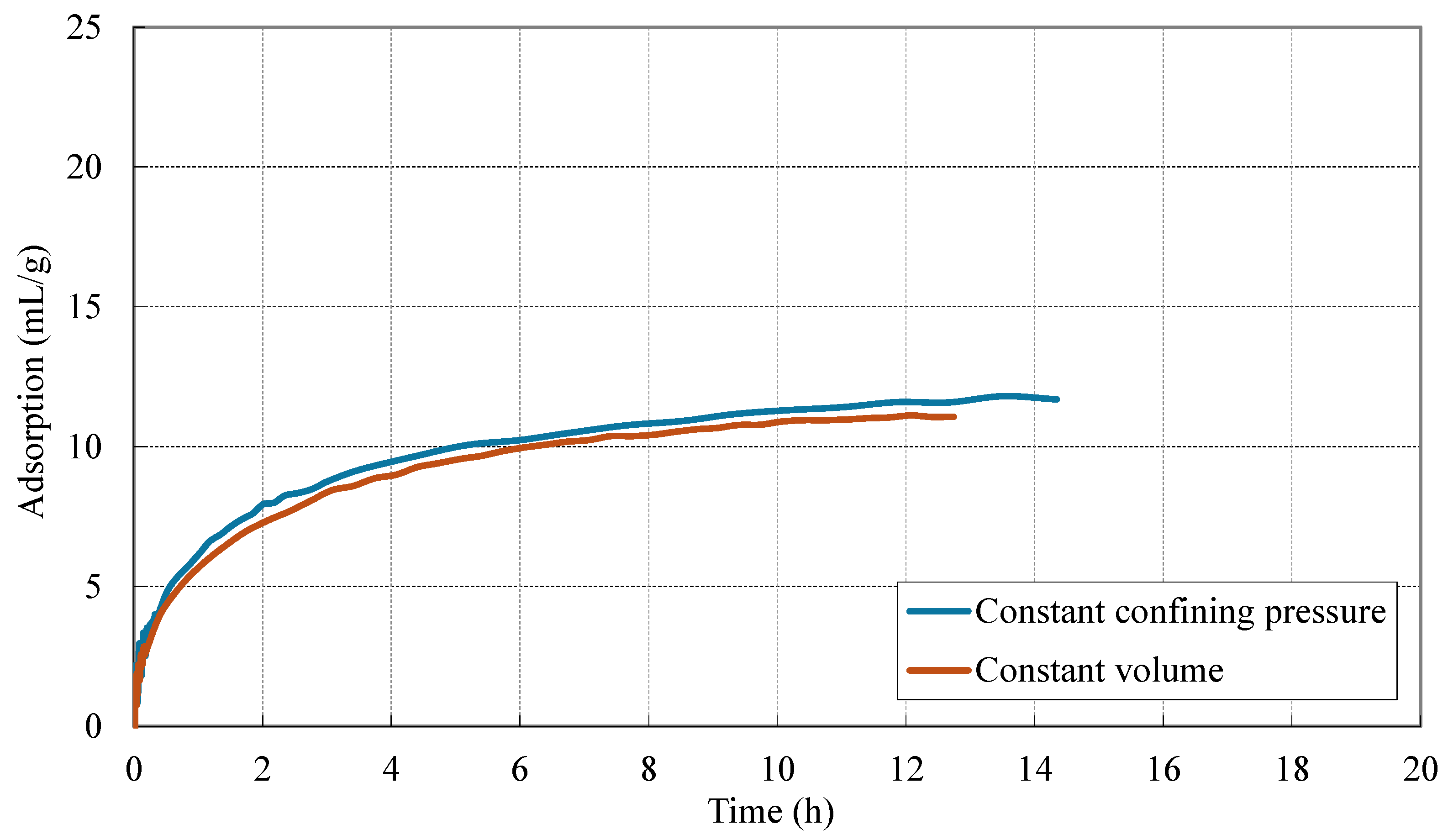
3.1. Adsorption Behavior of CO2 in Coal Under Different Stress Constraint Conditions
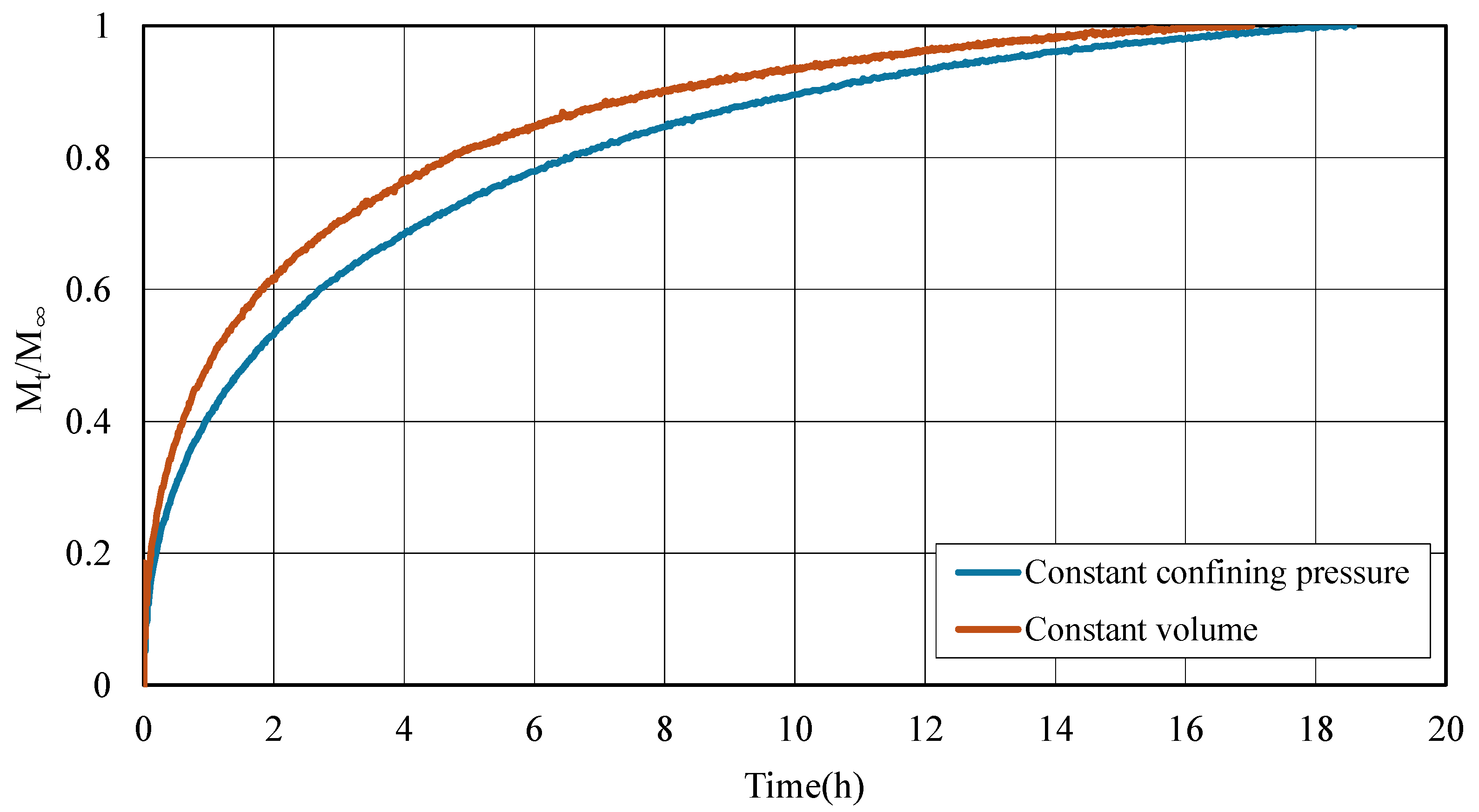
3.2. Adsorption Kinetics of CO2 in Coal Under Different Stress Constraint Conditions
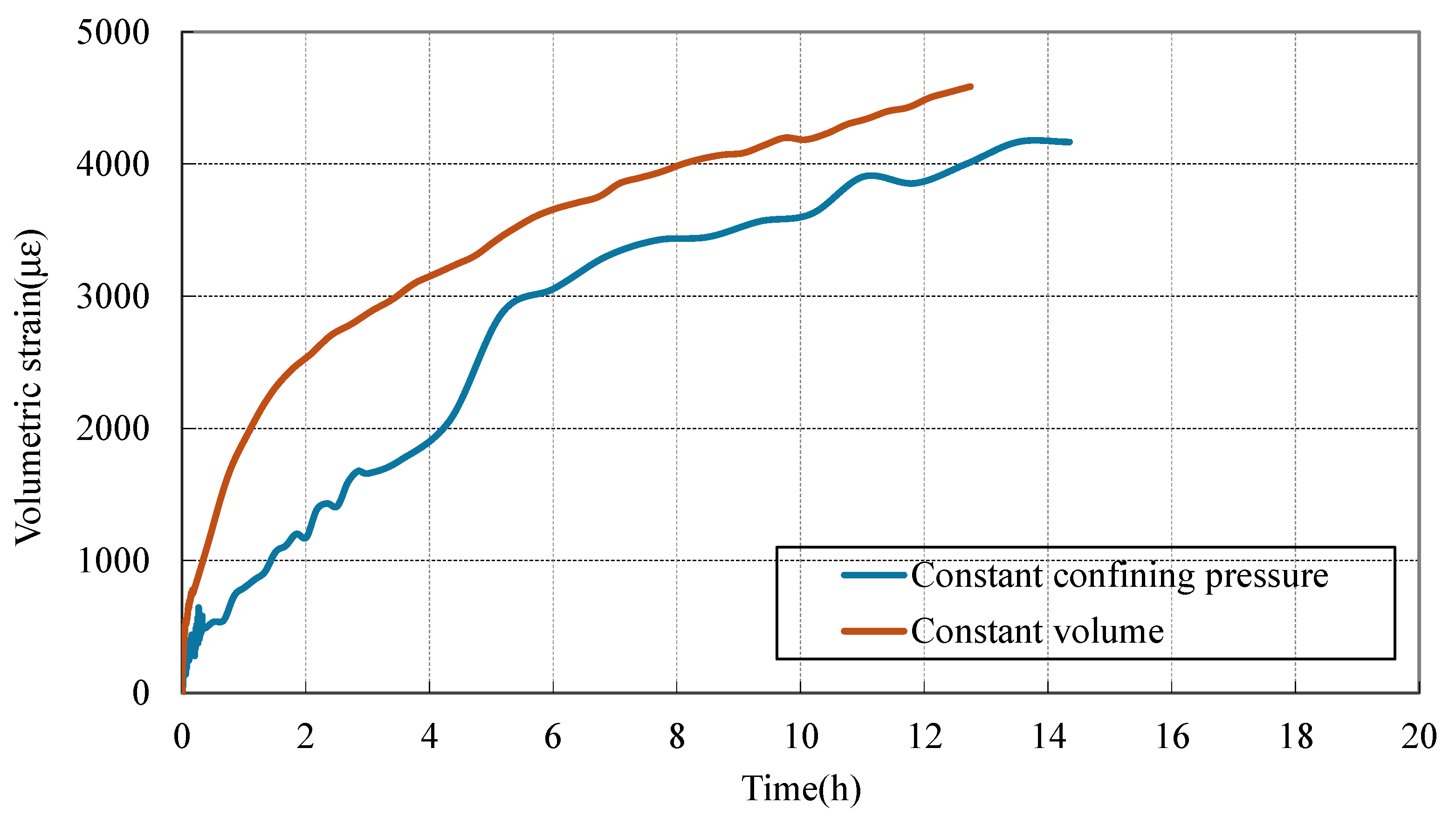
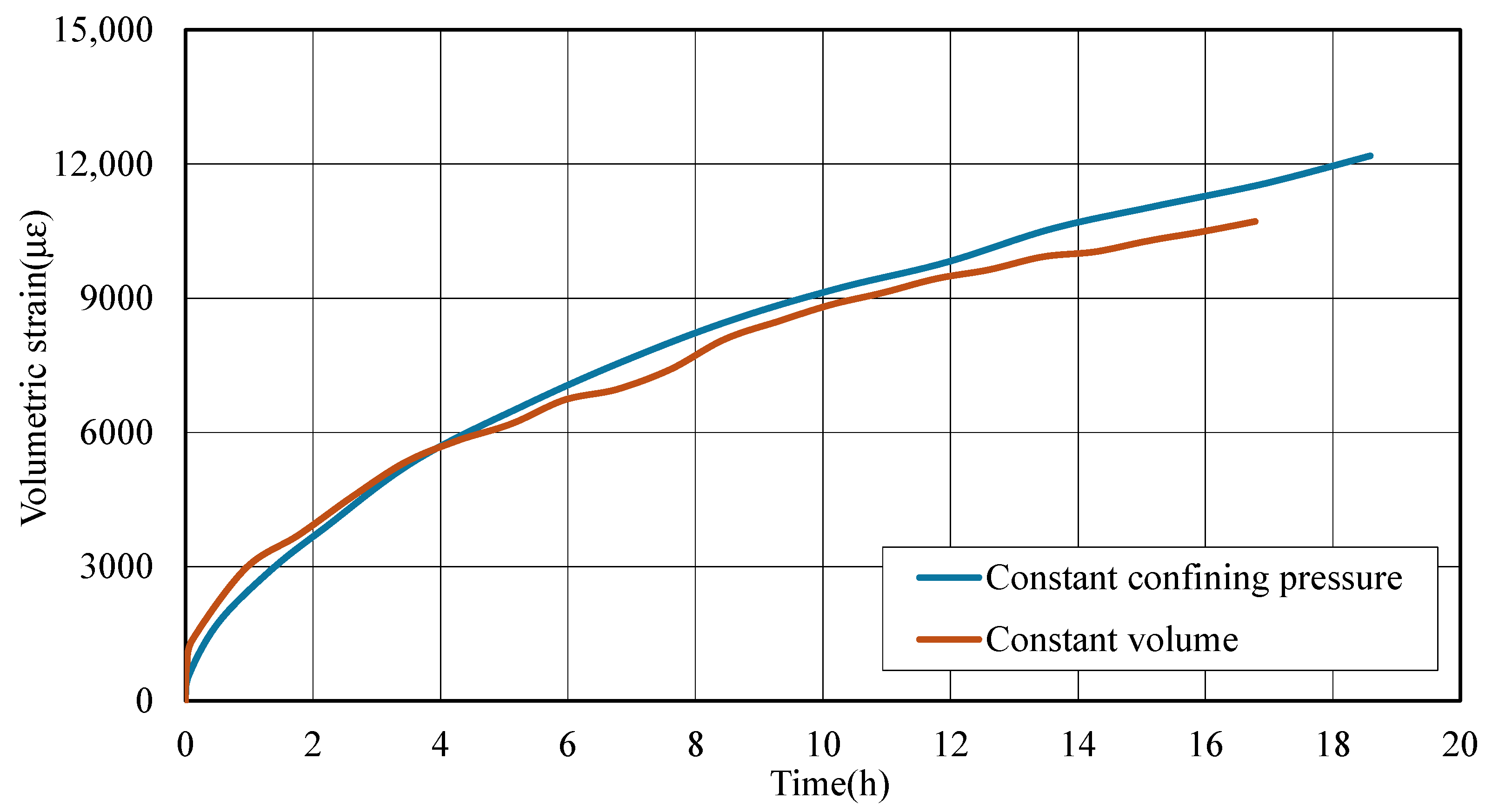
3.3. Deformation Behavior of Coal During CO2 Adsorption Under Different Stress Constraint Conditions
4. Implications for CO2 Geological Storage
- (1)
- Injection Strategy Optimization: The observed improvements in CO2 adsorption capacity under higher gas pressures suggest that moderate injection pressures can improve storage efficiency. However, the trade-off between increased adsorption and potential risk of fracture activation must be carefully evaluated to avoid compromising the integrity of the caprock.
- (2)
- Stress Constraint Management: The contrasting behaviors of coal under constant confining pressure (typical of stable, laterally unconfined reservoir regions) and constant volume (representative of rigid, low-permeability surrounding rock) highlight the critical influence of in situ stress states on CO2 injectivity and storage stability. Conditions of constant confining pressure (e.g., in coal seams overlain by deformable shale) facilitate controlled coal swelling, promote sustained gas uptake, and are more favorable for maintaining long-term injectivity. In contrast, constant volume scenarios (e.g., coal seams confined by rigid sandstone or carbonate rock) lead to rapid internal stress buildup, which may cause premature saturation and a reduction in effective storage capacity. However, due to the inherent complexity and heterogeneity of real geological conditions, actual reservoirs often exhibit more complex and variable constraint behaviors than the simplified binary scenarios of constant confining pressure and constant volume. This complexity represents a critical aspect that requires greater consideration in future practical geological storage engineering.
- (3)
- Deformation and Stability Prediction: The strong positive correlation between CO2 adsorption and coal strain, particularly under elevated pressure, highlights the necessity of incorporating poroelastic effects into reservoir models. Accurate prediction of adsorption-induced swelling and its consequences for permeability and fracture networks is crucial for evaluating reservoir stability and storage security. However, it is important to note that extrapolating lab-scale strain measurements to reservoir-scale predictions involves significant challenges and limitations. These include the effects of geological heterogeneity, differences in scale, and long-term creep behavior, which are difficult to fully capture in laboratory experiments. Heterogeneity at the field scale, such as variations in coal composition and the presence of natural fractures, may lead to non-uniform deformation and complex mechanical responses that differ from idealized laboratory conditions. Additionally, time-dependent effects such as creep and fatigue under sustained load and adsorption cycles could further alter deformation behavior over extended periods. To enhance predictive accuracy, it is essential to employ upscaled models that integrate geomechanical constraints with realistic reservoir architectures and temporal evolution.
- (4)
- Anisotropy and Heterogeneity Considerations: Although not explicitly addressed in this study, previous reports on stress-dependent anisotropy in coal swelling [23] and diffusion [24] indicate that site-specific structural features, such as bedding orientation and cleat geometry, should be integrated into large-scale storage simulations to improve predictive accuracy.
- (5)
- Long-Term Performance Monitoring: The persistence of strain and internal stress under constrained conditions emphasizes the need for continuous monitoring of geomechanical responses throughout both injection and post-injection phases. Techniques such as passive seismic monitoring and periodic strain mapping can aid in early detection of containment loss or structural deformation.
5. Conclusions
- (1)
- CO2 pressure enhances sorption capacity. Under identical stress conditions, CO2 adsorption capacity at 2 MPa is significantly higher than that at 1 MPa. This enhancement is attributed to the stronger mass transfer driving force provided by elevated pressure, which accelerates CO2 diffusion into the coal matrix and promotes more efficient pore filling.
- (2)
- Stress constraints modulate adsorption behavior and kinetics. Under constant confining pressure, coal exhibits consistently higher adsorption capacities compared to constant volume conditions. This enhanced adsorption is attributed to the ability of the coal matrix to swell volumetrically, which helps preserve pore accessibility and promotes continuous gas uptake. In contrast, under constant volume constraints, the restriction on expansion leads to a rapid buildup of internal stress. This stress accumulation not only hinders further adsorption but also accelerates the approach to saturation. Consequently, although initial gas uptake occurs more rapidly under constant volume conditions, early saturation is reached due to the limited capacity for matrix expansion.
- (3)
- Sorption-induced swelling is pressure- and constraint-dependent. Driven by increased CO2 adsorption, higher CO2 pressure (2 MPa) results in greater total strain compared to 1 MPa. Under constant volume, initial strain rises sharply due to rapid stress accumulation, while constant confining pressure enables more continuous, progressive swelling—especially at higher pressures, where adsorption-driven expansion dominates over mechanical restraint.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, S.C.; Fang, Z.M.; Li, X.C.; Jiang, Q.; Shen, H.M.; Shi, L. Enhanced coalbed methane recovery from lignite using CO2 and N2. Energy Fuels 2025, 39, 10394–10409. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, C.; Wang, R. Monitoring technologies for CO2 storage in coal seams and enhanced coalbed methane recovery: A review of field applications. Fuel Process. Technol. 2025, 276, 108274. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, C.; Shen, S. Experimental investigation of CO2-enhanced coalbed methane recovery using large intact coal cores. J. CO2 Util. 2025, 100, 103185. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery: A review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Bachu, S. CO2 storage in geological media: Role, means, status and barriers to deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- van Bergen, F.; Pagnier, H.; Krzystolik, P. Field experiment of enhanced coalbed methane–CO2 in the Upper Silesian Basin of Poland. Environ. Geosci. 2006, 13, 201–224. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D. A theoretical model for gas adsorption-induced coal swelling. Int. J. Coal Geol. 2007, 69, 243–252. [Google Scholar] [CrossRef]
- Vandamme, M.; Brochard, L.; Lecampion, B.; Coussy, O. Adsorption and strain: The CO2-induced swelling of coal. J. Mech. Phys. Solids 2010, 58, 1489–1505. [Google Scholar] [CrossRef]
- Karacan, C.O. Swelling-induced volumetric strains internal to a stressed coal associated with CO2 sorption. Int. J. Coal Geol. 2007, 72, 209–220. [Google Scholar] [CrossRef]
- Palmer, I.D.; Mansoori, J. How permeability depends on stress and pore pressure in coalbeds: A new model. SPE Reserv. Eval. Eng. 1998, 1, 539–544. [Google Scholar] [CrossRef]
- Shi, J.Q.; Durucan, S. A bidisperse pore diffusion model for methane displacement desorption in coal by CO2 injection. Fuel 2003, 82, 1219–1229. [Google Scholar] [CrossRef]
- Liu, J.; Peach, C.J.; Spiers, C.J. Anisotropic swelling behaviour of coal matrix cubes exposed to water vapour: Effects of relative humidity and sample size. Int. J. Coal Geol. 2016, 167, 119–135. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hol, S.; Peach, C.J.; Spiers, C.J. Applied stress reduces the CO2 sorption capacity of coal. Int. J. Coal Geol. 2011, 85, 128–142. [Google Scholar] [CrossRef]
- Pone, J.D.N.; Halleck, P.M.; Mathews, J.P. Sorption capacity and sorption kinetic measurements of CO2 and CH4 in confined and unconfined bituminous coal. Energy Fuels 2009, 23, 4688–4695. [Google Scholar] [CrossRef]
- Hol, S.; Peach, C.J.; Spiers, C.J. Effect of 3-D stress state on adsorption of CO2 by coal. Int. J. Coal Geol. 2012, 93, 1–15. [Google Scholar] [CrossRef]
- Liu, J.; Spiers, C.J.; Peach, C.J.; Vidal-Gilbert, S. Effect of lithostatic stress on methane sorption by coal: Theory vs. experiment and implications for predicting in-situ coalbed methane content. Int. J. Coal Geol. 2016, 167, 48–64. [Google Scholar] [CrossRef]
- Liu, J.; Fokker, P.A.; Peach, C.J.; Spiers, C.J. Applied stress reduces swelling of coal induced by adsorption of water. Geomech. Energy Environ. 2018, 16, 45–63. [Google Scholar] [CrossRef]
- Gensterblum, Y.; van Hemert, P.; Billemont, P.; Battistutta, E.; Busch, A.; Krooss, B.; De Weireld, G.; Wolf, K.-H. European inter-laboratory comparison of high pressure CO2 sorption isotherms II: Natural coals. Int. J. Coal Geol. 2010, 84, 115–124. [Google Scholar] [CrossRef]
- Day, S.; Fry, R.; Sakurovs, R.; Weir, S. Swelling of coals by supercritical gases and its relationship to sorption. Energy Fuels 2010, 24, 2777–2783. [Google Scholar] [CrossRef]
- Siriwardane, H.; Haljasmaa, I.; McLendon, R.; Irdi, G.; Soong, Y.; Bromhal, G. Influence of carbon dioxide on coal permeability determined by pressure transient methods. Int. J. Coal Geol. 2009, 77, 109–118. [Google Scholar] [CrossRef]
- Hol, S.; Spiers, C.J. Competition between adsorption-induced swelling and elastic compression of coal at CO2 pressures up to 100 MPa. J. Mech. Phys. Solids 2012, 60, 1862–1882. [Google Scholar] [CrossRef]
- Liu, J.; Fokker, P.A.; Spiers, C.J. Coupling of swelling, internal stress evolution, and diffusion in coal matrix material during exposure to methane. J. Geophys. Res. Solid Earth 2017, 122, 844–865. [Google Scholar] [CrossRef]
- Durucan, S.; Ahsanb, M.; Shi, J.-Q. Matrix shrinkage and swelling characteristics of European coals. Energy Procedia 2009, 1, 3055–3062. [Google Scholar] [CrossRef]
- van Bergen, F.; Spiers, C.; Floor, G.; Bots, P. Strain development in unconfined coals exposed to CO2, CH4 and Ar: Effect of moisture. Int. J. Coal Geol. 2009, 77, 43–53. [Google Scholar] [CrossRef]
- Harpalani, S.; Mitra, A. Impact of CO2 Injection on Flow Behavior of Coalbed Methane Reservoirs. Transp. Porous Media 2010, 82, 141–156. [Google Scholar] [CrossRef]
- Day, S.; Fry, R.; Sakurovs, R. Swelling of coal in carbon dioxide, methane and their mixtures. Int. J. Coal Geol. 2012, 93, 40–48. [Google Scholar] [CrossRef]
- Danesh, N.N.; Chen, Z.; Aminossadati, S.M.; Kizil, M.S.; Pan, Z.; Connell, L.D. Impact of creep on the evolution of coal permeability and gas drainage performance. J. Nat. Gas Sci. Eng. 2016, 33, 469–482. [Google Scholar] [CrossRef]
- Hol, S.; Spiers, C.J.; Peach, C.J. Microfracturing of coal due to interaction with CO2 under unconfined conditions. Fuel 2012, 97, 569–584. [Google Scholar] [CrossRef]
- Mazumder, S.; Karnik, A.A.; Wolf, K.-A.A. Swelling of coal in response to CO2 sequestration for ECBM and its effect on fracture permeability. SPE J. 2006, 11, 390–398. [Google Scholar] [CrossRef]
- Pillalamarry, M.; Harpalani, S.; Liu, S. Gas diffusion behavior of coal and its impact on production from coalbed methane reservoirs. Int. J. Coal Geol. 2011, 86, 342–348. [Google Scholar] [CrossRef]

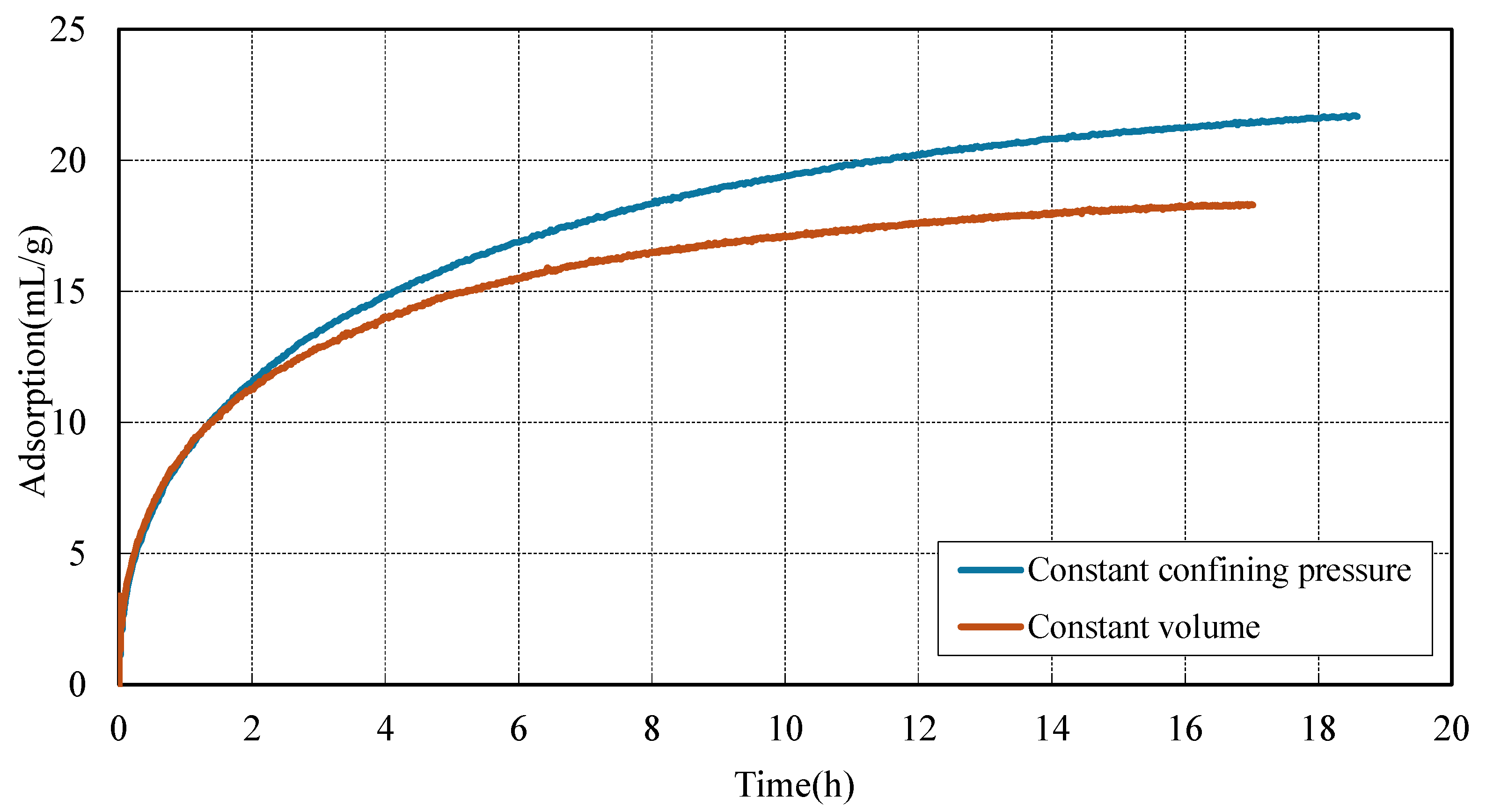

| CO2 Pressure (MPa) | Constraint Type | Equilibrium Adsorption Capacity (mL/g) |
|---|---|---|
| 1 | Constant Confining Pressure | 11.83 |
| 1 | Constant Volume | 11.13 |
| 2 | Constant Confining Pressure | 21.66 |
| 2 | Constant Volume | 18.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Yang, C.; Shen, S. Influence of Stress on Gas Sorption Behavior and Induced Swelling in Coal: Implications for Sustainable CO2 Geological Storage. Sustainability 2025, 17, 8990. https://doi.org/10.3390/su17208990
Fang Z, Yang C, Shen S. Influence of Stress on Gas Sorption Behavior and Induced Swelling in Coal: Implications for Sustainable CO2 Geological Storage. Sustainability. 2025; 17(20):8990. https://doi.org/10.3390/su17208990
Chicago/Turabian StyleFang, Zhiming, Chenlong Yang, and Shaicheng Shen. 2025. "Influence of Stress on Gas Sorption Behavior and Induced Swelling in Coal: Implications for Sustainable CO2 Geological Storage" Sustainability 17, no. 20: 8990. https://doi.org/10.3390/su17208990
APA StyleFang, Z., Yang, C., & Shen, S. (2025). Influence of Stress on Gas Sorption Behavior and Induced Swelling in Coal: Implications for Sustainable CO2 Geological Storage. Sustainability, 17(20), 8990. https://doi.org/10.3390/su17208990







