Comparison of Lichen and Moss Transplants for Monitoring the Deposition of Airborne Microfibers
Abstract
1. Introduction
2. Materials and Methods
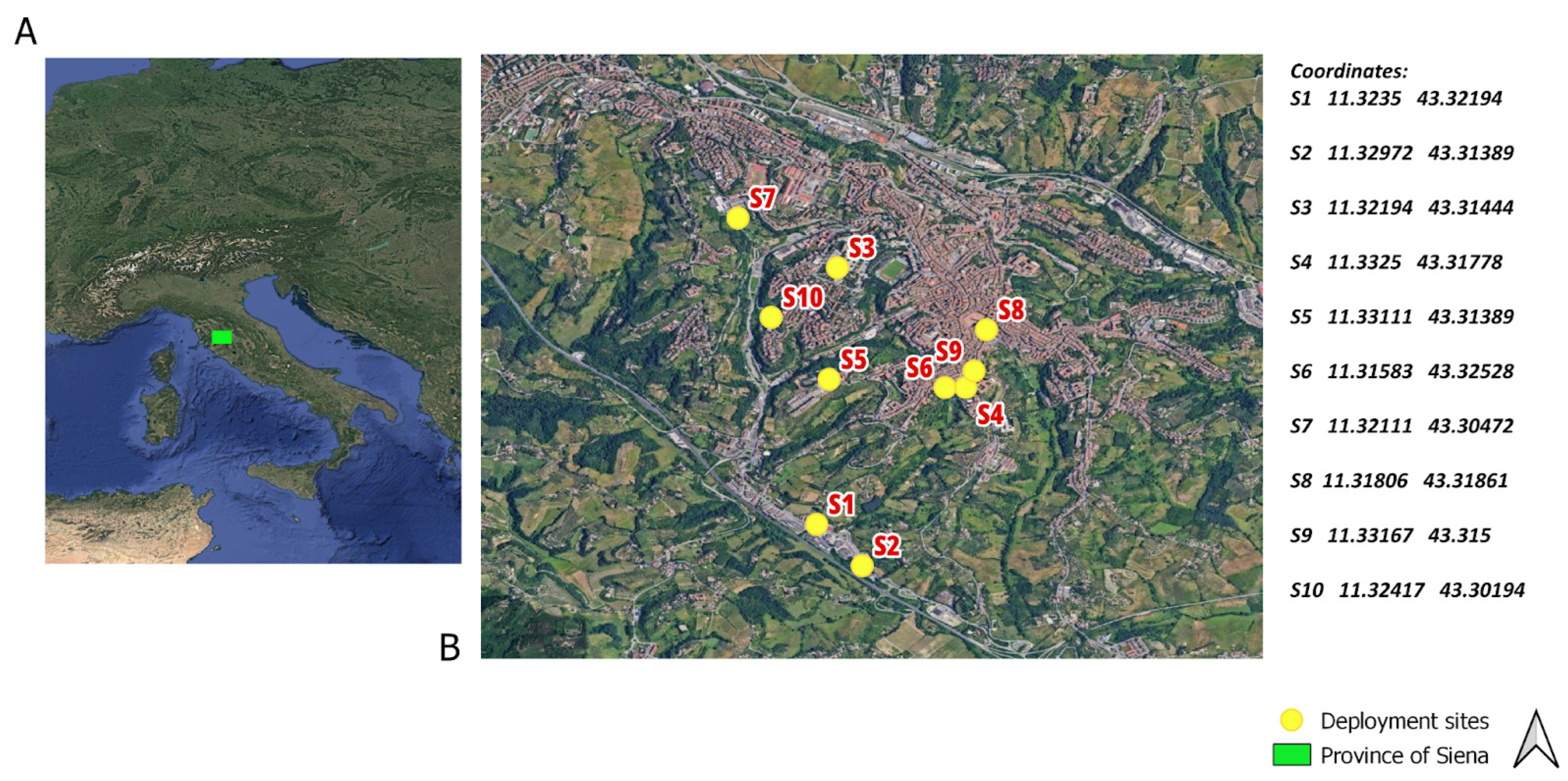
2.1. Experimental Design
2.2. Microplastic Extraction
2.3. Polymer Identification
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velis, C.A.; Lerpiniere, D.; Tsakona, M. Prevent Marine Plastic Litter-Now. An ISWA Facilitated Partnership to Prevent Marine Litter, With a Global Call to Action for Investing in Sustainable Waste and Resources Management Worldwide. Report Prepared on Behalf of the International Solid Waste Association (ISWA). An Output of ISWA Marine Litter Task Force, Vienna. 2017. Available online: https://www.eawag.ch/fileadmin/Domain1/Abteilungen/sandec/E-Learning/Moocs/Solid_Waste/Further_resources/Week_4/prevent_marine_plastic_litter_iswa.pdf (accessed on 19 October 2024).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Global concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.W. Marine debris and northern fur seals: A case study. Mar. Pollut. Bull. 1987, 18, 326–335. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith, K.L., Jr. Plastics on the Sargasso Sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Coe, J.; Rogers, D. Marine Debris Sources, Impacts, and Solutions; Springer: New York, NY, USA, 1996. [Google Scholar]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Surendran, U.; Jayakumar, M.; Raja, P.; Gopinath, G.; Chellam, P.V. Microplastics in terrestrial ecosystem: Sources and migration in soil environment. Chemosphere 2023, 318, 137946. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef] [PubMed]
- Zylstra, E.R. Accumulation of wind-dispersed trash in desert environments. J. Arid Environ. 2013, 89, 13–15. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Peijnenburg, W.J.; Li, R.; Yang, J.; Zhou, Q. Confocal measurement of microplastics uptake by plants. MethodsX 2020, 7, 100750. [Google Scholar] [CrossRef]
- Capozzi, F.; Granata, A.; Gaglione, A.; Gargiulo, T.; Rallo, A.; Marino, F.; Sorrentino, M.C.; Pironti, C.; Vergara, A.; Spagnuolo, V.; et al. Sequential extraction of anthropogenic microfibers from the leaves of Pittosporum tobira. Chemosphere 2024, 367, 143628. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef]
- Roblin, B.; Aherne, J. Moss as a biomonitor for the atmospheric deposition of anthropogenic microfibres. Sci. Total Environ. 2020, 715, 136973. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef] [PubMed]
- Din, K.S.; Khokhar, M.F.; Butt, S.I.; Qadir, A.; Younas, F. Exploration of microplastic concentration in indoor and outdoor air samples: Morphological, polymeric, and elemental analysis. Sci. Total Environ. 2024, 908, 168398. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Sun, D.; Pan, Z.; Zhou, A.; Xie, S.; Wang, J.; Zou, J. Microplastic pollution in surface water from east coastal areas of Guangdong, South China and preliminary study on microplastics biomonitoring using two marine fish. Chemosphere 2020, 256, 127202. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics the Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe; Association of Plastic Manufacturers: Brussels, Belgium, 2019; Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf (accessed on 19 October 2024).
- Branquinho, C.; Catarino, F.; Brown, D.H.; Pereira, M.J.; Soares, A. Improving the use of lichens as biomonitors of atmospheric metal pollution. Sci. Total Environ. 1999, 232, 67–77. [Google Scholar] [CrossRef]
- Abas, A. A systematic review on biomonitoring using lichen as the biological indicator: A decade of practices, progress and challenges. Ecol. Indic. 2021, 121, 107197. [Google Scholar] [CrossRef]
- Nimis, P.L.; Andreussi, S.; Pittao, E. The performance of two lichen species as bioaccumulators of trace metals. Sci. Total Environ. 2001, 275, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Al-Radady, A.S.; Davies, B.E.; French, M.J. A new design of moss bag to monitor metal deposition both indoors and outdoors. Sci. Total Environ. 1993, 133, 275–283. [Google Scholar] [CrossRef]
- Vukovic, G.; Urosevic, M.A.; Goryainova, Z.; Pergal, M.; Skrivanj, S.; Samson, R.; Popovic, A. Active moss biomonitoring for extensive screening of urban air pollution: Magnetic and chemical analyses. Sci. Total Environ. 2015, 521–522, 200–210. [Google Scholar] [CrossRef] [PubMed]
- DoŁęgowska, S.; Migaszewski, Z.M. PAH concentrations in the moss species Hylocomium splendens (Hedw.) BSG and Pleurozium schreberi (Brid.) Mitt. from the Kielce area (south-central Poland). Ecotoxicol. Environ. Saf. 2011, 74, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Landis, M.S.; Berryman, S.D.; White, E.M.; Graney, J.R.; Edgerton, E.S.; Studabaker, W.B. Use of an epiphytic lichen and a novel geostatistical approach to evaluate spatial and temporal changes in atmospheric deposition in the Athabasca Oil Sands Region, Alberta, Canada. Sci. Total Environ. 2019, 692, 1005–1021. [Google Scholar] [CrossRef] [PubMed]
- Uribe, D.M.; Ortega, L.M.; Grassi, M.T.; Dolatto, R.G.; Sánchez, N.E. Lichens as bio-monitors of polycyclic aromatic hydrocarbons: Measuring the impact of features and traffic patterns. Heliyon 2023, 9, e20087. [Google Scholar] [CrossRef] [PubMed]
- Root, H.T.; Jovan, S.; Fenn, M.; Amacher, M.; Hall, J.; Shaw, J.D. Lichen bioindicators of nitrogen and sulfur deposition in dry forests of Utah and New Mexico, USA. Ecol. Indic. 2021, 127, 107727. [Google Scholar] [CrossRef]
- Delves, J.; Lewis, J.E.J.; Ali, N.; Asad, S.A.; Chatterjee, S.; Crittenden, P.D.; Jones, M.; Kiran, A.; Pandey, B.P.; Reay, D.; et al. Lichens as spatially transferable bioindicators for monitoring nitrogen pollution. Environ. Pollut. 2023, 328, 121575. [Google Scholar] [CrossRef]
- Špirić, Z.; Stafilov, T.; Vučković, I.; Glad, M. Study of nitrogen pollution in Croatia by moss biomonitoring and Kjeldahl method. J. Environ. Sci. Health A Tox. 2014, 49, 1402–1408. [Google Scholar] [CrossRef]
- Rühling, A.; Tyler, G. Sorption and retention of heavy metals in the woodland moss Hylocomium splendens (Hedw). Oikos 1970, 21, 92–97. [Google Scholar] [CrossRef]
- Berg, T.; Royset, O.; Steinnes, E. Moss (Hylocomium splendens) used as biomonitor of atmospheric trace-element deposition-Estimation of uptake efficiencies. Atmos. Environ. 1995, 29, 353–360. [Google Scholar] [CrossRef]
- Jafarova, M.; Grifoni, L.; Aherne, J.; Loppi, S. Comparison of lichens and mosses as biomonitors of airborne microplastics. Atmosphere 2023, 14, 1007. [Google Scholar] [CrossRef]
- Bertrim, C.; Aherne, J. Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments. Biology 2023, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Sorrentino, M.C.; Granata, A.; Vergara, A.; Alberico, M.; Rossi, M.; Spagnuolo, V.; Giordano, S. Optimizing moss and lichen transplants as biomonitors of airborne anthropogenic microfibers. Biology 2023, 12, 1278. [Google Scholar] [CrossRef] [PubMed]
- Jafarova, M.; Contardo, T.; Aherne, J.; Loppi, S. Lichen biomonitoring of airborne microplastics in Milan (N Italy). Biology 2022, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Loppi, S.; Roblin, B.; Paoli, L.; Aherne, J. Accumulation of airborne microplastics in lichens from a landfill dumping site (Italy). Sci. Rep. 2021, 11, 4564. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, E.; Fortuna, L.; Benesperi, R.; Bianchi, E.; Brunialti, G.; Contardo, T.; Nuzzo L di Frati, L.; Monaci, F.; Munzi, S.; Nascimbene, J.; et al. New interpretative scales for lichen bioaccumulation data: The italian proposal. Atmosphere 2019, 10, 136. [Google Scholar] [CrossRef]
- Capozzi, F.; Giordano, S.; Aboal, J.R.; Adamo, P.; Bargagli, R.; Boquete, T.; Di Palma, A.; Real, C.; Reski, R.; Spagnuolo, V.; et al. Best options for the exposure of traditional and innovative moss bags: A systematic evaluation in three European countries. Environ. Pollut. 2016, 214, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Wayne, R. ImageJ; US National Institutes of Health: Bethesda, MD, USA, 2011; Available online: https://imagej.net/ij/ (accessed on 19 October 2024).
- Jafarova, M.; Grifoni, L.; Renzi, M.; Bentivoglio, T.; Anselmi, S.; Winkler, A.; Di Lella, L.A.; Spagnuolo, L.; Aherne, J.; Loppi, S. Robinia pseudoacacia L. (Black Locust) Leaflets as Biomonitors of Airborne Microplastics. Biology 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Sorrentino, M.C.; Di Palma, A.; Mele, F.; Arena, C.; Adamo, P.; Spagnuolo, V.; Giordano, S. Implication of vitality, seasonality and specific leaf area on PAH uptake in moss and lichen transplanted in bags. Ecol. Indic. 2020, 108, 105727. [Google Scholar] [CrossRef]
- Cowger, W.; Steinmetz, Z.; Gray, A.; Munno, K.; Lynch, J.; Hapich, H.; Primpke, S.; De Frond, H.; Rochman, C.; Herodotou, O. Microplastic Spectral Classification Needs an Open Source Community: Open Specy to the Rescue! Anal. Chem. 2021, 93, 7543–7548. [Google Scholar] [CrossRef]
- Dobson, A.J. An Introduction to Generalized Linear Models, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 19 October 2024).
- Jiao, M.; Wang, Y.; Yang, F.; Zhao, Z.; Wei, Y.; Li, R.; Wang, Y. Dynamic fluctuations in plant leaf interception of airborne microplastics. Sci. Total Environ. 2024, 906, 167877. [Google Scholar] [CrossRef]
- De Vos, L.; Van de Voorde, B.; Van Daele, L.; Dubruel, P.; Van Vlierberghe, S. Poly (alkylene terephthalate) s: From current developments in synthetic strategies towards applications. Eur. Polym. J. 2021, 161, 110840. [Google Scholar] [CrossRef]



| Site | Count | Mass (MF/g ± SE) | Surface Area (MF/g ± SE) | Fiber Length (μm) | ||||
|---|---|---|---|---|---|---|---|---|
| Moss | Lichen | Moss | Lichen | Moss | Lichen | Moss | Lichen | |
| S1 | 6 | 2 | 2.0 ± 0.8 | 0.7 ± 0.7 | 15 ± 6 | 56 ± 56 | 114–966 | 1082–1923 |
| S2 | 8 | 7 | 2.7 ± 0.9 | 2.3 ± 1.3 | 20 ± 7 | 194 ± 111 | 551–2340 | 430–3500 |
| S3 | 6 | 6 | 2.0 ± 0.6 | 2.0 ± 0.2 | 15 ± 4 | 167 ± 17 | 581–3671 | 426–1156 |
| S4 | 10 | 8 | 3.3 ± 1.2 | 2.7 ± 0.7 | 25 ± 9 | 222 ± 56 | 665–4530 | 393–2070 |
| S5 | 4 | 5 | 1.3 ± 0.7 | 1.7 ± 1.7 | 10 ± 5 | 139 ± 139 | 461–2740 | 722–3800 |
| S6 | 8 | 7 | 2.7 ± 1.3 | 2.3 ± 0.9 | 20 ± 10 | 194 ± 73 | 404–2870 | 533–2017 |
| S7 | 12 | 6 | 4.0 ± 2.0 | 2.0 ± 1.2 | 30 ± 15 | 167 ± 96 | 290–2200 | 562–4067 |
| S8 | 9 | 5 | 3.0 ± 1.5 | 1.7 ± 1.2 | 22 ± 11 | 139 ± 100 | 253–2294 | 462–2866 |
| S9 | 16 | 14 | 5.3 ± 0.9 | 4.7 ± 1.7 | 40 ± 7 | 389 ± 139 | 285–3526 | 320–4470 |
| S10 | 2 | 1 | 0.7 ± 0.3 | 0.3 ± 0.3 | 5 ± 2 | 28 ± 28 | 394–1240 | 1427 |
| Al | Ba | Cd | Cr | Cu | Fe | Sb | Zn | |
|---|---|---|---|---|---|---|---|---|
| Lichen | 0.80 ± 0.04 | 0.90 ± 0.02 | 1.23 ± 0.07 | 0.94 ± 0.03 | 1.08 ± 0.04 | 0.91 ± 0.80 | 0.44 ± 0.09 | 1.04 ± 0.04 |
| Moss | 2.03 ± 0.19 | 2.07 ± 0.06 | 0.90 ± 0.05 | 2.14 ± 0.07 | 1.91 ± 0.08 | 2.10 ± 0.07 | 1.47 ± 0.33 | 1.72 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grifoni, L.; Jafarova, M.; La Colla, N.S.; Aherne, J.; Raulli, A.; Loppi, S. Comparison of Lichen and Moss Transplants for Monitoring the Deposition of Airborne Microfibers. Sustainability 2025, 17, 537. https://doi.org/10.3390/su17020537
Grifoni L, Jafarova M, La Colla NS, Aherne J, Raulli A, Loppi S. Comparison of Lichen and Moss Transplants for Monitoring the Deposition of Airborne Microfibers. Sustainability. 2025; 17(2):537. https://doi.org/10.3390/su17020537
Chicago/Turabian StyleGrifoni, Lisa, Mehriban Jafarova, Noelia S. La Colla, Julian Aherne, Alessio Raulli, and Stefano Loppi. 2025. "Comparison of Lichen and Moss Transplants for Monitoring the Deposition of Airborne Microfibers" Sustainability 17, no. 2: 537. https://doi.org/10.3390/su17020537
APA StyleGrifoni, L., Jafarova, M., La Colla, N. S., Aherne, J., Raulli, A., & Loppi, S. (2025). Comparison of Lichen and Moss Transplants for Monitoring the Deposition of Airborne Microfibers. Sustainability, 17(2), 537. https://doi.org/10.3390/su17020537










