The Potential Role of Humic Substances in the Amelioration of Saline Soils and Its Affecting Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. HA Sources
2.2. Mitigation Capacity
2.3. Acid-Base Titrations
2.4. H-NMR Spectroscopy
2.5. Statistical Analysis
3. Results
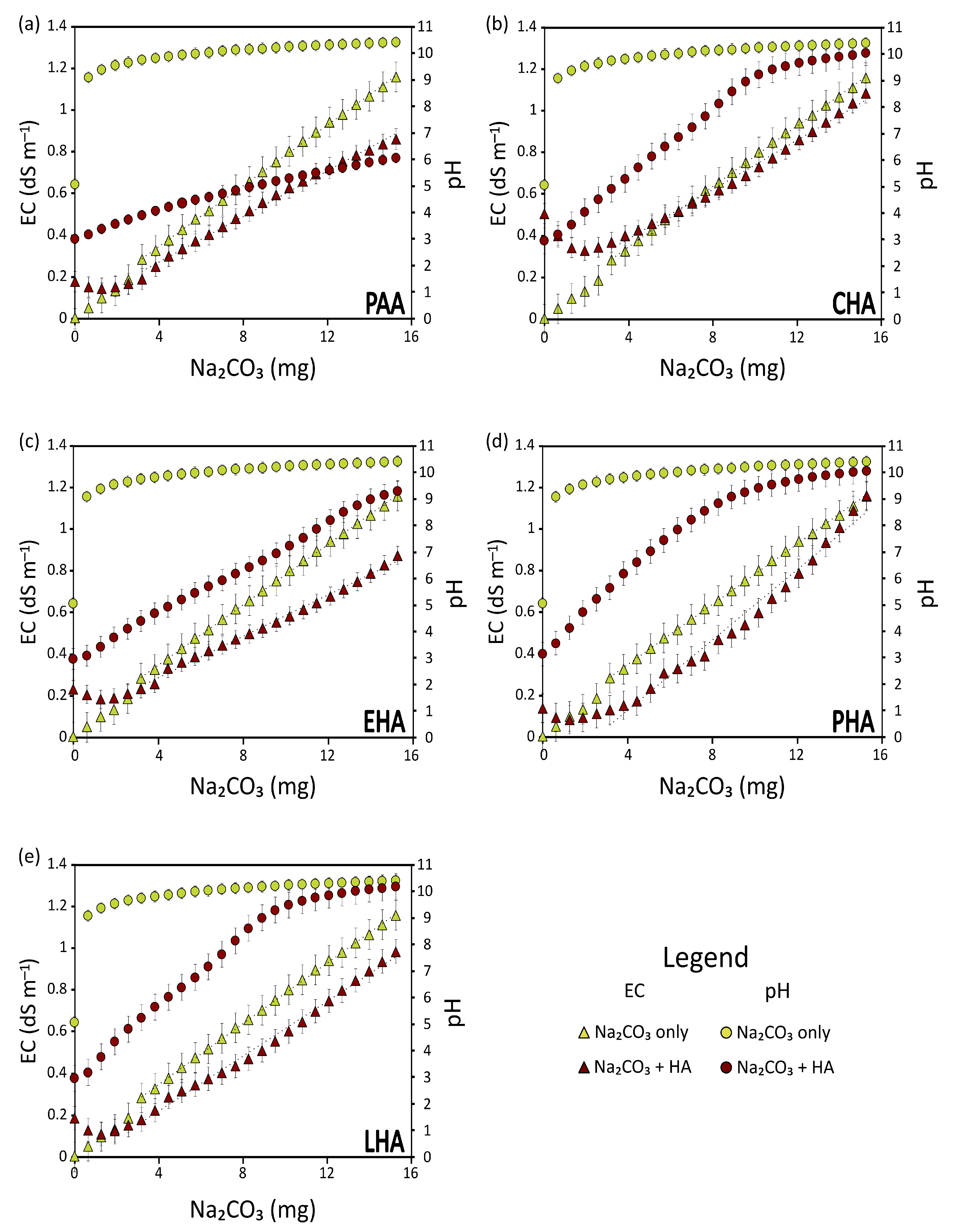
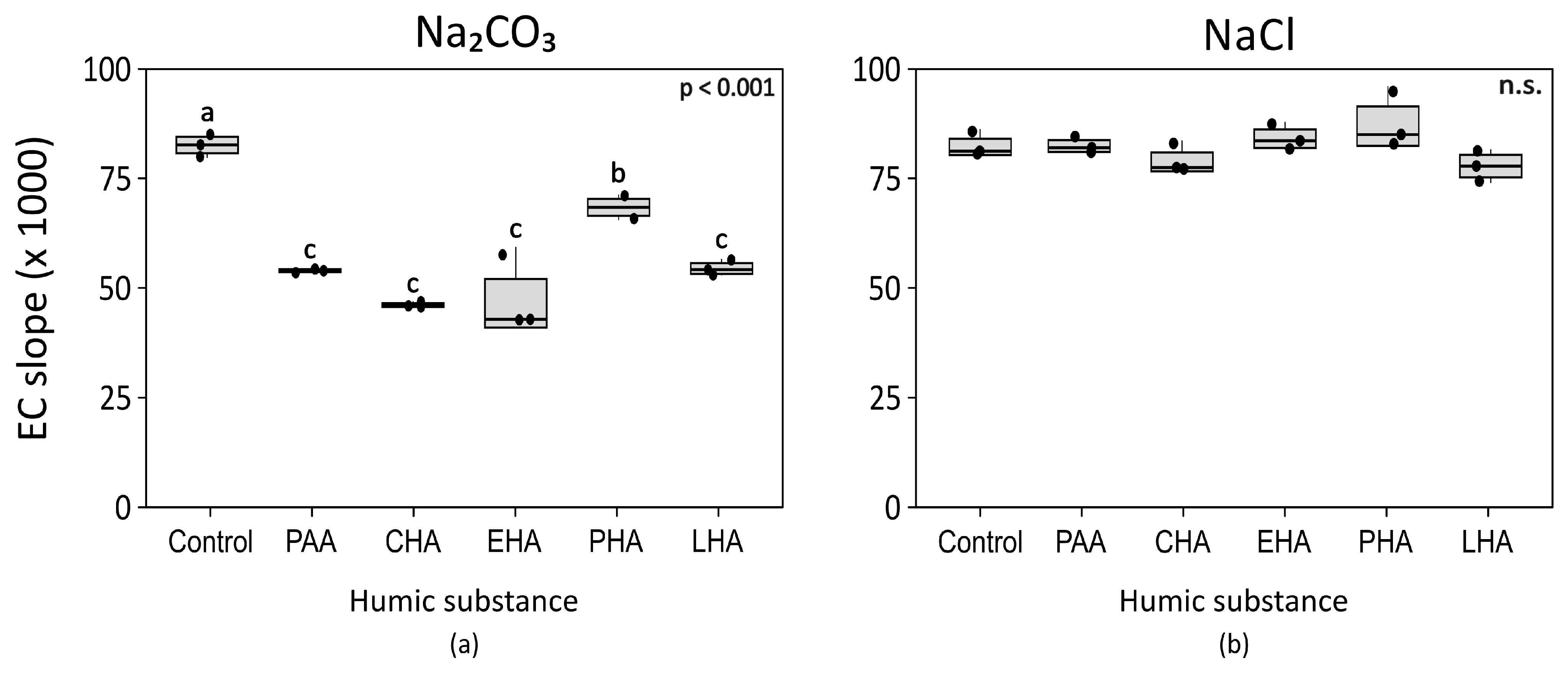
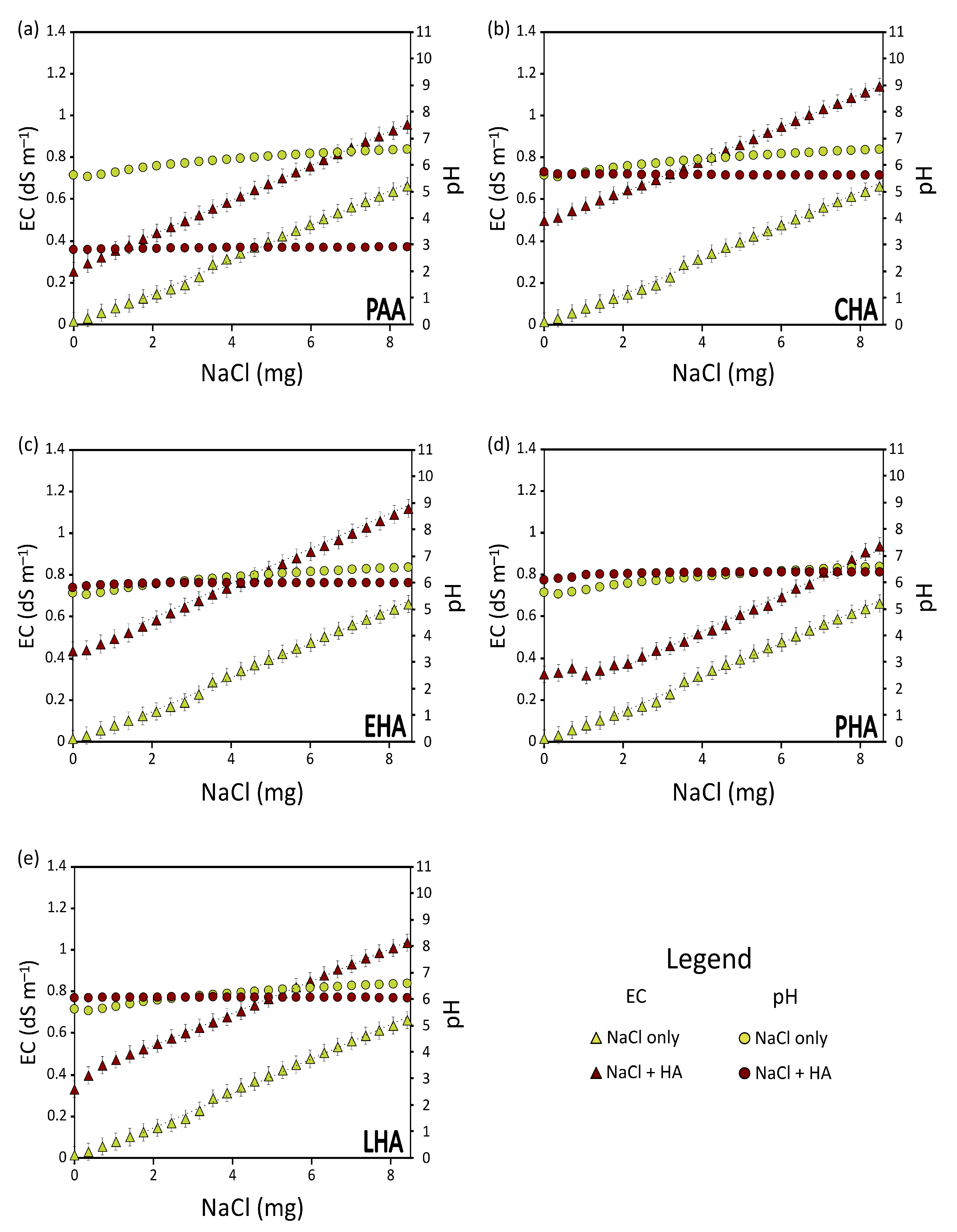
3.1. The Effect of the Type of Salt
3.2. The Effect of the Type of HA
- A is the amount of Na2CO3 that, in the water + HA solution, yields the pH experienced by the HA in the soil;
- A’ is the amount of Na2CO3 that, if added to water, would produce a solution with the same conductivity as A;
- WHA is the weight of HA expressed in mg.
- A is the amount of Na2CO3 that, in the water + HA solution, yields the pH experienced by the HA in the soil;
- A’ is the amount of Na2CO3 that, if added to water, would produce a solution with the same conductivity as A;
- B is the quantity of Na2CO3 added to HA to bring the acid-saturated HA to the pH of the soil at which they are applied;
- B’ is the quantity of Na2CO3 added to water to obtain a solution that, at the original pH of the HA, has the same conductivity as B.
3.3. HA Characterization
4. Discussion
4.1. Dependence of SAP on the Structural Traits of HA
4.2. Potential Practical Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Salinity and sodicity adaptation and mitigation options. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 55–89. ISBN 978-3-319-96190-3. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.-M.; Dietz, K.-J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Abd El-Samad, H.M.; Shaddad, M.A.K. Comparative effect of sodium carbonate, sodium sulphate, and sodium chloride on the growth and related metabolic activities of pea plants. J. Plant Nutr. 1996, 19, 717–728. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.-K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef]
- Betzen, B.M.; Smart, C.M.; Maricle, K.L.; Maricle, B.R. Effects of increasing salinity on photosynthesis and plant water potential in Kansas salt marsh species. Trans. Kans. Acad. Sci. 2019, 122, 49–58. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point; Synthesis Report; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Jordán, M.M.; Navarro-Pedreño, J.; García-Sánchez, E.; Mateu, J.; Juan, P. Spatial dynamics of soil salinity under arid and semi-arid conditions: Geological and environmental implications. Environ. Geol. 2004, 45, 448–456. [Google Scholar] [CrossRef]
- Naorem, A.; Jayaraman, S.; Dang, Y.P.; Dalal, R.C.; Sinha, N.K.; Rao, C.S.; Patra, A.K. Soil constraints in an arid environment—Challenges, prospects, and implications. Agronomy 2023, 13, 220. [Google Scholar] [CrossRef]
- Richards, L.A. (Ed.) Diagnosis and Improvement of Saline and Alkali Soils; USDA Handbook No. 60; U.S. Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Choudhary, O.P.; Kharche, V.K. Soil salinity and sodicity. In Soil Science: An Introduction; Indian Society of Soil Science: New Delhi, India, 2018; pp. 353–384. [Google Scholar]
- Chhabra, R. Classification of salt-affected soils. Arid Land Res. Manag. 2004, 19, 61–79. [Google Scholar] [CrossRef]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. (Eds.) Climate Change and Water; IPCC Technical Paper VI; IPCC Secretariat: Geneva, Switzerland, 2008; 210p, ISBN 978-92-9169-123-4. Available online: https://archive.ipcc.ch/pdf/technical-papers/climate-change-water-en.pdf (accessed on 17 June 2025).
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth-promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Mazhar, S.; Pellegrini, E.; Contin, M.; Bravo, C.; De Nobili, M. Impacts of salinization caused by sea level rise on the biological processes of coastal soils—A review. Front. Environ. Sci. 2022, 10, 909415. [Google Scholar] [CrossRef]
- Wu, Z.; Meng, R.; Feng, W.; Li, Z.; Lu, X.; Chen, Y.; Deng, X.; Chen, T.; Xue, Z.; Wang, X. Soil-Improving Effect of Sesbania–Sorghum Rotation in a Heavily Saline–Alkaline Coastal Region. Agronomy 2024, 14, 2139. [Google Scholar] [CrossRef]
- Lakhdar, A.; Rabhi, M.; Ghnaya, T.; Montemurro, F.; Jedidi, N.; Abdelly, C. Effectiveness of compost use in salt-affected soil. J. Hazard. Mater. 2009, 171, 29–37. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Ouni, Y.; Ghnaya, T.; Montemurro, F.; Abdelly, C.; Lakhdar, A. The role of humic substances in mitigating the harmful effects of soil salinity and improve plant productivity. Int. J. Plant Prod. 2014, 8, 353–374. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Li, J.; Liu, Z.; Gu, X.; Shi, L. Cow manure compost promotes maize growth and ameliorates soil quality in saline-alkali soil: Role of fertilizer addition rate and application depth. Sustainability 2022, 14, 10088. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble salts in compost and their effects on soil and plants: A review. Compost Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defence in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; Rabhi, N.E.H.; Belbahri, L. Mitigation of NaCl stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Hoque, M.N.; Hannan, A.; Imran, S.; Paul, N.C.; Mondal, M.F.; Sadhin, M.M.R.; Bristi, J.M.; Dola, F.S.; Hanif, M.A.; Ye, W.; et al. Plant growth-promoting rhizobacteria-mediated adaptive responses of plants under salinity stress. J. Plant Growth Regul. 2023, 42, 1307–1326. [Google Scholar] [CrossRef]
- Shukry, W.M.; Abu-Ria, M.E.; Abo-Hamed, S.A.; Anis, G.B.; Ibraheem, F. The efficiency of humic acid for improving salinity tolerance in salt-sensitive rice (Oryza sativa): Growth responses and physiological mechanisms. Gesunde Pflanzen 2023, 75, 2639–2653. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Magro, F.; Masetti, G.; Navarro-León, E.; Blasco, B.; Ruiz, J.M. Salinity stress mitigation by radicular and foliar humic substances application in lettuce plants. Plant Growth Regul. 2024, 104, 151–167. [Google Scholar] [CrossRef]
- Vitti, A.; Coviello, L.; Nuzzaci, M.; Vinci, G.; Deligiannakis, Y.; Giannakopoulos, E.; Ronga, D.; Piccolo, A.; Scopa, A.; Drosos, M. Biostimulation of humic acids on Lepidium sativum L. regulated by their content of stable phenolic O· radicals. Chem. Biol. Technol. Agric. 2024, 11, 92. [Google Scholar] [CrossRef]
- Abu-Ria, M.; Shukry, W.; Abo-Hamed, S.; Albaqami, M.; Almuqadam, L.; Ibraheem, F. Humic acid modulates ionic homeostasis, osmolytes content, and antioxidant defence to improve salt tolerance in rice. Plants 2023, 12, 1834. [Google Scholar] [CrossRef]
- Malik, Z.; Malik, N.; Noor, I.; Kamran, M.; Parveen, A.; Ali, M.; Sabir, F.; Elansary, H.O.; El-Abedin, T.K.Z.; Mahmoud, E.A.; et al. Combined effect of rice-straw biochar and humic acid on growth, antioxidative capacity, and ion uptake in maize (Zea mays L.) grown under saline soil conditions. J. Plant Growth Regul. 2023, 42, 3211–3228. [Google Scholar] [CrossRef]
- Delgado, A.; Madrid, A.; Kassem, S.; Andreu, L.; del Campillo, M.C. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 2002, 245, 277–286. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef]
- Dell’Amico, C.; Masciandaro, G.; Ganni, A.; Ceccanti, B.; Garcia, C.; Hernandez, T.; Costa, F. Effects of specific humic fractions on plant growth. In Humic Substances in the Global Environment and Implications on Human Health; Senesi, N., Miano, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 563–566. [Google Scholar]
- Saidimoradi, D.; Ghaderi, N.; Javadi, T. Salinity stress mitigation by humic acid application in strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2019, 256, 108594. [Google Scholar] [CrossRef]
- Mahdy, A.M.; Fathi, N.O. Interactive effects between biofertilizer and antioxidant on salinity mitigation and nutrition and yield of okra plants (Abelmoschus esculentus L.). J. Soil Sci. Agric. Eng. 2012, 3, 189–205. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Sonmez, O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res. Commun. 2018, 46, 67–78. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, I.; Ashraf, U.; Shahzad, T.; Hussain, S.; Shahid, M.; Abid, M.; Ullah, S. Effects of organic and inorganic manures on maize and their residual impact on soil physico-chemical properties. J. Soil Sci. Plant Nutr. 2017, 17, 22–32. [Google Scholar] [CrossRef]
- Maiwan, N.; Tunçtürk, M.; Tunçtürk, R. Effect of humic acid applications on physiological and biochemical properties of soybean (Glycine max L.) grown under salt stress conditions. YYU J. Agric. Sci. 2023, 33, 1–9. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Teodorescu, M.; Lungu, A.; Stanescu, P.O.; Neamţu, C. Preparation and properties of novel slow-release NPK agrochemical formulations based on poly(acrylic acid) hydrogels and liquid fertilizers. Ind. Eng. Chem. Res. 2009, 48, 6527–6534. [Google Scholar] [CrossRef]
- Wilske, B.; Bai, M.; Lindenstruth, B.; Bach, M.; Rezaie, Z.; Frede, H.G.; Breuer, L. Biodegradability of a polyacrylate superabsorbent in agricultural soil. Environ. Sci. Pollut. Res. 2014, 21, 9453–9460. [Google Scholar] [CrossRef]
- Chabreck, R.H. Vegetation, Water and Soil Characteristics of the Louisiana Coastal Region; Louisiana Agricultural Experiment Station Bulletin 664; Louisiana State University: Baton Rouge, LA, USA, 1972; 72p, Available online: https://repository.lsu.edu/agexp/147 (accessed on 1 July 2025).
- Weaver, A.R.; Kissel, D.E.; Chen, F.; West, L.T.; Adkins, W.; Rickman, D.; Luvall, J.C. Mapping soil pH buffering capacity of selected fields in the coastal plain. Soil Sci. Soc. Am. J. 2004, 68, 662–668. [Google Scholar] [CrossRef]
- Pettersson, C.; Arsenie, I.; Ephraim, J.; Boren, H.; Allard, B. Properties of fulvic acids from deep groundwaters. Sci. Total Environ. 1989, 81–82, 287–296. [Google Scholar] [CrossRef]
- Ritchie, J.D.; Perdue, E.M. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Wilson, M.A. Application of nuclear magnetic resonance spectroscopy to the study of the structure of soil organic matter. J. Soil Sci. 1981, 32, 167–186. [Google Scholar] [CrossRef]
- Gigliotti, G.; Giusquiani, P.L.; Businelli, D.; Macchioni, A. Composition changes of dissolved organic matter in a soil amended with municipal waste compost. Soil Sci. 1997, 162, 919–926. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 20 May 2025).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Chollakup, R.; Beck, J.B.; Dirnberger, K.; Tirrell, M.; Eisenbach, C.D. Polyelectrolyte molecular weight and salt effects on the phase behaviour and coacervation of aqueous solutions of poly(acrylic acid) sodium salt and poly(allylamine) hydrochloride. Macromolecules 2013, 46, 2376–2390. [Google Scholar] [CrossRef]
- Daba, A.W. Rehabilitation of soil salinity and sodicity using diverse amendments and plants: A critical review. Discov. Environ. 2025, 3, 53. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Li, S.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in North China: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, e89185. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M.; Šimůnek, J. Leaching and reclamation of a biochar and compost amended saline–sodic soil with moderate SAR reclaimed water. Agric. Water Manag. 2015, 158, 255–265. [Google Scholar] [CrossRef]
- Pertusatti, J.; Prado, A.G.S. Buffer capacity of humic acid: Thermodynamic approach. J. Colloid Interface Sci. 2007, 314, 484–489. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 15th ed.; Pearson: Columbus, OH, USA, 2016; ISBN 978-0-13-325448-8. [Google Scholar]
- Cooke, J.D.; Hamilton-Taylor, J.; Tipping, E. On the acid–base properties of humic acid in soil. Environ. Sci. Technol. 2007, 41, 465–470. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Brady, N.C.; Wail, R.R. Reclamation of saline and sodic soils. In The Nature and Properties of Soils, 13th ed.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2002; p. 442. [Google Scholar]
- Jovanović, U.D.; Marković, M.M.; Cupać, S.B.; Tomić, Z.P. Soil humic acid aggregation by dynamic light scattering and laser Doppler electrophoresis. J. Plant Nutr. Soil Sci. 2013, 176, 674–679. [Google Scholar] [CrossRef]
- Rengasamy, P.; Olsson, K.A. Sodicity and soil structure. Aust. J. Soil Res. 1991, 29, 935–952. [Google Scholar] [CrossRef]
- Shaaban, M.M.; El-Fouly, M.M. Nutrient contents and salt removal potential of some wild plants grown in salt-affected soils. Acta Hortic. 2002, 573, 377–385. [Google Scholar] [CrossRef]
- Bai, Y.; Yan, Y.; Zuo, W.; Gu, C.; Xue, W.; Mei, L.; Shan, Y.; Feng, K. Coastal Mudflat Saline Soil Amendment by Dairy Manure and Green Manuring. Int. J. Agron. 2017, 2017, 4635964. [Google Scholar] [CrossRef]
- Rai, A.K.; Basak, N.; Sundha, P. Chemistry of salt-affected soils. In Managing Salt-Affected Soils for Sustainable Agriculture; Minhas, P.S., Yadav, R.K., Eds.; ICAR: New Delhi, India, 2021; pp. 128–148. [Google Scholar]
- Villalobos, F.J.; Fereres, E. (Eds.) Principles of Agronomy for Sustainable Agriculture, 2nd ed.; Springer: Cham, Switzerland, 2024; ISBN 978-3-031-69149-2. [Google Scholar]
| Humic Acid | Origin | SAPHA (mg Na2CO3 g−1 HA) | SAPHA eff (mg Na2CO3 g−1 HA) |
|---|---|---|---|
| PAA | Polyacrylic Acid | 142.59 | 49.86 |
| CHA | Compost | 28.56 | 25.10 |
| PHA | Peat Soil | 155.65 | 43.60 |
| EHA | Elliott Soil | 164.55 | 44.35 |
| LHA | Leonardite | 142.62 | 46.44 |
| Humic Acid | Aromatic-H | H in α to Double Bonds | H in α to Carbons Bound to Oxygen | Methylene-H Associated with Carboxyl Groups | H in Terminal Methyl and Methylene Groups |
|---|---|---|---|---|---|
| PAA * | 0.31 | 0.00 | 61.58 | 36.92 | 1.19 |
| CHA | 10.43 | 0.16 | 21.86 | 30.64 | 37.19 |
| PHA | 11.82 | 2.79 | 29.25 | 19.50 | 36.62 |
| EHA | 14.70 | 1.64 | 25.53 | 23.82 | 34.30 |
| LHA | 19.15 | 3.54 | 5.38 | 33.56 | 38.35 |
| Humic Acid | Q1 (mmol g−1 C) | Q2 (mmol g−1 C) | Total Ionized Groups at pH 7 (mmol g−1 C) | Total Ionized Groups at pH 8.5 (mmol g−1 C) | Total Ionized Groups at pH 10 (mmol g−1 C) | Charge Increments from pH 7 to 8.5 (mmol g−1 C) | Charge Increments from pH 8.5 to 10 (mmol g−1 C) |
|---|---|---|---|---|---|---|---|
| PAA | 17.00 | 3.50 | 15.25 | 17.50 | 17.75 | 2.50 | 0.25 |
| CHA | 8.80 | 5.50 | 9.20 | 10.10 | 11.55 | 2.35 | 1.45 |
| EHA | 10.20 | 4.00 | 9.60 | 10.50 | 12.25 | 2.67 | 1.75 |
| PHA | 10.13 | 5.50 | 9.63 | 10.50 | 12.88 | 3.25 | 2.38 |
| LHA | 9.03 | 2.45 | 8.55 | 9.33 | 10.25 | 1.70 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moro, D.; Pellegrini, E.; Contin, M.; Zuccaccia, D.; Khakbaz, A.; De Nobili, M. The Potential Role of Humic Substances in the Amelioration of Saline Soils and Its Affecting Factors. Sustainability 2025, 17, 8621. https://doi.org/10.3390/su17198621
Moro D, Pellegrini E, Contin M, Zuccaccia D, Khakbaz A, De Nobili M. The Potential Role of Humic Substances in the Amelioration of Saline Soils and Its Affecting Factors. Sustainability. 2025; 17(19):8621. https://doi.org/10.3390/su17198621
Chicago/Turabian StyleMoro, Daniel, Elisa Pellegrini, Marco Contin, Daniele Zuccaccia, Ali Khakbaz, and Maria De Nobili. 2025. "The Potential Role of Humic Substances in the Amelioration of Saline Soils and Its Affecting Factors" Sustainability 17, no. 19: 8621. https://doi.org/10.3390/su17198621
APA StyleMoro, D., Pellegrini, E., Contin, M., Zuccaccia, D., Khakbaz, A., & De Nobili, M. (2025). The Potential Role of Humic Substances in the Amelioration of Saline Soils and Its Affecting Factors. Sustainability, 17(19), 8621. https://doi.org/10.3390/su17198621










