From Waste to Value: Recycling Industrial Waste into Functional ZnO Nanofibers
Abstract
1. Introduction
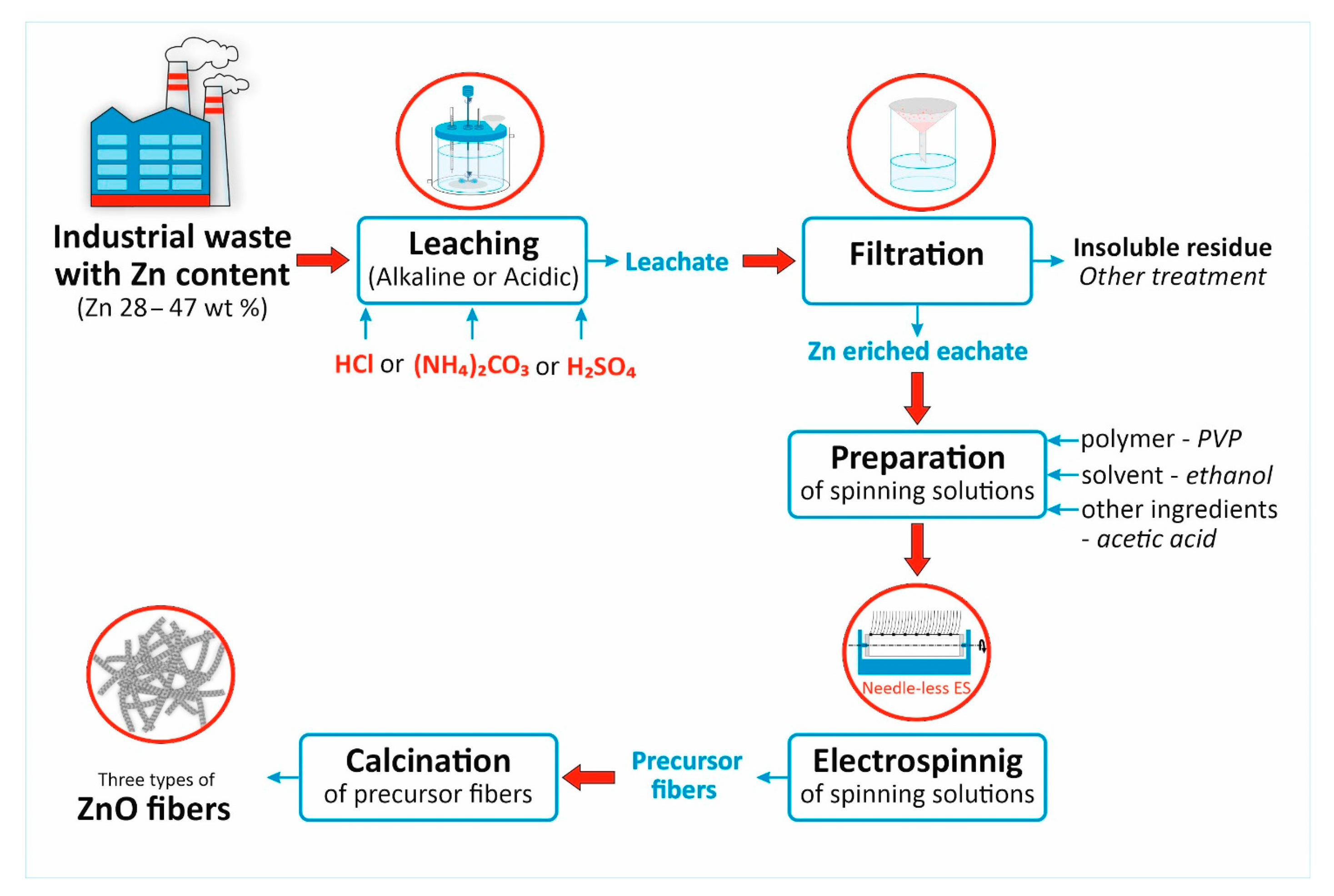
2. Materials and Methods
2.1. Preparation of Zn-Enriched Leachates
2.2. Preparation of the Electrospinning Precursor Solutions
3. Results
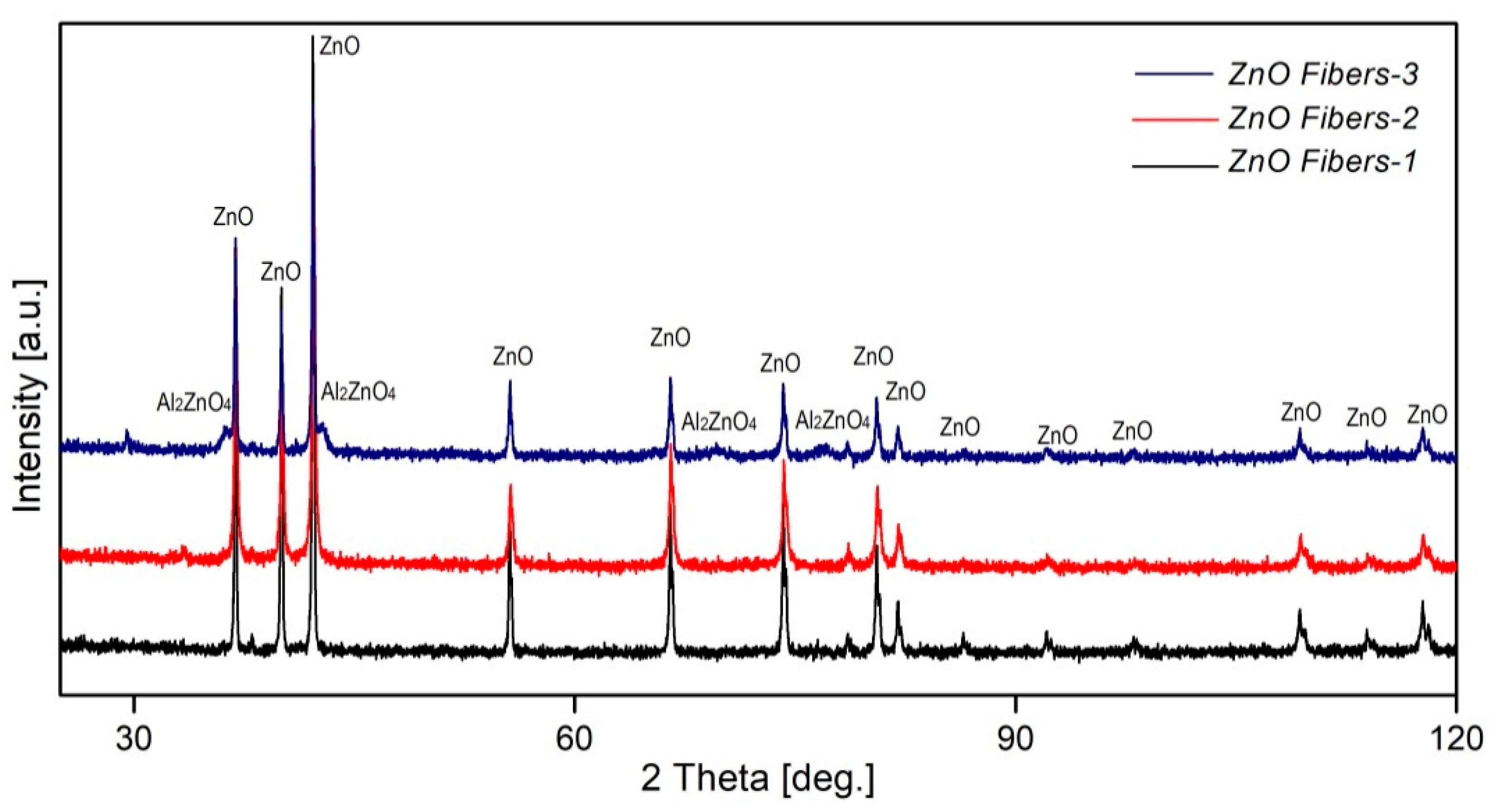
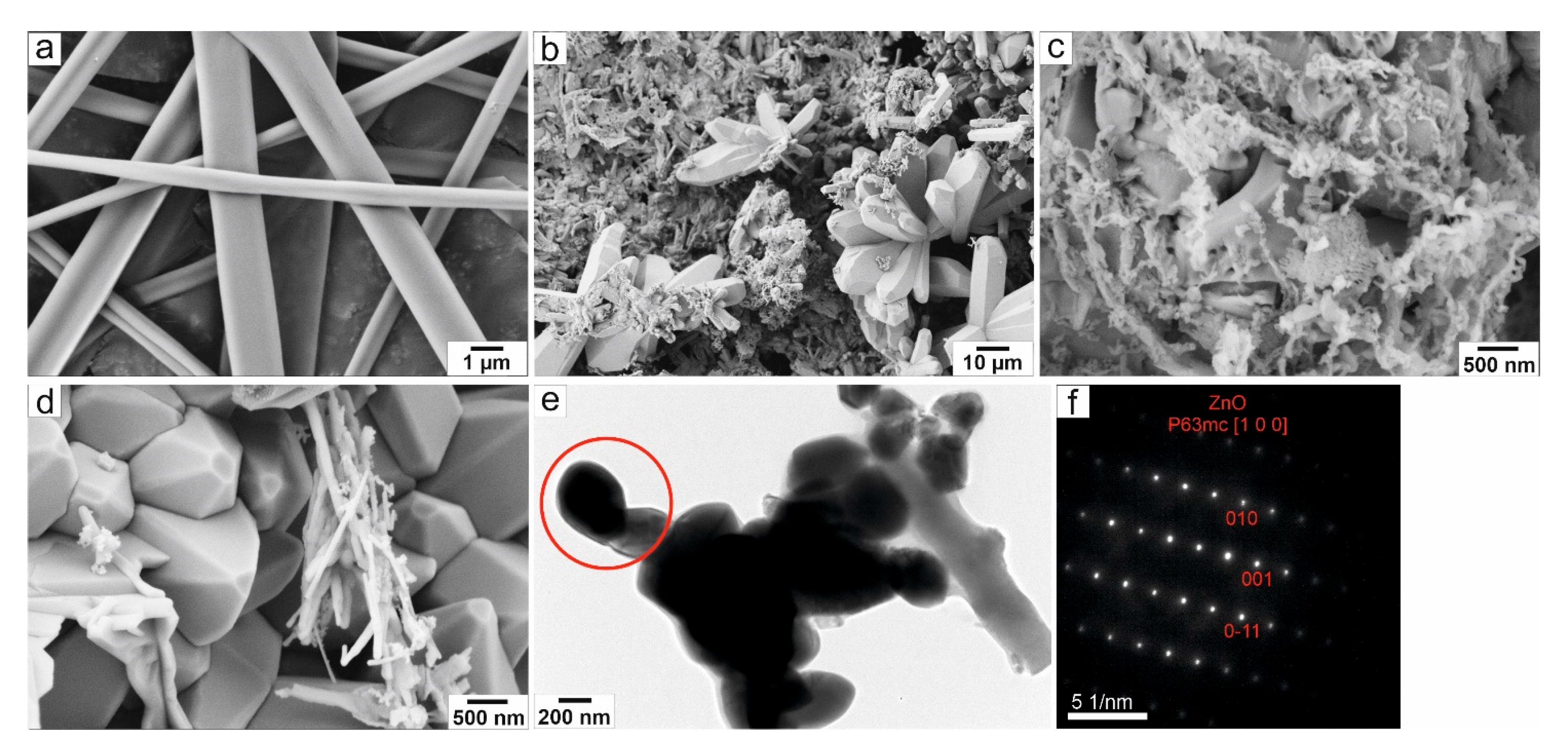
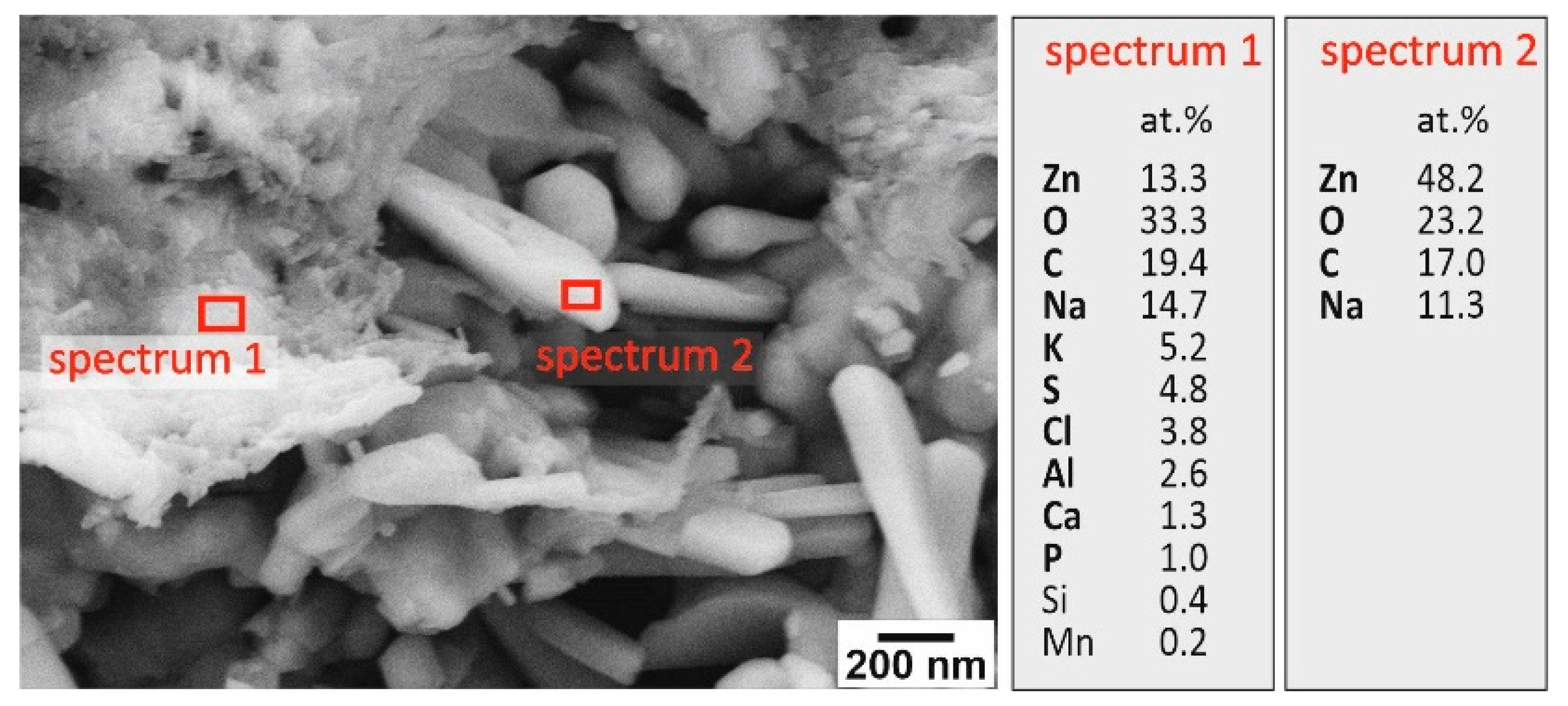
3.1. Structural and Morphological Characterization of Ceramic ZnO Fibers
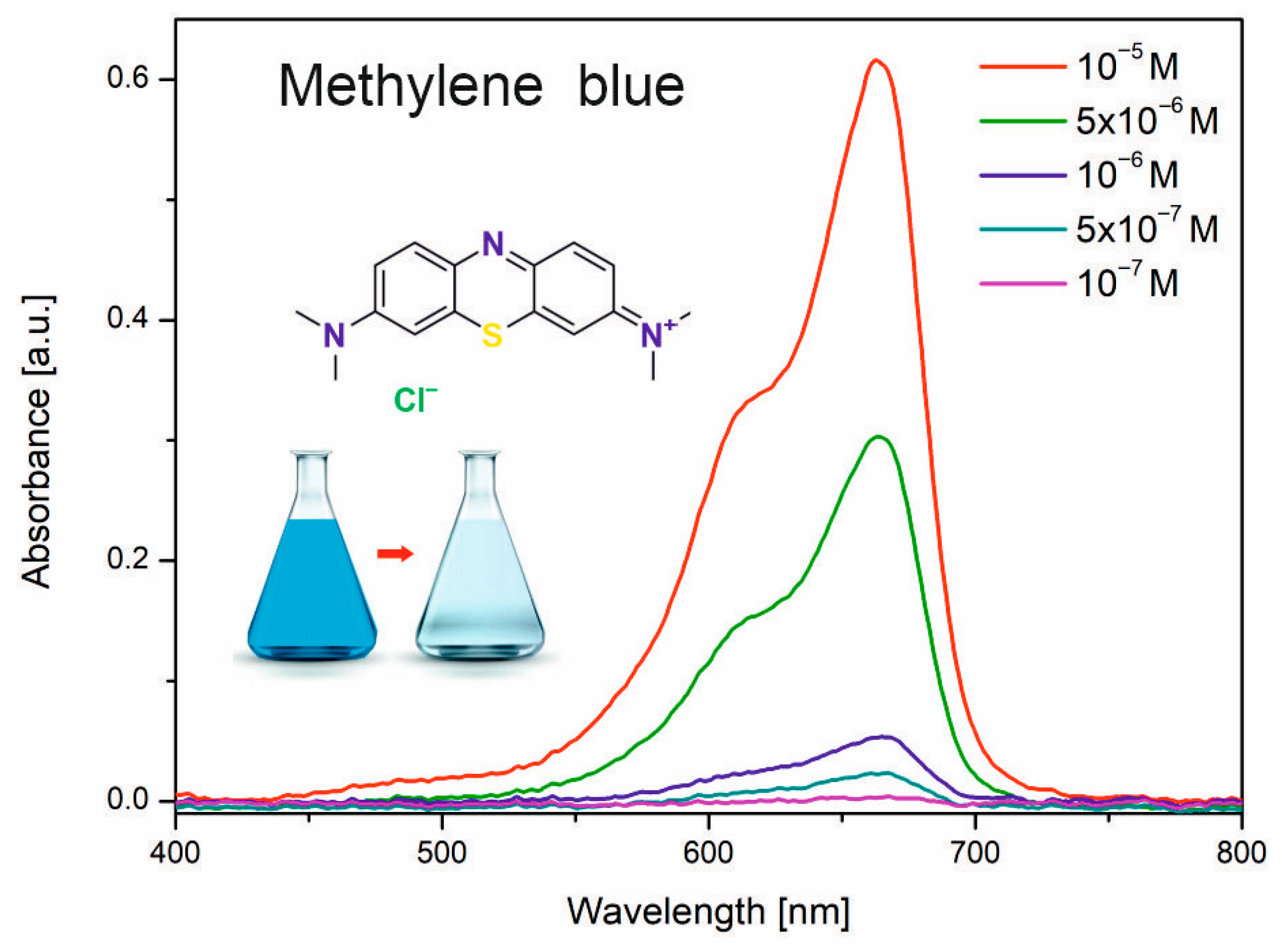
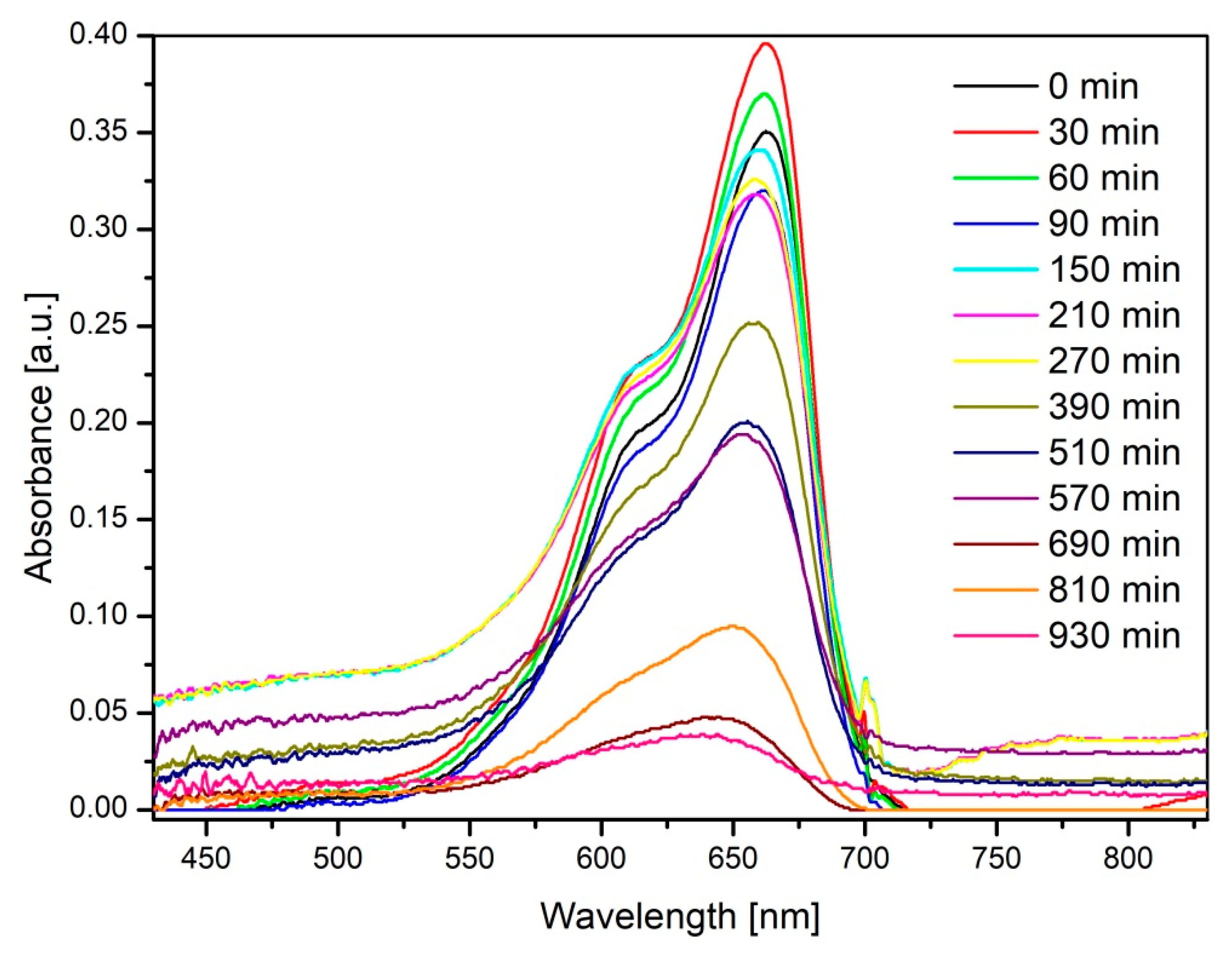
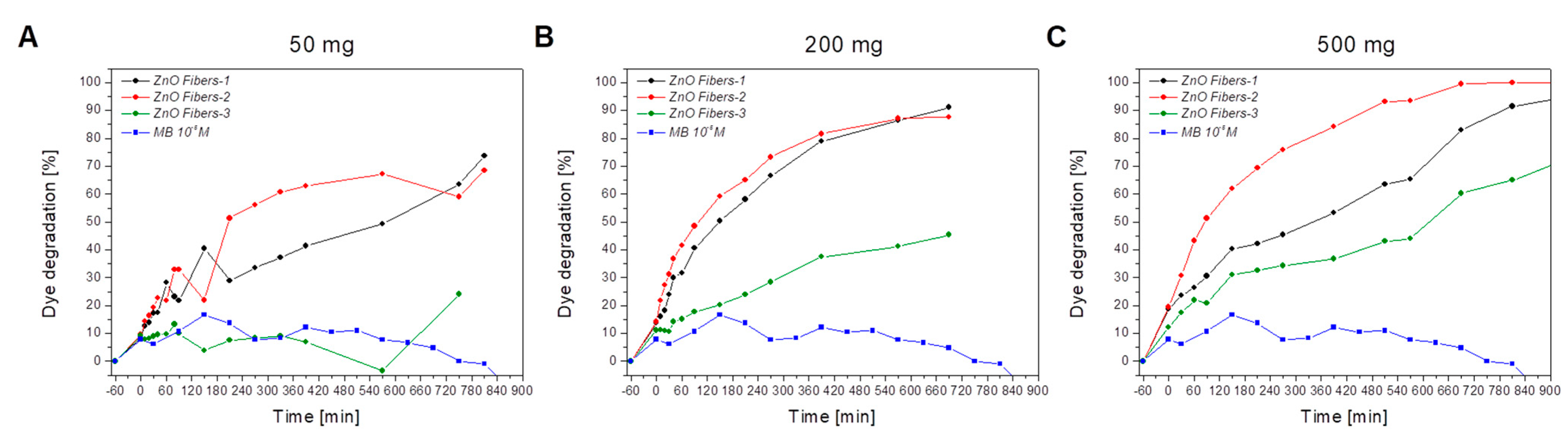
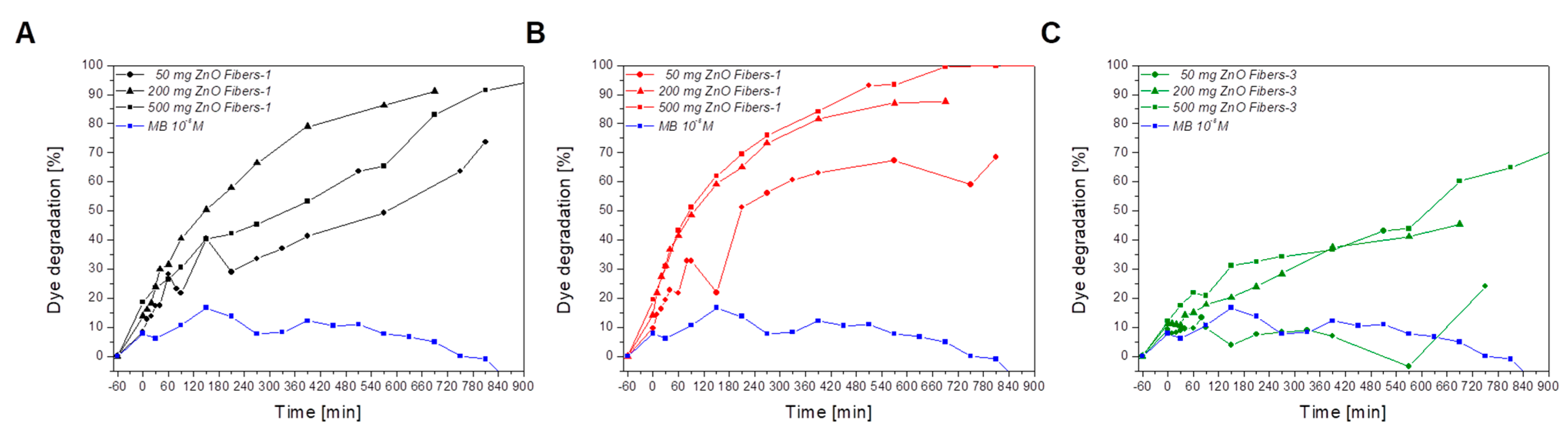
3.2. Characterization of the Photocatalytic Activity of Ceramic ZnO Fibers
4. Discussion
5. Conclusions
- In this work, three types of photocatalytically active ZnO fibers were successfully prepared from industrial waste products using three different leaching media: alkaline ((NH4)2CO3) and acidic (HCl, and H2SO4).
- The fibers were analyzed in detail using SEM, TEM, and XRD techniques, and their photocatalytic activity was tested by the common dye decolorization method, under the following conditions: 26 W UVA lamp with 365 nm irradiation wavelength, 50 mL of 10−5 M of methylene blue dye.
- The sample ZnO Fibers-1, formed by a mixture of doped nanofibers and pure ZnO micrograins, had excellent photocatalytic properties at higher catalyst-to-dye ratios—approaching 100% efficiency at 690 min of irradiation time. By contrast, the fine fibers of ZnO Fibers-2 showed better results at lower catalyst-to-dye ratios. The sample ZnO Fibers-3 had impaired photocatalytic properties due to the Zn depletion and the formation of a separate Al2ZnO4 phase. This is due to the high aluminum content in the input waste product, which is the biggest disadvantage of using recycled raw materials.
- The most suitable material, evaluated based on its photocatalytic activity, was zinc oxide fibers prepared from industrial waste leached in 0.01 M HCl, which were further electrospun and calcined at 600 °C for 1 h.
- The production of electrospun ZnO fibers from industrial waste products is important because of the acquisition of low-cost input material. At the same time, the added value is the reduction of environmental pollution and the creation of a new product with high added value.
- Among the main advantages of the application of ZnO-based nanofibers for photocatalytic wastewater treatment are low toxicity; antibacterial nature; low price due to recycling of waste materials; simple separation from the reactive media thanks to the fibrous morphology; and partial biodegradability of ZnO-based materials, which makes Zn bioavailable to living organisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takacova, Z.; Piroskova, J.; Miskufova, A.; Vindt, T.; Hezelova, M.; Orac, D. Removal of Impurities from EAFD Ammonium Carbonate Leachate and Upgrading the Purity of Prepared ZnO. Materials 2023, 16, 5004. [Google Scholar] [CrossRef] [PubMed]
- EAF Dust Recycling Market Size-Forecast to 2032|Report. Available online: https://www.businessresearchinsights.com/market-reports/eaf-dust-recycling-market-108880 (accessed on 25 November 2024).
- Al-Harahsheh, M.; Al-Nu’Airat, J.; Al-Otoom, A.; Al-Hammouri, I.; Al-Jabali, H.; Al-Zoubi, M.; Abu Al’Asal, S. Treatments of Electric Arc Furnace Dust and Halogenated Plastic Wastes: A Review. J. Environ. Chem. Eng. 2019, 7, 102856. [Google Scholar] [CrossRef]
- Kaya, M.; Hussaini, S.; Kursunoglu, S. Critical Review on Secondary Zinc Resources and Their Recycling Technologies. Hydrometallurgy 2020, 195, 105362. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Oustadakis, P.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical Process for Zinc Recovery from Electric Arc Furnace Dust (EAFD). Part II: Downstream Processing and Zinc Recovery by Electrowinning. J. Hazard. Mater. 2010, 179, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.Y.; Lee, H.S. Improved Hydrometallurgical Extraction of Zinc and Iron from Electric Arc Furnace (EAF) Dust Waste Using Hydrochloric Acid. In AIP Conference Proceedings; AIP: Perak, Malaysia, 2019; Volume 2157, p. 20017. [Google Scholar]
- Langová, Š.; Leško, J.; Matýsek, D. Selective Leaching of Zinc from Zinc Ferrite with Hydrochloric Acid. Hydrometallurgy 2009, 95, 179–182. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Manjón Fernández, Á.; Masaguer Torres, V. Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review; Springer International Publishing: Manhattan, NY, USA, 2020; Volume 6, ISBN 0123456789. [Google Scholar]
- Zoraga, M.; Ilhan, S.; Kalpakli, A.O. Leaching Kinetics of Electric Arc Furnace Dust in Nitric Acid Solutions. Int. J. Chem. Kinet. 2020, 52, 933–942. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Leikola, M.; Lundström, M. Developing a Sustainable Solution for Recycling Electric Arc Furnace Dust via Organic Acid Leaching. Miner. Eng. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Halli, P.; Agarwal, V.; Partinen, J.; Lundström, M. Recovery of Pb and Zn from a Citrate Leach Liquor of a Roasted EAF Dust Using Precipitation and Solvent Extraction. Sep. Purif. Technol. 2020, 236, 116264. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.M. Hydrometallurgical Extraction of Zinc from Zinc Ferrites. Hydrometallurgy 2003, 70, 175–183. [Google Scholar] [CrossRef]
- Miki, T.; Chairaksa-Fujimoto, R.; Maruyama, K.; Nagasaka, T. Hydrometallurgical Extraction of Zinc from CaO Treated EAF Dust in Ammonium Chloride Solution. J. Hazard. Mater. 2016, 302, 90–96. [Google Scholar] [CrossRef]
- Pirošková, J.; Klimko, J.; Trpčevská, J.; Laubertová, M.; Plešingerová, B.; Liptai, P.; Vindt, T.; Oráč, D. Characterization of Galvanizing Flue Dust and Recycling Possibilities. Metals 2022, 12, 744. [Google Scholar] [CrossRef]
- Pirošková, J.; Klimko, J.; Ružičková, S.; Laubertová, M.; Marcinov, V.; Múdra, E.; Vojtko, M.; Oráč, D. Utilization of Galvanizing Flue Dust Residue: A Sustainable Approach towards Complete Material Recycling. Metals 2024, 14, 253. [Google Scholar] [CrossRef]
- Cook, T.H. Composition, Testing, and Control of Hot Dip Galvanizing Flux. Met. Finish. 2003, 101, 22–35. [Google Scholar] [CrossRef]
- Leychkis, D.; Zervoudis, J. Flux and Process for Hot Dip Galvanization Flussmittel. EP 1 974 070 B1, 2010. Available online: https://lens.org/012-240-725-392-688 (accessed on 25 August 2025).
- Rahman, L.; Quddus, S.; Khanam, J.; Bilkis, K.; Rahman, M. Fabrication of Zinc Oxide from Zinc Dust and Its Characterization. IOSR J. Appl. Chem. 2017, 10, 21–26. [Google Scholar] [CrossRef]
- Bisol, F. Process for Treating Metallic Dust, Mostly Oxididised Waste in Particular Galvanising Dust and/or Steelworks Smoke. US 4169776 A, 1998. Available online: https://lens.org/073-460-353-409-131 (accessed on 25 August 2025).
- Piroskova, J.; Trpcevska, J.; Orac, D.; Laubertova, M.; Horvathova, H.; Holkova, B. Production of Zinc Oxide from Hazardous Waste - Sal Ammoniac Skimming. J. Min. Metall. Sect. B Metall. 2018, 54, 377–384. [Google Scholar] [CrossRef]
- Krištofová, D. Recyklace Neželezných Kovů; Skripta VŠB-TU Ostrava: Ostrava, Czech Republic, 2003. (In Czech) [Google Scholar]
- Dvořák, P.; Jandová, J. Hydrometallurgical Recovery of Zinc from Hot Dip Galvanizing Ash. Hydrometallurgy 2005, 77, 29–33. [Google Scholar] [CrossRef]
- Eddleman, W.L. Method for Recovery of Metallic Zinc from Chlorine Contaminated Skimmings. U.S. Patent 4,169,776, 2 October 1979. [Google Scholar]
- Tercero Espinoza, L.A. Critical Appraisal of Recycling Indicators Used in European Criticality Exercises and Circularity Monitoring. Resour. Policy 2021, 73, 102208. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Methaapanon, R.; Chutchakul, K.; Pavarajarn, V. Photocatalytic Zinc Oxide on Flexible Polyacrylonitrile Nanofibers via Sol–Gel Coaxial Electrospinning. Ceram. Int. 2020, 46, 8287–8292. [Google Scholar] [CrossRef]
- Roy, N.; Chakraborty, S. ZnO as Photocatalyst: An Approach to Waste Water Treatment. Mater. Today Proc. 2019, 46, 6399–6403. [Google Scholar] [CrossRef]
- Kosera, V.S.; Cruz, T.M.; Chaves, E.S.; Tiburtius, E.R.L. Triclosan Degradation by Heterogeneous Photocatalysis Using ZnO Immobilized in Biopolymer as Catalyst. J. Photochem. Photobiol. A Chem. 2017, 344, 184–191. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO-Nanostructures, Defects, and Devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Badri Narayan, R.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent Advancements in Supporting Materials for Immobilised Photocatalytic Applications in Waste Water Treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, M.J.; Lima, M.J.; Baptista, D.L.; Silva, A.M.T.; Silva, C.G.; Faria, J.L. Ag-Loaded ZnO Materials for Photocatalytic Water Treatment. Chem. Eng. J. 2017, 318, 95–102. [Google Scholar] [CrossRef]
- Liu, J.; Ma, N.; Wu, W.; He, Q. Recent Progress on Photocatalytic Heterostructures with Full Solar Spectral Responses. Chem. Eng. J. 2020, 393, 124719. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the Interparticle Electron Transfer Process in the Photocatalysed Oxidation of Phenol, 2-Chlorophenol and Pentachlorophenol: Chemical Evidence for Electron and Hole Transfer between Coupled Semiconductors. J. Photochem. Photobiol. A Chem. 1995, 85, 247–255. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Imran, M.; Haider, S.; Ahmad, K.; Mahmood, A.; Al-masry, W.A. Fabrication and Characterization of Zinc Oxide Nanofibers for Renewable Energy Applications. Arab. J. Chem. 2017, 10, S1067–S1072. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W. Preparation of Zinc Oxide Nanofibers by Electrospinning. J. Am. Ceram. Soc. 2006, 89, 699–701. [Google Scholar] [CrossRef]
- Wang, W.; Huang, H.; Li, Z.; Zhang, H.; Wang, Y.; Zheng, W.; Wang, C. Zinc Oxide Nanofiber Gas Sensors via Electrospinning. J. Am. Ceram. Soc. 2008, 91, 3817–3819. [Google Scholar] [CrossRef]
- Thangavel, K.; Balamurugan, A.; Venkatachalam, T.; Ranjith Kumar, E. Structural, Morphological and Optical Properties of ZnO Nano-Fibers. Superlattices Microstruct. 2016, 90, 45–52. [Google Scholar] [CrossRef]
- Piroskova, J.; Trpcevska, J.; Smincakova, E.; Holkova, B.; Laubertová, M.; Horvathova, H. Kinetic Study of Zinc Leaching from Flux Skimming. Metall 2016, 70, 28–32. [Google Scholar]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO 2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Ramesh, P.; Rajendran, A. Green Synthesis of Manganese Dioxide Nanoparticles: Photocatalytic and Antimicrobial Investigations. Int. J. Environ. Anal. Chem. 2023, 104, 8464–8476. [Google Scholar] [CrossRef]
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Bio-Inspired Green Synthesis of Zinc Oxide Nanoparticles Using Abelmoschus Esculentus Mucilage and Selective Degradation of Cationic Dye Pollutants. J. Phys. Chem. Solids 2019, 127, 265–274. [Google Scholar] [CrossRef]
- Venkatesan, S.; Suresh, S.; Ramu, P.; Arumugam, J.; Thambidurai, S.; Pugazhenthiran, N. Methylene Blue Dye Degradation Potential of Zinc Oxide Nanoparticles Bioreduced Using Solanum Trilobatum Leaf Extract. Results Chem. 2022, 4, 100637. [Google Scholar] [CrossRef]
- Mills, A. An Overview of the Methylene Blue ISO Test for Assessing the Activities of Photocatalytic Films. Appl. Catal. B Environ. 2012, 128, 144–149. [Google Scholar] [CrossRef]
- ISO ISO 10678:2010(En); Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10678:ed-1:v1:en (accessed on 1 September 2010).
- Bistas, E.; Sanghavi, D.K. Methylene Blue. Hist. Mod. Clin. Toxicol. 2023, 231–241. [Google Scholar] [CrossRef]
- Su, C.Y.; Lu, C.T.; Hsiao, W.T.; Liu, W.H.; Shieu, F.S. Evaluation of the Microstructural and Photocatalytic Properties of Aluminum-Doped Zinc Oxide Coatings Deposited by Plasma Spraying. Thin Solid Films 2013, 544, 170–174. [Google Scholar] [CrossRef]
- Bizarro, M.; Sánchez-Arzate, A.; Garduño-Wilches, I.; Alonso, J.C.; Ortiz, A. Synthesis and Characterization of ZnO and ZnO:Al by Spray Pyrolysis with High Photocatalytic Properties. Catal. Today 2011, 166, 129–134. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Zhang, Y.; Khalid, N.R.; Xu, J.; Ullah, M.; Hong, Z. Preparation of Highly Efficient Al-Doped ZnO Photocatalyst by Combustion Synthesis. Curr. Appl. Phys. 2013, 13, 697–704. [Google Scholar] [CrossRef]
- De Macedo, H.P.; De Araújo Medeiros, R.L.B.; De Medeiros, A.L.; De Oliveira, Â.A.S.; De Figueredo, G.P.; De Freitas Melo, M.A.; De Araújo Melo, D.M. Characterization of ZnAl2O4 Spinel Obtained by Hydrothermal and Microwave Assisted Combustion Method: A Comparative Study. Mater. Res. 2017, 20, 29–33. [Google Scholar] [CrossRef]
- Šebesta, M.; Kolenčík, M.; Ratna Sunil, B.; Illa, R.; Mosnáček, J.; Ingle, A.P.; Urík, M. Field Application of Zno and Tio2 Nanoparticles on Agricultural Plants. Agronomy 2021, 11, 2281. [Google Scholar] [CrossRef]
- Kurtinová, S.; Šebesta, M. Heavy Metal Stress Alleviation in Plants by ZnO and TiO2 Nanoparticles. Nanotechnol. Agric. Agroecosystems 2023, 347–365. [Google Scholar] [CrossRef]

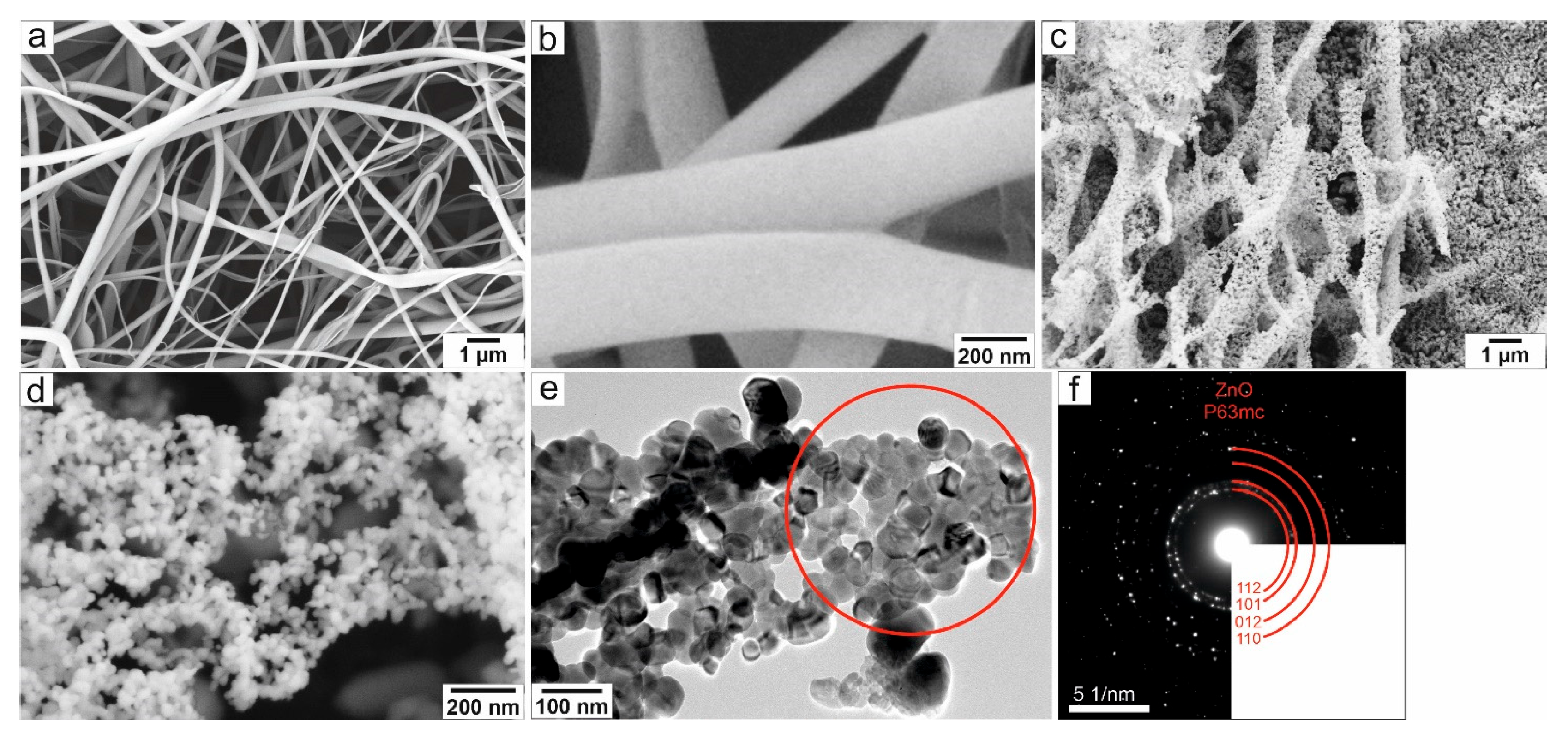
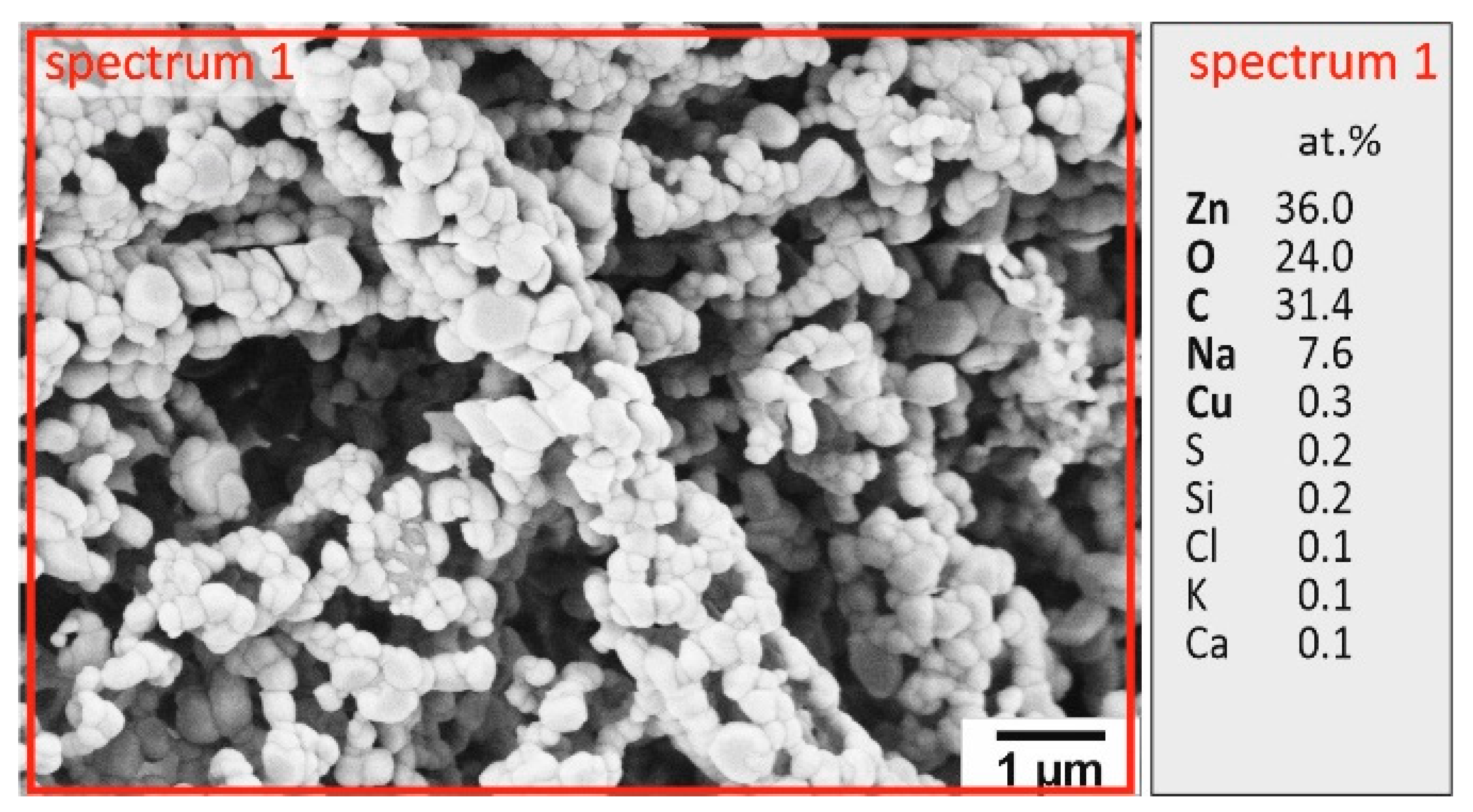
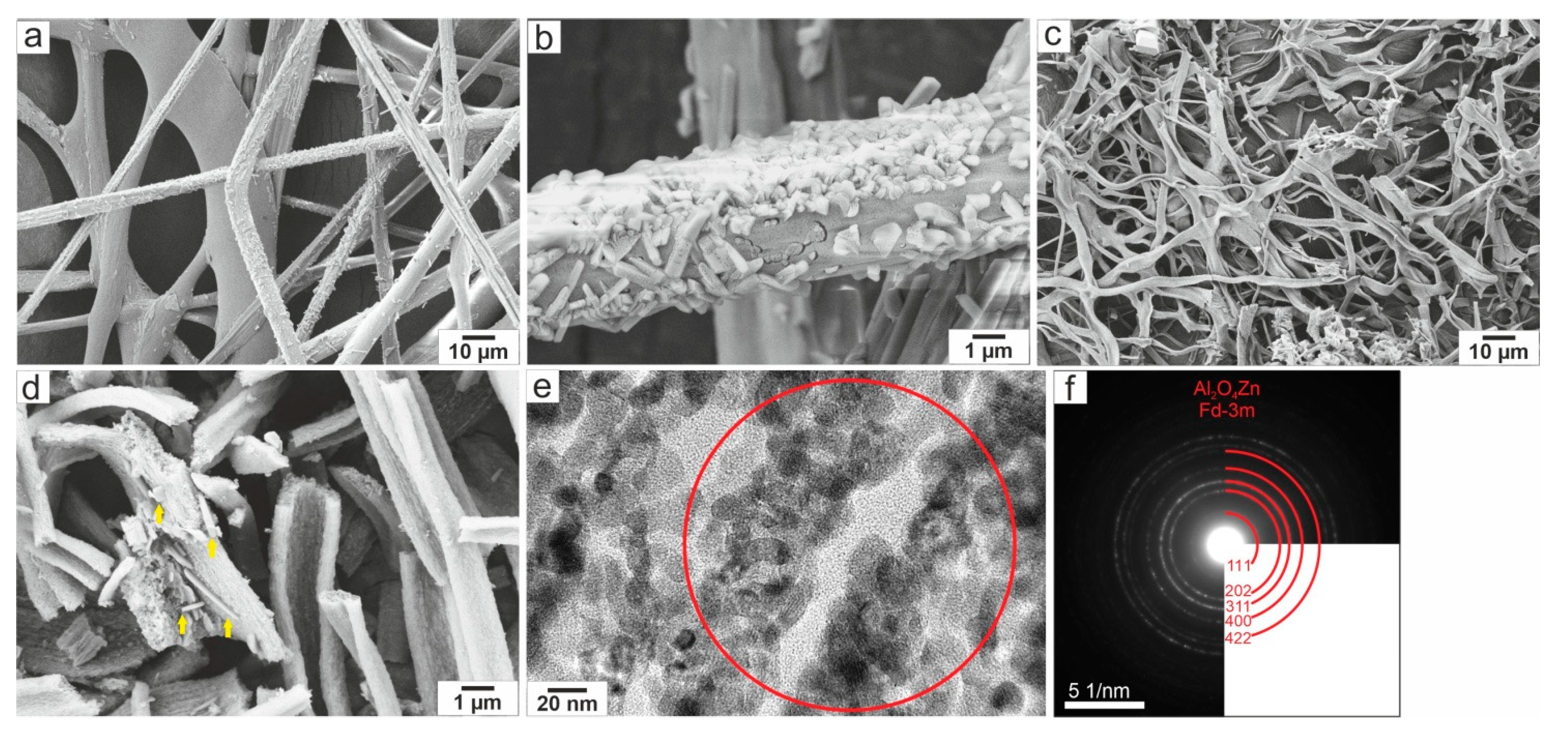

| Origin of the Liquid Sample | Content of Elements in Input Recycling Solution [g/L] | |||||||
|---|---|---|---|---|---|---|---|---|
| Zn | Pb | Fe | Cu | Al | Cr | Ca | Si | |
| Zn-enriched leachate-1: 0.01 M HCl; 50 °C, 30 min | 12.32 | 0.2 | 0.003 | 0.006 | - | 0.001 | 0.03 | 0.011 |
| Zn-enriched leachate-2: 25 g/L (NH4)2CO3; 20–60 °C, 30 min | 33.87 | 0.008 | - | - | - | - | - | - |
| Zn-enriched leachate-3: 0.5 M H2SO4; 20 °C, 30 min | 6.82 | 0.097 | 0.132 | 0.004 | 2.16 | - | - | - |
| Parameter | Sample | Amount of the Used ZnO Fibers for 50 mL of 10−5 M MB | ||
|---|---|---|---|---|
| 50 mg | 200 mg | 500 mg | ||
| Initial dye absorption, % | ZnO Fibers-1 | 8.5 | 13.8 | 18.6 |
| ZnO Fibers-2 | 9.6 | 14.2 | 19.5 | |
| ZnO Fibers-3 | 9.2 | 11.1 | 12.1 | |
| Dye degradation efficiency at 690 min, % | ZnO Fibers-1 | 61.8 | 87.6 | 99.4 |
| ZnO Fibers-2 | 59.0 | 91.1 | 83.0 | |
| ZnO Fibers-3 | 15.0 | 45.4 | 60.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudra, E.; Shepa, I.; Nemesh, K.; Piroskova, J.; Klimko, J.; Kundrakova, K.; Orac, D.; Kovalcikova, A.; Lisnichuk, M.; Kromka, F.; et al. From Waste to Value: Recycling Industrial Waste into Functional ZnO Nanofibers. Sustainability 2025, 17, 8373. https://doi.org/10.3390/su17188373
Mudra E, Shepa I, Nemesh K, Piroskova J, Klimko J, Kundrakova K, Orac D, Kovalcikova A, Lisnichuk M, Kromka F, et al. From Waste to Value: Recycling Industrial Waste into Functional ZnO Nanofibers. Sustainability. 2025; 17(18):8373. https://doi.org/10.3390/su17188373
Chicago/Turabian StyleMudra, Erika, Ivan Shepa, Kateryna Nemesh, Jana Piroskova, Jakub Klimko, Klaudia Kundrakova, Dusan Orac, Alexandra Kovalcikova, Maksym Lisnichuk, Frantisek Kromka, and et al. 2025. "From Waste to Value: Recycling Industrial Waste into Functional ZnO Nanofibers" Sustainability 17, no. 18: 8373. https://doi.org/10.3390/su17188373
APA StyleMudra, E., Shepa, I., Nemesh, K., Piroskova, J., Klimko, J., Kundrakova, K., Orac, D., Kovalcikova, A., Lisnichuk, M., Kromka, F., & Petrus, O. (2025). From Waste to Value: Recycling Industrial Waste into Functional ZnO Nanofibers. Sustainability, 17(18), 8373. https://doi.org/10.3390/su17188373










