Health and Environmental Risk Assessment of Utilization Products of Aluminum–Chromium Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Analysis
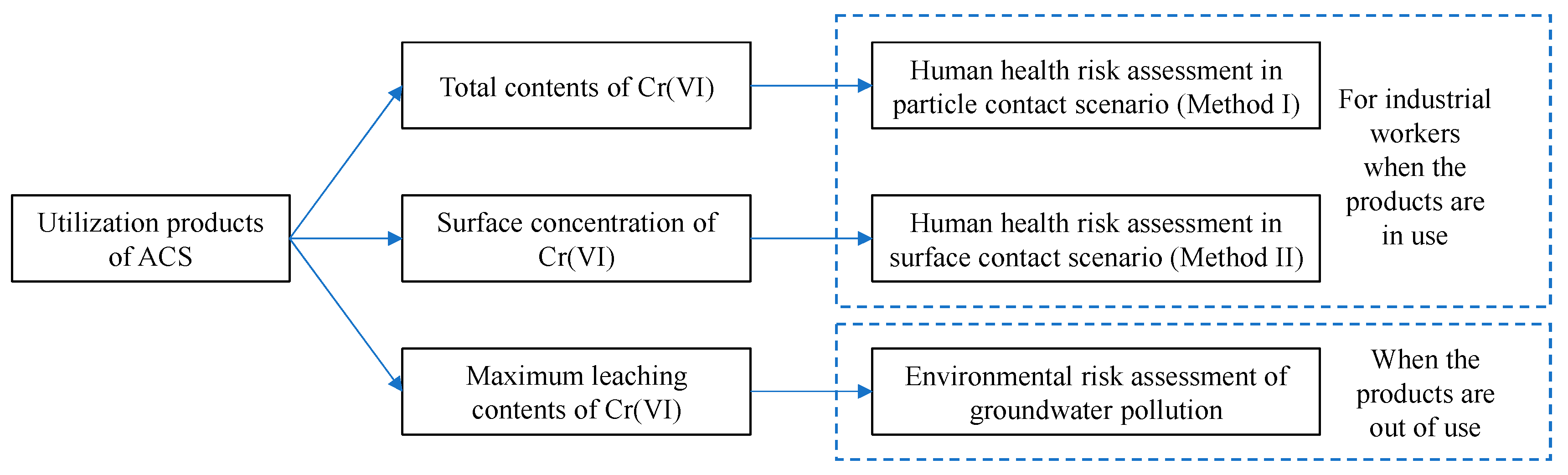
2.3. Health Risk Assessment
2.4. Environmental Risk Assessment
2.5. Quality Control and Data Analysis
3. Results and Discussion
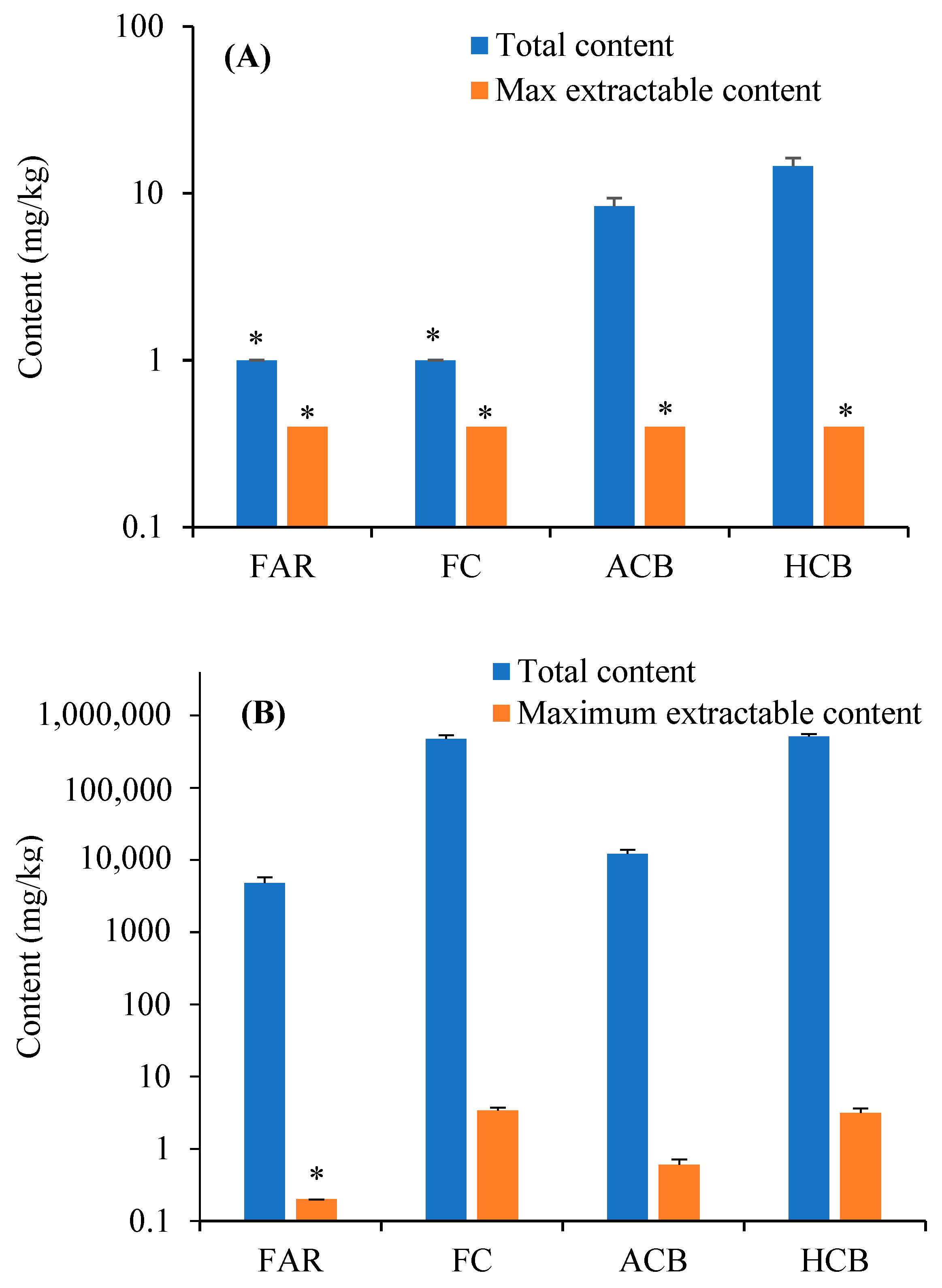
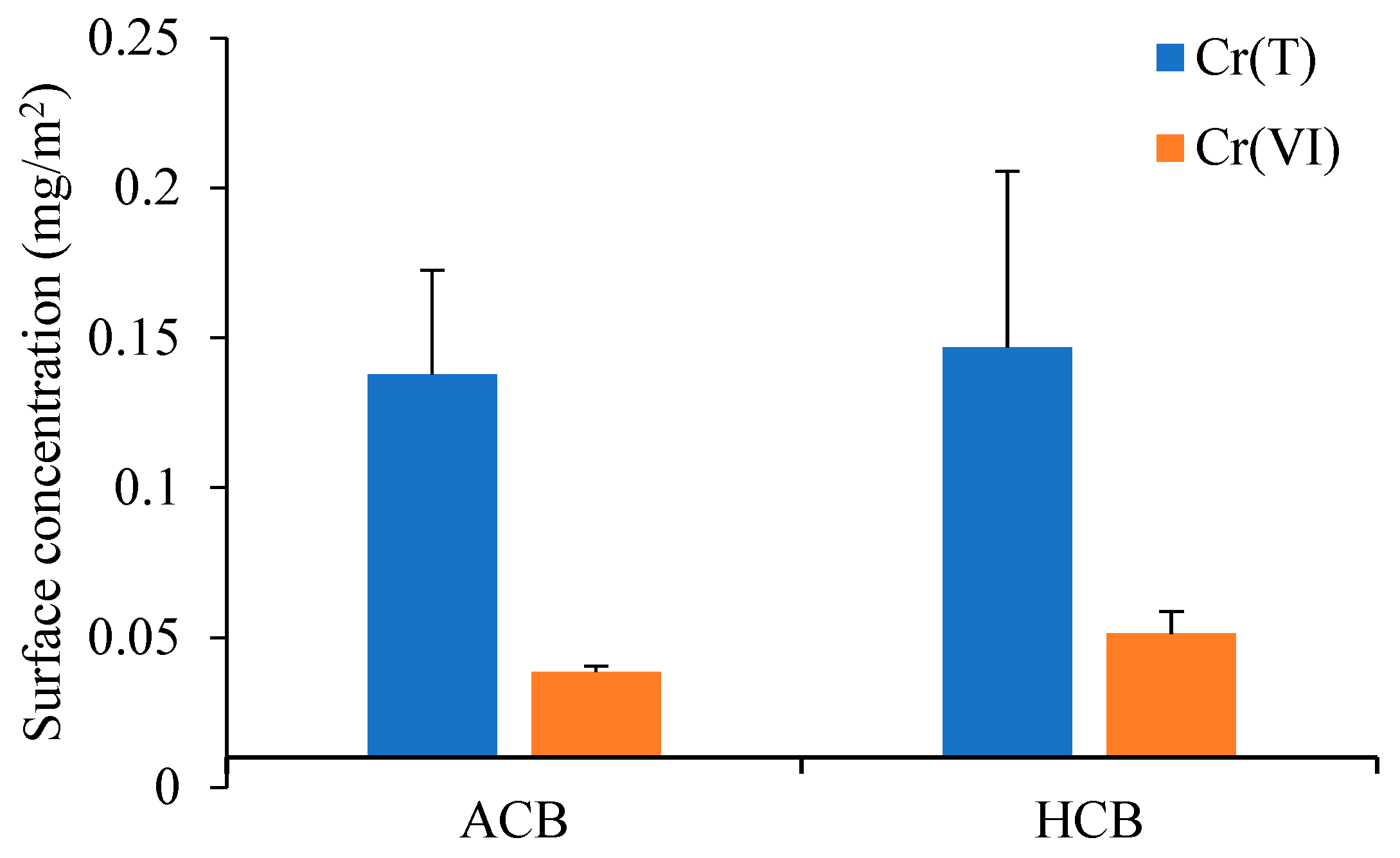
3.1. Heavy Metals in ACS and Utilization Products
3.2. Human Health Risk of ACSUtilization Products
3.3. Environmental Risk of ACS Utilization Products
3.4. Derivation of Limits for Cr(VI) in Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, P.; Zhang, H.; Yu, J.; Gao, H.; Cao, Y.; Zhu, Y.; Zhao, H. Conditions for mutual conversion of Cr(III) and Cr(VI) in aluminum chromium slag. J. Alloys Compd. 2019, 788, 506–513. [Google Scholar] [CrossRef]
- Fan, R.; Zhao, H.; Zhang, H.; Zhao, P.; Chen, J.; Wang, X. Effect of partial substitution of alumina–chromium slag for Al2O3 on microstructures and properties of Al2O3–SiC–C trough castables. Ceram. Int. 2019, 45, 11204–11215. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, H.; Gao, H.; Zhu, Y.; Yu, J.; Fan, R.; Zhao, H. Formation process and properties of aluminium–chromium slag. Ceram. Int. 2018, 44, 15730–15734. [Google Scholar] [CrossRef]
- Xu, J.; Ma, G.; Liu, M.; Zhang, X.; Zheng, D.; Du, T.; Luo, Y.; Zhang, W. Understanding chromium slag recycling with sintering–ironmaking processes: Influence of Cr2O3 on the sinter microstructure and mechanical properties of the silico–ferrite of calcium and aluminum (SFCA). Molecules 2024, 29, 2382. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Huang, S.; Yang, Z.; Peng, B.; Huang, Y.; Chen, Y. Cr(VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J. Hazard. Mater. 2009, 167, 516–522. [Google Scholar] [CrossRef]
- Xu, Z.; Yao, J.; Fu, R. Characteristic, resource approaches and safety utilization assessment of non-ferrous metal smelting slags: A literature review. J. Cent. South Univ. 2024, 31, 1178–1196. [Google Scholar] [CrossRef]
- Guo, X.; Wang, K.; He, M.; Liu, Z.; Yang, H.; Li, S. Antimony smelting process generating solid wastes and dust: Characterization and leaching behaviors. J. Environ. Sci. 2014, 26, 1549–1556. [Google Scholar] [CrossRef]
- Garrabrants, A.C.; Kosson, D.S.; Brown, K.G.; Fagnant, D.P.; Helms, G.; Thorneloe, S.A. Demonstration of the use of test results from the Leaching Environmental Assessment Framework (LEAF) to develop screening-level leaching assessments. Waste Manag. 2021, 121, 226–236. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Xu, Y.; Nie, Q.; Yang, Z.; Sheng, W.; Qian, G. Municipal solid waste incineration residues recycled for typical construction materials-a review. RSC Adv. 2022, 12, 6279–6291. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Tong, L.; He, J.; Wang, F.; Wang, Y.; Wang, L.; Tsang, D.C.W.; Hu, Q.; Hu, B.; Tang, Y. Evaluation of the BCR sequential extraction scheme for trace metal fractionation of alkaline municipal solid waste incineration fly ash. Chemosphere 2020, 249, 126115. [Google Scholar] [CrossRef]
- Liang, D.; Wang, F.; Lv, G. The resource utilization and environmental assessment of MSWI Fly ash with solidification and stabilization: A review. Waste Biomass Valoriz. 2024, 15, 37–56. [Google Scholar] [CrossRef]
- Men, D.; Yao, J.; Li, H.; Jordan, G.; Zhao, B.; Cao, Y.; Ma, B.; Liu, B.; Sun, Y.; Ban, J. The potential environmental risk implications of two typical non-ferrous metal smelting slags: Contrasting toxic metal(loid)s leaching behavior and geochemical characteristics. J. Soils Sediments 2023, 23, 1944–1959. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Li, Z.; Wen, H.; Yang, K.; Zhang, L. Characterization and environmental risk assessment of coal-based solid waste towards underground backfilling. KSCE J. Civ. Eng. 2024, 28, 1141–1150. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, X.; Wang, W.; Li, Z.; Zhang, L.; Cheng, S. Utilization of MSWI fly ash as partial cement or sand substitute with focus on cementing efficiency and health risk assessment. Front. Environ. Sci. Eng. 2019, 14, 5. [Google Scholar] [CrossRef]
- Xu, X.; Hua, J.; Zhang, H.; Zhao, Z.; Wang, Y.; Zhang, D.; Zhang, J.; Chen, X. Environmental risk assessment of recycled products of spent coppery etchant in Jiangsu Province, China. Int. J. Environ. Res. Public Health 2021, 18, 7881. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Tian, L.; Zhou, J.; Wang, Y. Risk and pollutant protective concentration levels of drilling waste used to pave oil and gas field well sites. Water 2025, 17, 30. [Google Scholar] [CrossRef]
- Spreadbury, C.J.; Clavier, K.A.; Lin, A.M.; Townsend, T.G. A critical analysis of leaching and environmental risk assessment for reclaimed asphalt pavement management. Sci. Total Environ. 2021, 775, 145741. [Google Scholar] [CrossRef]
- Arroyo, F.; Luna-Galiano, Y.; Leiva, C.; Vilches, L.F.; Fernández-Pereira, C. Environmental risks and mechanical evaluation of recycling red mud in bricks. Environ. Res. 2020, 186, 109537. [Google Scholar] [CrossRef] [PubMed]
- Gencel, O.; Munir, M.J.; Kazmi, S.M.S.; Sutcu, M.; Erdogmus, E.; Velasco, P.M.; Quesada, D.E. Recycling industrial slags in production of fired clay bricks for sustainable manufacturing. Ceram. Int. 2021, 47, 30425–30438. [Google Scholar] [CrossRef]
- HJ 766-2015; Solid Waste—Determination of Metals–Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Ministry of Environmental Protection of China: Beijing, China, 2015. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201511/t20151130_317999.shtml (accessed on 10 May 2023).
- HJ 702-2014; Solid Waste—Determination of Mercury, Arsenic, Selenium, Bismuth, Antimony—Microwave Dissolution/Atomic Fluorescence Spectrometry. Ministry of Environmental Protection of China: Beijing, China, 2014. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201409/t20140912_288936.shtml (accessed on 10 May 2023).
- HJ 687-2014; Solid Waste—Determination of Hexavalent Chromium—By Alkaline Digestion/Flame Atomic Absorption Spectrophotometic. Ministry of Environmental Protection of China: Beijing, China, 2014. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201401/t20140120_266671.shtml (accessed on 1 April 2014).
- HJ/T 299-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric acid Method. Environmental Protection Bureau of China: Beijing, China, 2007. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/200704/t20070418_102859.shtml (accessed on 10 May 2022).
- GB/T 15555.4-1995; Solid waste-Determination of Chromium(VI)-1,5-Diphenylcarbohydrazide Spectrophotometric Method. Environmental Protection Bureau of China: Beijing, China, 1995. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/199601/t19960101_82019.shtml (accessed on 10 May 2022).
- EPA/600/R-11/079; A Literature Review of Wipe Sampling Methods for Chemical Warfare Agents and Toxic Industrial Chemicals. United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2007.
- ASTM D6661-17; Standard Practice for Field Collection of Organic Compounds from Surfaces Using Wipe Sampling. Advancing Standards Transforming Markets (ASTM): West Conshohocken, PA, USA, 2006.
- HJ 25.3-2019; Technical Guidelines for Risk Assessment of Soil Contamination of Land for Construction. Ministry of Ecology and Environment of China: Beijing, China, 2019. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201912/t20191224_749893.shtml (accessed on 10 December 2019).
- Sun, Y.; Wang, J.; Guo, G.; Li, H.; Jones, K. A comprehensive comparison and analysis of soil screening values derived and used in China and the UK. Environ. Pollut. 2020, 256, 113404. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Regional Screening Levels (RSLs)—User’s Guide. 2021. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-users-guide (accessed on 8 August 2024).
- US EPA. Regional Screening Levels (RSLs)—Generic Tables. 2024. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 8 August 2024).
- Zhang, R.; Han, D.; Jiang, L.; Zhong, M.; Liang, J.; Xia, T.; Zhao, Y. Derivation of site-specific remediation goals by incorporating the bioaccessibility of polycyclic aromatic hydrocarbons with the probabilistic analysis method. J. Hazard. Mater. 2020, 384, 121239. [Google Scholar] [CrossRef] [PubMed]
- May, L.M.; Gaborek, B.; Pitrat, T.; Peters, L. Derivation of risk based wipe surface screening levels for industrial scenarios. Sci. Total Environ. 2002, 288, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wulijia, B.; Wang, L.; Li, Y.; Liao, X. New perspective on human health risk from polycyclic aromatic hydrocarbons (PAHs) on surfaces of structures and buildings for industrial legacy before and after remediation. J. Clean. Prod. 2022, 379, 134828. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Gu, R.; Liang, Z.; Xu, W.; Jat Baloch, M.Y. Health risk assessment during in situ remediation of Cr(VI)-contaminated groundwater by permeable reactive barriers: A field-scale study. Int. J. Environ. Res. Public Health 2022, 19, 13079. [Google Scholar] [CrossRef]
- Garrabrants, A.C.; Kosson, D.S.; Brown, K.G.; Fagnant, D.P.; Helms, G.; Thorneloe, S.A. Methodology for scenario-based assessments and demonstration of treatment effectiveness using the Leaching Environmental Assessment Framework (LEAF). J. Hazard. Mater. 2021, 406, 124635. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Guo, B. Revising the EPA dilution-attenuation soil screening model for PFAS. J. Hazard. Mater. Lett. 2023, 4, 100077. [Google Scholar] [CrossRef]
- GB 36600-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Development Land. Ministry of Ecology and Environment of China: Beijing, China, 2018. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201807/t20180703_446027.shtml (accessed on 1 August 2018).
- Xu, R.; Wang, Y.-N.; Li, S.; Sun, Y.; Gao, Y.; Guo, L.; Wang, H. Effective Cr(VI) reduction and immobilization in chromite ore processing residue (COPR) contaminated soils by ferrous sulfate and digestate: A comparative investigation with typical reducing agents. Ecotoxicol. Environ. Saf. 2023, 265, 115522. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Yang, Y.; Huang, Z.; Wang, Q. Formulation of criteria for pollution control on cement products produced from solid wastes in China. J. Environ. Manag. 2011, 92, 1931–1937. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yang, M.; Jiang, L.; Zhong, M.; Ma, L.; Wang, S.; Zhang, W.; Gong, Y.; Li, D. New insight into human health risk from polycyclic aromatic hydrocarbons on surfaces of buildings and facilities for industrial legacy regeneration. J. Hazard. Mater. 2022, 436, 129158. [Google Scholar] [CrossRef]
- Gaborek, B.J.; Mullikin, J.M.; Pitrat, T.; Cummings, L.; May, L.M. Pentagon surface wipe sampling health risk assessment. Toxicol. Ind. Health 2001, 17, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Khoshakhlagh, A.H.; Mohammadzadeh, M.; Manafi, S.S.; Yousefian, F.; Gruszecka-Kosowska, A. Inhalational exposure to formaldehyde, carcinogenic, and non-carcinogenic risk assessment: A systematic review. Environ. Pollut. 2023, 331, 121854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, P.; Yuan, B.; Zhang, Y.; Chen, J.; Liu, H.; Li, A.; Wei, Y.; Xiong, Y.; Zhao, B. Multifaceted insight into sensitivity analysis and environmental impact on human health of soil contamination risk assessment. Environ. Health 2023, 1, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chu, H.; Shi, W.; Wang, F.; Jiang, J. Utilization of municipal solid waste incineration fly ash in ecological concrete and pavement bricks: Mechanical properties and environmental impact. Case Stud. Constr. Mater. 2024, 21, e03999. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Hu, Y.; Cheng, H. Environmental performance of unfired bricks produced from co-disposal of mine tailings and municipal solid waste incineration fly ash based on comprehensive leaching tests. Environ. Pollut. 2024, 347, 123795. [Google Scholar] [CrossRef]
- Wang, C.; Liu, K.; Huang, D.; Huang, Q.; Wang, P.; Mei, X.; Li, S. Characteristic pollutants risk assessment of modified manganese residue utilization in sintered product. Environ. Sci. Pollut. Res. 2022, 29, 88369–88382. [Google Scholar] [CrossRef]
- US EPA. Exposure Factors Handbook. EPA/600/R-09/052F. 2011. Available online: https://www.epa.gov/expobox/exposure-factors-handbook-2011-edition (accessed on 8 August 2024).
- US EPA. ProUCL: Statistical Software for Environmental Applications for Data Sets with and Without Nondetect Observations. Version 5.2. 2022. Available online: https://www.epa.gov/land-research/proucl-software (accessed on 4 June 2023).
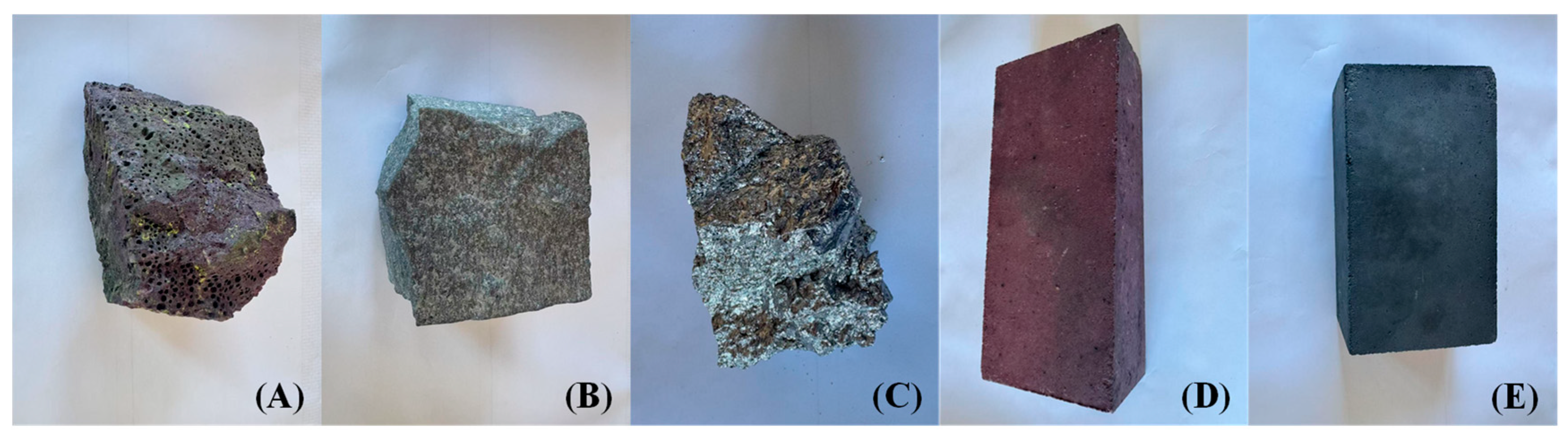


| Parameter | Unit | Value |
|---|---|---|
| SFo | (mg/kg/d)−1 | 0.5 |
| SFd | (mg/kg/d)−1 | 20 |
| SFi | (mg/kg/d)−1 | 51.1 |
| RfDo | mg/kg/d | 0.003 |
| RfDd | mg/kg/d | 7.5 × 10−5 |
| RfDi | mg/kg/d | 2.35 × 10−5 |
| ABSd | dimensionless | 0 |
| Parameter | Definition and Unit | Value |
|---|---|---|
| EDa | Exposure duration of adults, a | 25 |
| EFa | Exposure frequency of adults, d/a | 250 |
| BWa | Body weight of adults, kg | 61.8 |
| IRAa | Daily air inhalation rate of adults, m3/d | 14.5 |
| OIRa | Oral ingestion rate of dusts of adults, mg/d | 50 |
| Ev | Contact frequency with surface, d−1 | 12 |
| AFa | Adherence rate of soil on skin for adults, mg/m2 | 0.2 |
| SAd | Dermal contact area, cm2 | 800 |
| PM | Content of inhalable particulates in air, mg/m3 | 0.425 |
| PIAF | Retention fraction of inhaled particulates in body, dimensionless | 0.75 |
| fspi | Workshop airborne particulate fraction, dimensionless | 0.8 |
| EFIa | Exposure frequency of workers to particles in a workshop, d/a | 84 |
| ABSo | Absorption factor of oral ingestion, dimensionless | 1 |
| CF | Convention factor, kg/mg | 10−6 |
| ATca | Time for carcinogenic effect, d | 27,740 |
| ATnc | Time for non-carcinogenic effect, d | 9125 |
| Parameter | Definition and Unit | Value |
|---|---|---|
| SAd | Dermal contact area, m2 | 0.08 |
| Fd | Fraction of available dermal area that contacts the surface, dimensionless | 0.25 |
| SAg | Dermal surface available for ingestion, m2 | 0.28 |
| Fg | Fraction of available dermal area that contacts mouth, dimensionless | 0.1 |
| FTss | Fraction of dust transferred from surface to skin, dimensionless | 0.1 |
| FTsm | Fraction of dust transferred from skin to mouth, dimensionless | 0.3 |
| HTME | Hand to mouth events, dimensionless | 3 |
| K | Resuspension factor, m−1 | 5 × 10−8 |
| Products | Ct (mg/kg) | Carcinogenic Risk | Non-Carcinogenic Risk | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CRo1 | CRd1 | CRi1 | CR1 | HIo1 | HId1 | HIi1 | HI1 | ||
| FAR | 1 * | 9.11 × 10−8 | 0 | 2.32 × 10−7 | 3.23 × 10−7 | 1.85 × 10−4 | 0 | 5.87 × 10−4 | 7.72 × 10−4 |
| FC | 1 * | 9.11 × 10−8 | 0 | 2.32 × 10−7 | 3.23 × 10−7 | 1.85 × 10−4 | 0 | 5.87 × 10−4 | 7.72 × 10−4 |
| ACB | 7 10 8.3 1.0 | 6.38 × 10−7 9.11 × 10−7 7.59 × 10−7 9.41 × 10−8 | 0 0 0 0 | 1.62 × 10−6 2.32 × 10−6 1.93 × 10−6 2.40 × 10−7 | 2.26 × 10−6 3.23 × 10−6 2.69 × 10−6 3.34 × 10−7 | 1.30 × 10−3 1.85 × 10−3 1.54 × 10−3 1.91 × 10−4 | 0 0 0 0 | 4.11 × 10−3 5.87 × 10−3 4.89 × 10−3 6.06 × 10−4 | 5.40 × 10−3 7.72 × 10−3 6.43 × 10−3 7.97 × 10−4 |
| HCB | 12 16 14.5 1.8 | 1.10 × 10−6 1.46 × 10−6 1.32 × 10−6 1.61 × 10−7 | 0 0 0 0 | 2.78 × 10−6 3.71 × 10−6 3.36 × 10−6 4.08 × 10−7 | 3.87 × 10−6 5.16 × 10−6 4.68 × 10−6 5.68 × 10−7 | 2.22 × 10−3 2.96 × 10−3 2.68 × 10−3 3.26 × 10−4 | 0 0 0 0 | 7.04 × 10−3 9.39 × 10−3 8.51 × 10−3 1.03 × 10−3 | 9.26 × 10−3 1.24 × 10−2 1.12 × 10−2 1.36 × 10−3 |
| Products | Csurf (mg/m2) | Carcinogenic Risk | Non-Carcinogenic Risk | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CRo2 | CRd2 | CRi2 | CR2 | HIo2 | HId2 | HIi2 | HI2 | ||
| ACB | 0.035 | 1.85 × 10−6 | 0 | 4.54 × 10−9 | 1.86 × 10−6 | 3.76 × 10−3 | 0 | 1.15 × 10−5 | 3.77 × 10−3 |
| 0.041 | 2.17 × 10−6 | 0 | 5.32 × 10−9 | 2.18 × 10−6 | 4.40 × 10−3 | 0 | 1.35 × 10−5 | 4.41 × 10−3 | |
| 0.039 | 2.04 × 10−6 | 0 | 5.00 × 10−9 | 2.04 × 10−6 | 4.13 × 10−3 | 0 | 1.27 × 10−5 | 4.14 × 10−3 | |
| 0.002 | 1.10 × 10−7 | 0 | 2.69 × 10−10 | 1.10 × 10−7 | 2.23 × 10−4 | 0 | 6.83 × 10−7 | 2.23 × 10−4 | |
| HCB | 0.042 | 2.22 × 10−6 | 0 | 5.45 × 10−9 | 2.23 × 10−6 | 4.50 × 10−3 | 0 | 1.38 × 10−5 | 4.52 × 10−3 |
| 0.061 | 3.23 × 10−6 | 0 | 7.92 × 10−9 | 3.24 × 10−6 | 6.54 × 10−3 | 0 | 2.01 × 10−5 | 6.56 × 10−3 | |
| 0.051 | 2.72 × 10−6 | 0 | 6.66 × 10−9 | 2.72 × 10−6 | 5.50 × 10−3 | 0 | 1.69 × 10−5 | 5.52 × 10−3 | |
| 0.007 | 3.92 × 10−7 | 0 | 9.60 × 10−10 | 3.92 × 10−7 | 7.93 × 10−4 | 0 | 2.44 × 10−6 | 7.95 × 10−4 | |
| Type of Cr(VI) Limit for Utilization Products | Value |
|---|---|
| Limit of Cr(VI) content in product (mg/kg) | 31 |
| Limit of Cr(VI) concentration on surface of refractory brick (mg/m2) | 0.189 |
| Limit of Cr(VI) leaching concentration in product (mg/L) | 0.259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, H.; Wang, J.; Jia, S.; Xu, Y. Health and Environmental Risk Assessment of Utilization Products of Aluminum–Chromium Slag. Sustainability 2025, 17, 8852. https://doi.org/10.3390/su17198852
Hou H, Wang J, Jia S, Xu Y. Health and Environmental Risk Assessment of Utilization Products of Aluminum–Chromium Slag. Sustainability. 2025; 17(19):8852. https://doi.org/10.3390/su17198852
Chicago/Turabian StyleHou, Haimeng, Jian Wang, Shu Jia, and Yong Xu. 2025. "Health and Environmental Risk Assessment of Utilization Products of Aluminum–Chromium Slag" Sustainability 17, no. 19: 8852. https://doi.org/10.3390/su17198852
APA StyleHou, H., Wang, J., Jia, S., & Xu, Y. (2025). Health and Environmental Risk Assessment of Utilization Products of Aluminum–Chromium Slag. Sustainability, 17(19), 8852. https://doi.org/10.3390/su17198852






