Biofortification of Common Bean: Critical Analysis of Genetic and Agronomic Strategies as Viable Alternatives to Tackling Zinc Deficiency in Developing Countries
Abstract
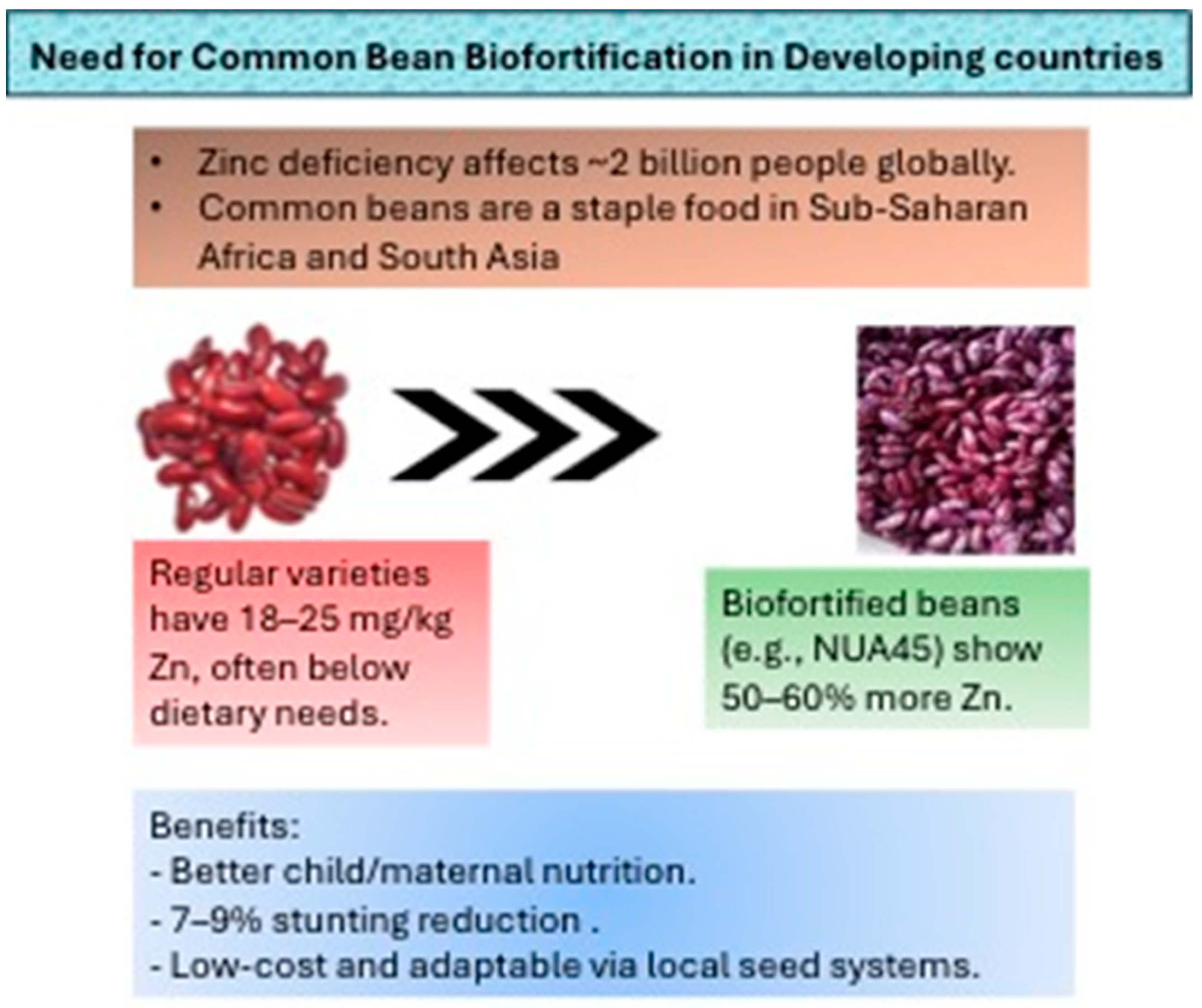
1. Introduction
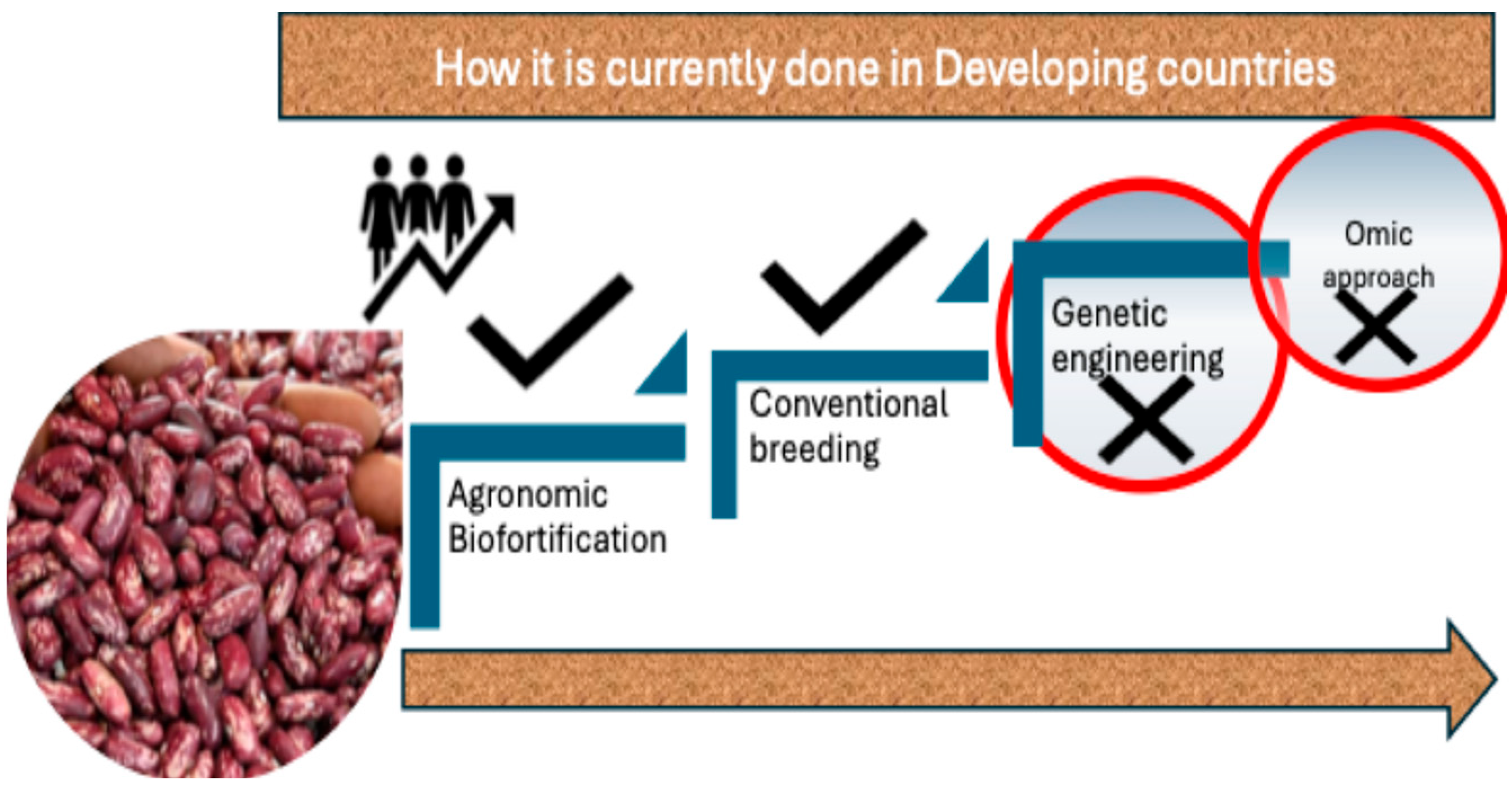
2. Biofortification Methods
2.1. Agronomic Biofortification
2.2. Genetic Biofortification
3. Comparative Evaluation of Biofortification Approaches
4. Status of Biofortification in Developing Countries: Case of Common Bean in Malawi
5. Promising Implementation Strategies to Enhance Biofortification of Common Bean in Developing Countries
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Myers, J.R.; Kmiecik, K. Common Bean: Economic Importance and Relevance to Biological Science Research; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar] [CrossRef]
- Aseete, P.; Katungi, E.; Bonabana-Wabbi, J.; Birachi, E.; Ugen, M.A. Consumer demand heterogeneity and valuation of value-added pulse products: A case of precooked beans in Uganda. Agric. Food Secur. 2018, 7, 51. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil. 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Bulyaba, R.; Winham, D.M.; Lenssen, A.W.; Moore, K.J.; Kelly, J.D.; Brick, M.A.; Wright, E.M.; Ogg, J.B. Genotype by Location Effects on Yield and Seed Nutrient Composition of Common Bean. Agronomy 2020, 10, 347. [Google Scholar] [CrossRef]
- Mecha, E.; Natalello, S.; Carbas, B.; da Silva, A.B.; Leitão, S.T.; Brites, C.; Veloso, M.M.; Rubiales, D.; Costa, J.; Cabral Mde, F.; et al. Disclosing the Nutritional Quality Diversity of Portuguese Common Beans—The Missing Link for Their Effective Use in Protein Quality Breeding Programs. Agronomy 2021, 11, 221. [Google Scholar] [CrossRef]
- Frossard, E.; Bucher, M.; Machler, F.; Mozafar, A.; Hurrell, R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar] [CrossRef]
- Xue, Y.; Xia, H.; Christie, P.; Zhang, Z.; Li, L.; Tang, C. Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: A critical review. Ann. Bot. 2016, 117, 363–377. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef]
- Blair, M.W. Mineral Biofortification Strategies for Food Staples: The Example of Common Bean. J. Agric. Food Chem. 2013, 61, 8287–8294. [Google Scholar] [CrossRef]
- Meenakshi, J.V. Best Practice Paper Cost-Effectiveness of Biofortification; Copenhagen Consensus Center: Lowell, MA, USA, 2008. [Google Scholar]
- de Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Sec. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Ntoupa, P.-S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Devarshi, P.P.; Mao, Q.; Grant, R.W.; Hazels Mitmesser, S. Comparative Absorption and Bioavailability of Various Chemical Forms of Zinc in Humans: A Narrative Review. Nutrients 2024, 16, 4269. [Google Scholar] [CrossRef]
- Gibson, R.S.; Ferguson, E.L. Nutrition intervention strategies to combat zinc deficiency in developing countries. Nutr Res Rev. 1998, 11, 115–131. [Google Scholar] [CrossRef]
- Likoswe, B.H.; Phiri, F.P.; Broadley, M.R.; Joy, E.J.M.; Patson, N.; Maleta, K.M.; Phuka, J.C. Inflammation Adjustment by Two Methods Decreases the Estimated Prevalence of Zinc Deficiency in Malawi. Nutrients 2020, 12, 1563. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Ander, E.L.; Young, S.D.; Black, C.R.; Watts, M.J.; Chilimba, A.D.C.; Chilima, B.; Siyame, E.W.P.; Kalimbira, A.A.; Hurst, R.; et al. Dietary mineral supplies in Africa. Physiol. Plant 2014, 151, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; King, L.E.; Laakko, T.; Vollmer, T.L. The Dynamic Link between the Integrity of the Immune System and Zinc Status. J. Nutr. 2000, 130, 1399S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fang, T.; Chen, H. Zinc and Central Nervous System Disorders. Nutrients 2023, 15, 2140. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil. Sci. Plant Nutr. 2013, 14, 905–927. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Ozturk, L.; Cakmak, I. Biofortification of Durum Wheat with Zinc Through Soil and Foliar Applications of Nitrogen. Cereal Chem. 2010, 87, 1–9. [Google Scholar] [CrossRef]
- Ofori, K.F.; Antoniello, S.; English, M.M.; Aryee, A.N.A. Improving nutrition through biofortification–A systematic review. Front. Nutr. 2022, 9, 1043655. [Google Scholar] [CrossRef]
- Singh, U.; Praharaj, C.S.; Singh, S.S.; Singh, N.P. Biofortification: Introduction, Approaches, Limitations, and Challenges. In Biofortification of Food Crops; Springer: New Delhi, India, 2016; pp. 3–18. [Google Scholar] [CrossRef]
- Nankya, E.; Tenywa, J.; Nkalubo, S.; Mulumba, L. Response of Tissue Zinc to Zinc Fertilisation by Zinc Biofortifier Bush Bean Genotypes Targeted for Low Income Communities. Int. J. Plant Soil. Sci. 2015, 7, 172–179. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil. Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological Limits to Zinc Biofortification of Edible Crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Teklu, D.; Gashu, D.; Joy, E.J.M.; Amede, T.; Broadley, M.R. Effectiveness of Agronomic Biofortification Strategy in Fighting against Hidden Hunger. Agronomy 2023, 13, 2173. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons. Agronomy 2019, 10, 39. [Google Scholar] [CrossRef]
- Açık, A.; Öncan Sümer, F. Foliar Application of Zinc Improves Agronomical and Quality Parameters and Biofortification of Cowpea (Vigna sinensis) under Deficit Irrigation. Agronomy 2023, 13, 1021. [Google Scholar] [CrossRef]
- Cambraia, T.L.L.; Fontes, R.L.F.; Vergütz, L.; Vieira, R.F.; Neves, J.C.L.; Corrêa Netto, P.S.; Dias, R.F.N. Agronomic biofortification of common bean grain with zinc. Pesqui. Agropecu. Bras. 2019, 54, e01003. [Google Scholar] [CrossRef]
- Montalvo, D.; Degryse, F.; da Silva, R.C.; Baird, R.; McLaughlin, M.J. Agronomic Effectiveness of Zinc Sources as Micronutrient Fertilizer. Adv. Agron. 2016, 139, 215–267. [Google Scholar] [CrossRef]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.d.S.; Reis, A.R.d.; Nogueira, T.A.R.; Moretti Neto, M.J.; Mortinho, E.S.; Fernandes, G.C.; Teixeira Filho, M.C.M. Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Baligah, H.U.; Mir, S.A.; Sofi, P.; Mir, A.H. Grain Zinc Phytic Acid and Nutrient Uptake in Common Bean is Influenced by Sources and Concentrations of Zinc Fertilization. Int. Res. J. Pure Appl. Chem. 2020, 21, 9–17. [Google Scholar] [CrossRef]
- Mutambu, D.; Mucheru-Muna, M.; Kihara, L.; Bolo, P.; Kinyua, M. Response of Common Bean (Phaseolus vulgaris L.) and Maize (Zea mays) to Zinc Fertilizers in Acidic Ferralsols of Western Kenya. Available online: https://doi.org/10.21203/rs.3.rs-3388458/v1 (accessed on 10 August 2025).
- Ram, H.; Rashid, A.; Zhang, W.; Duarte, A.P.; Phattarakul, N.; Simunji, S.; Kalayci, M.; Freitas, R.; Rerkasem, B.; Bal, R.S.; et al. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant Soil. 2016, 403, 389–401. [Google Scholar] [CrossRef]
- Kachinski, W.D.; Ávila, F.W.; dos Reis, A.R.; Muller, M.M.L.; Mendes, M.C.; Petranski, P.H. Agronomic biofortification increases concentrations of zinc and storage proteins in common bean (Phaseolus vulgaris L.) grains. Food Res. Int. 2022, 155, 111105. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Kiani, S.; Khoshgoftarmanesh, A.H. The effectiveness of seed priming and foliar application of zinc- amino acid chelates in comparison with zinc sulfate on yield and grain nutritional quality of common bean. J. Plant Nutr. 2020, 43, 2106–2116. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Chejara, S.; Malik, K.; Kumar, R.; Kumar, A.; Yadav, R.K. Agronomic biofortification of food crops: An emerging opportunity for global food and nutritional security. Front. Plant Sci. 2022, 13, 1055278. [Google Scholar] [CrossRef]
- Hussain, S.; Maqsood, M.A.; Rengel, Z.; Aziz, T. Biofortification and Estimated Human Bioavailability of Zinc in Wheat Grains as Influenced by Methods of Zinc Application. Plant Soil 2012, 361, 279–290. [Google Scholar] [CrossRef]
- Joy, E.J.; Stein, A.J.; Young, S.D.; Ander, E.L.; Watts, M.J.; Broadley, M.R. Zinc-enriched fertilisers as a potential public health intervention in Africa. Plant Soil 2015, 389, 1–24. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.P.B.; Moreira, A.; Guimarães, C.M. Foliar Fertilization of Crop Plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, M.A.; Yucel, C.; Torun, A.; Cekic, C.; Bagci, A.; Ozkan, H.; Braun, H.; Sayers, Z.; Cakmak, I. Concentration and localization of zinc during seed development and germination in wheat. Physiol. Plant 2006, 128, 144–152. [Google Scholar] [CrossRef]
- Woolfolk, C.W.; Raun, W.R.; Johnson, G.V.; Thomason, W.E.; Mullen, R.W.; Wynn, K.J.; Freeman, K.W. Influence of Late-Season Foliar Nitrogen Applications on Yield and Grain Nitrogen in Winter Wheat. Agron. J. 2002, 94, 429–434. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kiran, A.; ur Rehman, H.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- Montanha, G.S.; Rodrigues, E.S.; Romeu, S.L.Z.; de Almeida, E.; Reis, A.R.; Lavres, J.; Pereira de Carvalho, H.W. Zinc uptake from ZnSO4 (aq) and Zn-EDTA (aq) and its root-to-shoot transport in soybean plants (Glycine max) probed by time-resolved in vivo X-ray spectroscopy. Plant Sci. 2020, 292, 110370. [Google Scholar] [CrossRef]
- Prom-u-thai, C.; Rerkasem, B.; Yazici, A.; Cakmak, I. Zinc priming promotes seed germination and seedling vigor of rice. J. Plant Nutr. Soil. Sci. 2012, 175, 482–488. [Google Scholar] [CrossRef]
- Poudel, P.; Di Gioia, F.; Lambert, J.D.; Connolly, E.L. Zinc biofortification through seed nutri-priming using alternative zinc sources and concentration levels in pea and sunflower microgreens. Front. Plant Sci. 2023, 14, 1177844. [Google Scholar] [CrossRef]
- Veena, M.; Puthur, J.T. Seed nutripriming with zinc is an apt tool to alleviate malnutrition. Environ. Geochem. Health 2022, 44, 2355–2373. [Google Scholar] [CrossRef]
- Elhaj Baddar, Z.; Unrine, J.M. Functionalized-ZnO-Nanoparticle Seed Treatments to Enhance Growth and Zn Content of Wheat (Triticum aestivum) Seedlings. J. Agric. Food Chem. 2018, 66, 12166–12178. [Google Scholar] [CrossRef]
- Rameshraddy Pavithra, G.J.; Rajashekar Reddy, B.H.; Salimath, M.; Geetha, K.N.; Shankar, A.G. Zinc oxide nano particles increases Zn uptake, translocation in rice with positive effect on growth, yield and moisture stress tolerance. Indian J. Plant Physiol. 2017, 22, 287–294. [Google Scholar] [CrossRef]
- Bala, R.; Kalia, A.; Dhaliwal, S.S. Evaluation of Efficacy of ZnO Nanoparticles as Remedial Zinc Nanofertilizer for Rice. J. Soil. Sci. Plant Nutr. 2019, 19, 379–389. [Google Scholar] [CrossRef]
- Marques, E.; Darby, H.M.; Kraft, J. Benefits and Limitations of Non-Transgenic Micronutrient Biofortification Approaches. Agronomy 2021, 11, 464. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Maqsood, M.; Hussain, S. Biofortification of rice with iron and zinc: Progress and prospects. In Rice Research for Quality Improvement: Genomics and Genetic Engineering; Springer: Singapore, 2020; pp. 605–627. [Google Scholar]
- Huey, S.L.; Krisher, J.T.; Bhargava, A.; Friesen, V.M.; Konieczynski, E.M.; Mbuya, M.N.N.; Mehta, N.H.; Monterrosa, E.; Nyangaresi, A.M.; Mehta, S. Review of the Impact Pathways of Biofortified Foods and Food Products. Nutrients 2022, 14, 1200. [Google Scholar] [CrossRef]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding Crops for Better Nutrition. Crop Sci. 2007, 47, S-88–S-105. [Google Scholar] [CrossRef]
- Nkhata, W.; Shimelis, H.; Chirwa, R. Productivity of newly released common bean (Phaseolus vulgaris L.) varieties under sole cropping and intercropping with maize (Zea mays L.). Front. Sustain. Food Syst. 2021, 5, 741177. [Google Scholar] [CrossRef]
- Amongi, W.; Kato, F.; Male, A.; Nakyanzi, B.; Sebuliba, S.; Kabwama, A.; Mbiu, J.; Williams, M.; Baguma, G.; Mukankusi, C. Gain and performance in yield and micronutrient concentration in common bean improvement. Afr. Crop Sci. J. 1970, 31, 85–111. [Google Scholar] [CrossRef]
- Muroki, M.W.; Waswa, L.M.; Fungo, R.; Kabwama, A.; Eric, N.; Nepomuscene, N.; Ndabashinze, B.; Mahungu, S.M. Sensory properties of selected biofortified common bean (Phaseolus vulgaris) varieties grown in Burundi. Food Sci. Nutr. 2024, 12, 3199–3213. [Google Scholar] [CrossRef] [PubMed]
- Mughi, I.; Ochwo-Ssemakula, M.; Edema, R.; Mukankusi, C. Variability in Cooking Time, Iron and Zinc Content in Common Bean (Phaseolus Vulgaris L.) Genotypes. J. Sci. Agric. 2021, 5, 6–11. [Google Scholar] [CrossRef]
- Blair, M.W.; Medina, J.I.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Machado, G.; Graham, R. QTL for seed iron and zinc concentration and content in a Mesoamerican common bean (Phaseolus vulgaris L.) population. Theor. Appl. Genet. 2010, 121, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Gelin, J.R.; Forster, S.; Grafton, K.F.; McClean, P.E.; Rojas-Cifuentes, G.A. Analysis of Seed Zinc and Other Minerals in a Recombinant Inbred Population of Navy Bean (Phaseolus vulgaris L.). Crop Sci. 2007, 47, 1361–1366. [Google Scholar] [CrossRef]
- Dreher, K.; Khairallah, M.; Ribaut, J.-M.; Morris, M. Money matters (I): Costs of field and laboratory procedures associated with conventional and marker-assisted maize breeding at CIMMYT. Mol. Breed. 2003, 11, 221–234. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Das, S.; Kumar, V.; Gowda, C.L.L.; Sharma, S.; Tyagi, A.K.; Parida, S.K. Genetic dissection of seed-iron and zinc concentrations in chickpea. Sci. Rep. 2016, 6, 24050. [Google Scholar] [CrossRef]
- Contreras-Soto, R.I.; Mora, F.; de Oliveira, M.A.R.; Higashi, W.; Scapim, C.A.; Schuster, I. A Genome-Wide Association Study for Agronomic Traits in Soybean Using SNP Markers and SNP-Based Haplotype Analysis. PLoS ONE 2017, 12, e0171105. [Google Scholar] [CrossRef]
- Hnatuszko-Konka, K.; Kowalczyk, T.; Gerszberg, A.; Wiktorek-Smagur, A.; Kononowicz, A.K. Phaseolus vulgaris—Recalcitrant potential. Biotechnol. Adv. 2014, 32, 1205–1215. [Google Scholar] [CrossRef]
- Sperotto, R.A.; Ricachenevsky, F.K. Common Bean Fe Biofortification Using Model Species’ Lessons. Front. Plant Sci. 2017, 8, 2187. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; González, L.F.; Kimani, P.M.; Butare, L. Genetic diversity, inter-gene pool introgression and nutritional quality of common beans (Phaseolus vulgaris L.) from Central Africa. Theor. Appl. Genet. 2010, 121, 237–248. [Google Scholar] [CrossRef]
- Astudillo-Reyes, C.; Fernandez, A.C.; Cichy, K.A. Transcriptome Characterization of Developing Bean (Phaseolus vulgaris L.) Pods from Two Genotypes with Contrasting Seed Zinc Concentrations. PLoS ONE 2015, 10, e0137157. [Google Scholar] [CrossRef] [PubMed]
- Cvitanich, C.; Przybyłowicz, W.J.; Mesjasz-Przybyłowicz, J.; Blair, M.W.; Astudillo, C.; Orłowska, E.; Jurkiewicz, A.M.; Jensen, E.Ø.; Stougaard, J. Micro-PIXE investigation of bean seeds to assist micronutrient biofortification. Nucl. Instrum. Methods Phys. Res. B 2011, 269, 2297–2302. [Google Scholar] [CrossRef]
- Nestel, P.; Bouis, H.E.; Meenakshi, J.; Pfeiffer, W. Biofortification of Staple Food Crops. J. Nutr. 2006, 136, 1064–1067. [Google Scholar] [CrossRef]
- Pérez, S.; Oparinde, A.; Birol, E.; Funes, J. Consumer Acceptance of an Iron Bean Variety in Northwest Guatemala: The Role of Information and Repeated Messaging. Agric. Food Econ. 2018, 6, 14. [Google Scholar] [CrossRef]
- Vaiknoras, K.; Larochelle, C. The Impact of Iron-Biofortified Bean Adoption on Bean Productivity, Consumption, Purchases and Sales. World Dev. 2021, 139, 105260. [Google Scholar] [CrossRef]
- Shi, G.; Chavas, J.-P.; Lauer, J. Commercialized transgenic traits, maize productivity and yield risk. Nat. Biotechnol. 2013, 31, 111–114. [Google Scholar] [CrossRef]
- Fan, M.; Cui, Z.; Chen, X.; Jiang, R.; Zhang, F. Integrated nutrient management for improving crop yields and nutrient utilization efficiencies in China. J. Soil. Water Conserv. 2008, 63, 126A–128A. [Google Scholar] [CrossRef]
- Shi, R.; Li, H.; Tong, Y.; Jing, R.; Zhang, F.; Zou, C. Identification of quantitative trait locus of Zn and phosphorus density in wheat (Triticum aestivum L.) grain. Plant Soil. 2008, 306, 95–104. [Google Scholar] [CrossRef]
- Martinelli, L.; Karbarz, M.; Siipi, H. Science, safety, and trust: The case of transgenic food. Croat. Med. J. 2013, 54, 91–96. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Johnson, N.; Qaim, M. Consumer acceptance of second-generation GM foods: The case of biofortified cassava in the northeast of Brazil. J. Agric. Econ. 2009, 60, 604–624. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef]
- Stein, A.J.; Sachdev, H.P.S.; Qaim, M. Potential impact and cost-effectiveness of Golden Rice. Nat. Biotechnol. 2006, 24, 1200–1201. [Google Scholar] [CrossRef]
- Mari, G.F.; Prado, R.M.; Soares, A.A.V.L.; Caione, G.; Campos, C.N.S. Residual effect of zinc application doses and methods on nutrition and productivity of corn. Am. J. Plant Sci. 2015, 6, 298–305. [Google Scholar] [CrossRef]
- Zia, M.H.; Ahmed, I.; Bailey, E.H.; Lark, R.M.; Young, S.D.; Lowe, N.M.; Joy, E.J.M.; Broadley, M.R. Site-specific factors influence the field performance of a Zn-biofortified wheat variety. Front. Sustain. Food Syst. 2020, 4, 135. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Phiri, D. Case Study of Malawi: Scaling Biofortification Through Home-Grown School Feeding. Available online: https://www.harvestplus.org/wp-content/uploads/2023/11/Malawi-Case-Study.pdf (accessed on 10 August 2025).
- Foley, J.K.; Michaux, K.D.; Mudyahoto, B.; Kyazike, L.; Cherian, B.; Kalejaiye, O.; Ifeoma, O.; Ilona, P.; Reinberg, C.; Mavindidze, D.; et al. Scaling Up Delivery of Biofortified Staple Food Crops Globally: Paths to Nourishing Millions. Food Nutr. Bull. 2021, 42, 116–132. [Google Scholar] [CrossRef]
- Huey, S.L.; Islam, S.; Mehta, N.H.; Konieczynski, E.M.; Friesen, V.M.; Krisher, J.T.; Mbuya, M.N.N.; Monterrosa, E.C.; Nyangaresi, A.M.; Mehta, S. Review of the facilitators and barriers to adoption of biofortified foods and food products. Nutr. Res. Rev. 2025, 38, 371–392. [Google Scholar] [CrossRef]
- Das, A.; Thakur, S.; Soren, K.R.; Datta, S.; Singh, N.P. Transgenic Strategies Towards Nutritional Enrichment of Crops. In Biofortification of Food Crops; Springer: New Delhi, India, 2016; pp. 105–113. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur. J. Soil. Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Petry, N.; Boy, E.; Wirth, J.; Hurrell, R. Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification. Nutrients 2015, 7, 1144–1173. [Google Scholar] [CrossRef] [PubMed]

| Zinc Application Method | Application Rate | Fertilizer Used | Baseline (mg kg−1) | Final (mg kg−1) | % Increase | Country | Reference |
|---|---|---|---|---|---|---|---|
| Soil application | 8 kg/ha | Zinc sulphate | 42.7 | 46.4 | 8.7 | Brazil | [36] |
| Soil application | 15 kg/ha | Zinc sulphate | 36.6 | 58.2 | 59.0 | Tanzania | [37] |
| Soil application | 20 kg/ha | Zinc sulphate | 23.0 | 28.7 | 24.8 | Kenya | [38] |
| Soil application | 7.5 kg/ha | Zinc sulphate | 27.0 | 43.0 | 59.3 | Kenya | [27] |
| Foliar application | 1.2 kg/ha | Zinc sulphate | 15.3 | 20.7 | 35.3 | Brazil | [34] |
| Foliar application | 4 kg/ha | Zinc sulphate | 68 | 78 | 15 | China, India, and Zambia | [39] |
| Foliar application | 0.6 kg/ha | Zinc sulphate | 11.6 | 29.0 | 150 | Brazil | [40] |
| Seed priming | 0.7 mg/mL | Zn chelated by histidine | 16.4 | 45.1 | 175 | Iran | [41] |
| Variety | Countries of Release | Zn Content (mg kg−1) | References |
|---|---|---|---|
| NUA35 | Rwanda, Colombia, Democratic Republic of Congo, Malawi | 31–41 | [61] |
| NUA45 | Rwanda, Democratic Republic of Congo, Kenya, Malawi, Zambia, Eswatini, Mauritius, Mozambique, and Zimbabwe | 10–40 | [62] |
| RWR 2245 | Rwanda, Democratic Republic of Congo, Uganda | 34 | [63] |
| CODMLB 001 | Democratic Republic of Congo | * | [64] |
| MAC 44 | Burundi | 32 | [65] |
| RWR 1129 | Burundi | * | [65] |
| Consideration | Transgenic Breeding | Conventional Breeding | Agronomic Biofortification | |||
|---|---|---|---|---|---|---|
| Benefit | Limitation | Benefit | Limitation | Benefit | Limitation | |
| Geographical applicability | Works where soils inherently have Zn minerals | Works where the soils are inherently rich in zinc | Works even where soils are Zn-deficient | |||
| Cost of developing technology | Relatively expensive | Relatively cheaper compared to genetic biofortification | Cheaper compared to genetic and conventional breeding | |||
| Application cost | Cheaper | Cheaper | Costly | |||
| Acceptability | Mostly not acceptable | Easily acceptable | Easily acceptable | |||
| Farmers’ Accessibility Time | Takes several years before a cultivar is developed and assessed | Slower process, requiring multiple generations to achieve significant zinc biofortification | Less time-consuming approach | |||
| Sustainability | Very sustainable, as the same genetic material can be used for years | Sustainable | Not sustainable | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matumba, A.; Nalivata, P.C.; Bailey, E.H.; Lark, M.R.; Broadley, M.R.; Ander, L.E.; Chimungu, J.G. Biofortification of Common Bean: Critical Analysis of Genetic and Agronomic Strategies as Viable Alternatives to Tackling Zinc Deficiency in Developing Countries. Sustainability 2025, 17, 8510. https://doi.org/10.3390/su17188510
Matumba A, Nalivata PC, Bailey EH, Lark MR, Broadley MR, Ander LE, Chimungu JG. Biofortification of Common Bean: Critical Analysis of Genetic and Agronomic Strategies as Viable Alternatives to Tackling Zinc Deficiency in Developing Countries. Sustainability. 2025; 17(18):8510. https://doi.org/10.3390/su17188510
Chicago/Turabian StyleMatumba, Annie, Patson C. Nalivata, Elizabeth H. Bailey, Murray R. Lark, Martin R. Broadley, Louise E. Ander, and Joseph G. Chimungu. 2025. "Biofortification of Common Bean: Critical Analysis of Genetic and Agronomic Strategies as Viable Alternatives to Tackling Zinc Deficiency in Developing Countries" Sustainability 17, no. 18: 8510. https://doi.org/10.3390/su17188510
APA StyleMatumba, A., Nalivata, P. C., Bailey, E. H., Lark, M. R., Broadley, M. R., Ander, L. E., & Chimungu, J. G. (2025). Biofortification of Common Bean: Critical Analysis of Genetic and Agronomic Strategies as Viable Alternatives to Tackling Zinc Deficiency in Developing Countries. Sustainability, 17(18), 8510. https://doi.org/10.3390/su17188510






