1. Introduction
Fluctuating market prices of metals and metalloids complicate lithium-ion batteries’ (LIBs) production and affect their overall demand, especially with the growing demand for electronics and electric vehicles [
1,
2]. Despite the many benefits that can be provided by LIBs, their relatively short life cycle means that environmental disposal and recycling plans must be employed. Spent LIBs contain dangerous metal elements such as cobalt, nickel, and lithium, which could find their way into the surrounding soil and water and endanger the lives of living organisms [
3,
4]. In addition, spent LIBs consist of critical raw materials such as lithium, cobalt, nickel, and iron, which are used for manufacturing new batteries and in other technologies [
1]. The disposal of LIBs leads to pollution and depletion of the available materials; therefore, it is important to recover them for optimal recycling [
5].
Given that resource recycling and environmental management are two major concerns, research on problems associated with spent LIBs remains a critical area of interest to establish efficient techniques for their effective recycling [
2,
6]. It should be noted that the traditional methods of recycling, including pyrometallurgical and hydrometallurgical recycling, require circuitous processes, a substantial amount of energy, and may result in secondary pollution [
7]. Therefore, potential approaches to new and environmentally sound recycling methods should be investigated.
Cathode-active materials (CAM) used in LIBs attract great attention with their promising catalytic properties in environmental remediation applications. These materials can be evaluated in catalytic decontamination processes prior to disposal or recycling thanks to their redox-active transition metals such as nickel, cobalt, manganese, and iron. Transition metal oxides play a critical role in catalytic processes thanks to their regular crystal structures, providing high surface area and high surface energies of transition metal atoms [
7,
8]. Therefore, using expired LIB CAMs as a catalyst in water treatment applications finds its application in the management of LIB wastes and the cost-effective method of controlling wastewater.
One type of LIBs containing valuable CAMs is LiFePO
4 (LFP) batteries. Among various cathode chemistries, LFP has attracted considerable attention in the field of energy storage due to its long cycle life and excellent thermal stability, resisting temperatures as high as 340 °C without any adverse reactions, hence improving safety during operation [
9]. Moreover, LFP batteries are nontoxic and environmentally friendly, possessing minimal environmental hazards, hence meeting the global sustainability goals [
10]. Therefore, the use of LFP batteries in the industry is becoming increasingly widespread. According to Global EV Outlook 2024 data, LFP’s share in the electric vehicle market has doubled compared to 2020 and reached 40% [
11]. This rapid increase in demand for LFP in the battery market brings with it the question of how to recycle battery components that have reached the end of their service life. Using LFP-based cathode active material (CAM) wastes in wastewater treatment is a more economical and easier process than LiCoO
2 (LCO) and LiNi
xCo
yMn
1−x−yO
2 (NMC) based CAMs. The main reason for this is that the main components of LFP, iron (Fe) and phosphorus (P), are more abundant in nature and cheaper compared to the components of LCO and NMC, nickel (Ni), cobalt (Co), and manganese (Mn). Although recycling of LCO and NMC components for reuse in the battery industry is costly and requires complex processes, it is necessary for the efficient use of critical waste CAM components for battery production. On the other hand, the recovery of LFP CAM components to the battery industry does not carry the same level of necessity. Therefore, LFP wastes stand out as a suitable material source that can be evaluated in alternative applications such as water treatment [
10]. In addition, the presence of iron in LFP makes it a suitable activator for peroxymonosulfate (PMS), a strong oxidant used in advanced oxidation processes (AOPs) [
12].
The textile industry uses various synthetic dyes to color textile products, resulting in wastewaters containing high amounts of dyes [
13,
14]. When the dye wastewaters are discharged into water environments without treatment, they cause serious environmental pollution [
13,
15]. In addition, they threaten human health and other living organisms due to their carcinogenicity and toxicity [
16,
17]. Therefore, such wastewaters must be effectively treated before discharge. Advanced oxidation processes (AOPs) are one of the most effective methods for removing such pollutants [
6]. PMS-based AOPs depend on the activation of PMS to produce reactive oxygen species (ROS), including sulfate radicals (
) and hydroxyl radicals (
), which are highly effective in organic pollutant degradation [
12,
18]. Recent studies have shown that LFP is a potential catalyst for PMS activation in the degradation of organic pollutants [
7,
12,
19]. LFP showed excellent catalytic activity, attributed to lithium defects and oxygen vacancies produced during battery cycling [
20]. These defects could enhance the adsorption and activation of PMS, leading to efficient ROS generation and subsequent pollutant degradation [
12]. However, it is well known that the application of nanomaterials without immobilization on a suitable supported material is limited by drawbacks such as aggregation, difficulties in separation, environmental risks, and technical challenges [
21,
22,
23,
24]. To eliminate these drawbacks, as polymeric membranes can be easily modified with catalytic materials using simple methods, and these membranes are generally resistant to acids, bases, and oxidants, they have been used as a support material [
25,
26,
27]. Wastewater treatment applications of the PMS-based AOPs using composite catalytic membranes modified with LFP have not been carried out.
This work presents the preparation of a novel LFP-modified polyvinylidene fluoride microfiltration catalytic membrane (LFP@PVDF) to remove the organic dyes in wastewater. The opinion is to load LFP on the surface of the PVDF membrane to fabricate a catalytic membrane system of high efficiency. The proposed LFP@PVDF membrane offers several advantages over conventional powder-based catalysts: enhanced stability, reduced catalyst loss, and easier separation from the treated effluent. The activation of persulfate on the LFP@PVDF membrane and its effectiveness in the degradation of organic dyes were examined. This study investigated the impact of operational parameters, such as LFP loading, PMS concentration, solution pH, and dye concentration, on degradation efficiency. In addition, the reactive radicals involved and their contribution to the dye removal process were studied, along with the reusability of the material. Results obtained from this study will contribute to advancing appropriate wastewater treatment technologies and the circular economy by demonstrating the potential of LIB active materials for environmental remediation.
2. Materials and Methods
2.1. Materials
Commercial polymeric polyvinylidene difluoride (PVDF) membrane with 0.2 μm average pore diameter was purchased from Microdyn-Nadir membranes, Wiesbaden, Germany. The LFP was purchased from MTI Corporation (Salt Lake City, UT, USA), having a particle size of D50 ~3.5 µm. Tert-butyl alcohol (TBA ≥ 99.7) and ethanol (emsure) were purchased from Merck (Darmstadt, Germany). Potassium peroxymonosulfate, commercially named oxone (KHSO5·0.5KHSO4·0.5K2SO4), was purchased from Merck. Reactive Black 5 dye (RB5, C26H21N5Na4O19S6) was obtained from DyStar (Türkiye).
2.2. Modification of the LFP@PVDF Catalytic Membrane
The iron-based LFP catalytic membrane (LFP@PVDF) was fabricated using a straightforward and convenient filter press coating method. In the method, a 100 mg/L LFP stock solution was first prepared by mixing the LFP catalytic material with deionized water. Solutions with varying concentrations were prepared by diluting the stock solution, and then produced LFP@PVDF membranes with different loading amounts of LFP using these solutions under a vacuum filtration system. In this process, a flat sheet PVDF membrane with an active surface area of 11.94 cm2 was placed in a cup filter device, and the LFP catalytic material was uniformly loaded onto the PVDF membrane surface under a vacuum pressure of −250 mm Hg at room temperature (25 ± 1 °C). The PVDF membranes loaded with LFP catalytic material were subsequently dried at 40 °C in a vacuum oven for at least 30 min. Following this procedure, LFP@PVDF membranes with varying loading amounts of LFP catalytic material were fabricated (0.2, 0.3, and 0.4 mg/cm2).
2.3. Characterization
To confirm that the LFP catalytic materials were loaded on the surface of the PVDF membrane, infrared spectrophotometer (FT-IR), scanning electron microscopy (SEM), and energy dispersive X-ray (EDX) analyses were conducted. The LFP catalytic material, the unmodified membrane, and the LFP@PVDF membrane were analyzed by a Perkin Elmer Pyris infrared spectrophotometer (FT-IR) between 550 and 4000 cm−1 wavenumber at room temperature (PerkinElmer, Shelton, CT, USA). The structure of the unmodified PVDF membrane and the LFP@PVDF membrane was analyzed by a field emission scanning electron microscope (SEM, FEI Quanta 400F, FEI, Houston, TX, USA) at an operating voltage of 5 kV. Energy dispersive X-ray (EDX) analysis was performed in the attachment in SEM-Ametek EDAX Genesis at the operating voltage of 15 kV (Edax Inc., Berwyn, PA, USA). The crystal structures of samples were characterized by an X-ray diffractometer (XRD, Panalytical Empyrean, Almelo, The Netherlands), equipped with 2θ range = 10–90° Cu-Kα radiation (λ = 1.54 Å).
2.4. RB5 Dye Removal Performance Tests
The catalytic performance of the LFP@PVDF membrane with/without the PMS activator on the RB5 dye wastewater was investigated using different experimental tests. The experiments were conducted using a vacuum filtration system (dead-end filtration system) (
Table 1,
Figure S1) under −250 mm Hg vacuum pressure at room temperature (25 ± 1 °C). A stock RB5 dye wastewater of 500 mg/L was prepared with deionized water and then diluted to the desired concentrations for the experiments. In the RB5 dye removal experiments, LFP@PVDF membranes loaded with the target LFP dosages were placed in the dead-end filtration system. After adding a given concentration of PMS to the 50 mL RB5 dye solution and stirring it with a magnetic stirrer for 60 s to fully dissolve the PMS, the dye solution was poured into the filtration system cup. To determine the absorbance of the residual RB5 dye after the filtration, samples were taken from the filtrate every 5 min for 30 min, and the absorbance was measured immediately using a UV-visible spectrophotometer (Shimadzu-1800, Shimadzu, Duisburg, Germany) at the maximum absorption wavelength (λmax = 597 nm). The percentage of RB5 dye removal efficiency was described in Equation (1).
where at 597 nm of the reaction solution, A
0 is the initial absorbance value of RB5, and A
t is the absorbance value of RB5 at a time, t.
The impacts of various parameters on RB5 dye removal were investigated. To find the optimum loading dosage, the dosage of LFP on the PVDF membrane surface was applied in different amounts from 0 to 0.4 mg/cm2. The effects of PMS dosage (0–500 mg/L), RB5 dye concentration (20–80 mg/L), and pH level (3, 7, 11) were also investigated to determine the optimal conditions. Adjustment of pH was made using HCI and NaOH solutions. To identify the reactive species, experiments were carried out during the catalytic reaction by adding various quenchers, such as EtOH, TBA, and NaN3, at a concentration of 1500 mM. Additionally, the recyclability of the LFP@PVDF membrane was studied to assess its potential for reuse. In detail, the contaminated catalytic membrane was cleaned using distilled water and then dried at 40 °C in the vacuum oven before reuse.
2.5. Decolorization Rate
In the study, Pseudo-first-order and Pseudo-second-order kinetic models were used to determine the decolorization kinetic rate of RB5 dye by the oxidation process. The pseudo-first and second-order reaction kinetic models were described in Equations (2) and (3), respectively [
28,
29]. The calibration curve of RB5 dye was prepared to determine the value of RB5 dye concentration using the RB5 dye solutions from 0 to 80 mg/L, and the calibration curve was found to be linear (R
2 values > 0.99).
where C
0 (mg/L) and C
t (mg/L) are concentration values of the RB5 dye at the initial and at a certain time t, respectively; K
1 (1/min) and K
2 (L/mg·min) are kinetic rate constants of pseudo-first-order reaction and pseudo-second-order reaction, respectively. After integrating Equation (3), Equation (4) can be obtained [
28,
29].
2.6. Statistical Analysis
All of the experimental results were subjected to one-way analysis of variance (ANOVA) with Tukey post hoc tests. SPSS software (v.20.0) was used, and the significance was attested by
p < 0.05. ANOVA results are provided as tables in the
Supplementary Material (Tables S2–S13).
3. Results and Discussion
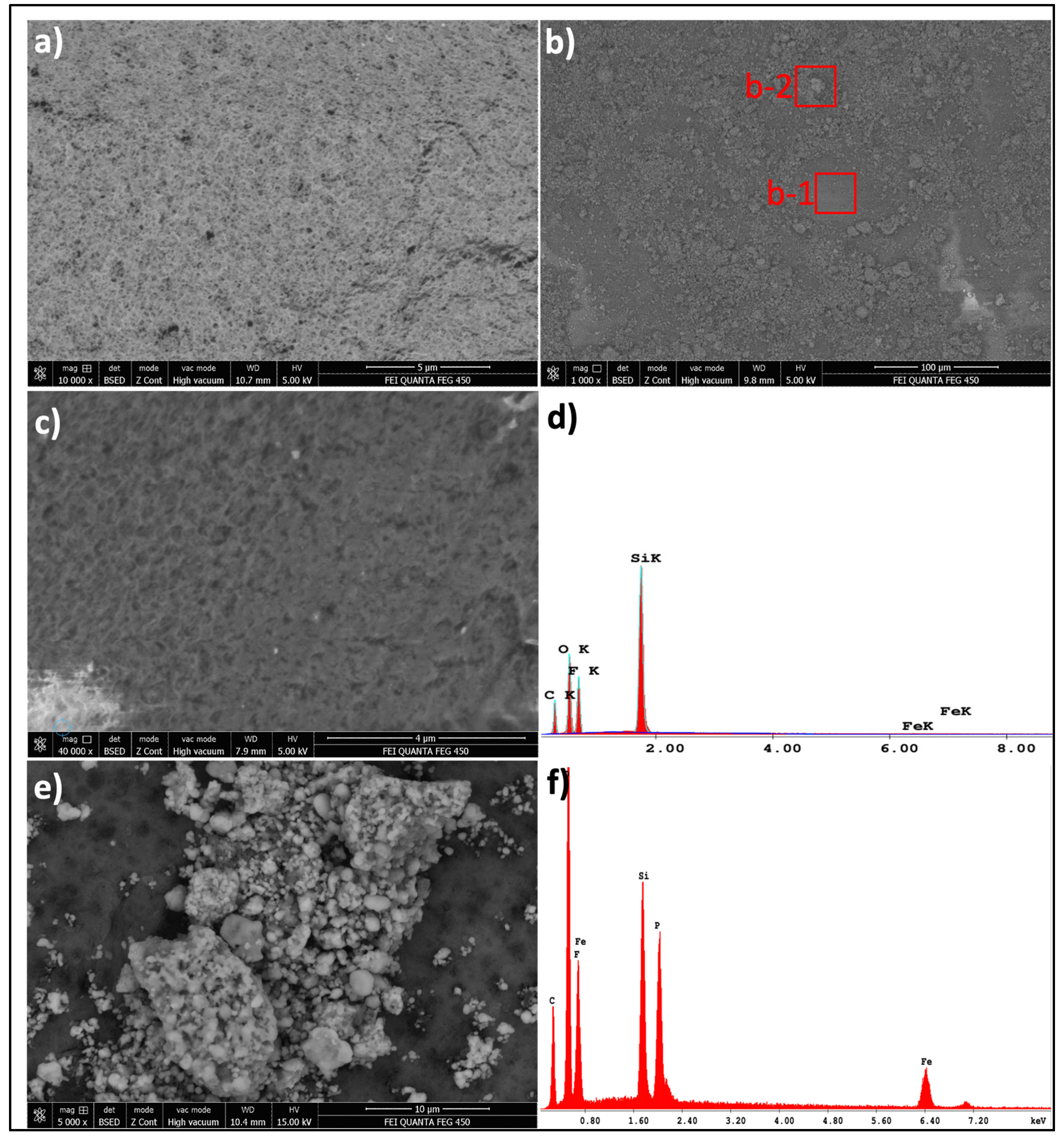
The microstructures of unmodified PVDF and LFP-loaded PVDF (LFP@PVDF) membranes were examined by scanning electron microscopy (SEM); the chemical analyses of the elements forming the PVDF and LFP materials were examined by energy dispersive X-ray spectrum (EDX), and the obtained results are shown in
Figure 1. When the unmodified PVDF micrograph is examined, it is seen that it consists of homogeneously distributed pores with a size distribution ranging from 100 to 300 nm (
Figure 1a). The homogeneity of the pore distribution indicates that the solvent/PVDF balance is at an appropriate ratio, and it is seen that the PVDF membrane is in the microfiltration membrane range in terms of the pore size of the membrane [
30,
31]. The SEM image obtained after loading the PVDF membrane with LFP catalytic material (
Figure 1b) shows that the LFP particles are distributed on the membrane surface. It is seen that the LFP particles have a wide particle size range of 400 nm–3 µm and are held on the PVDF surface in the form of agglomerations consisting of secondary particles (
Figure S2). As seen in the SEM images (
Figure 1c), the membrane maintains its porous structure. Partial contraction due to LFP loading did not negatively affect target pollutant removal. On the contrary, it increased the contact time between the wastewater and the LFP catalytic material, contributing positively to PMS activation and catalytic efficiency.
The EDX area analysis of the particle-loaded membrane surface (
Figure 1f), together with the detailed spectra shown in
Supplementary Materials (Figure S3), confirmed the presence of Fe and P atoms originating from LFP. The Fe
2+/Fe
3+ redox couples in LFP are therefore expected to act as catalytic activators of PMS, thereby enabling the membrane/persulfate activation process.
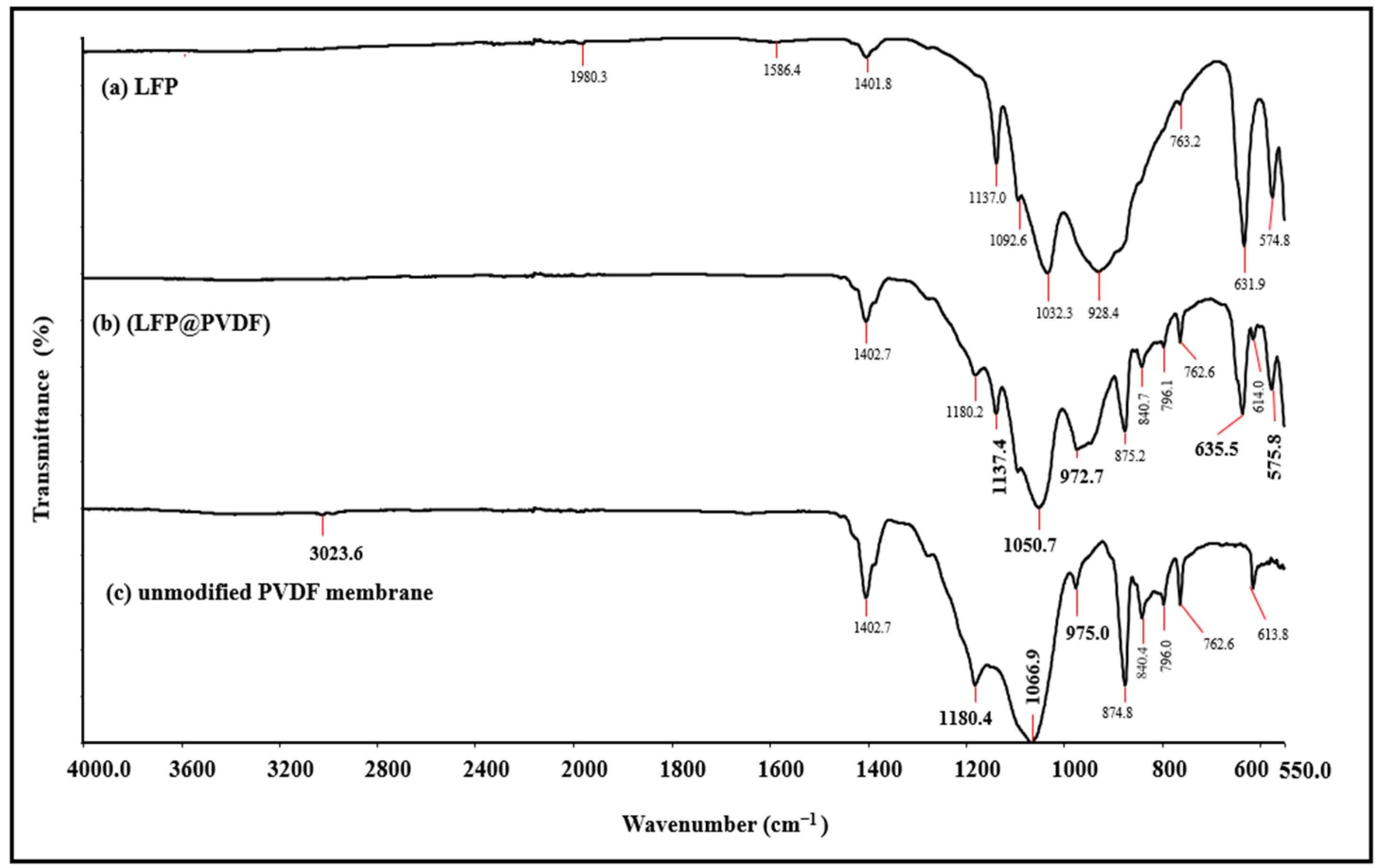
The chemical structures of unmodified PVDF, LFP-loaded PVDF (LFP@PVDF), and LFP materials were investigated by Fourier Transform Infrared Spectroscopy (FT-IR) analysis, and the obtained spectra are presented in
Figure 2. The FTIR spectra of the unmodified PVDF material indicate the characteristic PVDF properties. That is because the peaks obtained at 3023.6 cm
−1 and 1402.7 cm
−1 are related to C–H vibrations and indicate the presence of CH
2 groups in PVDF [
32]. Similarly, the peak at 1180.4 cm
−1 reflects the basic properties of C-F stretching vibrations in the 1400–1000 cm
−1 range in the literature, while the peaks in the 830–520 cm
−1 range are a clear indicator of C-F deformation vibration modes [
33]. Turning now to the FT-IR spectrums of LFP-loaded PVDF membrane and pristine LFP, the LFP@PVDF membrane has the characteristic peaks of the pristine LFP, which were at 575.8 and 635.5 cm
−1 associated with the vibrations of the (P-O-P) [
34] and (Fe–O) [
35] and at 1137.4 cm
−1 related to the (PO
4)
3− vibrations [
34]. Furthermore, the 972.7 and 1050.7 cm
−1 wavenumbers should be associated with the vibrations of the (PO
4)
3− [
34]. This is because the two peaks of the LFP@PVDF membrane shifted and changed significantly in terms of shape and wavenumber when compared with the two characteristic peaks of 975.0 and 1066.9 cm
−1 in the PVDF membrane (
Figure 2).
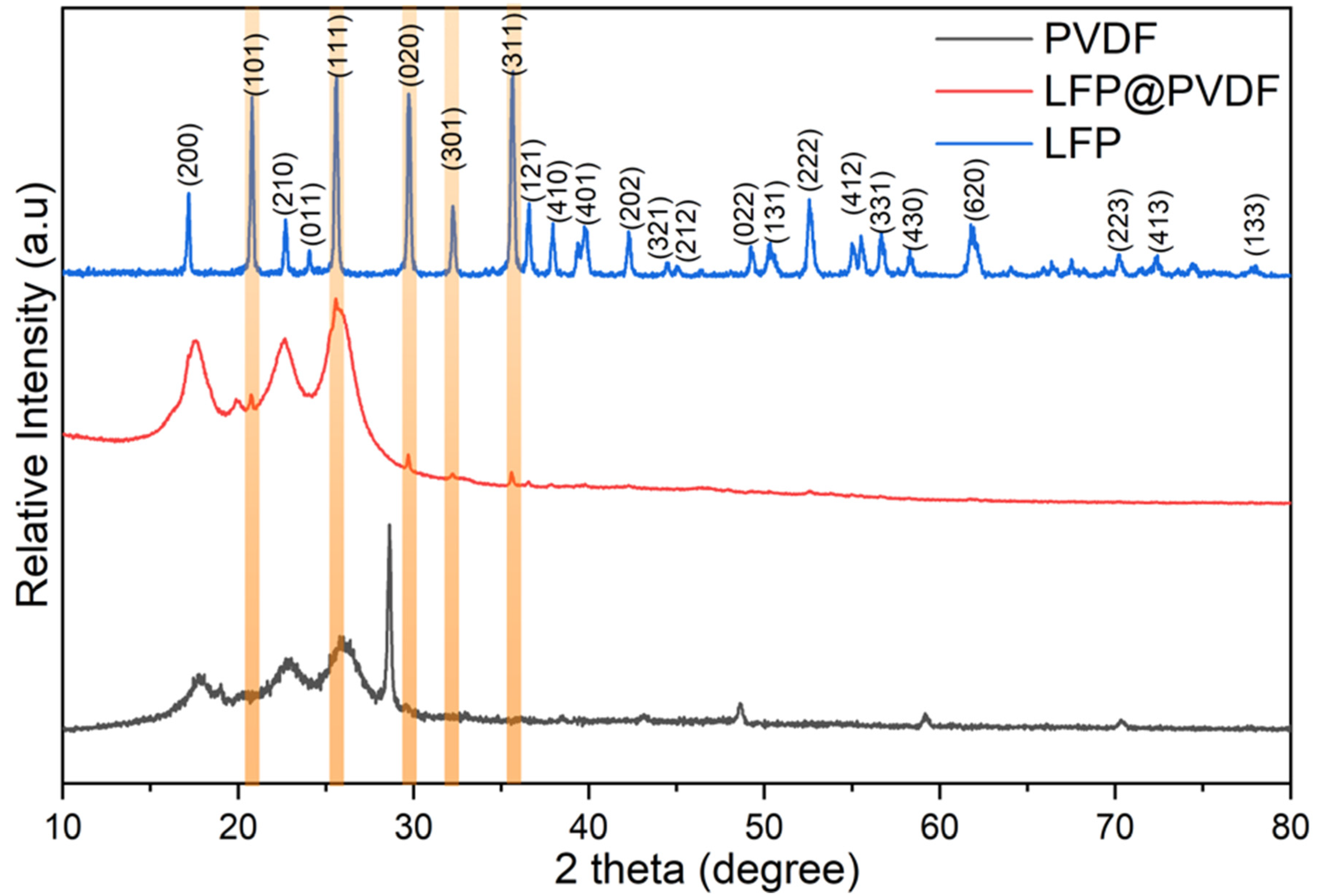
XRD analysis was used to characterize the loading of LFP catalytic material onto the PVDF membrane surface, and the obtained diffraction patterns are presented in
Figure 3. In the XRD analysis of commercial LFP powder, all diffraction peaks were found to be compatible with the orthorhombic Pnma (62) crystal structure (JCPDS 83-2092). The absence of any impurities indicates that the commercial LFP has high purity, which is necessary for providing the required catalytic properties during water treatment. On the other hand, the sharp and broad diffraction patterns observed in the unmodified PVDF membrane reveal that the structure contains both crystalline and amorphous phases. Among these structures, a peak observed at approximately 28 degrees corresponds to monoclinic SiO
2 (PDF 98-016-2627) with the C12/c1 space group. Modification of PVDF membranes with silica nanoparticles improves mechanical properties, permeability, and degradation resistance compared to standard PVDF membranes [
36]. Finally, XRD analyses of LFP-loaded PVDF membranes show that the major peaks of LFP are present, indicating that LFP is successfully loaded onto the PVDF surface. The preservation of the LFP crystal structure suggests that no chemical reaction occurs between PVDF and LFP. However, in the LFP-modified PVDF membrane, the SiO
2 peak observed at approximately 28 degrees in the unmodified membrane is no longer visible. This suggests that the diffraction signals from nano-sized SiO
2 are masked by the micro-sized LFP signals. EDX analyses confirm the presence of Si on the LFP-modified membrane surface, supporting this interpretation. As a result, the FT-IR, SEM, SEM-EDX, and XRD analyses confirmed that the LFP catalytic material was successfully loaded onto the surface of the PVDF membrane.
3.1. The Performance of the RB5 Dye Removal Using PMS Activator Agent with LFP@PVDF Catalytic Membranes
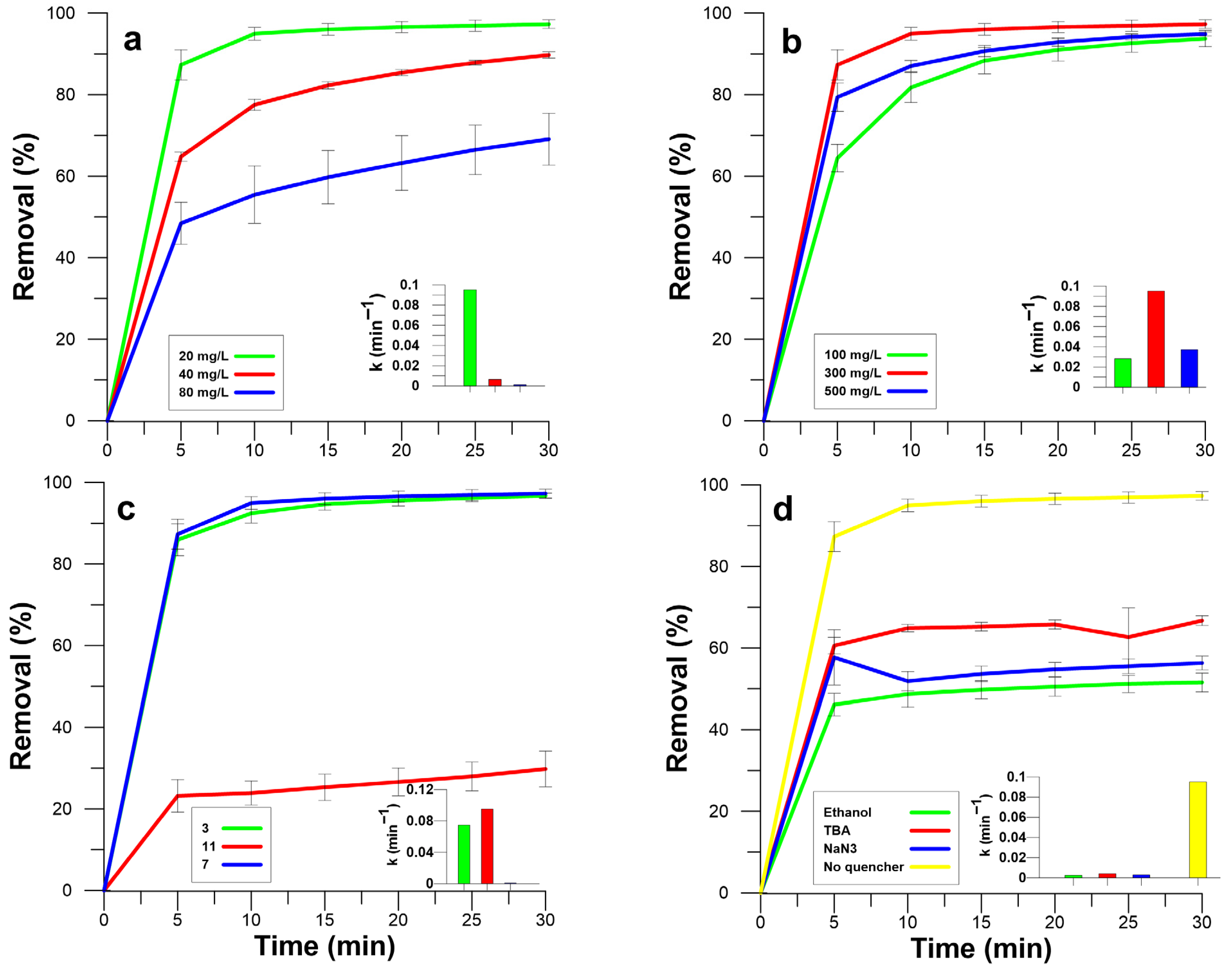
3.1.1. Effect of LFP Catalyst Dosage on the RB5 Dye Removal
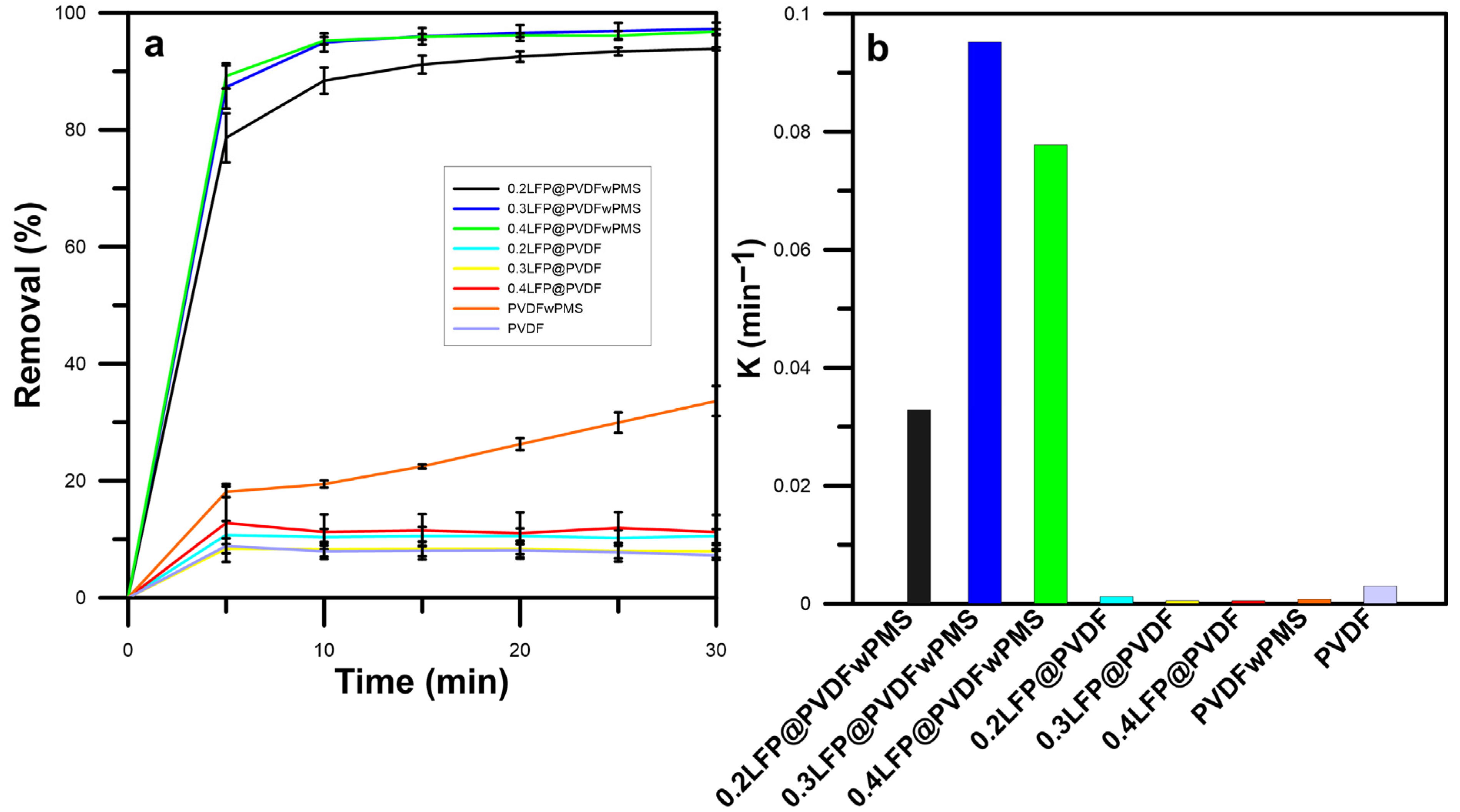
Figure 4a shows RB5 dye removal efficiencies obtained using various loading amounts of LFP from 0 to 0.4 mg/cm
2 on the PVDF membrane surface and 300 mg/L PMS activator agent. When the unmodified PVDF membrane was used, the final RB5 dye removal efficiency increased from 7.25% to 33.62% after PMS was added to the RB5 dye solution. It was demonstrated that PMS has the potential to oxidize a range of organic pollutants to some extent, including antibiotics and pharmaceuticals, without the need for activation [
37]. The degradation process primarily involves the generation of singlet oxygen (
1O
2) through PMS self-decomposition, although its role is limited due to rapid quenching by water [
37]. Modified membranes (0.2LFP@PVDF, 0.3LFP@PVDF, and 0.4LFP@PVDF) resulted in limited RB5 dye removal efficiencies around 10% without PMS application. This suggests that it might be due to the limited adsorption to the lithium-ion battery material [
38,
39].
When PMS was added to the dye solutions, RB5 dye removal efficiency for 0.2LFP@PVDF, 0.3LFP@PVDF, and 0.4LFP@PVDF membranes was strongly improved to 93.8%, 97.3%, and 96.8%, respectively, because the LFP could activate the PMS effectively, which enhances the removal of dye [
40]. In the first ten minutes, removal efficiencies of 0.3LFP@PVDF and 0.4LFP@PVDF were significantly higher (
p < 0.05) than 0.2LFP@PVDF. This continued in the following phases of the experiment, but at the end of the experiment, there was no significant difference between the three treatments (
p > 0.05). As the LFP dosage increased, it offered more active sites for the reaction with PMS [
40]. However, when the amount of catalyst exceeds a certain level, the removal efficiency might decrease due to a self-quenching mechanism. This is the case when an excessively high catalytic dosage causes the catalyst to react with sulfate radicals, which increases the rate of self-quenching and decreases the degradation of organic substances [
7,
41,
42].
Moreover, the RB5 dye removal by LFP@PVDF membranes with varying LFP concentrations was found to fit the pseudo-second-order kinetic model well (
Figure 4b and
Table S1). For the different LFP loading amounts of 0.2, 0.3, and 0.4 mg/cm
2 on the PVDF membrane surface, the rate constant K
2 values of RB5 dye removal were 0.033, 0.095, and 0.078 L/mg·min, respectively. Notably, the kinetic rate of RB5 dye removal was highest for the 0.3 mg/cm
2 LFP loading, which aligns with the trend observed in the dye removal efficiencies. As a result, the loading amount of LFP with 0.3 mg/cm
2 on the PVDF membrane surface was selected for further experiments, as it has the highest kinetic constant as well as a high removal efficiency and thus provides a balance between economic and practical considerations compared to the other options.
Examples of advanced oxidation studies adopting persulfates and various catalysts together with their experimental conditions and performances were presented in
Supplementary Materials (Table S14). Removal efficiencies reported are under conditions optimized for each catalyst. Despite variations in experimental parameters, iron-based catalysts activating PMS consistently demonstrate high dye removal efficiencies above 90%. LFP@PVDF catalytic membrane achieved a dye removal efficiency of approximately 97% for RB5 dye, which is comparable to or better than many reported catalytic AOP systems. These results indicate that the LFP@PVDF membrane is an effective catalyst for dye degradation.
3.1.2. Effect of Initial RB5 Dye Concentrations on the RB5 Dye Removal
The effect of RB5 dye removal at various initial RB5 concentrations was studied using LFP@PVDF membrane with a loading capacity of 0.3 mg/cm
2 and 300 mg/L of PMS. As shown in
Figure 5, by increasing the initial concentration of RB5 dye from 20 to 80 mg/L, the efficiency of RB5 dye removal decreased from 97.1% to 69.1%. This trend was further proved by the pseudo-second-order kinetic model, in which the associated rate constant was calculated to drop dramatically from 0.095 to 0.0011 L/mg·min (
Figure 5a and
Table S1). This can be ascribed to the insufficient supply of radicals produced, which were insufficient for the efficient degradation of dye solutions with very high concentrations of RB5 dye [
12,
41]. The removal efficiencies of 20 and 40 mg/L were 97.3 and 89.7%, respectively, and they were not significantly different (
p > 0.05). However, the rate constants were 0.095 to 0.006 L mg
−1 min
−1, respectively. Therefore, it was concluded that the optimal RB5 dye concentration for further experiments was 20 mg/L.
It was observed that increasing the initial RB5 dye concentration from 20 mg/L to 80 mg/L led to a decrease in removal efficiency from 97.1% to 69.1%. Simultaneously, the associated pseudo-second-order kinetic rate constant dropped dramatically. On the other hand, 20, 40, and 80 mg/L initial concentrations led to 0.97, 1.79, and 2.76 mg dye removal at the end of the experimental period. When we calculate the absolute degraded amounts, we expect to illustrate that while the percentage efficiency declines due to the system being overwhelmed, the total mass of dye removed may still increase, up to a certain point. This phenomenon is, as previously stated, primarily ascribed to the insufficient supply of radicals produced, which becomes inadequate for the efficient degradation of dye solutions at very high concentrations.
3.1.3. Effect of the PMS Dosage on the RB5 Dye Removal
The amount of the persulfate dosage affects the efficiency of the treatment process and operating costs [
43]. To determine the effect of PMS dosage on the RB5 dye removal, different initial PMS concentrations from 100 to 500 mg/L were tested (
Figure 5b). Increasing the initial PMS concentration from 100 to 300 mg/L, the RB5 dye removal efficiency increased from 91.4 to 97.3% with a significant difference (
p < 0.05) and similarly the Pseudo-second-order kinetic rate constant K
2 values of RB5 dye removal rose from 0.0281 to 0.0952 L/mg·min. This phenomenon may be due to the fact that when PMS concentration increases, the contact opportunities for catalytic material and PMS increase more, which generates more radicals [
40]. However, when the initial PMS concentration increased to 500 mg/L, the RB5 dye removal efficiency decreased to 94.8% and there was no difference between 300 and 500 mg/L PMS statistically (
p > 0.05). The result was consistent with the Pseudo-second-order kinetic model (
Figure 5b and
Table S1), in which the corresponding kinetic constant value declined to 0.0372 L/mg·min. The reason can be attributed to the self-quenching of excess ROS because of the excessive PMS concentration (Equations (5) and (6)) [
38]. Additionally, using excessive PMS concentration can lead to a high sulfate concentration in the wastewater, potentially resulting in undesirable secondary contamination [
44]. According to these results, and minimize operating costs, a 300 mg/L PMS concentration was chosen for the following experiments.
3.1.4. Effect of Initial pH on the RB5 Dye Removal
The pH level of the solution had a significant impact on the removal of the RB5 dye from the wastewater. To investigate the effect of initial pH, the RB5 dye removal was studied across a range of pH values from 3 to 11 (
Figure 5c). As the initial pH increased from 3 to 7, the RB5 dye removal efficiency remained relatively consistent at around 97% with no significant difference (
p > 0.05). This shows the ability of the material to operate under neutral pH levels without the need for pH adjustment. However, the efficiency decreased dramatically to 29.8% when the pH reached 11. These results were confirmed by the pseudo-second-order kinetic model, which showed the corresponding rate constant values increasing from 0.0747 to 0.0952 L/mg·min as the pH increased from 3 to 7, before dropping to 0.0009 L/mg·min at a pH of 11 (
Figure 5c,
Table S1).
Persulfates are more effectively activated at lower pH values; the formed sulfate radicals, which have a high reactivity for organic dye decomposition, depend strongly on this parameter. The literature states that if an initial pH value is less than 3, there will be a higher rate of reaction due to a high amount of hydrogen ions that may easily help generate reactive sulfate radicals [
40]. When the pH is increased from neutral values, the activation efficiency decreases. It was suggested that when the initial pH value is above 3.5, it may rise very fast as a result of the increase in OH
− ions that come from the dissociation of impurities like Li
3PO
4 present in solution. This can be a consequence of the stability of PMS. Persulfate has lower stability at alkaline conditions, which causes it to break down automatically and hence is less available for the next reactions [
45]. Moreover, another factor is that when pH becomes higher, there is a tendency for the radicals generated (e.g.,
and
) to recombine rather than reacting with organic pollutants, thus further decreasing the degradation rate [
46].
3.2. Identification of Reactive Oxygen Species
To examine the effect of the dominant reactive oxygen species, such as
,
and
1O
2 generated during the reaction on the RB5 dye removal, the scavenging experiments were carried out by using various quenchers. The TBA, EtOH, and NaN3 were added to the RB5 dye solution to inhibit the reactive oxygen species during the removal of the RB5 dye. The RB5 dye removal efficiency was 97.3% without the addition of any quenchers, but decreased to 66.7, 56.3, and 51.5% after the introduction of 1500 mM TBA, NaN
3, and EtOH, respectively (
Figure 5d). All of the quenchers led to significant differences (
p < 0.05) in removal efficiencies, confirming the contribution of reactive species in treatments. Also, the corresponding kinetic constant value was 0.0952 L mg
−1 min
−1 without the addition of any quenchers, but decreased to 0.0043, 0.0031, and 0.0026 L mg
−1 min
−1 after the introduction of the TBA, NaN
3, and EtOH, respectively (
Figure 5d,
Table S1). Based on these results, it was concluded that
(approximately 30.3% contribution) appeared to be more dominant than
(approximately 15.2% contribution). Previous studies show that either
or
could contribute more to the oxidation process upon activation of PMS by LiFePO
4 [
12,
40]. This difference might be related to other experimental factors. Moreover, the results of the NaN
3 quenching suggest the presence of
1O
2, which reveals the contribution of a non-radical pathway. Several other studies in which LiFePO
4 and other cathode materials have been used as PMS activator have reported the presence of
1O
2 [
6,
39,
47]. For instance, Xu et al. (2023) [
39] found
1O
2 to be the primary reactive oxygen species after using LiNixCoyMn
1−x−yO
2, NCM as an activator for PMS. The results in this study indicated that reactive oxygen species.
,
and
1O
2 were all responsible for the RB5 dye removal.
3.3. Catalytic Mechanisms of LFP@PVDF/PMS System
The LFP@PVDF/PMS system’s potential reaction pathways have been demonstrated (Equations (7)–(12)). It can be inferred that reactive radicals (
and
) are originated from the activation of PMS by LFP surface ≡Fe(II) (Equations (7) and (8)) [
12,
48]. Furthermore, the ≡Fe
2+ sites on LFP are able to form a ≡Fe
2+-OH complex in aqueous environments. This complex subsequently reacts with PMS, yielding the ≡Fe
2+-OH-
complex (Equation (9)). This complex might generate ≡Fe
3+-OH and
(Equation (10)) [
49] which leads to the reaction of
with OH or H
2O in solution to form
(Equations (11) and (12)) [
12,
50,
51]. In conclusion,
and
radicals generated during PMS activation of the LFP membrane catalyst played an important role in RB5 dye removal.
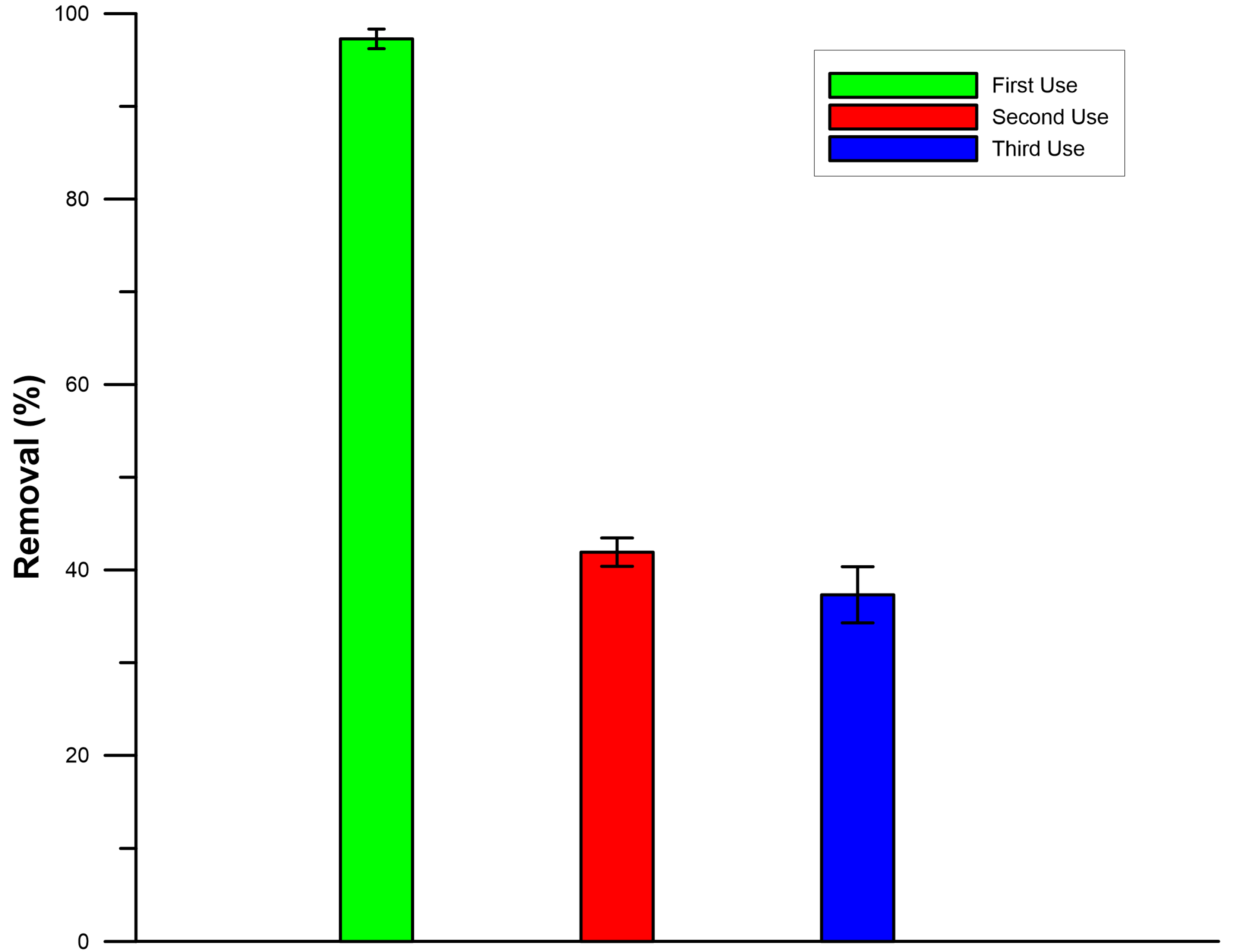
3.4. Reusability of the Catalytic Membrane and Cost Analysis
Reusability tests were conducted for three successive rounds to determine the reusability of the LFP@PVDF catalytic membrane. The RB5 dye removal efficiency was 97.3% for the first cycle; then, it decreased dramatically to 41.9% for the second one and further reduced to 37.3% in the third cycle (
Figure 6). With increasing number of cycles, the efficiency of dye removal decreases for two possible main reasons: One might be that the active sites within the catalytic material are occupied after repeated cycles [
40]. Also, the by-products generated during the reaction of dye removal could deposit on the surface of the catalytic material, thus blocking its active sites [
6]. After the first cycle of the Rb5 dye experiment, FT-IR analysis of the LFP@PVDF membrane revealed a new peak at 682.5 cm
−1 (
Supplementary Materials, Figure S5). This peak may be due to SO
4 formed as a result of RB5/LFP/PMS interactions [
52,
53], which might have accumulated on the catalyst surface and reduced its efficiency. The LFP catalytic material, with PMS as an activator, showed a good result in the removal of dye in the first cycle, but could not be reused efficiently to remove dye after that. A similar result was obtained with the LFP catalytic material using persulfate as an activating agent in the breakdown of orange G dye [
40]. Lin et al. [
40] also reported that the primary reason for the decreased removal efficiency of the LFP material is the partial degradation of the catalyst’s active sites as the reaction is repeated and the dissolution and leaching of small amounts of Fe, PO
43−, and Li ions from the surface. This leaching, even at low levels, weakens the structural stability of the catalyst and partially limits the heterogeneous activation process. Moreover, regeneration using an acid (HCl) or a base (NaOH) also did not improve the reusability of the membrane. Further research is needed to improve the stability and reusability of LFP-based materials. Alternative regeneration methods that may be more effective in membrane reuse should be investigated.
Table 2 shows the cost calculation of the materials used in catalytic membrane production and the amount of PMS used in the experiments. For example, in the treatment of a 20 mg/L RB5 solution in a volume of 50 mL using a 0.3 mg/cm
2 LFP@PVDF catalytic membrane and 300 mg/L PMS, the chemical cost is approximately 0.029 €. These experiments were conducted under low vacuum pressure (−250 mm Hg; equivalent to −0.33 bar), and the removal efficiency of the system was 97.3%. Similar efficiencies can also be achieved with nanofiltration (NF) or reverse osmosis (RO) membrane systems. However, NF membranes generally require high pressures in the range of 5–20 bar, while RO membranes require high pressures in the range of 7–60 bar [
54]. Therefore, these membranes require higher initial investment and operating costs compared to MF membranes. The high removal efficiency obtained in this study suggests that this method could be considered for industrial-scale applications in the future.