Activation of Persulfate by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar for 2,4-Dichlorophenol Degradation: Efficiency, Sustainability, and Mechanism Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of S-nZVI@BC
2.3. Experimental Processes
2.4. Analytical Methods
3. Results and Discussion
3.1. Characterization of Prepared Materials
3.2. Effects of Different Degradation Systems
3.3. Effects of Different Influencing Factors
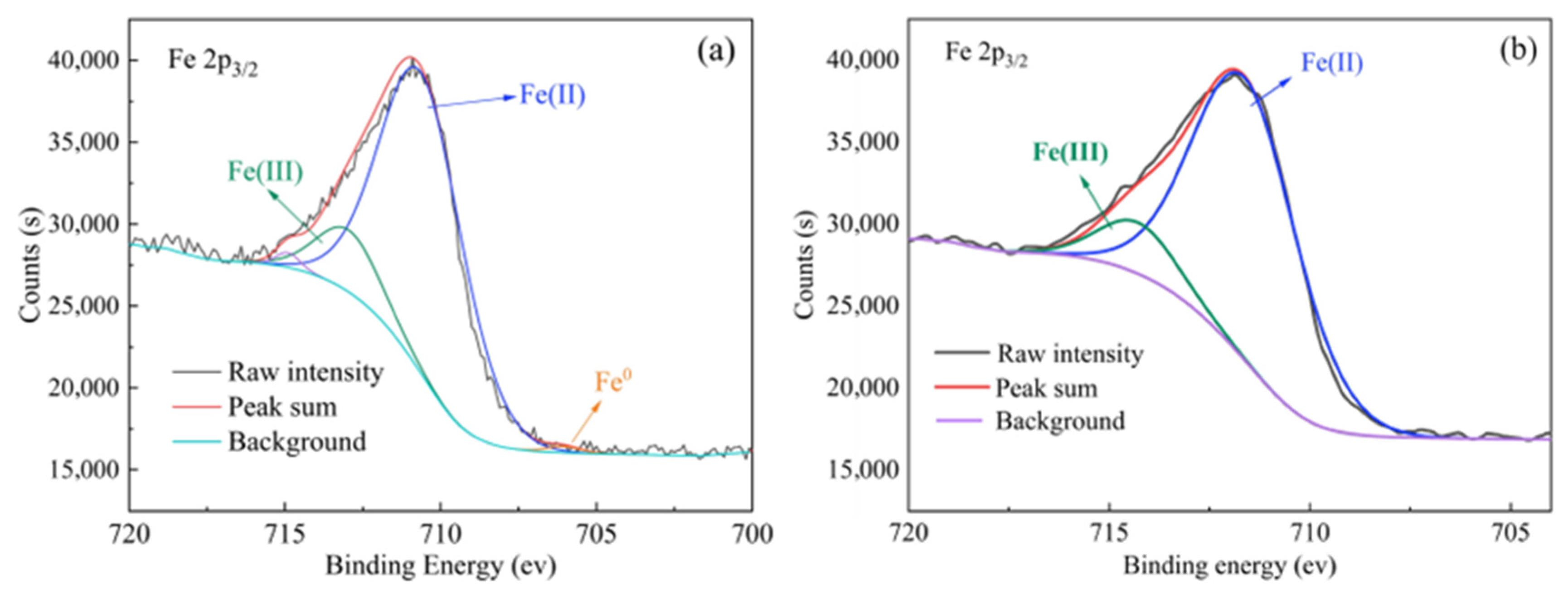
3.4. Analysis of XPS
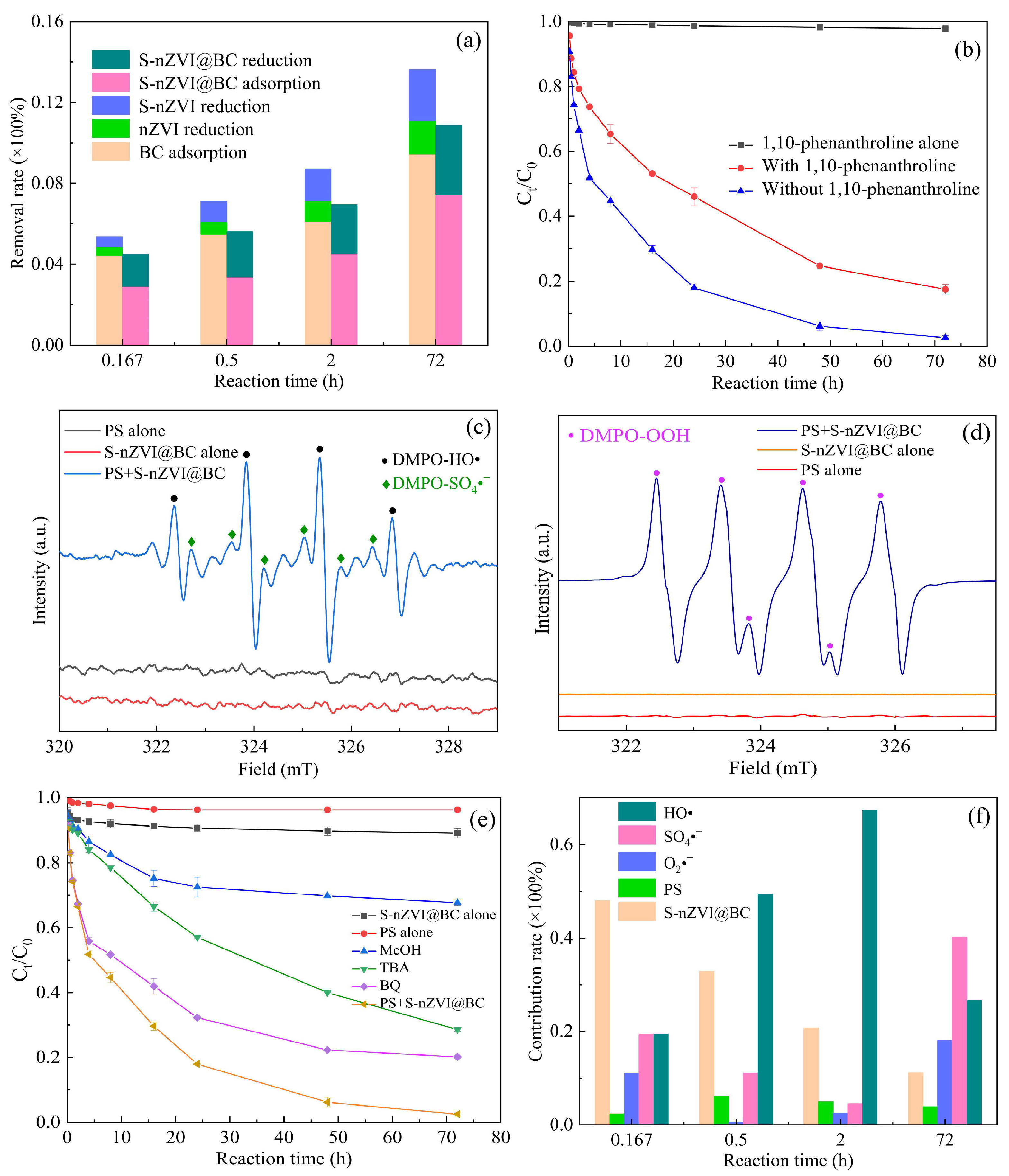
3.5. Degradation Mechanisms Analysis
3.6. Analysis of Degradation Pathway
3.7. Summary and Reflection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, S.; Kumar, S.; Haritash, A.K. A comprehensive review of chlorophenols: Fate, toxicology and its treatment. J. Environ. Manag. 2023, 342, 118254. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Lee, C.M. Pentachlorophenol contaminated groundwater bioremediation using immobilized Sphingomonas cells inoculation in the bioreactor system. J. Hazard. Mater. 2008, 152, 159–165. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological profile of chlorophenols and their derivatives in the environment: The public health perspective. Sci. World J. 2013, 2013, 460215. [Google Scholar] [CrossRef] [PubMed]
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts. Environ. Res. 2021, 195, 110742. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Jin, X.Y.; Megharaj, M.; Naidu, R.; Chen, Z.L. Heterogeneous Fenton oxidation of 2,4-dichlorophenol using iron-based nanoparticles and persulfate system. Chem. Eng. J. 2015, 264, 587–594. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.M.; Lawan, I.; Xiao, W.; Zhang, M.; Wang, L.; Chen, L.; Yuan, Z. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef]
- Ma, T.G. Efficiency and Mechanism of Persulfate Activation by Polyaniline-Derived Carbon Materials for Chlorophenols Degradation in Water. Ph.D. Thesis, Jilin University, Changchun, China, November 2023. [Google Scholar]
- Wang, J.L.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Anjali, R.; Shanthakumar, S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manag. 2019, 246, 51–62. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Zhang, Y.L.; Hu, X.M. Enhanced activation of peroxymonosulfate using oxygen vacancy-enriched FeCo2O4-x spinel for 2,4-dichlorophenol removal: Singlet oxygen-dominated nonradical process. Colloids Surf. A 2020, 597, 124568. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbaek, H.; Siegrist, R.L.; Bjerg, P.L. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Ling, S.K.; Wang, S.; Peng, Y. Oxidative degradation of dyes in water using Co2+/H2O2 and Co2+/peroxymonosulfate. J. Hazard. Mater. 2010, 178, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Zhang, J.; Xiao, Y.; Chang, W.C.; Lim, T.T. Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H2O2 and UV/persulfate. Chem. Eng. J. 2016, 302, 526–534. [Google Scholar] [CrossRef]
- Lee, J.; Von, G.U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Wu, G.Y.; Li, N.; Lu, X.K.; Zhao, J.H.; He, M.T.; Yan, B.B.; Zhang, H.Q.; Duan, X.G.; Wang, S.B. Landfill leachate treatment by persulphate related advanced oxidation technologies. J. Hazard. Mater. 2021, 418, 126355. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.L.; Feng, M.B.; Liu, W.; Wang, W.; Yang, Q.; Hu, Y. Degradation of 2,4-dichlorophenoxyacetic acid in water by persulfate activated with FeS (mackinawite). Chem. Eng. J. 2017, 313, 498–507. [Google Scholar] [CrossRef]
- Kim, C.L.; Ahn, J.Y.; Kim, T.Y.; Shin, W.S.; Hwang, I. Activation of Persulfate by Nanosized Zero-Valent Iron (NZVI): Mechanisms and Transformation Products of NZVI. Environ. Sci. Technol. 2018, 52, 3625–3633. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhao, M.Y.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.Q.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Ouyang, Z.Z.; Zhang, Z.F.; Yang, C.; Li, X.Q.; Dang, Z.; Wu, P.X. Mechanism of glyphosate removal by biochar supported nano-zero-valent iron in aqueous solutions. Colloids Surf. A 2018, 547, 64–72. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Y. Enhanced degradation of bisphenol S by persulfate activated with sulfide-modified nanoscale zero-valent iron. Environ. Sci. Pollut. Res. 2022, 29, 8281–8293. [Google Scholar] [CrossRef]
- Wu, G.C.; Kong, W.J.; Gao, Y.; Kong, Y.; Dai, Z.G.; Dan, H.B.; Shang, Y.N.; Wang, S.Q.; Yin, F.J.; Yue, Q.Y.; et al. Removal of chloramphenicol by sulfide-modified nanoscale zero-valent iron activated persulfate: Performance, salt resistance, and reaction mechanisms. Chemosphere 2021, 286, 131876. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Hu, S.D.; Chen, J.H.; Muller, K.; Li, Y.C.; Fu, W.J.; Lin, Z.W.; Wang, H.L. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Xu, J.; Avellan, A.; Li, H.; Liu, X.T.; Noël, V.; Lou, Z.; Wang, Y.; Kaegi, R.; Henkelman, G.; Lowry, G.V. Sulfur Loading and Speciation Control the Hydrophobicity, Electron Transfer, Reactivity, and Selectivity of Sulfidized Nanoscale Zerovalent Iron. Adv. Mater. 2020, 32, e1906910. [Google Scholar] [CrossRef]
- Chen, J.; Dong, H.R.; Tian, R.; Li, R.; Xie, Q.Q. Remediation of trichloroethylene-contaminated groundwater by sulfide-modified nanoscale zero-valent iron supported on biochar: Investigation of critical factors. Water Air Soil Pollut. 2020, 231, 432. [Google Scholar] [CrossRef]
- Xie, R.H.; Wang, M.; Li, W.P.; Song, J.J. Degradation of 2-Chlorophenol in aqueous solutions using persulfate activated by biochar supported sulfide-modified nanoscale zero-valent iron: Performance and mechanisms. Water 2023, 15, 2805. [Google Scholar] [CrossRef]
- Taiwo, K.J.; Oancea, A.V.; Kotha, N.S.; Usack, J.G. Enhancing wastewater treatment sustainability through integrated anaerobic digestion and hydrothermal carbonization: A life-cycle perspective. Sustainability 2025, 17, 7545. [Google Scholar] [CrossRef]
- Kaynak, E.; Piri, I.S.; Das, O. Revisiting the Basics of Life Cycle Assessment and Lifecycle Thinking. Sustainability 2025, 17, 7444. [Google Scholar] [CrossRef]
- Su, Y.M.; Jassby, D.; Song, S.K.; Zhou, X.F.; Zhao, H.Y.; Filip, J.; Petala, E.; Zhang, Y.L. Enhanced oxidative and adsorptive removal of diclofenac in heterogeneous Fenton-like reaction with sulfide modified nanoscale zerovalent iron. Environ. Sci. Technol. 2018, 52, 6466–6475. [Google Scholar] [CrossRef]
- Jin, H.; Cang, Z.Z.; Ding, W.; Wu, W.T.; Ma, H.K.; Wang, C.X.; Qi, Z.W.; Li, Z.F.; Zhang, L.L. Oxidative removal of antibiotic resistant E. coli by sulfidated zero-valent iron: Homogeneous vs heterogeneous activation. J. Hazard. Mater. 2021, 408, 124411. [Google Scholar] [CrossRef]
- Wang, X.Q.; Guo, Z.Z.; Hu, Z.; Ngo, H.H.; Liang, S.; Zhang, J. Adsorption of phenanthrene from aqueous solutions by biochar derived from anammoniation-hydrothermal method. Sci. Total Environ. 2020, 733, 139267. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, Q.N.; Chen, Y.; Tian, Q.L.; Zhao, G.H. Efficient removal of dimethyl phthalate with activated iron-doped carbon aerogel through an integrated adsorption and electro-Fenton oxidation process. Carbon 2017, 124, 111–122. [Google Scholar] [CrossRef]
- Zhang, P.; Song, D.; Xu, X.; Hao, Y.; Shang, X.; Wang, C.; Tang, J.; Sun, H. Sulfidated zero valent iron as a persulfate activator for oxidizing organo-phosphorus pesticides (OPPs) in aqueous solution and aged contaminated soil columns. Chemosphere 2021, 281, 130760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, L.; Zhang, Y.L.; Mao, X.H.; Tan, W.B.; Zhang, Y.L.; Wang, X.S.; Chang, M.; Guo, R.N.; Xi, B.D. Perdisulfate-assisted advanced oxidation of 2,4-dichlorophenol by bio-inspired iron encapsulated biochar catalyst. J. Colloid Interface Sci. 2021, 592, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.W.; Shen, M.X.; Liu, J.H.; Ma, Y.J.; Gong, B.N.; Liu, H.Q.; Huang, Z.J. Resource utilization of piggery sludge to prepare recyclable magnetic biochar for highly efficient degradation of tetracycline through peroxymonosulfate activation. J. Clean. Prod. 2021, 294, 126372. [Google Scholar] [CrossRef]
- Song, S.K.; Su, Y.M.; Adeleye, A.S.; Zhang, Y.L.; Zhou, X.F. Optimal design and characterization of sulfide-modified nanoscale zerovalent iron for diclofenac removal. Appl. Catal. B-Environ. 2017, 201, 211–220. [Google Scholar] [CrossRef]
- Manz, K.E.; Carter, K.E. Investigating the effects of heat activated persulfate on the degradation of furfural, a component of hydraulic fracturing fluid chemical additives. Chem. Eng. J. 2017, 327, 1021–1032. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Zhang, B.-T.; Li, J.; Shi, Y.; Zhang, Y. Oxidative degradation of chloroxylenol in aqueous solution by thermally activated persulfate: Kinetics, mechanisms and toxicities. Chem. Eng. J. 2019, 368, 553–563. [Google Scholar] [CrossRef]
- Wang, Y.R.; Tian, D.F.; Chu, W.; Li, M.R.; Lu, X.W. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Gao, N.Y.; Wang, W.; Kang, S.F.; Xu, J.H.; Xiang, H.M.; Yin, D.Q. Ultrasound-assisted heterogeneous activation of persulfate by nano zero-valent iron (nZVI) for the propranolol degradation in water. Ultrason. Sonochem. 2018, 49, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Lin, Y.C. Phototransformation of cephalosporin antibiotics in an aqueous environment results in higher toxicity. Environ. Sci. Technol. 2012, 46, 12417–12426. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Luo, H.Y.; Luo, D.Y.; Chen, Y.; Tang, J.; Ma, H.; Pu, S.Y. New insights into the degradation of nitrobenzene by activated persulfate with sulfidated nanoscale zero-valent iron: Synergistic effects of reduction and reactive oxygen species oxidation. Sep. Purif. Technol. 2023, 322, 124252. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Lu, C.; Werner, D. Reductive sequestration of chromate by hierarchical FeS@Fe0 particles. Water Res. 2016, 102, 73–81. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.B.; Ma, Y.; Xing, S.T. Enhanced superoxide radical production for ofloxacin removal via persulfate activation with Cu-Fe oxide. Chem. Eng. J. 2018, 354, 473–480. [Google Scholar] [CrossRef]
- Webster, R.D. Electrochemistry combined with electron paramagnetic resonance (EPR) spectroscopy for studying catalytic and energy storage processes. Curr. Opin. Electrochem. 2023, 40, 101308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Activation of peroxydisulfate by a novel Cu0-Cu2O@CNTs composite for 2, 4-dichlorophenol degradation. Sci. Total Environ. 2021, 754, 141883. [Google Scholar] [CrossRef]




| Contents | This Research | The Established Literatures |
|---|---|---|
| To prepare S-nZVI@BC and characterize its microscopic shape | S-nZVI@BC was synthesized with two-step method in liquid phase; analyzed the microscopic structures, functional groups and chemical bonds of it | The preparation methods were mature, morphological characterization more comprehensive, and test equipment more advanced |
| To establish an S-nZVI@BC/PS reaction system for 2,4-DCP degradation | The optimal PS:2,4-DCP mass ratio was 70:1 and S-nZVI@BC:PS of 1.5:1; the degradation effect was better than that of either one or more components of the S-nZVI@BC/PS system | Due to the differences in material composition and degradable pollutants, the optimal ratios were not the same. However, the highest degradation rate of the entire system was consistent |
| To analyze different influencing factors of the system | The reusability of S-nZVI@BC, pH, temperature, and anions in solution was evaluated | The conventional influencing factors studied were the same |
| To quantify the contribution of S-nZVI@BC | Component contribution analysis quantified the adsorption and reduction in S-nZVI@BC; this was a notable highlight in this research | Some studies focused on the adsorption properties of S-nZVI@BC, but very little literature has paid attention to and quantitatively analyzed other chemical effects |
| To explore the PS activation mechanism | Fe0 played a dominant role in the PS activation process, HO•, SO4•−, and O2•− were key agents in subsequent degradation stages | The methods for determining the activation mode and identifying the active substances were basically the same |
| To analyze the degradation pathway | The degradation products and pathways were analyzed, but propionic acid and maleic acid were predicted products based on chemical equations | Every degradation product could be detected, and determined the clear degradation pathway |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhao, Y.; An, Z.; Dou, C. Activation of Persulfate by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar for 2,4-Dichlorophenol Degradation: Efficiency, Sustainability, and Mechanism Investigation. Sustainability 2025, 17, 8721. https://doi.org/10.3390/su17198721
Wang M, Zhao Y, An Z, Dou C. Activation of Persulfate by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar for 2,4-Dichlorophenol Degradation: Efficiency, Sustainability, and Mechanism Investigation. Sustainability. 2025; 17(19):8721. https://doi.org/10.3390/su17198721
Chicago/Turabian StyleWang, Mu, Yan Zhao, Zongsheng An, and Changming Dou. 2025. "Activation of Persulfate by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar for 2,4-Dichlorophenol Degradation: Efficiency, Sustainability, and Mechanism Investigation" Sustainability 17, no. 19: 8721. https://doi.org/10.3390/su17198721
APA StyleWang, M., Zhao, Y., An, Z., & Dou, C. (2025). Activation of Persulfate by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar for 2,4-Dichlorophenol Degradation: Efficiency, Sustainability, and Mechanism Investigation. Sustainability, 17(19), 8721. https://doi.org/10.3390/su17198721







