1. Introduction
The mass use of plastics in everyday social practices presents environmental challenges for terrestrial and marine ecosystems. The rapid rise in plastic pollution is largely driven by the widespread use of single-use plastics. Globally, over 380 million tons of plastic are produced each year, with nearly half designed for single-use applications [
1]. The food and beverage sector is the largest consumer of such packaging, generating about 35% of worldwide packaging waste. Notably, around 95% of food packaging is discarded after just one use [
2].
This short life cycle of single-use plastic raises various environmental impacts in the form of greenhouse gas emissions, blockage of drainage systems, soil contamination, and diseases due to ingestion of microplastics [
2]. In spite of the various disadvantages, food packaging is absolutely essential as it extends the shelf life of food and maintains its organoleptic properties, such as flavor, texture, and color [
3,
4], and is lightweight compared to alternative packaging materials. Moreover, food packaging enables safe, hygienic transportation and reduces food loss [
5]. The benefits of plastic food packaging make it challenging for businesses and communities to reduce its use. Hence, the need arises to transition from a linear economy to a circular economy, where products and materials are kept in cyclic use with little loss of value.
The model of the circular economy includes extending the life of products to minimize waste. Despite advancements in waste management technologies, recycling, and biodegradable alternatives, the total amount of plastic packaging can be reduced more effectively by following the sequence of the waste management hierarchy, which outlines the preferred order for managing waste based on their potential environmental impact as described in
Figure 1. The hierarchy prioritizes the prevention and reuse of materials over recycling. Notably, disposal is the last and least favorable waste management option.
The model of the circular economy builds on the waste management hierarchy by promoting effective ways to retain resources in a continuous regenerative system and by eliminating the option of disposal [
8]. The CE model follows an “extraction–production–reuse–recovery” approach to extend the lifespan of existing products and reduce waste generation [
9,
10].
The physical form, chemical nature of the plastics, and the surrounding environment greatly influence the breakdown rates of waste polymers with the passage of time due to the actions of microorganisms in soil, aquatic, landfill, and compost environments [
11,
12]. While biodegradation can in principle resolve the problem of landfill sites and huge plastic waste accumulation, the synthetic plastic PET—commonly used in food packaging—is considered to be bio-inert because its repeat unit is a rigid aromatic compound that does not provide active sites for enzymatic catalysis enabling degradation [
11].
Therefore, in line with the waste management hierarchy, an effective alternative solution to mitigate the effect of plastic food packaging is to adopt a circular, closed-loop strategy that allows for multiple reuses of the packaging before end-of-life recycling. However, in the scenario where packaging is used several times, new challenges must be addressed to ensure food-health safety [
13]. Due to a current lack of appropriate circular economy systems for this type of product, implementation on an industrial scale will require advancements in logistics, cleaning, and quality assurance technologies [
14].
As awareness of the environmental consequences of plastic waste continues to grow, significant efforts are being directed toward enhancing the reusability of plastics, particularly in food packaging. Establishing effective reuse systems requires the creation of appropriate business models.
Table 1 outlines four core business models that have been designed to facilitate the reuse of plastic food packaging.
A key concern with reusable packaging is hygiene, particularly regarding aging containers that may become scratched and scuffed after multiple uses. Recently, the initiative to reuse plastic food packaging on a small hospitality and food services scale has been taken by some companies such as Barepack
®, Just Salad
®, Starbucks
®, Reuser
®, and Junee
®, etc. Most of the companies employed the return-on-the-go business model (
Table 1). However, none of these companies have made available details of any mechanism that keeps track of the number of times the packaging has been (re)used or details of any post-cleaning assessment method to verify that each reusable packaging has met appropriate safety standards after cleaning.
Recent research has compared the use of ultraviolet (UV) fluorescence imaging and the current industry standard, i.e., the adenosine triphosphate (ATP) swabbing technique to detect residual food contamination on plastic food packaging surfaces. The research highlights several advantages of ultraviolet (UV) fluorescence imaging in terms of sensitivity level, comprehensive inspection, quicker results, and compatibility in an automated system [
17].
However, the concept of the reuse of plastic food packaging raises many challenges due to insufficient infrastructure, resistance to change, risk to human health, difficulty in understanding the impact on the overall food system, and complications in testing and validating new package designs [
18]. Therefore, it is important for consumers and the industry to address these major concerns.
A PESTEL analysis outlines the challenges associated with transitioning plastic packaging from a single-use business model to one of reuse across multiple areas. Politically, government policies and political stability play a crucial role in supporting or hindering adoption. Economically, costs for infrastructure, cleaning, and logistics need to be considered for long-term feasibility. Social acceptance depends on consumer perceptions of hygiene, convenience, and pricing. Technically, efficient design, collection, cleaning, and testing processes are necessary for success. Environmentally, while reuse reduces waste, its overall impact should be evaluated through a comprehensive Life Cycle Assessment (LCA) in accordance with ISO 14040 standards to determine their broader ecological implications [
19,
20]. Legally, compliance with national and international food safety regulations is essential. These challenges can be addressed by detailed planning, innovation, and regulatory support [
21,
22,
23].
Of all the challenges identified in the PESTEL analysis, challenges associated with protecting the hygiene food and safety of the consumers are the most important concern [
13,
22]. Of primary concern is the potential of cross contamination of product between pack use cycles.
Reusable packaging is intended for multiple uses, but it is only considered more environmentally friendly than single-use alternatives after a certain number of reuses; however, repeated washing, transportation, and refilling can lead to noticeable signs of wear and surface damage over time. This wear and tear may stimulate contaminants accumulation, raise health and safety concerns, and ultimately reduce acceptance or lead to the packaging being replaced [
24].
The connatural complexity of “circular” reuse systems in comparison to linear systems might also be challenging to track and control outbreaks of foodborne illnesses. Therefore, implementing a comprehensive cleaning process to eliminate bacterial contaminants and reduce the risk of foodborne illnesses is essential [
25].
In this context, it is crucial to understand the impact of microscopic surface characteristics, such as the surface roughness of plastic food packaging, because higher surface roughness provides more surface area for bacterial attachment. Moreover, rougher surfaces promote greater microbial colonization by offering protection from shear forces, particularly during the initial attachment phase as they are harder to clean than smooth surfaces [
26,
27].
Moreover, surface roughness of the plastic food packaging may potentially change after multiple cleaning cycles due to the application of heat, detergent, water pressure, washing time, and during the usage cycle due to mechanical abrasion by the use of cutlery, reverse logistics, and industrial operations. This could make the cleaning process more challenging and negatively impact the reusability of the packaging.
Therefore, this research aims to examine the impact of repeated use–clean cycles on the surface roughness of food packaging and how alterations in surface texture may influence subsequent cleaning and fouling detection, ensuring compliance with relevant food safety standards.
This study specifically investigates how the condition of collected and cleaned post-use packaging can be qualified for food safety before refilling. The findings contribute to SDG 3 (Good Health and Well-being) by ensuring safer food consumption, SDG 9 (Industry, Innovation, and Infrastructure) by promoting innovative cleaning and monitoring methods, SDG 12 (Responsible Consumption and Production) through supporting circular packaging systems, and SDG 13 (Climate Action) by reducing single-use plastic waste and associated environmental impacts.
To our knowledge, this is the first experimental study that examines effect of repeated washing and associated surface roughness on the most widely used food packaging material, polyethylene terephthalate (PET). The study subjected commercially available single-use packaging to industrial washing procedures to determine the changes in surface roughness and the impact this has on detecting residual fouling. The study further incorporates simulated consumer use by controlled abrasion to understand the impact of a wide range of roughness on the cleaning and assessment of cleanliness.
The cleanliness results were obtained from three different methods, namely, changes in mass, UV fluorescence imaging and adenosine triphosphate (ATP) assay. The manuscript concludes with a discussion on the implications of our findings on the future circular economy for packaging industries.
2. Materials and Methods
The investigative procedure comprised two phases. The first phase sought to establish the effect of a number of wash cycles on the roughness of rPET packaging, which may change due to industrial washing standard parameters, i.e., high temperature, water pressure, time of washing, and amount of detergent.
In addition to washing, reusable food packaging may also pass through some uncontrolled environments during consumers use and during reverse logistics where the surface roughness could also be impacted. This includes actions such as, but not limited to, nesting and de-nesting, transit along conveyor systems, vibration during logistics transport, the refilling process, and use of cutlery during consumption. Therefore, the research incorporated a second phase aimed at investigating how artificially induced high surface roughness impacts the cleaning efficiency of PET packaging. Additionally, this phase evaluated the effectiveness of detecting trace levels of fouling on these roughened surfaces.
2.1. Materials
The packaging used in this study were vacuum-formed rPET trays of nominal dimensions 150 × 210 × 40 mm (W × L × H), with 0.30–0.35 mm nominal thickness, provided by Klöckner Pentaplast, Featherstone, UK. An image of a new pack is shown in
Figure 2. As no suitable reusable PET packs could be sourced for the study, the packs investigated are intended for single use but considered viable for this study, which utilized controlled washing and scratching of surfaces, rather than investigating prolonged use of the packs. Furthermore, PET and its variants are very popular packaging material choices and likely play a major role in the shift towards the reusable packaging; hence, the focus on rPET in this study is relevant. The packs used in the study were new and unused as provided by the manufacturer.
2.2. Washing Parameters
The packaging was washed in a 400 mm × 400 mm two-tiered commercial ware washer (Classeq® Glasswasher G400 Duo purchased from Nisbets Plc, Catering Equipment Supplies, Bristol, UK) utilizing Jantex Pro Glass Wash Detergent 0.25% caustic soda. During the washing cycles, the packs were held firmly on the bottom shelf of the warewasher rack with the help of a customized silicone strap bracing system to avoid any movement of the packaging.
A total of two rPET containers underwent 20 consecutive washing cycles without any intervening use or contact with food. For this phase, only two containers were selected to enable consistent measurement of surface roughness at the same location on each. Surface roughness was assessed using two different methods: a mechanical technique for one container and an optical technique for the other. This controlled setup eliminated variability arising from differences between individual containers. The washing parameters were as follows:
Detergent dose: The containers were washed with and without 3 mL per liter of water (3 mL/L) detergent dosage to examine the effect of the detergent on the surface properties of containers. This concentration corresponds to standard practices in commercial dishwashing operations.
Washing cycle duration: rPET packs were washed at standard washing cycle i.e., 102 s washing followed by a 10 s sanitizing rinse phase.
Washing temperature: The washing and rinsing temperature were kept constant at the standard operating temperature of the device, i.e., 55 °C (±2 °C) and 70 °C (±2 °C), respectively, during each washing cycle.
Before any further investigation, packs were air-dried after each wash cycle to avoid any error from the presence of moisture during roughness measurement.
2.3. Roughness Measurement (Mechanical Method)
A Taylor Hobson Form Intra 50 apparatus equipped with a 2-μm radius conisphere stylus featuring a 90° tip angle was employed to measure the average surface roughness of washed packs. Prior to conducting the roughness measurements, the washed packs were air dried, labelled, and one side of each pack was cut off to enable the stylus arm to access the inner base surface. The packs were set on a stage and held in place with soft clay to avoid any movement during the measurement. The height of the stylus was manually adjusted before being drawn across the surface of the pack such that the transverse displacement of the stylus was able to measure the roughness of the material surface. The stylus was then moved along a 5 mm profile length of the containers at a speed of 0.5 mm/s, and the roughness data was automatically logged by the system software. For accurate results, surface roughness was measured at the same location five times following each wash cycle, and the average of these measurements was calculated.
2.4. Roughness Test Measurement (Optical Method)
The roughness profile of the surface of the packaging was also measured using an optical interferometry technique after washing the packs for various numbers of wash cycles. Optical interferometry enabled precise, non-contact roughness measurements, overcoming the contact inconsistencies of the stylus method on deformed rPET surfaces after repeated washing. For this purpose, a Bruker’s NPFLEXᵀᴹ White Light Interferometer was used with optical resolution of 2.2 μm and a vertical resolution of <1.5 nm. The samples were scanned with a ×5 magnification objective lens over the sampling area of 1200 μm × 945 μm. All sides of the pack were removed so that the objective lens could reach the inner surface of the base of the pack. The base of the packaging was placed on a metal surface having with a hole in the support located under the measurement area to ensure the roughness measurement was of the surface of the rPET packaging rather than the metal base surface. In order to attain accuracy, the surface roughness measurement was repeated at the same location after each wash cycle and then the average of three readings was calculated.
2.5. Synthesis of Contrived Rough Surfaces of the Packaging
For the second phase of experimental study, a controlled surface abrasion of PET was carried out to replicate mechanical wear of the packaging during repeated consumer usage and industrial processing cycles. For this purpose, the base of the rPET packs was made artificially rough using a Draperᵀᴹ Mini Air orbital sander (50 mm pad size, 15,000 rpm) purchased from Draper Tools Ltd., Chandler’s Ford, Hampshire, UK. Firstly, the sides of the packaging were removed, and the base of the packaging was firmly held with adhesive tape on a weighing balance, which enabled, to some extent, the gauging of the applied force on the packaging. The air pressure of the sander was maintained at 60 psi using a pneumatic regulator.
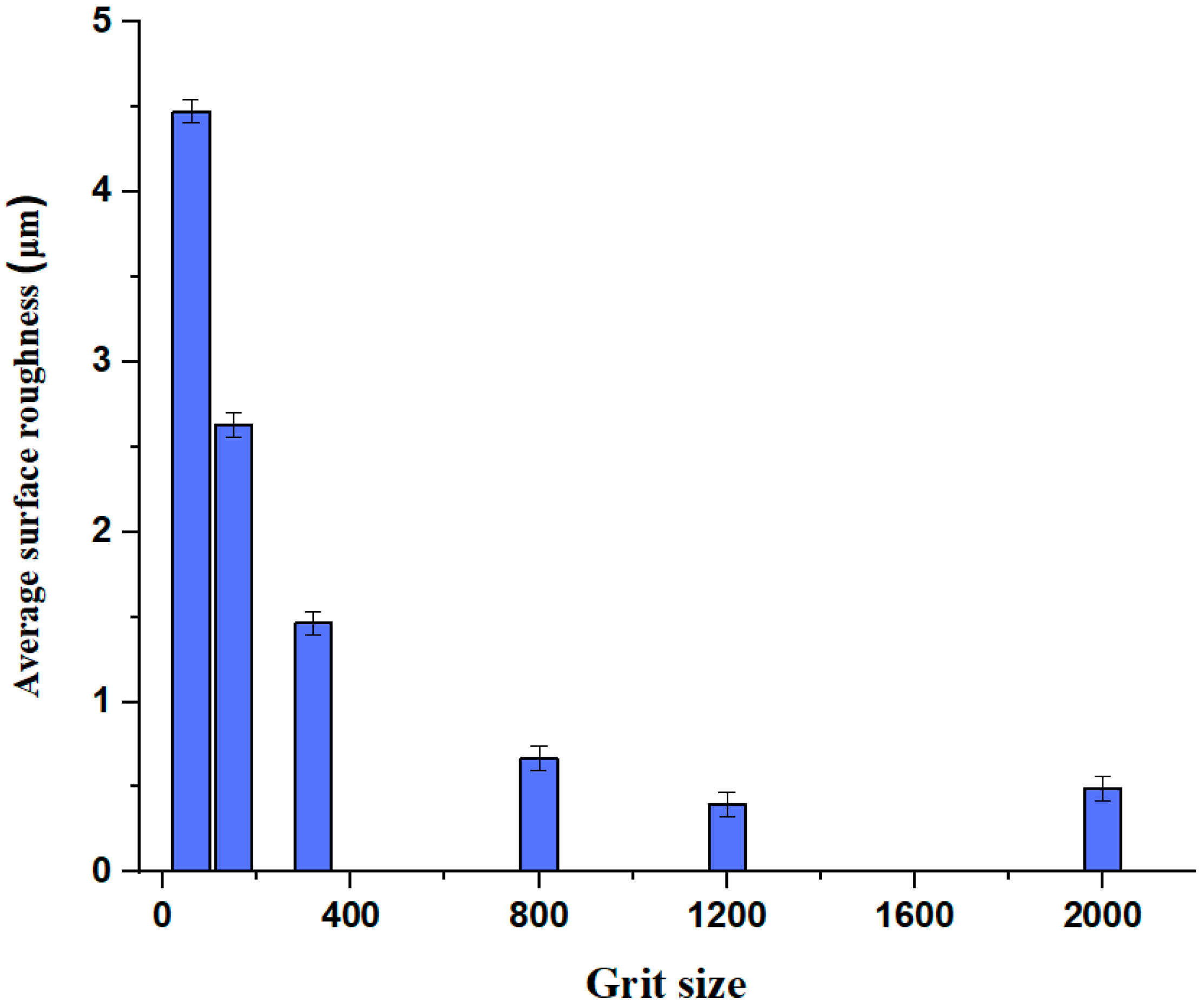
Thirty samples were created using six different sandpaper grit sizes ranging between 60-grit and 2000-grit with five samples of each grit designation prepared in order to characterize the repeatability of the results. The validity of the abrasion process was confirmed by mechanical measurement of the mechanically roughened samples (
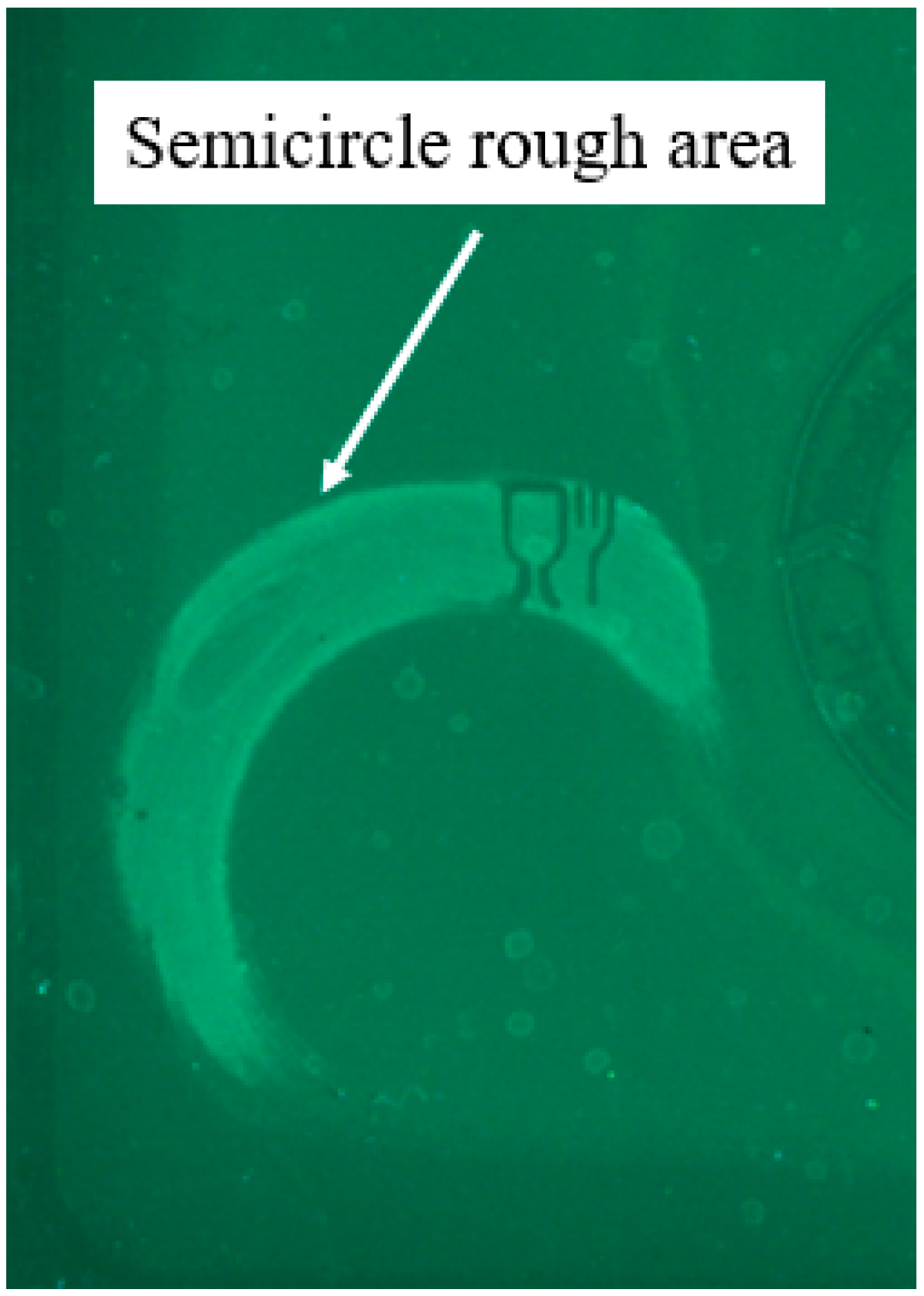
Figure 3). Error bars represent the margin of uncertainty in results, since during this process it was attempted to keep the applied force constant at 2 N ± 20% for each sample; however, this did not prove practical, largely due to the short contact time (2 s) between the sander and pack, and the relatively slow response rate of the weighing balance. Moreover, the surface of the rPET pack was not completely flat, which also caused difficulty in creating uniform areas of roughness. Therefore, most of the samples resulted in a semi-circle shaped rough area on the packaging surface instead of the ideal result, which would have been a homogenously abraded circular area (
Figure 4). However, the resulted samples confirm the reliability of the process as the grit size indicates the nominal number of sharp particles per square inch of sandpaper, the lower grit number provides a coarser finish and hence a rougher surface has been obtained, while a higher grit number provides a smooth finish and therefore a fine surface is achieved.
2.6. Selection of Food Fouling Materials
The influence of surface roughness on the removal of food fouling was analyzed by applying mayonnaise (Hellmann’s
®, original) and peanut butter (Sun-Pat
® smooth) on the rough surfaces. Mayonnaise is a lipid-based food material, containing 70–80% of oil [
28], while peanut butter is a plant-based protein that contains 49.4% fat and 27.8% protein [
29]. These food materials are hydrophobic in nature [
28], and as a result they act as a water repellent. Furthermore, peanut butter has high oleic acid content that imparts high viscosity [
30]. These properties make them more difficult to wash off of surfaces [
31] and hence make them suitable to study cleaning effectiveness.
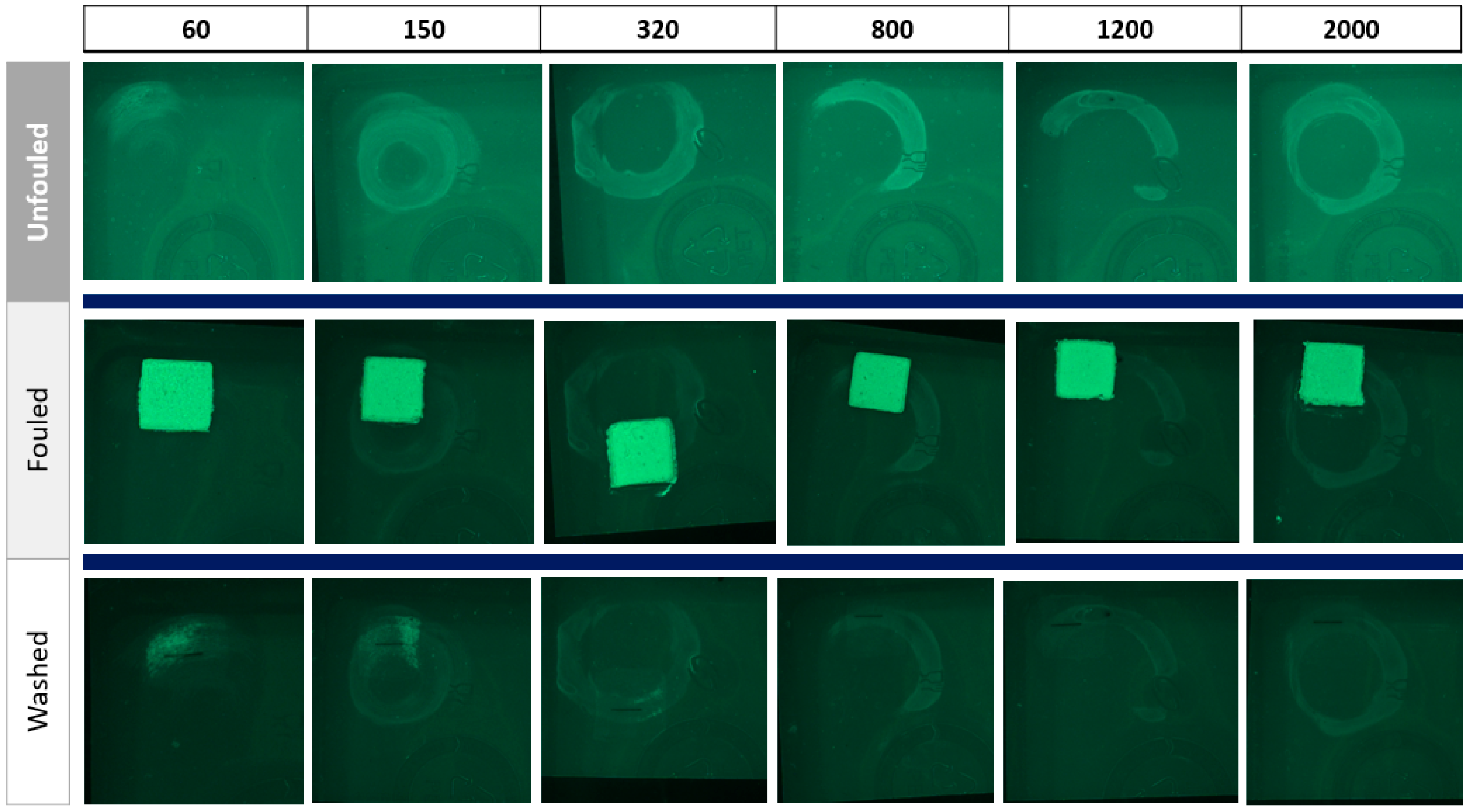
The artificially created rough samples were first weighed, imaged through a UV fluorescence imaging system (
Section 2.7), and then fouled with mayonnaise using a mask with a 20 × 20 mm and 2 mm thick aperture. To replicate dried-on fouling, the samples were subjected to drying in an incubator for 15 min at 25 °C, and the weight of the wet and dried fouling was measured to compute any weight loss during the drying process. The images of the fouled samples were again taken using the UV fluorescence imaging system. The samples were then washed with 3 mL/L of the detergent in the commercial ware washer and dried in the incubator at previously described conditions. Subsequently, the washed samples were reweighed, imaged using the UV fluorescence imaging system, and analyzed via ATP swab testing (see
Section 2.8).
An identical procedure was carried out for peanut butter fouling except for the drying step, which was excluded because peanut butter behaves as a pseudoplastic fluid. Its viscosity decreases at elevated temperatures [
32,
33], which may cause spread of the fouling beyond the masked area.
2.7. Fluorescence Imaging
Ultraviolet fluorescence imaging was utilized to study clean PET packaging and residual fouling on the PET packaging following artificial fouling and cleaning. The lighting and fluorescence imaging apparatus was housed in a steel box (1.2 m × 1.2 m × 1.2 m), providing optical isolation of the UV light. Optical excitation was provided by two sets of dual 18 W, 370 nm (nominal) fluorescent lamps (UV18W BLDTU, UV Light Technology Limited, Sudbury, Suffolk, UK). Samples were positioned directly under the light sources on a manual height-adjustable stage. The light sources emit a peak wavelength of 370 nm with a range of wavelengths from approximately 344–412 nm. Blue food grade PVC conveyor material (Beeston Belting, Sandiacre, Nottinghamshire, UK) was used as the background for the imaging. Image acquisition utilized an AV ALVIUM® 1800U-240C-CH-C, 2.4 MP Color area scan camera, Stemmer Imaging Ltd., Tongham, Guilford, UK. The camera was fitted with a MIDOPT® FIL BI550/25.5 narrow pass filter, Stemmer Imaging Ltd., Tongham, Guilford, UK, which allowed the passage of light in the 535–558 nm wavelength range. This filter served to block unwanted wavelengths of non-fluorescent light, thereby improving the specificity of the captured fluorescence signal.
After optimizing the camera and lighting parameters, all system settings were kept constant (gain = 0, intensity controller target = 50, black level = 0, gamma = 1) and no image processing was performed to ensure consistency during data collection for each sample set. The only exception was exposure time, which was manually adjusted for mayonnaise (1.5 s) and peanut butter (1.0 s) to maximize captured image intensity.
2.8. ATP Swab Test
To chemically assess residual fouling, adenosine triphosphate (ATP) swab tests were applied, which has been widely utilized in the food industry to ensure the effectiveness of cleaning procedures [
34]. Tests were performed using a handheld Hygiena SystemSURE Plus
TM luminometer purchased from Hygiena International Ltd., Guilford, UK, with UltraSnap swabs, held upright in accordance with user guidelines. The Hygiena luminometers have a measurement range of 0 to 9999, displaying Pass and Fail relative light unit (RLU) limits of 10 and 30 RLU for the SystemSURE Plus. Scores below 30 RLU are classified as passing, whereas scores of 30 RLU or higher indicate a failure. Although the manufacturer guideline suggests swabbing a 100 mm × 100 mm area, in this study, the fouling was applied at a known 20 mm × 20 mm area with a controlled process. Therefore, that particular area was swabbed following the washing of the fouled samples. Swabbing a larger region would go beyond the region of interest potentially leading to inaccurate results. Whilst this chemical assay was performed in this research, microbial growth tests, which often underpin cleanliness validation in the food industry, were not used due to resource constraints. However, ATP assay is often the chosen method of routine assessment utilized in the industry and provides a valid summative test for the current study.
4. Conclusions
This study represents and signifies a notable step forward in understanding the relationship between surface roughness and the cleanability of food-packaging surfaces. The three most important contributions are as follows:
Firstly, it demonstrates that a standard industrial washing process shows no notable change in surface roughness for single-use packs, even after 20 wash cycles. Additionally, it evaluates the effectiveness of the cleaning process in removing fouling from surfaces with increased roughness levels. This is a critical assurance for the reusability and durability of rPET materials in food packaging applications.
Secondly, the research introduces a UV fluorescence imaging system as an advanced method to identify fouling traces and rough areas. Its ability to detect residual fouling in scenarios where the industry-standard ATP assay passes, as seen with peanut butter residues, underscores its better sensitivity and presents an opportunity to refine the technique with enhanced image processing for microscale contamination detection.
Thirdly, an important contribution of this study is defining a process for creating artificial surface roughness. Despite challenges in achieving precise control, this method yielded a reliable range of roughness levels, validated through mechanical assessments. This innovation provides a valuable foundation for future investigations into the effects of roughness on cleaning efficacy.
These findings provide actionable insights for regulators and industry. The superior detection capability of UV fluorescence imaging supports its implementation in hygiene verification protocols for enhanced food safety. The findings suggest that the current washing process is highly effective for easier-to-remove fouling such as mayonnaise but may be insufficient for more stubborn material fouling. Therefore, industrial cleaning processes need to be optimized to effectively remove a variety of fouling types and levels for ensuring the success of reusable packaging within a circular economy framework.
The requirement to avoid distortion in packaging form may present an industrial challenge if rPET were to be used for reusable packaging to supply a wide range of food products. This emphasizes the need for design guidelines for packaging manufacturers that can maintain performance through multiple wash cycles. While the study focused on mayonnaise and peanut butter as test materials on laboratory-made artificial rough samples, the insights gained opens avenues for future research, including extending investigations to other types of fouling and substances that may interact with packaging, which can guide logistics companies in developing standardized cleaning protocols. Different types of fouling will clearly behave differently to each other and across the range of common packaging polymer materials. These varied behaviors will need to be understood in order to support industrial acceptance of reusable packaging. It is also worth noting that it may not be appropriate to directly associate laboratory-made artificial roughness with that which may be generated from many cycles of reuse, but this point serves to emphasize the importance of accurate reuse tracking to support future studies in this area. Importantly, the research underscores the potential for refining washing processes by tailoring parameters such as washing time and water pressure to the specific characteristics of fouling. These insights pave the way for resource-efficient cleaning strategies that balance high hygiene standards with sustainability goals. Whilst microbial growth testing was not performed during this study, the results could enable health authorities to strengthen verification protocols by integrating advanced techniques such as UV fluorescence imaging, while considering that long-term use may involve factors like increased number of scratches, presence of deep scratches, discoloration, and environmental conditions that could lead to different outcomes. Moreover, the methodology used in this research could be adapted for other reusable plastic packaging materials, such as polypropylene, high-density polyethylene, polycarbonate, and even extended to industries where surface hygiene is critical, such as medical devices or food processing equipment.