Design and Implementation of a Small-Scale Hydroponic Chamber for Sustainable Vegetative Propagation from Cuttings: A Basil (Ocimum basilicum L.)
Abstract
1. Introduction
2. Materials and Methods
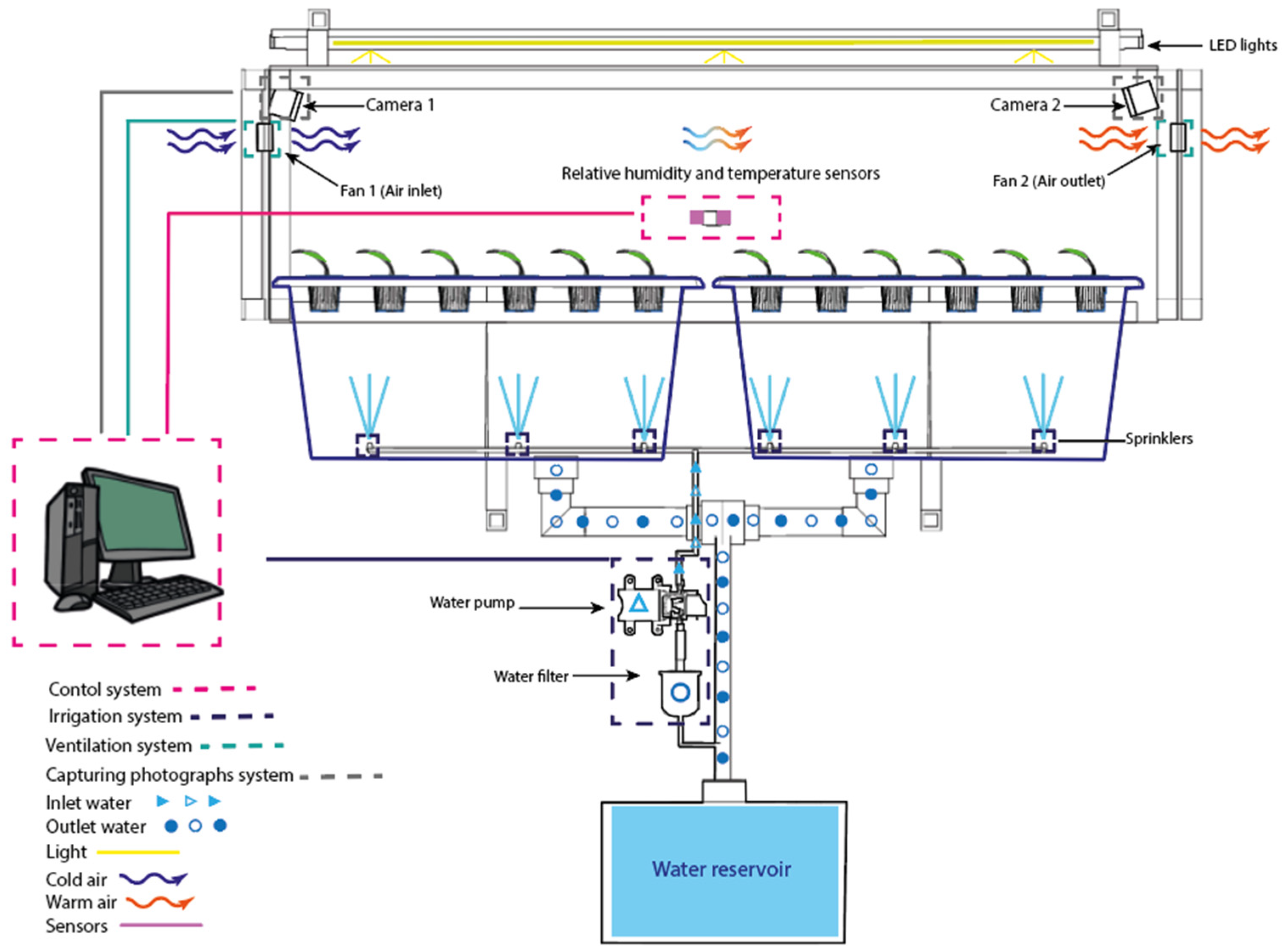
2.1. Structural Design and Construction of the SSHG
2.2. Vegetative Propagation Protocol and Control Parameters
3. Results
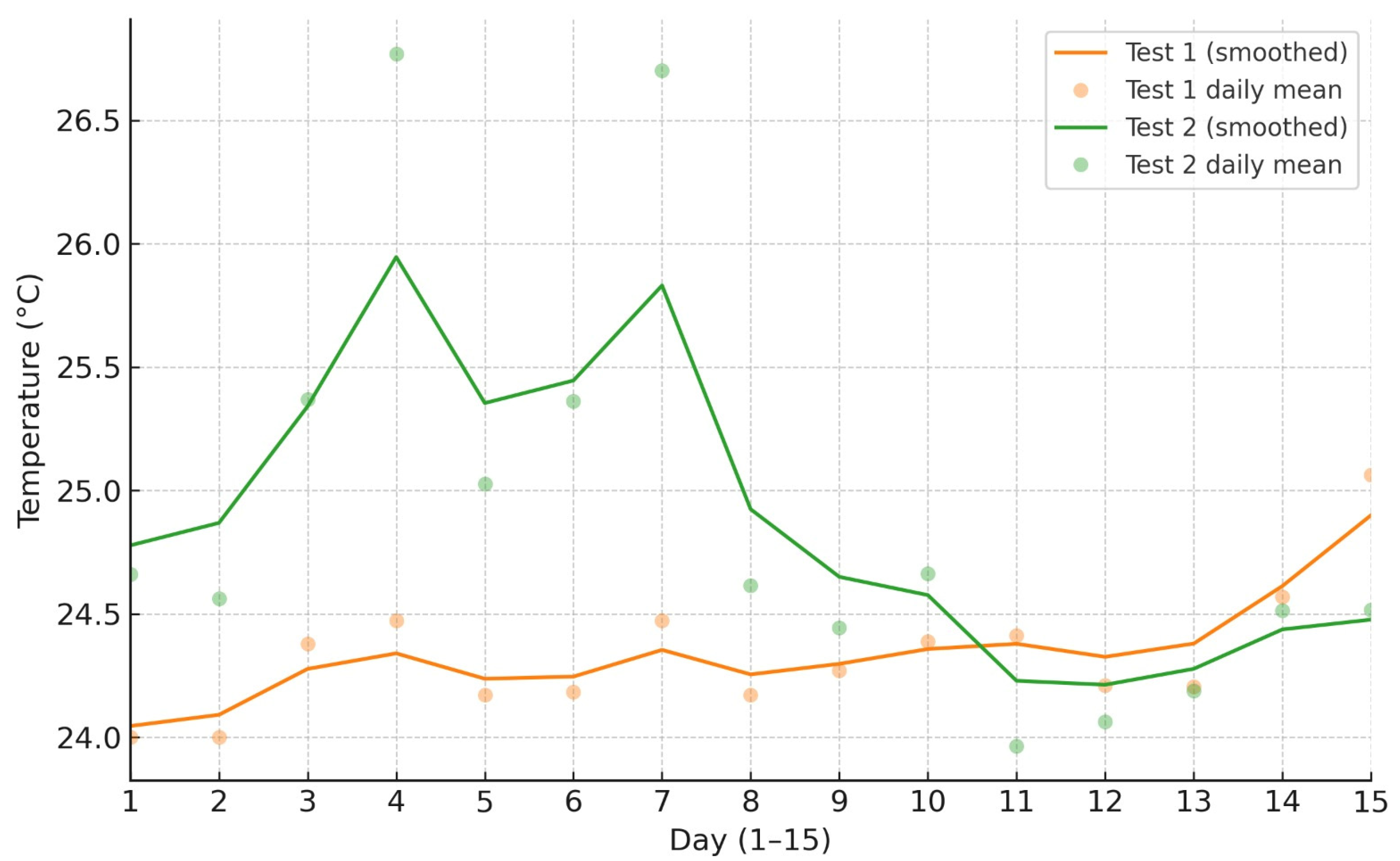
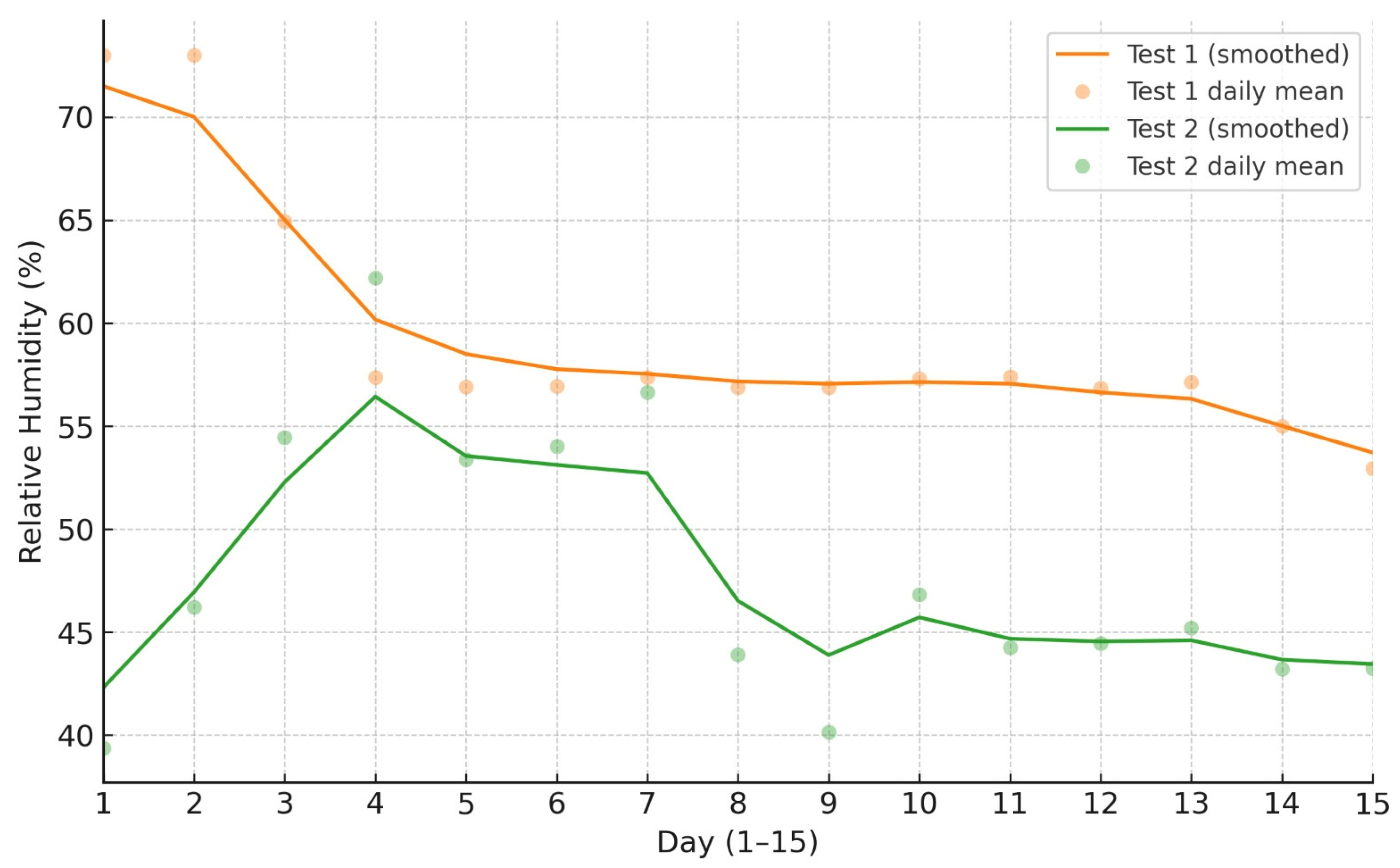
3.1. Enviromental Control and Monitoring
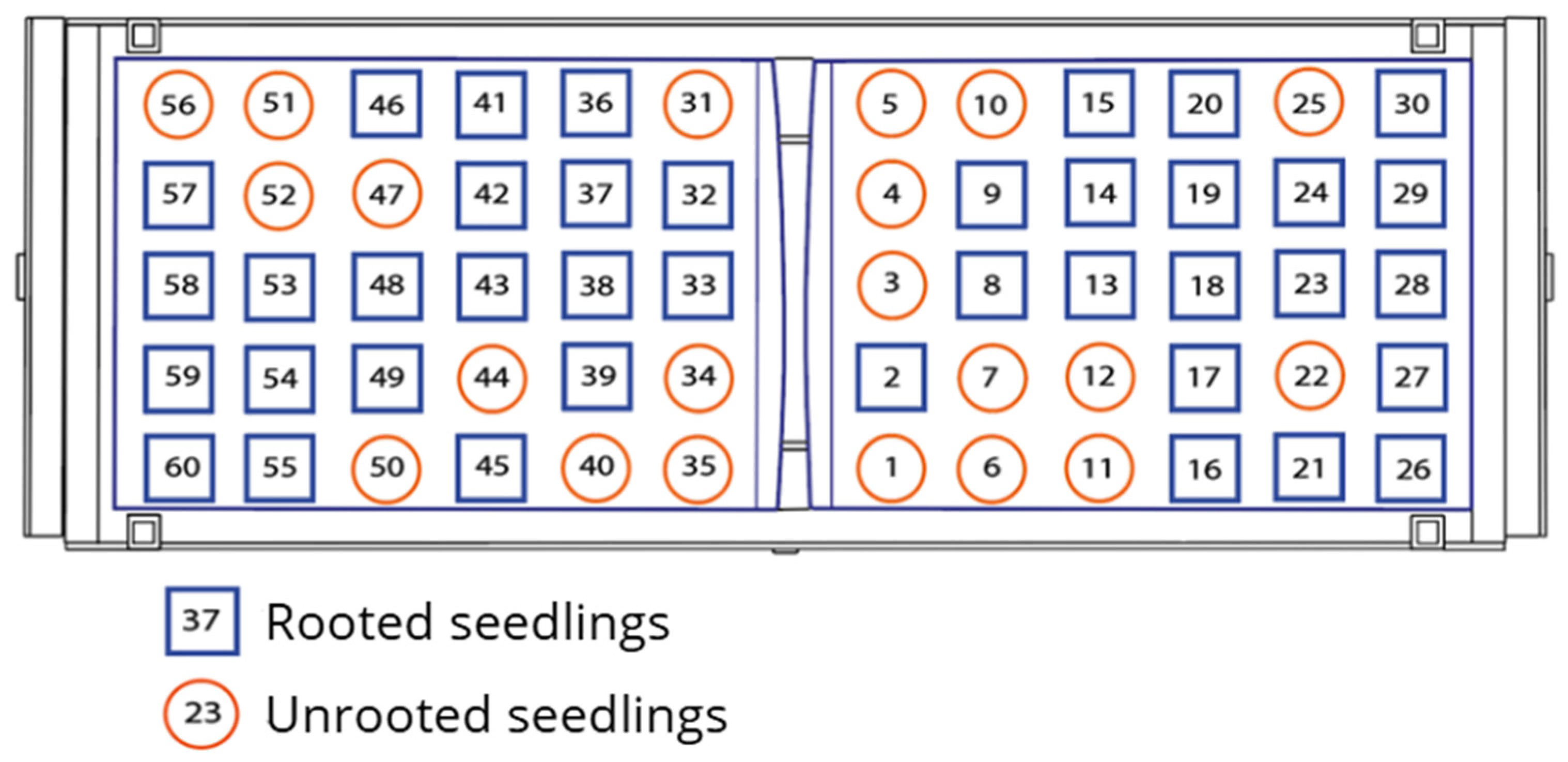
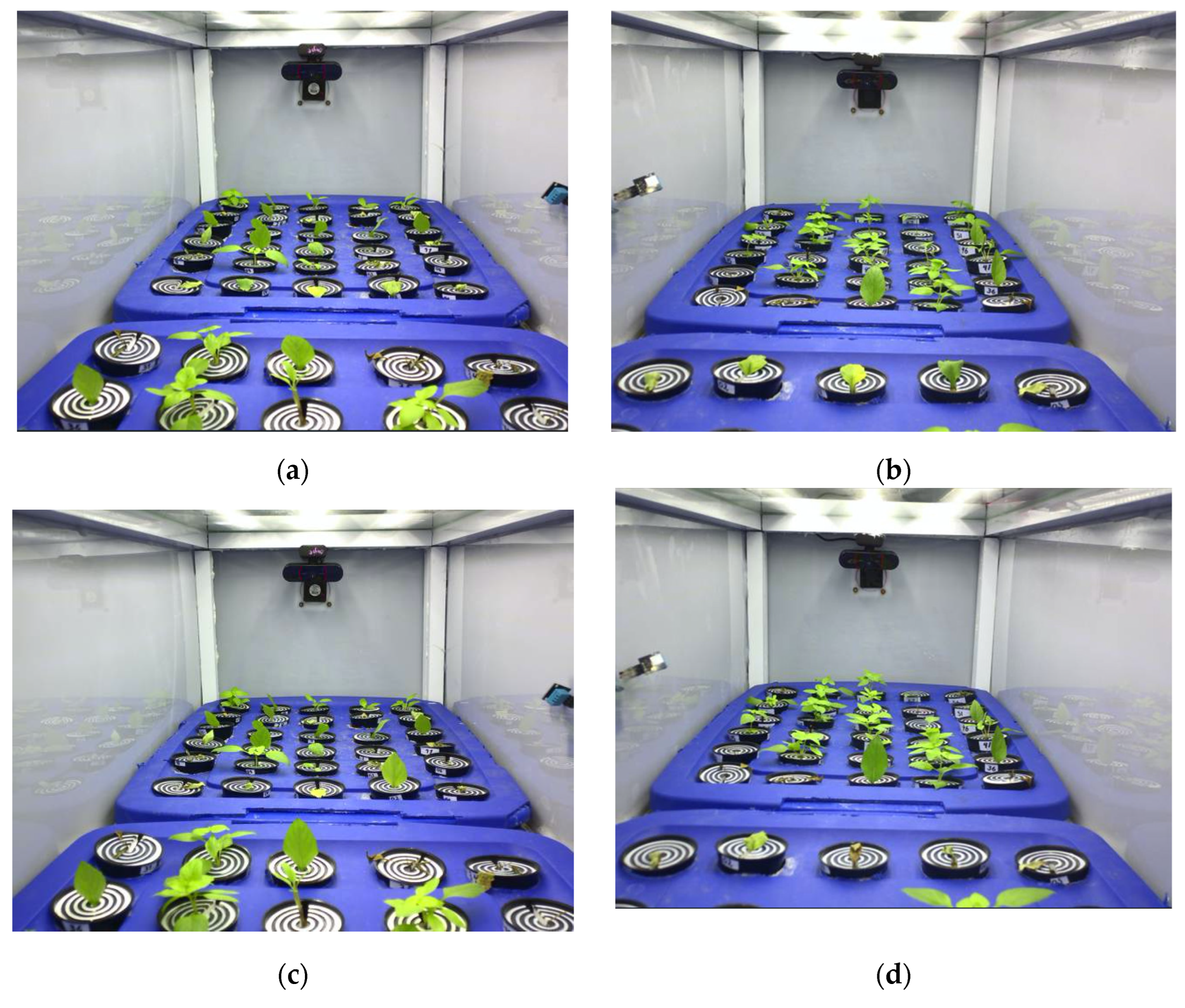
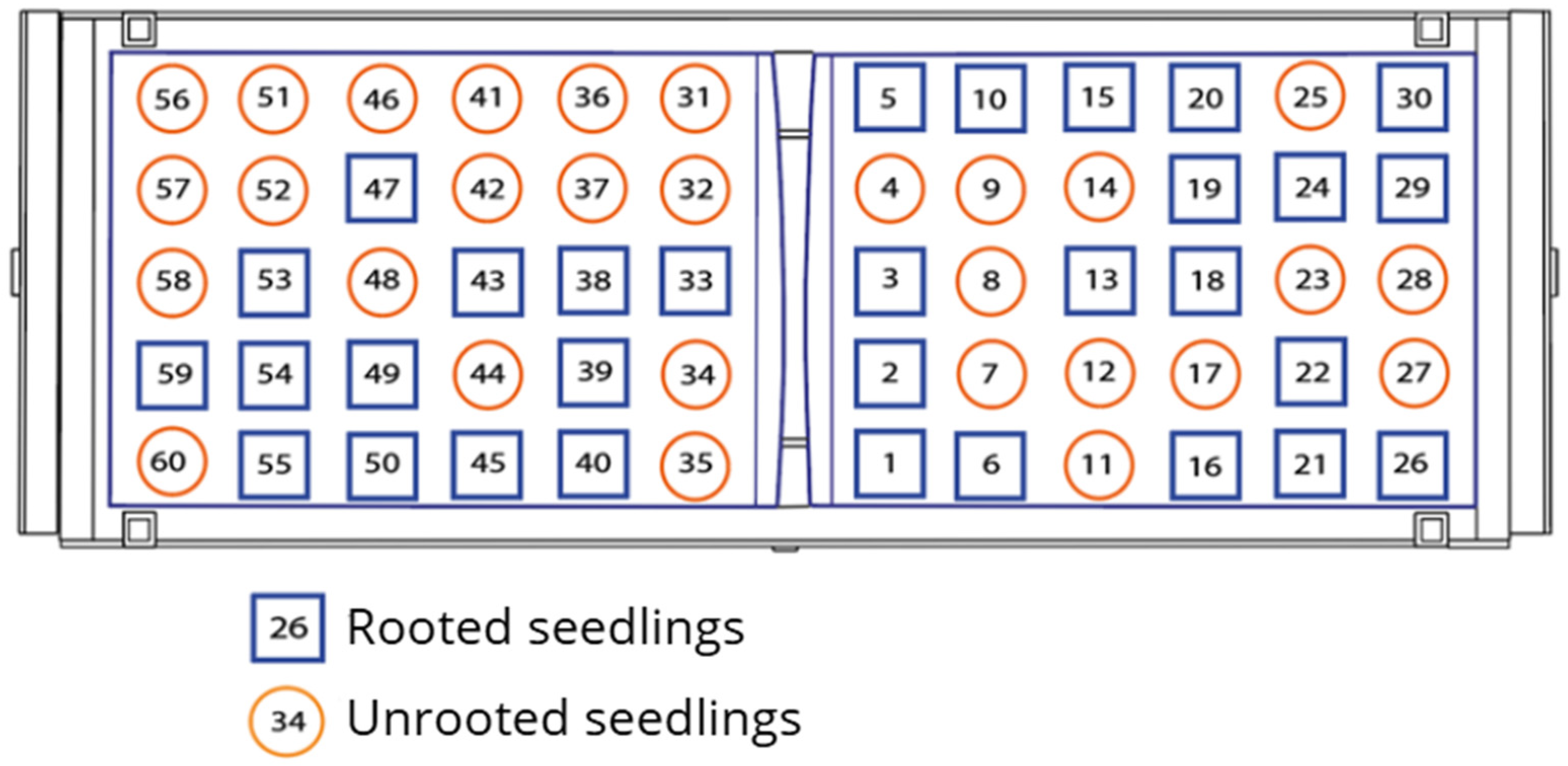
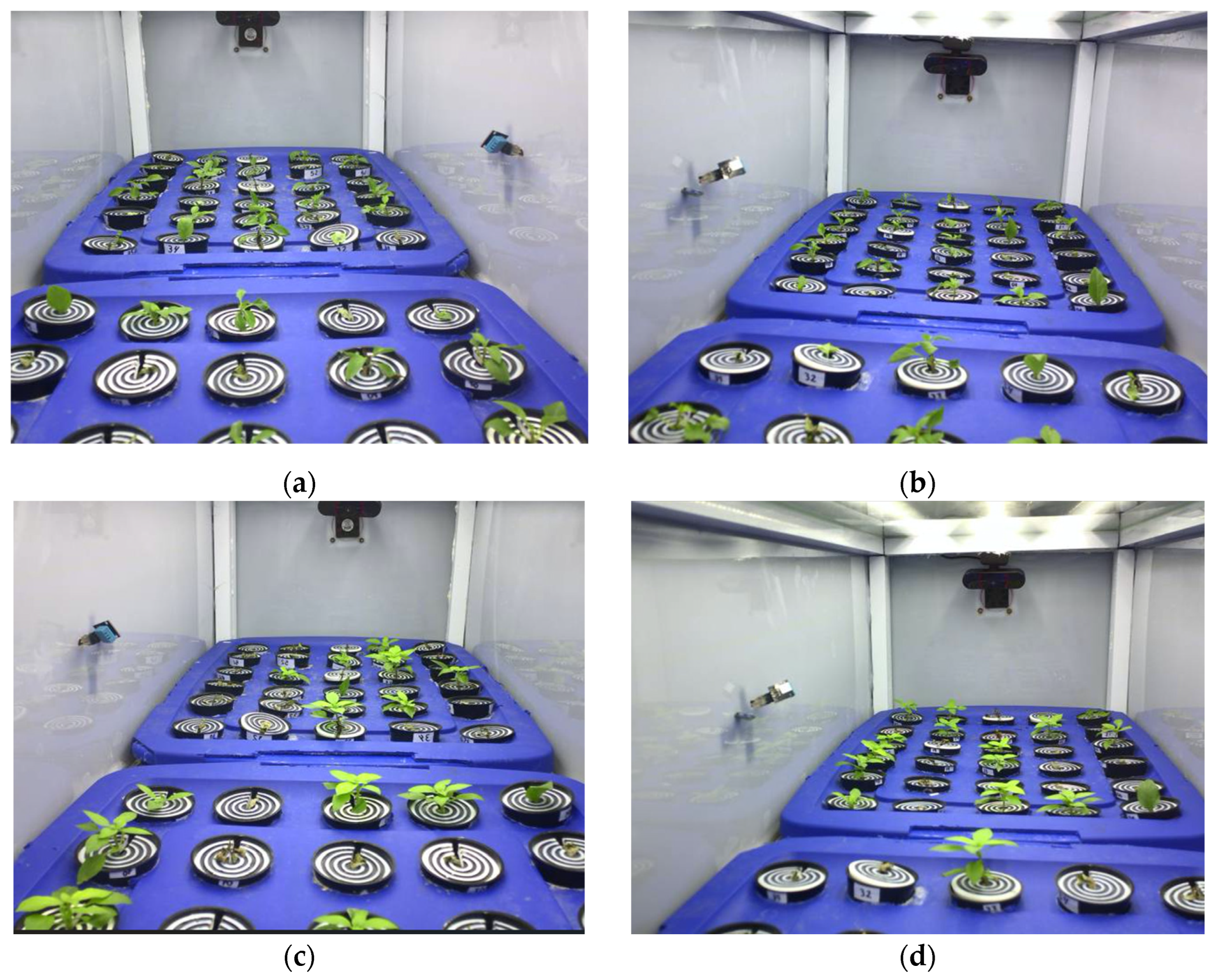
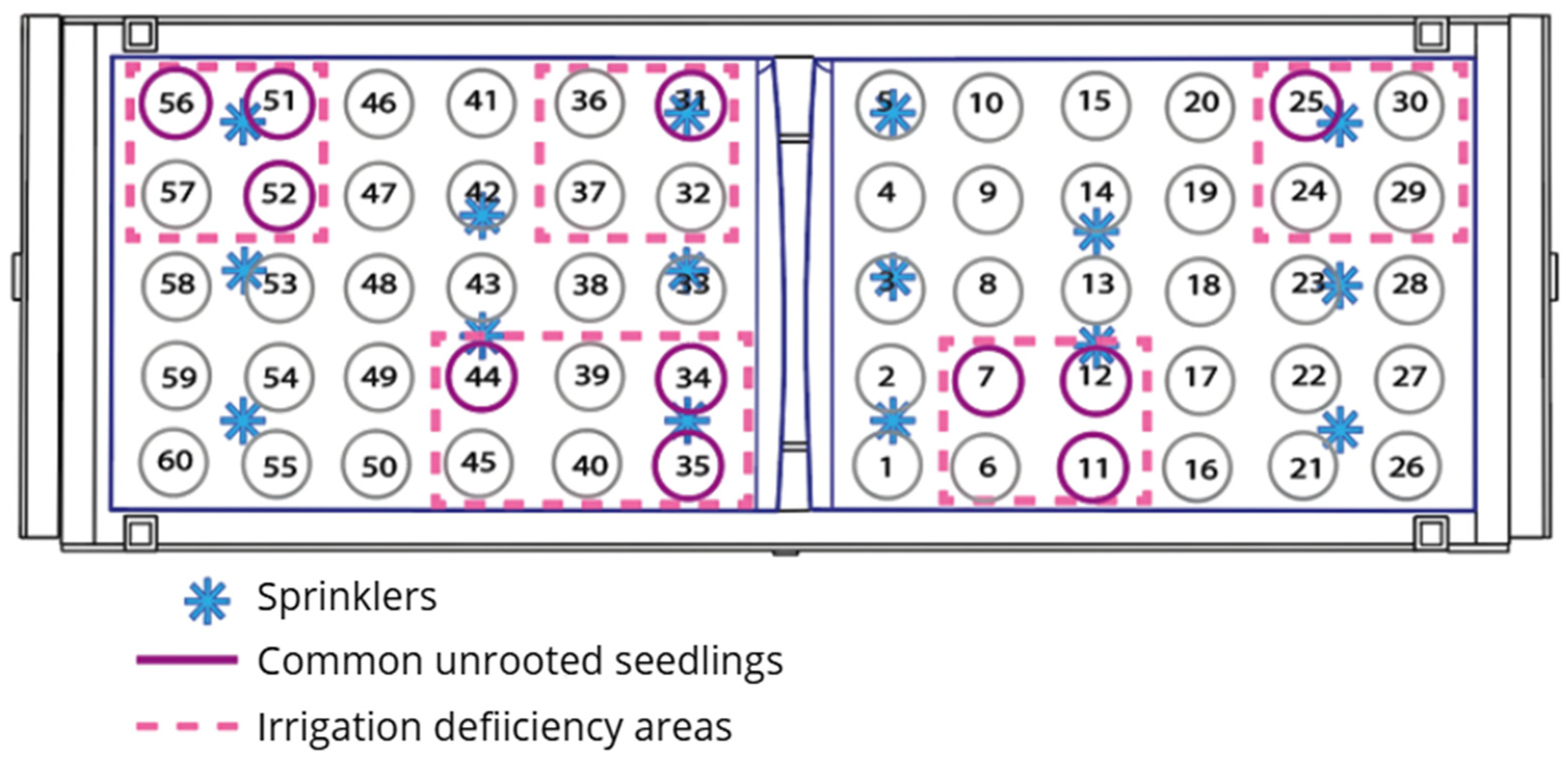
3.2. Vegetative Propagation Outcomes and Survival Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Additional Adaptability Test with Poinsettia Cuttings

| Parameter 1 | Minimum | Maximum | Average |
|---|---|---|---|
| Temperature (°C) | 23.1 | 28.2 | 24.92 |
| Relative Humidity (%) | 42.0 | 76.0 | 55.64 |
References
- Avgoustaki, D.D.; Xydis, G. Indoor Vertical Farming in the Urban Nexus Context: Business Growth and Resource Savings. Sustainability 2020, 12, 1965. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture—Trends and Challenges, 1st ed.; FAO: Rome, Italy, 2017; p. 12. [Google Scholar]
- Teoh, S.H.; Wong, G.R.; Mazumdar, P. A review on urban farming: Potential, challenges and opportunities. Innov. Agric. 2024, 7, 1–11. [Google Scholar] [CrossRef]
- Sustainable Development Goals. Goal 11: Make Cities Inclusive, Safe, Resilient and Sustainable. Available online: https://www.un.org/sustainabledevelopment/cities/ (accessed on 10 January 2025).
- Maxime Market Research. Available online: https://www.maximizemarketresearch.com/market-report/global-commercial-greenhouse-market/110591/ (accessed on 23 April 2025).
- Specht, K.; Zoll, F.; Schümann, H.; Bela, J.; Kachel, J.; Robischon, M. How Will We Eat and Produce in the Cities of the Future? From Edible Insects to Vertical Farming—A Study on the Perception and Acceptability of New Approaches. Sustainability 2019, 11, 4315. [Google Scholar] [CrossRef]
- Sanjuan-Delmás, D.; Llorach-Massana, P.; Nadal, A.; Ercilla-Montserrat, M.; Muñoz, P.; Montero, J.I.; Josa, A.; Gabarrell, X.; Rieradevall, J. Environmental Assessment of an Integrated Rooftop Greenhouse for Food Production in Cities. J. Clean. Prod. 2018, 177, 326–337. [Google Scholar] [CrossRef]
- Yuan, G.N.; Marquez, G.P.B.; Deng, H.; Iu, A.; Fabella, M.; Salonga, R.B.; Ashardiono, F.; Cartagena, J.A. A Review on Urban Agriculture: Technology, Socio-economy, and Policy. Heliyon 2022, 8, e11583. [Google Scholar] [CrossRef]
- Specht, K.; Siebert, R.; Hartman, I.; Freisinger, U.B.; Sawicka, M.; Werner, A.; Thomaier, S.; Henckel, D.; Walk, H.; Dierich, A. Urban Agriculture of the Future: An Overview of Sustainability Aspects of Food Production in And on Buildings. Agric. Hum. Values 2013, 31, 33–51. [Google Scholar] [CrossRef]
- Martin, M.; Molin, E. Enviromental Assessment of an Urban Vertical Hydroponic Farming System in Sweden. Sustainability 2019, 11, 4124. [Google Scholar] [CrossRef]
- Gumisiriza, M.S.; Ndakidemi, P.A.; Mbega, E.R. A Simplified non-Greenhouse Hydroponic System for Small-scale Soilless Urban Vegetable farming. Methods X 2022, 9, 101882. [Google Scholar] [CrossRef] [PubMed]
- Földhazy, E. Smart Hydroponics Conceptual Design of Hydroponic Plant System for Home Enviroment. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, February 2018. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1187320&dswid=320 (accessed on 24 April 2025).
- Barret, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Solis-Toapanta, E.; Fisher, P.; Gómez, C. Growth Rate and Nutrient Uptake of Basil in Small-scale Hydroponics. HortScience 2020, 55, 507–514. [Google Scholar] [CrossRef]
- Nikolov, N.V.; Atanasov, A.Z.; Evstatiev, B.I.; Vladut, V.N.; Biris, S.S. Design of a Small-Scale Hydroponic System for Indoor Farming of Leafy Vegetables. Agriculture 2023, 13, 1191. [Google Scholar] [CrossRef]
- Borrero, J.D. Expanding the Level of Technological Readiness for a Low-Cost Vertical Hydroponic System. Inventions 2021, 6, 68. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of Red and Blue Light on Cucumber Seedlings Grown in a Plant Factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Feng, J.; Hui, X. An IoT-based Hierarchical Control Method for Greenhouse Seedling Production. Procedia Comput. Sci. 2021, 192, 1954–1963. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Nursery Manual for Native Plants, 1st ed.; United States Department of Agriculture, Forest Service: Washington, DC, USA, 2009; pp. 154, 174.
- Tang, L.; Syed, A.; Otho, A.R.; Junejo, A.R.; Tunio, M.H.; Hao, L.; Ali, M.N.H.A.; Brohi, S.A.; Othoand, S.A.; Channa, J.A. Intelligent Rapid Asexual Propagation Technology—A Novel Aeroponics Propagation Approach. Agronomy 2024, 14, 2289. [Google Scholar] [CrossRef]
- Byeon, S.; Lee, H. Transcriptome of Two-Hybrid Poplar (Populus alba × P. tomentiglandulosa) During Adventitious Root Formation After Stem Cutting. Biology 2025, 14, 751. [Google Scholar] [CrossRef]
- Guerrero-Sánchez, J.; Olvera-Olvera, C.A.; Solis-Sánchez, L.O.; Martínez-Blanco, M.D.R.; López-Martínez, M.d.J.; Castañeda-Miranda, C.L.; Soto-Zarazúa, G.M.; Díaz-Flórez, G. Design and Implementation of a Low-Cost Controlled-Environment Growth Chamber for Vegetative Propagation of Mother Plants. AgriEngineering 2025, 7, 177. [Google Scholar] [CrossRef]
- Daas, K.; Daas, T.; Van Der Drift, P. Apparatus for Breeding Plants. Patent 10076086, 22 September 2011. [Google Scholar]
- Kirci, P.; Ozturk, E.; Celik, Y. A Novel Approach for Monitoring of Smart Greenhouse and Flowerpot Parameters and Detection of Plant Growth with Sensors. Agriculture 2022, 12, 1705. [Google Scholar] [CrossRef]
- Bouzas Abad, V.M. Household Hydroponics System. Spain Patent WO/2017/212080, 8 August 2016. [Google Scholar]
- Sustainable Development Goals. Goal 2: Zero Hunger. Available online: https://www.un.org/sustainabledevelopment/hunger/ (accessed on 12 June 2025).
- Goal 12: Responsible Consumption and Production. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 12 June 2025).
- Goal 13: Climate Action. Available online: https://www.un.org/sustainabledevelopment/climate-change/ (accessed on 12 June 2025).
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an Advanced Technique for Vegetable Production: An Overview. J. Soil Water Conserv. 2018, 17, 364–370. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Ronzón-Ortega, M.; Hernández-Vergara, M.P.; Pérez-Rostro, C.I. Hydroponic and Aquaponic Production of Sweet Basil (Ocimum basilicum) and Giant River Prawn (Macrobrachium rosenbergii). Trop. Subtrop. Agroecosyst. 2012, 15, 63–71. [Google Scholar]
- Modarelli, G.C.; Vanacore, L.; Rouphael, Y.; Langellotti, A.L.; Masi, P.; De Pascale, S.; Cirillo, C. Hydroponic and Aquaponic Floating Raft Systems Elicit Differential Growth and Quality Responses to Consecutive Cuts of Basil Crop. Plants 2023, 12, 1355. [Google Scholar] [CrossRef]
- Zaho, Z.; Zhang, W.; Liu, Y.; Li, S.; Yao, W.; Sun, X.; Li, S.; Ma, L.; Sun, J.; Yang, Q.; et al. De Novo Hydroponics System Efficiency for the Cuttings of Alfalfa (Medicago sativa L.). Physiol. Mol. Biol. Plants 2021, 27, 1414–1420. [Google Scholar] [CrossRef]
- Propagation by Cuttings, Layering and Division. Available online: https://www.pubs.ext.vt.edu/426/426-002/426-002.html (accessed on 3 July 2025).
- Hao, W.; Chen, L.; Byoung, R.J. An Optimal Combination of the Propagation Medium and Fogging Duration Enhances the Survival, Rooting and Early Growth of Strawberry Daughter Plants. Agronomy 2020, 10, 577. [Google Scholar] [CrossRef]
- Lázló, S.; László, B.; Géza, S.; Jung, A.; Sárosi, S.; Radácsi, P.; László, C. Optimization of basil (Ocimum basilicum L.) production in LED light environments—A review. Sci. Hortic. 2021, 289, 110486. [Google Scholar]
- Song, J.; Yang, J.; Jeong, B.R. Characterization of Physiology, Photosynthesis, and Nutrition Based on Induced Deficiencies of Macro- and Micronutrients in Basil (Ocimum basilicum L.). Agronomy 2024, 14, 208. [Google Scholar] [CrossRef]
- Mejía Londoño, H.A.; Barrera Sánchez, C.F.; Córdoba Gaona, O.J. Asexual Propagation in Female Plants of Cannabis (Cannabis sativa L.). Rev. Colomb. Cienc. Hortíc. 2023, 17, 1–8. [Google Scholar]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative Propagation of Cannabis by Stem Cuttings: Effects of Leaf Number, Cutting Position, Rooting Hormone, and Leaf Tip Removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Rajaseger, G.; Chan, K.L.; Tan, K.Y.; Ramasamy, S.; Khin, M.C.; Amaladoss, A.; Haribhai, P.K. Hydroponics: Current Trends in Sustainable Crop Poduction. Bionformation 2023, 19, 925–938. [Google Scholar] [CrossRef] [PubMed]
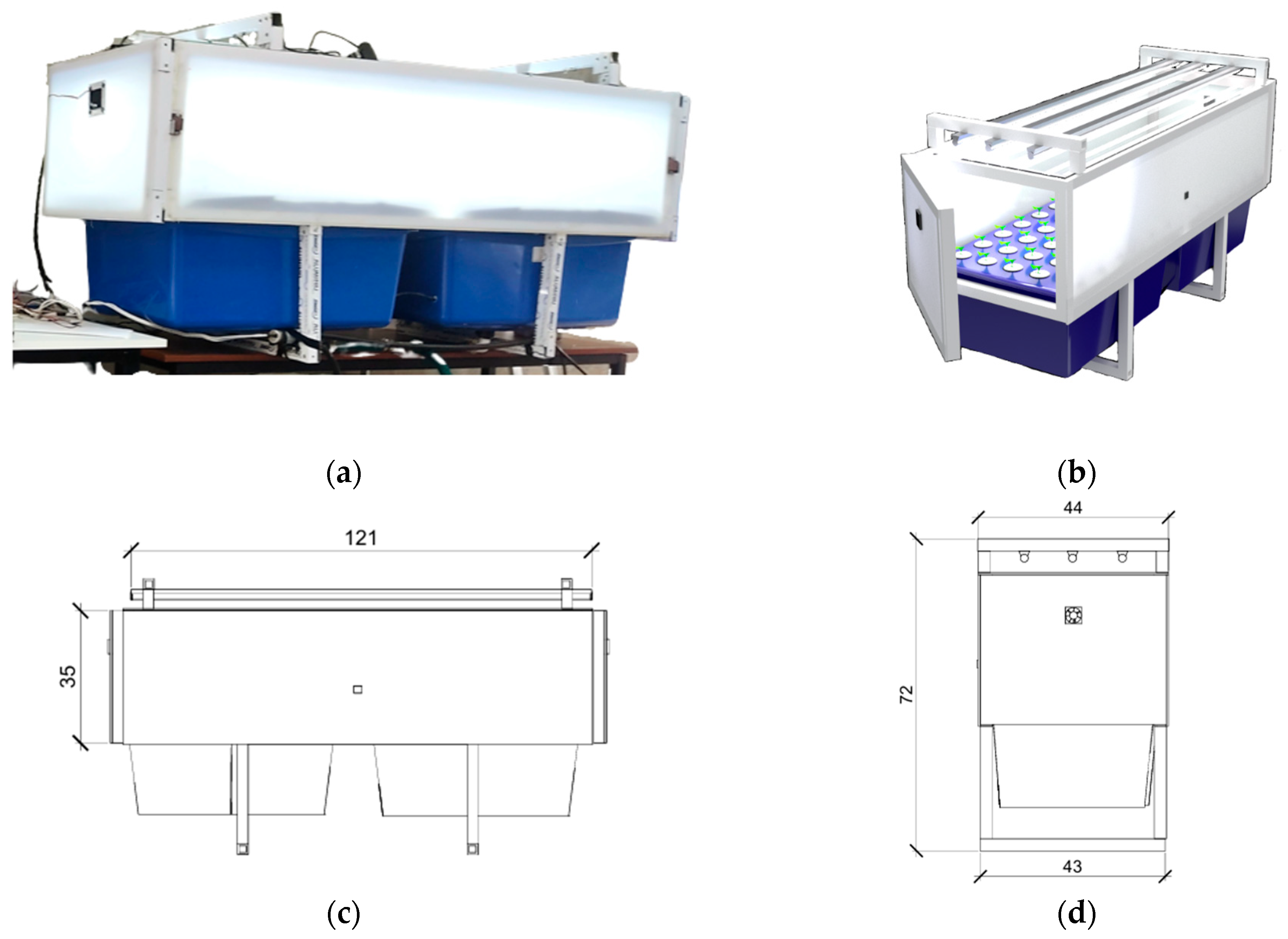
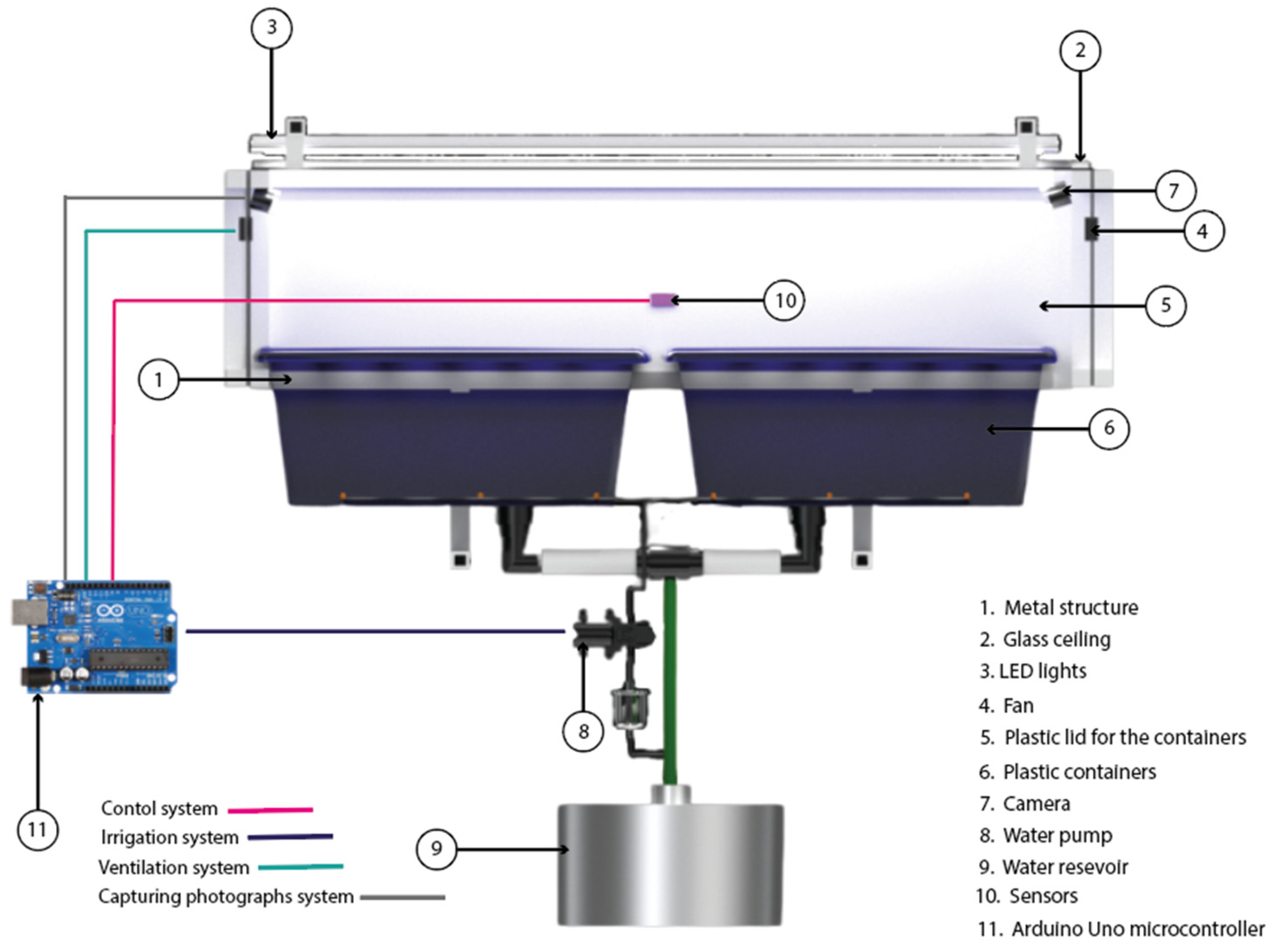
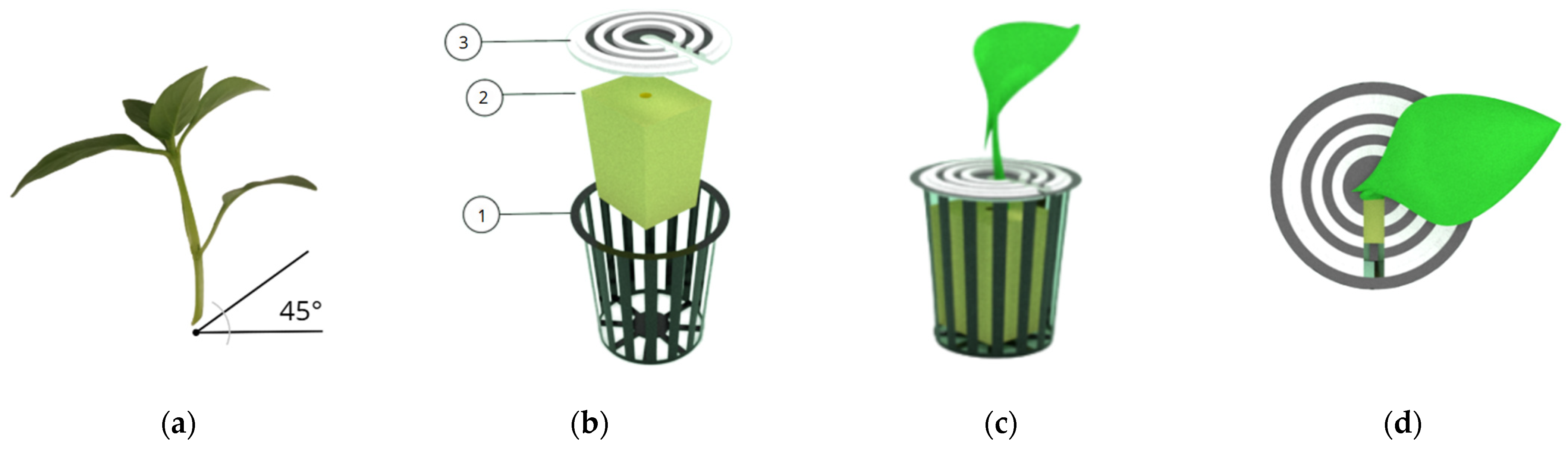
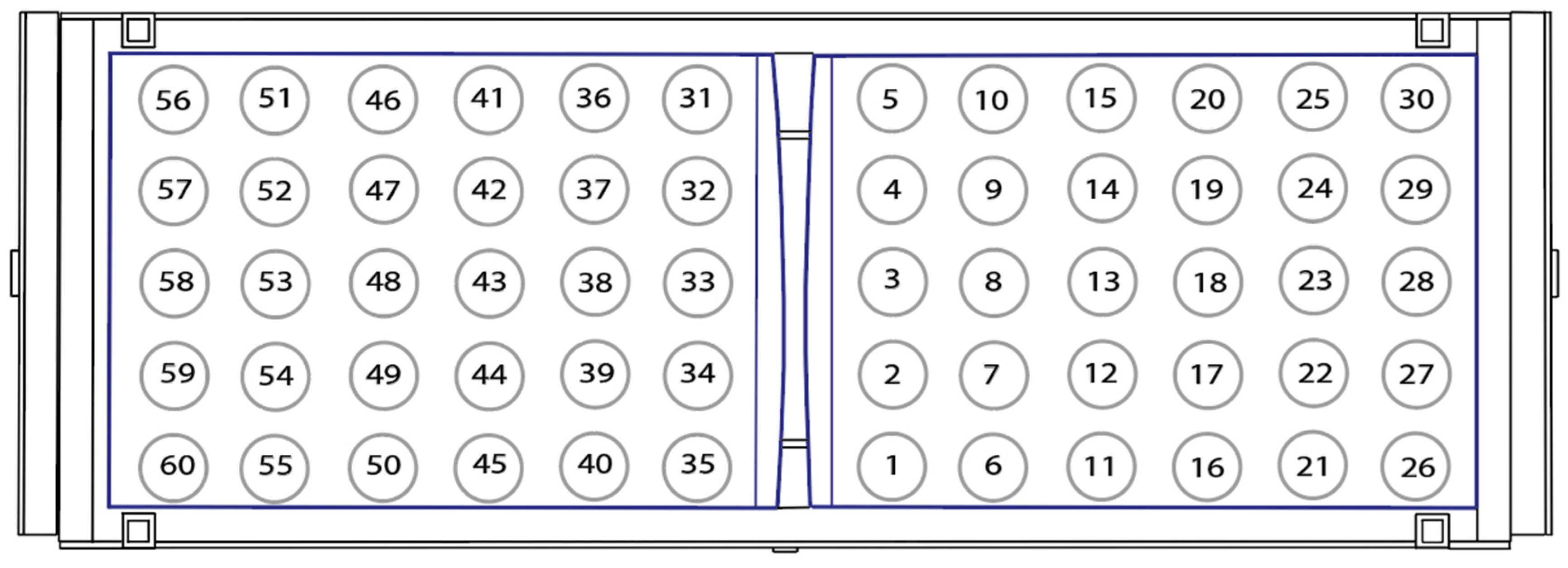


| Component | Specification | Manufacturer/Model (Location) | Estimated Cost (USD) |
|---|---|---|---|
| Structural frame | Aluminum profiles (6 mm thick) | Generic/OEM | 78.00 |
| Covering panels | Polycarbonate (lateral), glass (top) | Generic/OEM | 42.00 |
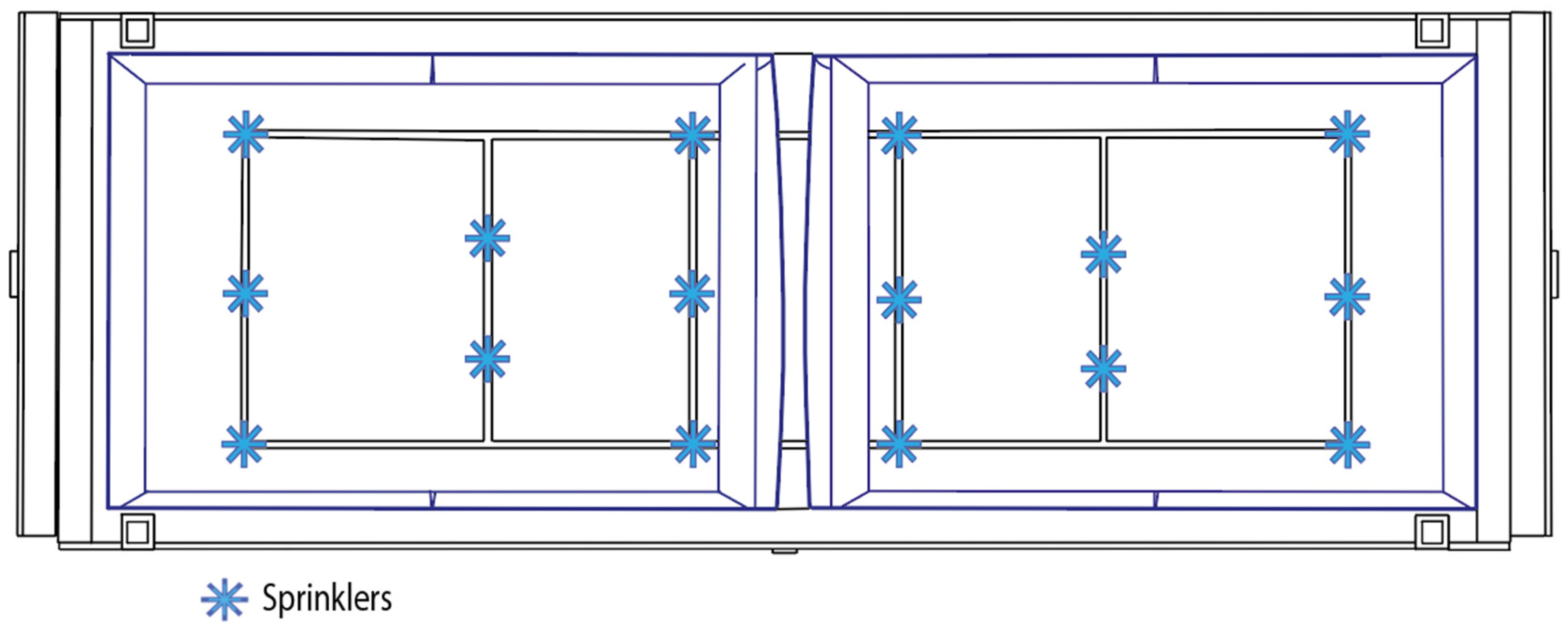
| Irrigation system | 16 sprinklers, diaphragm pump 72 W (12 V) | Generic/OEM | 23.00 |
| Lighting system | 3 x LED lamps (30 W each) | 25.00 | |
| Ventilation | 2 × DC fans, 4 × 4 cm, 12 V, 2.4 W each | Generic/OEM (VN4-012P) | 9.00 |
| Control and sensors | Arduino Uno Microcontroller, DHT11 temp/RH, relay module | Lionchip (Arduino Uno); ASAIR (DHT11); Generic/OEM (Nuevo Leon, Mexico) | 12.00 |
| Image capture | USB webcams (2 unit) | EMEET SmartCam C960 (Hong Kong, China) | 62.00 1 |
| Hydroponic pots + lids | Net pots Ø 5 cm | Generic/OEM (pots); In-house 3D-printed lids | 18.00 |
| Rockwool | Commercial rooting substrate cubes | Owens Corning (Ohio, United States of America) | 10.00 |
| Total Estimated Cost | 279.00 2 | ||
| Subsystem | Activation Logic | Schedule or Condition |
|---|---|---|
| Irrigation | Timer-controlled | Four 4 times per day: 00:00–00:15 06:00–06:10 12:00–12:15 18:00–18:15 |
| Ventilation | Temperature-controlled (conditional) | Fan 1 (inlet): activates if T ≤ 26 °C Fan 2 (exhaust): activates if T 26 °C |
| Characteristic | Range | Accuracy |
|---|---|---|
| Temperature | 0–50 °C | ±2 °C |
| Humidity | 20–90% RH | ±5% RH |
| Parameter | Minimum | Maximum | Average | SD (±) |
|---|---|---|---|---|
| Temperature (°C) | 23.0 | 28.0 | 24.47 | 2.76 |
| Relative Humidity (%) | 47 | 73 | 59.19 | 13.00 |
| Parameter | Minimum | Maximum | Average | SD (±) |
|---|---|---|---|---|
| Temperature (°C) | 23.0 | 29.0 | 24.76 | 3.00 |
| Relative Humidity (%) | 33 | 98 | 47.71 | 32.50 |
| Test | Cuttings Planted (P) | Rooted Seedlings (R) | Success Rate (%) (R/P × 100) | 95% CI (Wilson) |
|---|---|---|---|---|
| Test 1 | 60 | 37 | 61.7% | 49.0–72.9 |
| Test 2 | 60 | 26 | 43.3% | 31.6–55.9 |
| Feature | SSHG (This Study) | Zhao et al. (2021) [33] | Mejía-Londoño et al. (2023) [38] | Caplan et al. (2018) [39] |
|---|---|---|---|---|
| Target species | Ocimum basilicum (Basil) | Medicago sativa (Alfalfa) | Cannabis sativa (Cannabis) | Cannabis sativa (Cannabis) |
| Type of propagation | Asexual (cuttings) | Asexual (cuttings) | Asexual (cuttings) | Asexual (cuttings) |
| Substrate | Rockwool | NR | Peat | NR |
| Nutrient solution/rooting aids | None (tap water only) | Yes (specific solution) | No regulators, peat (salts NR) | Fertilizers used |
| Automation | Yes (irrigation, ventilation) | No | No | No |
| Environmental monitoring | Yes (temperature, RH) | No | No | Yes (temperature control) |
| Lighting control | Not controlled/analyzed | NR | NR | NR |
| Image capture | Yes (2 internal cameras, qualitative) | No | No | No |
| Replication (n, cycles) | n = 60, 2 × 15-day cycles | NR | No | NR |
| Rooting criterion | Roots ≥ 2 mm + anchorage | NR | NR | NR |
| Rooting rate (%) | 61.7% (95% CI: 49.0–72.9); 43.3% (95% CI: 31.6–55.9) | (with solution) | 73.33% (no regulators; peat) | 84% (with fertilizers) |
| Estimated cost | Low (accessible, local materials, see BoM) | Medium | Medium | Medium |
| Scope/applicability | Functional validation (minimal inputs); educational and research; replicable | Germplasm conservation | Cannabis research | Cannabis research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardona Rodríguez, A.N.; Olvera-Olvera, C.A.; Villagrana-Barraza, S.; Araiza-Ezquivel, M.A.; Ortíz-Esquivel, D.I.; Solís-Sánchez, L.O.; Díaz-Flórez, G. Design and Implementation of a Small-Scale Hydroponic Chamber for Sustainable Vegetative Propagation from Cuttings: A Basil (Ocimum basilicum L.). Sustainability 2025, 17, 8773. https://doi.org/10.3390/su17198773
Cardona Rodríguez AN, Olvera-Olvera CA, Villagrana-Barraza S, Araiza-Ezquivel MA, Ortíz-Esquivel DI, Solís-Sánchez LO, Díaz-Flórez G. Design and Implementation of a Small-Scale Hydroponic Chamber for Sustainable Vegetative Propagation from Cuttings: A Basil (Ocimum basilicum L.). Sustainability. 2025; 17(19):8773. https://doi.org/10.3390/su17198773
Chicago/Turabian StyleCardona Rodríguez, Angélica Nohemí, Carlos Alberto Olvera-Olvera, Santiago Villagrana-Barraza, Ma. Auxiliadora Araiza-Ezquivel, Diana I. Ortíz-Esquivel, Luis Octavio Solís-Sánchez, and Germán Díaz-Flórez. 2025. "Design and Implementation of a Small-Scale Hydroponic Chamber for Sustainable Vegetative Propagation from Cuttings: A Basil (Ocimum basilicum L.)" Sustainability 17, no. 19: 8773. https://doi.org/10.3390/su17198773
APA StyleCardona Rodríguez, A. N., Olvera-Olvera, C. A., Villagrana-Barraza, S., Araiza-Ezquivel, M. A., Ortíz-Esquivel, D. I., Solís-Sánchez, L. O., & Díaz-Flórez, G. (2025). Design and Implementation of a Small-Scale Hydroponic Chamber for Sustainable Vegetative Propagation from Cuttings: A Basil (Ocimum basilicum L.). Sustainability, 17(19), 8773. https://doi.org/10.3390/su17198773









